Natural History of Stargardt Disease: The Longest Follow-Up Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Clinical Analysis
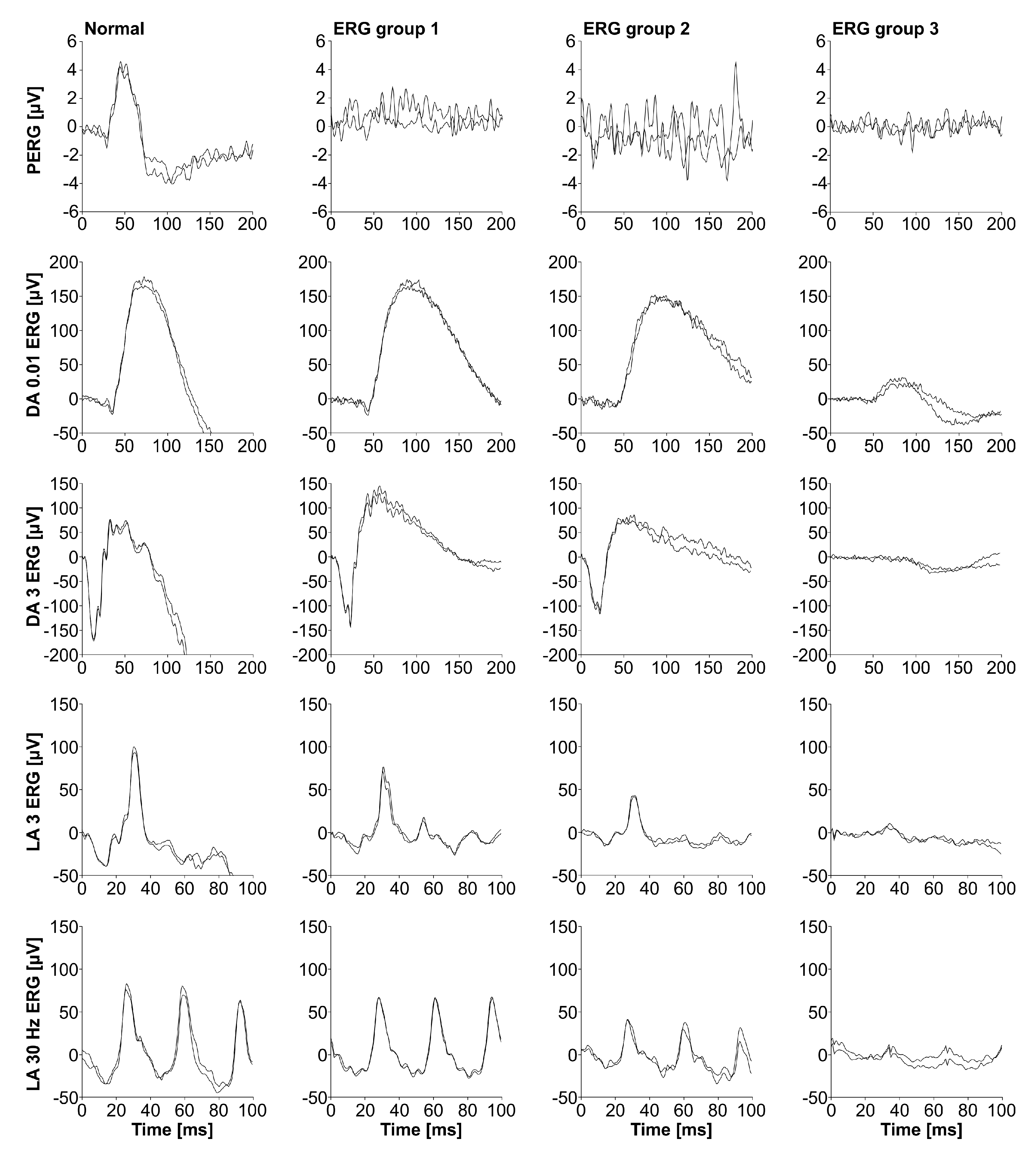
2.3. Analysis of Electroretinography
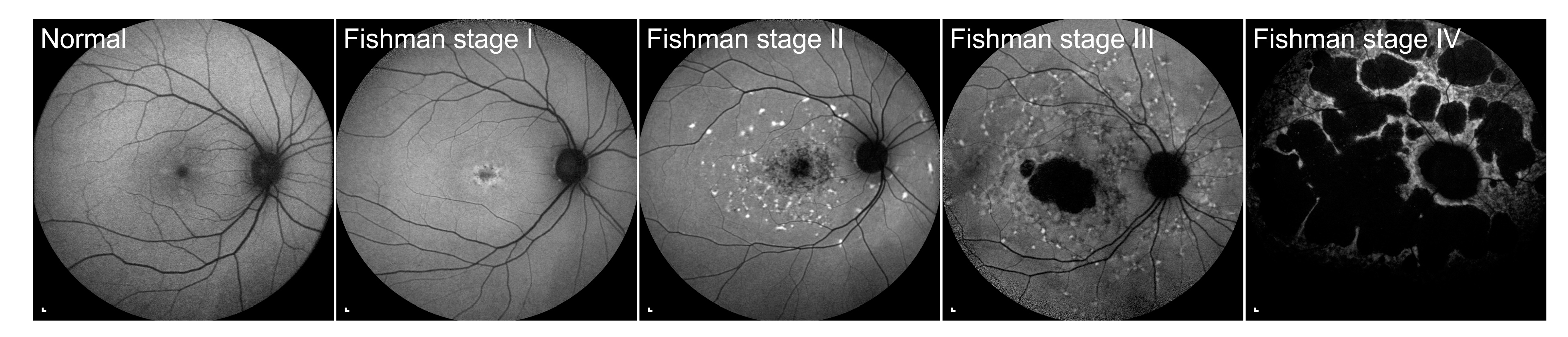
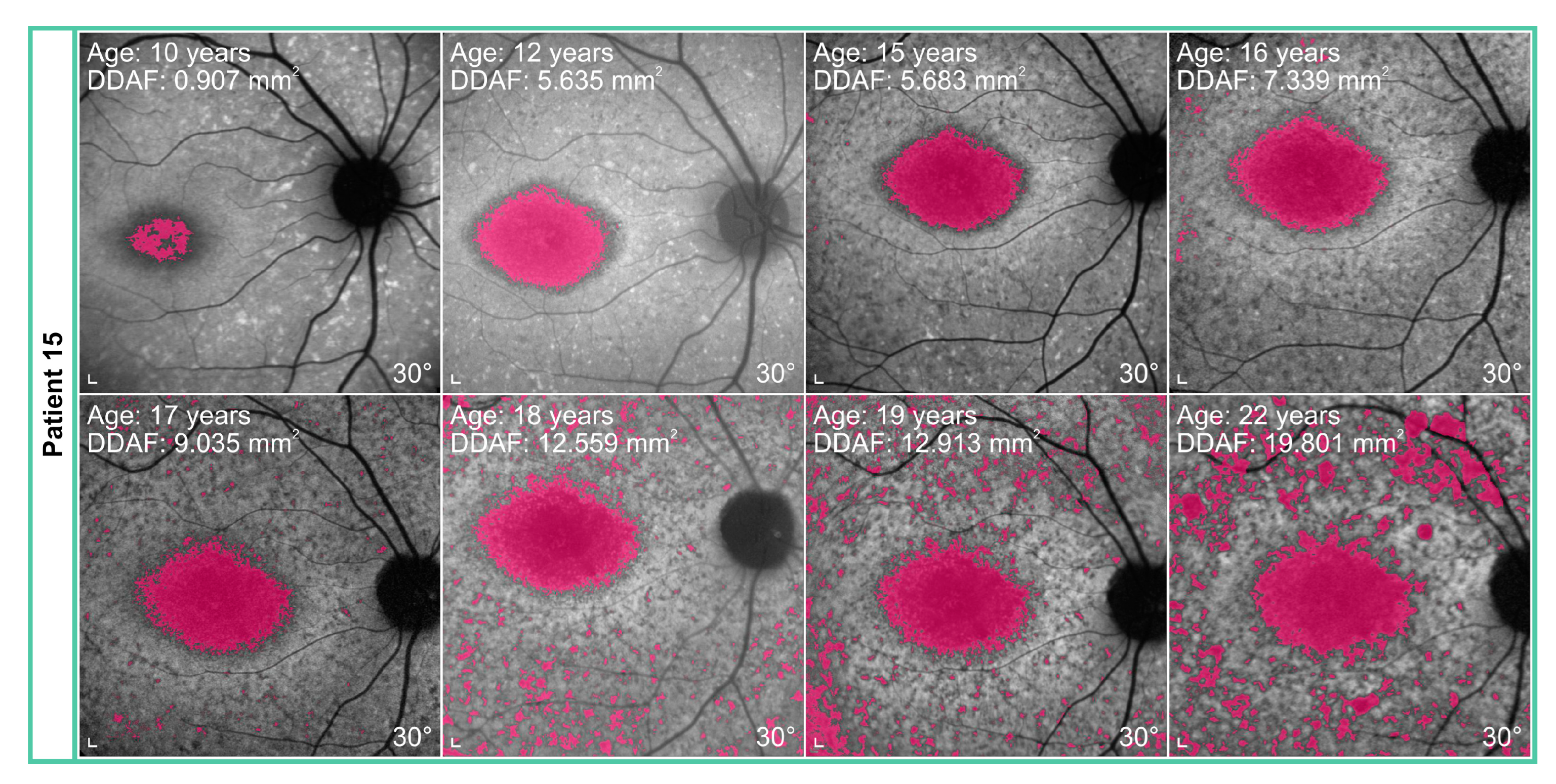
2.4. Analysis of Fundus Autofluorescence Images
2.5. Genetic Analysis
2.6. Statistical Analysis
3. Results
3.1. Clinical Findings
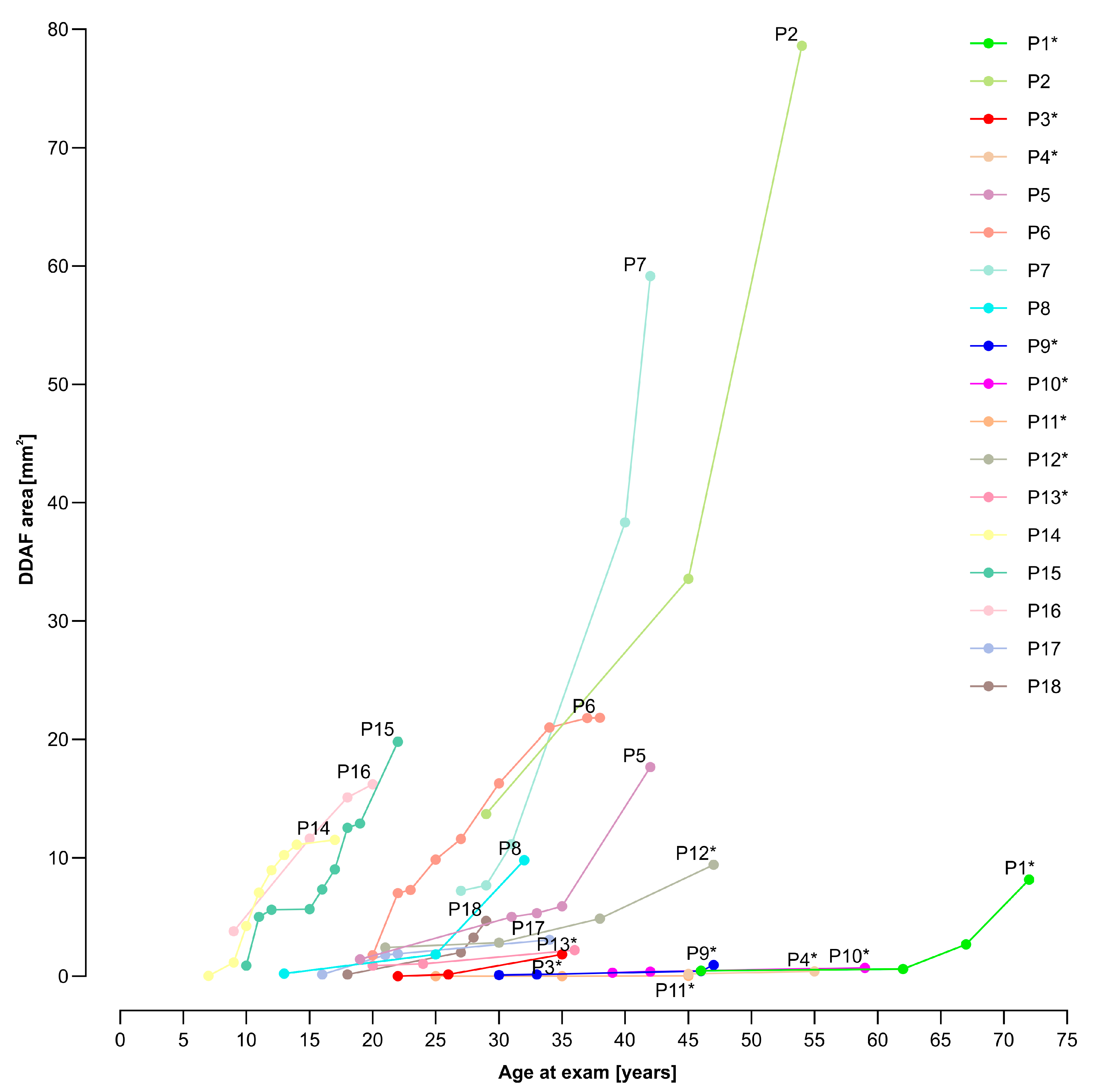
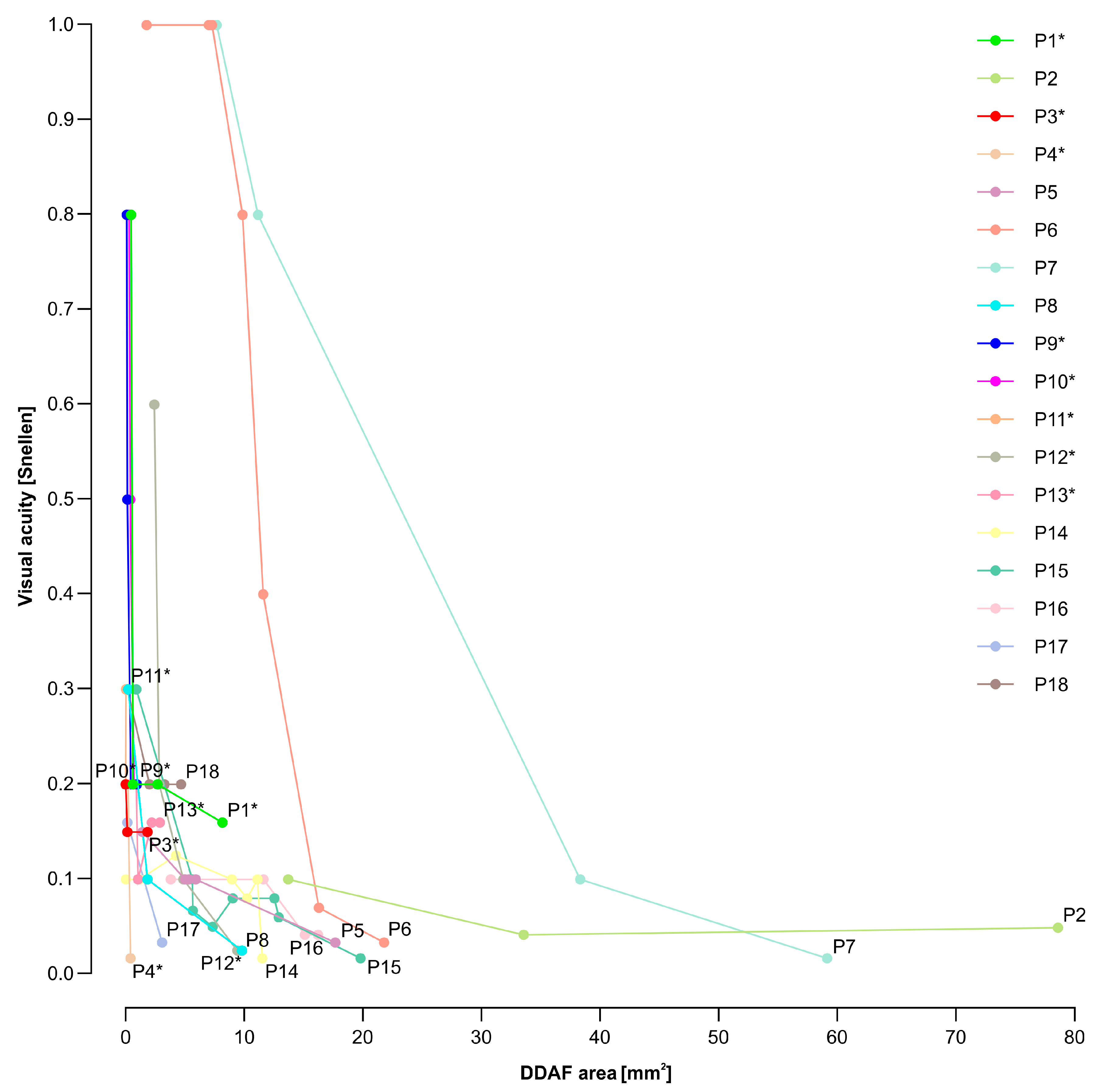
3.2. Electrophysiological and Fundus Autofluorescence Progression
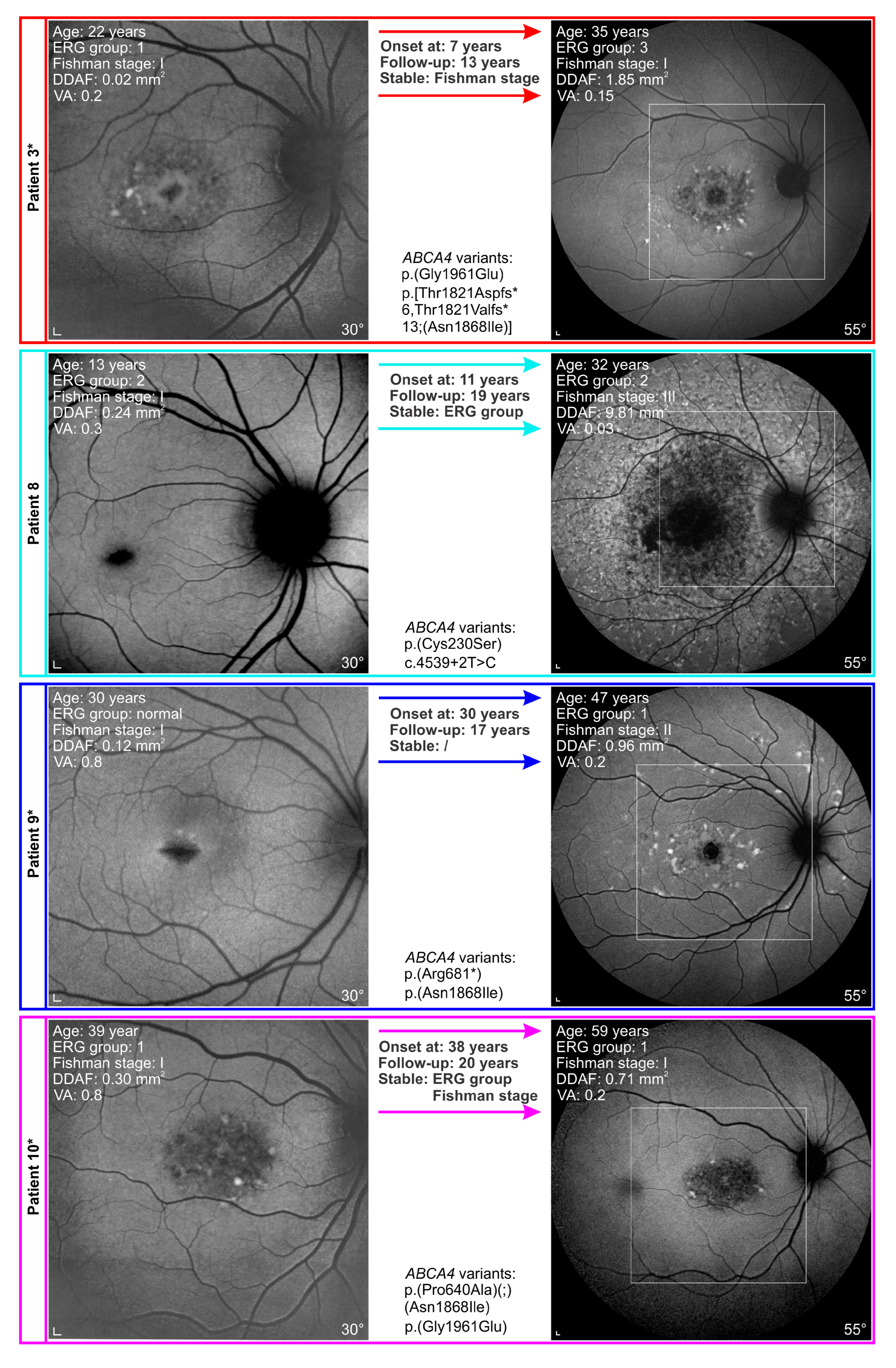
3.3. Genotype-Phenotype Correlations
3.4. Analysis of Siblings
4. Discussion
4.1. Electrophysiological and Fundus Autofluorescence (FAF) Progression Rate
4.2. Progression of Definitely Decreased Autofluorescence Area
4.3. Visual Acuity Decline
4.4. Disease Course in Patients Harbouring p.(Gly1961Glu) or p.(Asn1868Ile) Allele
4.5. Variable Disease Courses among Siblings
4.6. Study Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cremers, F.P.M.; Lee, W.; Collin, R.W.J.; Allikmets, R. Clinical spectrum, genetic complexity and therapeutic approaches for retinal disease caused by ABCA4 mutations. Prog. Retin. Eye Res. 2020, 79, 100861. [Google Scholar] [CrossRef] [PubMed]
- Blacharski, P. Fundus flavimaculatus. In Retinal Dystrophies and Degenerations; Newsome, D., Ed.; Raven Press: New York, NY, USA, 1988; pp. 135–159. [Google Scholar]
- Lee, W.; Zernant, J.; Nagasaki, T.; Molday, L.L.; Su, P.Y.; Fishman, G.A.; Tsang, S.H.; Molday, R.S.; Allikmets, R. Cis-acting modifiers in the ABCA4 locus contribute to the penetrance of the major disease-causing variant in Stargardt disease. Hum. Mol. Genet 2021, 30, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Zernant, J.; Lee, W.; Collison, F.T.; Fishman, G.A.; Sergeev, Y.V.; Schuerch, K.; Sparrow, J.R.; Tsang, S.H.; Allikmets, R. Frequent hypomorphic alleles account for a significant fraction of ABCA4 disease and distinguish it from age-related macular degeneration. J. Med. Genet. 2017, 54, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Tanna, P.; Strauss, R.W.; Fujinami, K.; Michaelides, M. Stargardt disease: Clinical features, molecular genetics, animal models and therapeutic options. Br. J. Ophthalmol. 2017, 101, 25–30. [Google Scholar] [CrossRef]
- Jarc-Vidmar, M.; Perovšek, D.; Glavač, D.; Brecelj, J.; Hawlina, M.; Stirn Kranjc, B. Morphology and function of the retina in children and young adults with Stargardt dystrophy. Zdrav. Vestn. 2012, 81 (Suppl. S1), 51–60. [Google Scholar]
- Strauss, R.W.; Ho, A.; Muñoz, B.; Cideciyan, A.V.; Sahel, J.A.; Sunness, J.S.; Birch, D.G.; Bernstein, P.S.; Michaelides, M.; Traboulsi, E.I.; et al. The Natural History of the Progression of Atrophy Secondary to Stargardt Disease (ProgStar) Studies: Design and Baseline Characteristics: ProgStar Report No. 1. Ophthalmology 2016, 123, 817–828. [Google Scholar] [CrossRef]
- Strauss, R.W.; Kong, X.; Ho, A.; Jha, A.; West, S.; Ip, M.; Bernstein, P.S.; Birch, D.G.; Cideciyan, A.V.; Michaelides, M.; et al. Progression of Stargardt Disease as Determined by Fundus Autofluorescence Over a 12-Month Period: ProgStar Report No. 11. JAMA Ophthalmol. 2019, 137, 1134–1145. [Google Scholar] [CrossRef]
- Strauss, R.W.; Muñoz, B.; Ho, A.; Jha, A.; Michaelides, M.; Cideciyan, A.V.; Audo, I.; Birch, D.G.; Hariri, A.H.; Nittala, M.G.; et al. Progression of Stargardt Disease as Determined by Fundus Autofluorescence in the Retrospective Progression of Stargardt Disease Study (ProgStar Report No. 9). JAMA Ophthalmol. 2017, 135, 1232–1241. [Google Scholar] [CrossRef]
- Strauss, R.W.; Muñoz, B.; Ho, A.; Jha, A.; Michaelides, M.; Mohand-Said, S.; Cideciyan, A.V.; Birch, D.; Hariri, A.H.; Nittala, M.G.; et al. Incidence of Atrophic Lesions in Stargardt Disease in the Progression of Atrophy Secondary to Stargardt Disease (ProgStar) Study: Report No. 5. JAMA Ophthalmol. 2017, 135, 687–695. [Google Scholar] [CrossRef]
- Fujinami, K.; Lois, N.; Davidson, A.E.; Mackay, D.S.; Hogg, C.R.; Stone, E.M.; Tsunoda, K.; Tsubota, K.; Bunce, C.; Robson, A.G.; et al. A longitudinal Study of Stargardt Disease: Clinical and Electrophysiologic Assessment, Progression, and Genotype Correlations. Am. J. Oophthalmol. 2013, 155, 1075–1088.e13. [Google Scholar] [CrossRef]
- Fujinami, K.; Zernant, J.; Chana, R.K.; Wright, G.A.; Tsunoda, K.; Ozawa, Y.; Tsubota, K.; Robson, A.G.; Holder, G.E.; Allikmets, R.; et al. Clinical and Molecular Characteristics of Childhood-Onset Stargardt Disease. Ophthalmology 2015, 122, 326–334. [Google Scholar] [CrossRef]
- Rotenstreich, Y.; Fishman, G.A.; Anderson, R.J. Visual acuity loss and clinical observations in a large series of patients with Stargardt disease. Ophthalmology 2003, 110, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Cideciyan, A.V.; Swider, M.; Schwartz, S.B.; Stone, E.M.; Jacobson, S.G. Predicting Progression of ABCA4-Associated Retinal Degenerations Based on Longitudinal Measurements of the Leading Disease Front. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5946–5955. [Google Scholar] [CrossRef] [PubMed]
- Fujinami, K.; Lois, N.; Mukherjee, R.; McBain, V.A.; Tsunoda, K.; Tsubota, K.; Stone, E.M.; Fitzke, F.W.; Bunce, C.; Moore, A.T.; et al. A Longitudinal Study of Stargardt Disease: Quantitative Assessment of Fundus Autofluorescence, Progression, and Genotype Correlations. Investig. Ophthalmol. Vis. Sci. 2013, 54, 8181–8190. [Google Scholar] [CrossRef] [PubMed]
- Testa, F.; Melillo, P.; Di Iorio, V.; Orrico, A.; Attanasio, M.; Rossi, S.; Simonelli, F. Macular Function and Morphologic Features in Juvenile Stargardt Disease: Longitudinal Study. Ophthalmology 2014, 121, 2399–2405. [Google Scholar] [CrossRef] [PubMed]
- Green, J.S.; O’Rielly, D.D.; Pater, J.A.; Houston, J.; Rajabi, H.; Galutira, D.; Benteau, T.; Sheaves, A.; Abdelfatah, N.; Bautista, D.; et al. The genetic architecture of Stargardt macular dystrophy (STGD1): A longitudinal 40-year study in a genetic isolate. Eur. J. Hum. Genet. 2020, 28, 925–937. [Google Scholar] [CrossRef]
- Fishman, G.A. Fundus Flavimaculatus. A Clinical Classification. Arch. Ophthalmol. 1976, 94, 2061–2067. [Google Scholar] [CrossRef]
- Lois, N.; Holder, G.E.; Bunce, C.; Fitzke, F.W.; Bird, A.C. Phenotypic Subtypes of Stargardt Macular Dystrophy-Fundus Flavimaculatus. Arch. Ophthalmol. 2001, 119, 359–369. [Google Scholar] [CrossRef]
- Bach, M.; Brigell, M.G.; Hawlina, M.; Holder, G.E.; Johnson, M.A.; McCulloch, D.L.; Meigen, T.; Viswanathan, S. ISCEV standard for clinical pattern electroretinography (PERG): 2012 update. Doc. Ophthalmol. 2013, 126, 1–7. [Google Scholar] [CrossRef]
- Robson, A.G.; Frishman, L.J.; Grigg, J.; Hamilton, R.; Jeffrey, B.G.; Kondo, M.; Li, S.; McCulloch, D.L. ISCEV Standard for full-field clinical electroretinography (2022 update). Doc. Ophthalmol. 2022, 144, 165–177. [Google Scholar] [CrossRef]
- Hawlina, M.; Konec, B. New noncorneal HK-loop electrode for clinical electroretinography. Doc. Ophthalmol. 1992, 81, 253–259. [Google Scholar] [CrossRef]
- Lenassi, E.; Jarc-Vidmar, M.; Glavac, D.; Hawlina, M. Pattern electroretinography of larger stimulus field size and spectral-domain optical coherence tomography in patients with Stargardt disease. British J. Ophthalmol. 2009, 93, 1600–1605. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Cornelis, S.S.; Pozo-Valero, M.D.; Whelan, L.; Runhart, E.H.; Mishra, K.; Bults, F.; AlSwaiti, Y.; AlTalbishi, A.; De Baere, E.; et al. Resolving the dark matter of ABCA4 for 1054 Stargardt disease probands through integrated genomics and transcriptomics. Genet. Med. 2020, 22, 1235–1246. [Google Scholar] [CrossRef]
- Jaakson, K.; Zernant, J.; Külm, M.; Hutchinson, A.; Tonisson, N.; Glavac, D.; Ravnik-Glavac, M.; Hawlina, M.; Meltzer, M.R.; Caruso, R.C.; et al. Genotyping microarray (gene chip) for the ABCR (ABCA4) gene. Hum. Mutat. 2003, 22, 395–403. [Google Scholar] [CrossRef]
- Valkenburg, D.; Runhart, E.H.; Bax, N.M.; Liefers, B.; Lambertus, S.L.; Sánchez, C.I.; Cremers, F.P.M.; Hoyng, C.B. Highly Variable Disease Courses in Siblings with Stargardt Disease. Ophthalmology 2019, 126, 1712–1721. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, W.; Zhou, J.; Li, X.; Jiang, Y.; Li, S.; Jia, X.; Xiao, X.; Ouyang, J.; Wang, Y.; et al. Different Phenotypes Represent Advancing Stages of ABCA4-Associated Retinopathy: A Longitudinal Study of 212 Chinese Families From a Tertiary Center. Investig. Ophthalmol. Vis. Sci. 2022, 63, 28. [Google Scholar] [CrossRef]
- van Huet, R.A.; Bax, N.M.; Westeneng-Van Haaften, S.C.; Muhamad, M.; Zonneveld-Vrieling, M.N.; Hoefsloot, L.H.; Cremers, F.P.; Boon, C.J.; Klevering, B.J.; Hoyng, C.B. Foveal sparing in Stargardt disease. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7467–7478. [Google Scholar] [CrossRef]
- Lambertus, S.; Lindner, M.; Bax, N.M.; Mauschitz, M.M.; Nadal, J.; Schmid, M.; Schmitz-Valckenberg, S.; den Hollander, A.I.; Weber, B.H.F.; Holz, F.G.; et al. Progression of Late-Onset Stargardt Disease. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5186–5191. [Google Scholar] [CrossRef] [PubMed]
- Burke, T.R.; Tsang, S.H. Allelic and phenotypic heterogeneity in ABCA4 mutations. Ophthalmic. Genet. 2011, 32, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Ritter, M.; Zotter, S.; Schmidt, W.M.; Bittner, R.E.; Deak, G.G.; Pircher, M.; Sacu, S.; Hitzenberger, C.K.; Schmidt-Erfurth, U.M. Characterization of Stargardt Disease Using Polarization-Sensitive Optical Coherence Tomography and Fundus Autofluorescence Imaging. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6416–6425. [Google Scholar] [CrossRef]
- Lambertus, S.; van Huet, R.A.; Bax, N.M.; Hoefsloot, L.H.; Cremers, F.P.; Boon, C.J.; Klevering, B.J.; Hoyng, C.B. Early-Onset Stargardt Disease: Phenotypic and Genotypic Characteristics. Ophthalmology 2015, 122, 335–344. [Google Scholar] [CrossRef]
- Georgiou, M.; Kane, T.; Tanna, P.; Bouzia, Z.; Singh, N.; Kalitzeos, A.; Strauss, R.W.; Fujinami, K.; Michaelides, M. Prospective Cohort Study of Childhood-Onset Stargardt Disease: Fundus Autofluorescence Imaging, Progression, Comparison with Adult-Onset Disease, and Disease Symmetry. Am. J. Ophthalmol. 2020, 211, 159–175. [Google Scholar] [CrossRef]
- Glazer, L.C.; Dryja, T.P. Understanding the etiology of Stargardt’s disease. Ophthalmol. Clin. N. Am. 2002, 15, 93–100, viii. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.; Mata, N.L.; Azarian, S.M.; Tzekov, R.T.; Birch, D.G.; Travis, G.H. Insights into the Function of Rim Protein in Photoreceptors and Etiology of Stargardt’s Disease from the Phenotype in abcr Knockout Mice. Cell 1999, 98, 13–23. [Google Scholar] [CrossRef]
- Sajovic, J.; Meglič, A.; Hawlina, M.; Fakin, A. Electroretinography as a Biomarker to Monitor the Progression of Stargardt Disease. Int. J. Mol. Sci. 2022, 23, 16161. [Google Scholar] [CrossRef]
- Jauregui, R.; Nuzbrokh, Y.; Su, P.-Y.; Zernant, J.; Allikmets, R.; Tsang, S.H.; Sparrow, J.R. Retinal Pigment Epithelium Atrophy in Recessive Stargardt Disease as Measured by Short-Wavelength and Near-Infrared Autofluorescence. Transl. Vis. Sci. Technol. 2021, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Tosha, C.; Gorin, M.B.; Nusinowitz, S. Analysis of Autofluorescent retinal images and measurement of atrophic lesion growth in Stargardt disease. Exp. Eye Res. 2010, 91, 143–152. [Google Scholar] [CrossRef]
- Cicinelli, M.V.; Rabiolo, A.; Brambati, M.; Viganò, C.; Bandello, F.; Battaglia Parodi, M. Factors Influencing Retinal Pigment Epithelium-Atrophy Progression Rate in Stargardt Disease. Transl. Vis. Sci. Technol. 2020, 9, 33. [Google Scholar] [CrossRef]
- Lambertus, S.; Bax, N.M.; Fakin, A.; Groenewoud, J.M.M.; Klevering, B.J.; Moore, A.T.; Michaelides, M.; Webster, A.R.; van der Wilt, G.J.; Hoyng, C.B. Highly sensitive measurements of disease progression in rare disorders: Developing and validating a multimodal model of retinal degeneration in Stargardt disease. PLoS ONE 2017, 12, e0174020. [Google Scholar] [CrossRef]
- McBain, V.A.; Townend, J.; Lois, N. Progression of Retinal Pigment Epithelial Atrophy in Stargardt Disease. Am. J. Ophthalmol. 2012, 154, 146–154. [Google Scholar] [CrossRef]
- Strauss, R.W.; Ho, A.; Jha, A.; Fujinami, K.; Michaelides, M.; Cideciyan, A.V.; Audo, I.; Birch, D.G.; Sadda, S.; Ip, M.; et al. Progression of Stargardt Disease as Determined by Fundus Autofluorescence Over a 24-Month Period (ProgStar Report No. 17). Am. J. Ophthalmol. 2023, 250, 157–170. [Google Scholar] [CrossRef]
- Cukras, C.A.; Wong, W.T.; Caruso, R.; Cunningham, D.; Zein, W.; Sieving, P.A. Centrifugal Expansion of Fundus Autofluorescence Patterns in Stargardt Disease Over Time. Arch. Ophthalmol. 2012, 130, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Strauss, R.W.; Michaelides, M.; Cideciyan, A.V.; Sahel, J.A.; Muñoz, B.; West, S.; Scholl, H.P. Visual Acuity Loss and Associated Risk Factors in the Retrospective Progression of Stargardt Disease Study (ProgStar Report No. 2). Ophthalmology 2016, 123, 1887–1897. [Google Scholar] [CrossRef] [PubMed]
- Runhart, E.H.; Sangermano, R.; Cornelis, S.S.; Verheij, J.; Plomp, A.S.; Boon, C.J.F.; Lugtenberg, D.; Roosing, S.; Bax, N.M.; Blokland, E.A.W.; et al. The Common ABCA4 Variant p.Asn1868Ile Shows Nonpenetrance and Variable Expression of Stargardt Disease When Present in trans With Severe Variants. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3220–3231. [Google Scholar] [CrossRef]
- Fakin, A.; Robson, A.G.; Chiang, J.P.; Fujinami, K.; Moore, A.T.; Michaelides, M.; Holder, G.E.; Webster, A.R. The Effect on Retinal Structure and Function of 15 Specific ABCA4 Mutations: A Detailed Examination of 82 Hemizygous Patients. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5963–5973. [Google Scholar] [CrossRef] [PubMed]
- Burke, T.R.; Fishman, G.A.; Zernant, J.; Schubert, C.; Tsang, S.H.; Smith, R.T.; Ayyagari, R.; Koenekoop, R.K.; Umfress, A.; Ciccarelli, M.L.; et al. Retinal Phenotypes in Patients Homozygous for the G1961E Mutation in the ABCA4 Gene. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4458–4467. [Google Scholar] [CrossRef] [PubMed]
- Cella, W.; Greenstein, V.C.; Zernant-Rajang, J.; Smith, T.R.; Barile, G.; Allikmets, R.; Tsang, S.H. G1961E mutant allele in the Stargardt disease gene ABCA4 causes bull’s eye maculopathy. Exp. Eye Res. 2009, 89, 16–24. [Google Scholar] [CrossRef]
- Burke, T.R.; Tsang, S.H.; Zernant, J.; Smith, R.T.; Allikmets, R. Familial discordance in Stargardt disease. Mol. Vis. 2012, 18, 227–233. [Google Scholar]
- Lois, N.; Holder, G.E.; Fitzke, F.W.; Plant, C.; Bird, A.C. Intrafamilial Variation of Phenotype in Stargardt Macular Dystrophy-Fundus Flavimaculatus. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2668–2675. [Google Scholar]
- Heath Jeffery, R.C.; Thompson, J.A.; Lo, J.; Lamey, T.M.; McLaren, T.L.; De Roach, J.N.; Azamanov, D.N.; McAllister, I.L.; Constable, I.J.; Chen, F.K. SIBLING CONCORDANCE IN SYMPTOM ONSET AND ATROPHY GROWTH RATES IN STARGARDT DISEASE USING ULTRA-WIDEFIELD FUNDUS AUTOFLUORESCENCE. Retina 2022, 42, 1545–1559. [Google Scholar] [CrossRef]
- Teussink, M.M.; Lee, M.D.; Smith, R.T.; van Huet, R.A.C.; Klaver, C.C.; Klevering, B.J.; Theelen, T.; Hoyng, C.B. The Effect of Light Deprivation in Patients With Stargardt Disease. Am. J. Ophthalmol. 2015, 159, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Sodi, A.; Franco, F.; Murro, V.; Biagini, D.; Miele, A.; Abbruzzese, G.; Mucciolo, D.P.; Virgili, G.; Menchini, U.; et al. Dietary profile of patients with Stargardt’s disease and Retinitis Pigmentosa: Is there a role for a nutritional approach? BMC Ophthalmol. 2016, 16, 13. [Google Scholar] [CrossRef] [PubMed]
- Zernant, J.; Lee, W.; Wang, J.; Goetz, K.; Ullah, E.; Nagasaki, T.; Su, P.Y.; Fishman, G.A.; Tsang, S.H.; Tumminia, S.J.; et al. Rare and common variants in ROM1 and PRPH2 genes trans-modify Stargardt/ABCA4 disease. PLoS Genet. 2022, 18, e1010129. [Google Scholar] [CrossRef] [PubMed]
- Whelan, L.; Dockery, A.; Stephenson, K.A.J.; Zhu, J.; Kopčić, E.; Post, I.J.M.; Khan, M.; Corradi, Z.; Wynne, N.; O’ Byrne, J.J.; et al. Detailed analysis of an enriched deep intronic ABCA4 variant in Irish Stargardt disease patients. Sci. Rep. 2023, 13, 9380. [Google Scholar] [CrossRef] [PubMed]
- Donato, L.; Alibrandi, S.; Scimone, C.; Rinaldi, C.; Dascola, A.; Calamuneri, A.; D’Angelo, R.; Sidoti, A. The impact of modifier genes on cone-rod dystrophy heterogeneity: An explorative familial pilot study and a hypothesis on neurotransmission impairment. PLoS ONE 2022, 17, e0278857. [Google Scholar] [CrossRef]



| Parameter | Hypomorphic Group (n = 8) (Median, Range) | Other Patients (n = 10) (Median, Range) | Mann–Whitney Test (p-Value) |
|---|---|---|---|
| Age at the first exam [years] | 28 (20–46) | 17 (7–31) | p = 0.016 |
| Age at the last exam [years] | 47 (35–72) | 33 (17–54) | p = 0.009 |
| Age at onset [years] | 20 (7–46) | 13 (7–25) | p = 0.043 |
| Duration of follow-up [years] | 20 (13–26) | 12 (10–25) | p = 0.055 |
| The time between onset and the first exam [years] | 1 (0–30) | 3 (0–14) | p = 0.633 |
| DDAF area at the first exam [mm2] | 0.25 (0.00–2.43) | 1.17 (0.03–13.70) | p = 0.122 |
| DDAF area at the last exam [mm2] | 1.41 (0.03–9.43) | 16.96 (3.09–78.61) | p = 0.001 |
| The yearly rate of DDAF area increase [mm2] | 0.07 (0.0017–0.30) | 1.03 (0.16–4.36) | p < 0.001 |
| VA at the first exam [Snellen] | 0.40 (0.20–0.80) | 0.23 (0.10–0.80) | p = 0.122 |
| VA at the last exam [Snellen] | 0.16 (0.02–0.30) | 0.03 (0.02–0.20) | p = 0.068 |
| The yearly rate of VA loss [Snellen] | 0.02 (0.0025–0.035) | 0.01 (0.0020–0.07) | p = 0.740 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sajovic, J.; Meglič, A.; Fakin, A.; Brecelj, J.; Šuštar Habjan, M.; Hawlina, M.; Jarc Vidmar, M. Natural History of Stargardt Disease: The Longest Follow-Up Cohort Study. Genes 2023, 14, 1394. https://doi.org/10.3390/genes14071394
Sajovic J, Meglič A, Fakin A, Brecelj J, Šuštar Habjan M, Hawlina M, Jarc Vidmar M. Natural History of Stargardt Disease: The Longest Follow-Up Cohort Study. Genes. 2023; 14(7):1394. https://doi.org/10.3390/genes14071394
Chicago/Turabian StyleSajovic, Jana, Andrej Meglič, Ana Fakin, Jelka Brecelj, Maja Šuštar Habjan, Marko Hawlina, and Martina Jarc Vidmar. 2023. "Natural History of Stargardt Disease: The Longest Follow-Up Cohort Study" Genes 14, no. 7: 1394. https://doi.org/10.3390/genes14071394
APA StyleSajovic, J., Meglič, A., Fakin, A., Brecelj, J., Šuštar Habjan, M., Hawlina, M., & Jarc Vidmar, M. (2023). Natural History of Stargardt Disease: The Longest Follow-Up Cohort Study. Genes, 14(7), 1394. https://doi.org/10.3390/genes14071394







