The Role of PLA2R in Primary Membranous Nephropathy: Do We Still Need a Kidney Biopsy?
Abstract
1. Introduction: The Historical Evolvement of Defining Primary MN
2. PLA2R and Antibody-Mediated MN
3. Traditional Workup for Primary MN
4. Summary of Evidence Evaluating PLA2RAb as a Diagnostic Tool for Primary MN
5. The Significance of an FSGS Diagnosis on Kidney Biopsy
6. Should We Biopsy the Diabetic Patients Suspected with MN?
7. Utility of Glomerular PLA2R Staining with Undetectable Serum PLA2RAb
8. The Different Methods of Testing for PLA2RAb
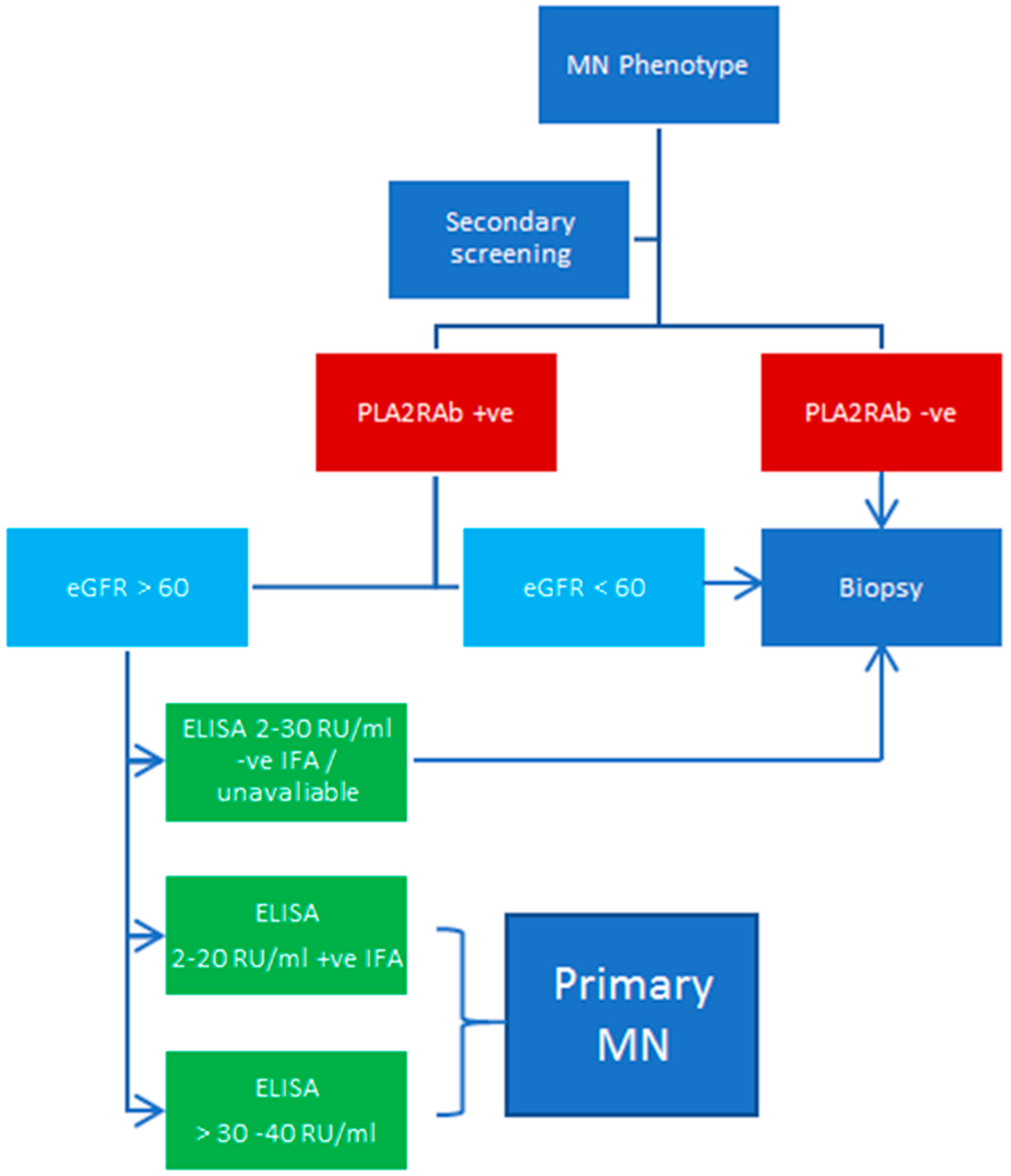
9. Considerations for Secondary Screening in MN
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruggenenti, P.; Remuzzi, G. A First Step toward a New Approach to Treating Membranous Nephropathy. N. Engl. J. Med. 2019, 381, 86–88. [Google Scholar] [CrossRef]
- Keri, K.C.; Blumenthal, S.; Kulkarni, V.; Beck, L.; Chongkrairatanakul, T. Primary membranous nephropathy: Comprehensive review and historical perspective. Postgrad. Med. J. 2019, 95, 23–31. [Google Scholar] [CrossRef]
- Ronco, P.; Debiec, H. Pathophysiological advances in membranous nephropathy: Time for a shift in patient’s care. Lancet 2015, 385, 1983–1992. [Google Scholar] [CrossRef]
- Beck, L.H.; Bonegio, R.G.B.; Lambeau, G.; Beck, D.M.; Powell, D.W.; Cummins, T.D.; Klein, J.B.; Salant, D.J. M-Type Phospholipase A 2 Receptor as Target Antigen in Idiopathic Membranous Nephropathy. N. Engl. J. Med. 2009, 361, 11–21. [Google Scholar] [CrossRef]
- Jones, D.B. Nephrotic glomerulonephritis. Am. J. Pathol. 1957, 33, 313–329. [Google Scholar]
- Heymann, W.; Hackel, D.B.; Harwood, S.; Wilson, S.G.F.; Hunter, J.L.P. Production of Nephrotic Syndrome in Rats by Freund’s Adjuvants and Rat Kidney Suspensions. Exp. Biol. Med. 1959, 100, 660–664. [Google Scholar] [CrossRef]
- Debiec, H.; Guigonis, V.; Mougenot, B.; Decobert, F.; Haymann, J.P.; Bensman, A.; Deschênes, G.; Ronco, P.M. Antenatal Membranous Glomerulonephritis Due to Anti–Neutral Endopeptidase Antibodies. N. Engl. J. Med. 2002, 346, 2053–2060. [Google Scholar] [CrossRef]
- Lambeau, G. Receptors for a growing family of secreted phospholipases A2. Trends Pharmacol. Sci. 1999, 20, 162–170. [Google Scholar] [CrossRef]
- Fresquet, M.; Jowitt, T.A.; Gummadova, J.; Collins, R.; O’Cualain, R.; McKenzie, E.A.; Lennon, R.; Brenchley, P.E. Identification of a Major Epitope Recognized by PLA2R Autoantibodies in Primary Membranous Nephropathy. J. Am. Soc. Nephrol. 2015, 26, 302–313. [Google Scholar] [CrossRef]
- Doi, T.; Mayumi, M.; Kanatsu, K.; Suehiro, F.; Hamashima, Y. Distribution of IgG subclasses in membranous nephropathy. Clin. Exp. Immunol. 1984, 58, 57–62. [Google Scholar]
- Meyer-Schwesinger, C.; Tomas, N.M.; Dehde, S.; Seifert, L.; Hermans-Borgmeyer, I.; Wiech, T.; Koch-Nolte, F.; Huber, T.B.; Zahner, G. A novel mouse model of phospholipase A2 receptor 1-associated membranous nephropathy mimics podocyte injury in patients. Kidney Int. 2020, 97, 913–919. [Google Scholar] [CrossRef]
- Hofstra, J.M.; Beck, L.H.; Beck, D.M.; Wetzels, J.F.; Salant, D.J. Anti-Phospholipase A2 Receptor Antibodies Correlate with Clinical Status in Idiopathic Membranous Nephropathy. Clin. J. Am. Soc. Nephrol. 2011, 6, 1286–1291. [Google Scholar] [CrossRef]
- Hofstra, J.M.; Debiec, H.; Short, C.D.; Pellé, T.; Kleta, R.; Mathieson, P.W.; Ronco, P.; Brenchley, P.E.; Wetzels, J.F. Antiphospholipase A2 Receptor Antibody Titer and Subclass in Idiopathic Membranous Nephropathy. J. Am. Soc. Nephrol. 2012, 23, 1735–1743. [Google Scholar] [CrossRef]
- Beck, L.H.; Fervenza, F.C.; Beck, D.M.; Bonegio, R.G.B.; Malik, F.A.; Erickson, S.B.; Cosio, F.G.; Cattran, D.C.; Salant, D.J. Rituximab-Induced Depletion of Anti-PLA2R Autoantibodies Predicts Response in Membranous Nephropathy. J. Am. Soc. Nephrol. 2011, 22, 1543–1550. [Google Scholar] [CrossRef]
- Dahan, K.; Debiec, H.; Plaisier, E.; Cachanado, M.; Rousseau, A.; Wakselman, L.; Michel, P.-A.; Mihout, F.; Dussol, B.; Matignon, M.; et al. Rituximab for Severe Membranous Nephropathy: A 6-Month Trial with Extended Follow-Up. J. Am. Soc. Nephrol. 2017, 28, 348–358. [Google Scholar] [CrossRef]
- Fervenza, F.C.; Appel, G.B.; Barbour, S.J.; Rovin, B.H.; Lafayette, R.A.; Aslam, N.; Jefferson, J.A.; Gipson, P.E.; Rizk, D.V.; Sedor, J.R.; et al. Rituximab or Cyclosporine in the Treatment of Membranous Nephropathy. N. Engl. J. Med. 2019, 381, 36–46. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021, 100, S1–S276. [Google Scholar] [CrossRef]
- Kanigicherla, D.; Gummadova, J.; McKenzie, E.A.; Roberts, S.A.; Harris, S.; Nikam, M.; Poulton, K.; McWilliam, L.; Short, C.D.; Venning, M.; et al. Anti-PLA2R antibodies measured by ELISA predict long-term outcome in a prevalent population of patients with idiopathic membranous nephropathy. Kidney Int. 2013, 83, 940–948. [Google Scholar] [CrossRef]
- Sethi, S.; Debiec, H.; Madden, B.; Charlesworth, M.C.; Morelle, J.; Gross, L.; Ravindran, A.; Buob, D.; Jadoul, M.; Fervenza, F.C.; et al. Neural epidermal growth factor-like 1 protein (NELL-1) associated membranous nephropathy. Kidney Int. 2020, 97, 163–174. [Google Scholar] [CrossRef]
- Caza, T.N.; Hassen, S.I.; Dvanajscak, Z.; Kuperman, M.; Edmondson, R.; Herzog, C.; Storey, A.; Arthur, J.; Cossey, L.N.; Sharma, S.G.; et al. NELL1 is a target antigen in malignancy-associated membranous nephropathy. Kidney Int. 2021, 99, 967–976. [Google Scholar] [CrossRef]
- Tomas, N.M.; Beck, L.H.; Meyer-Schwesinger, C.; Seitz-Polski, B.; Ma, H.; Zahner, G.; Dolla, G.; Hoxha, E.; Helmchen, U.; Dabert-Gay, A.-S.; et al. Thrombospondin Type-1 Domain-Containing 7A in Idiopathic Membranous Nephropathy. N. Engl. J. Med. 2014, 371, 2277–2287. [Google Scholar] [CrossRef]
- Hoxha, E.; Beck, L.H.; Wiech, T.; Tomas, N.M.; Probst, C.; Mindorf, S.; Meyer-Schwesinger, C.; Zahner, G.; Stahl, P.R.; Schöpper, R.; et al. An Indirect Immunofluorescence Method Facilitates Detection of Thrombospondin Type 1 Domain–Containing 7A–Specific Antibodies in Membranous Nephropathy. J. Am. Soc. Nephrol. 2017, 28, 520–531. [Google Scholar] [CrossRef]
- Hanset, N.; Aydin, S.; Demoulin, N.; Cosyns, J.P.; Castanares-Zapatero, D.; Crott, R.; Cambier, J.-F.; Pochet, J.-M.; Gillerot, G.; Reginster, F.; et al. Podocyte Antigen Staining to Identify Distinct Phenotypes and Outcomes in Membranous Nephropathy: A Retrospective Multicenter Cohort Study. Am. J. Kidney Dis. 2020, 76, 624–635. [Google Scholar] [CrossRef]
- Sethi, S.; Madden, B.J.; Debiec, H.; Charlesworth, M.C.; Gross, L.; Ravindran, A.; Hummel, A.M.; Specks, U.; Fervenza, F.C.; Ronco, P. Exostosin 1/Exostosin 2–Associated Membranous Nephropathy. J. Am. Soc. Nephrol. 2019, 30, 1123–1136. [Google Scholar] [CrossRef]
- Sethi, S.; Madden, B.; Debiec, H.; Morelle, J.; Charlesworth, M.C.; Gross, L.; Negron, V.; Buob, D.; Chaudhry, S.; Jadoul, M.; et al. Protocadherin 7–Associated Membranous Nephropathy. J. Am. Soc. Nephrol. 2021, 32, 1249–1261. [Google Scholar] [CrossRef]
- Sethi, S.; Debiec, H.; Madden, B.; Vivarelli, M.; Charlesworth, M.C.; Ravindran, A.; Gross, L.; Ulinski, T.; Buob, D.; Tran, C.L.; et al. Semaphorin 3B–associated membranous nephropathy is a distinct type of disease predominantly present in pediatric patients. Kidney Int. 2020, 98, 1253–1264. [Google Scholar] [CrossRef]
- Al-Rabadi, L.F.; Caza, T.; Trivin-Avillach, C.; Rodan, A.R.; Andeen, N.; Hayashi, N.; Williams, B.; Revelo, M.P.; Clayton, F.; Abraham, J.; et al. Serine Protease HTRA1 as a Novel Target Antigen in Primary Membranous Nephropathy. J. Am. Soc. Nephrol. 2021, 32, 1666–1681. [Google Scholar] [CrossRef]
- Debiec, H.; Nauta, J.; Coulet, F.; van der Burg, M.; Guigonisy, V.; Schurmans, T.; de Heer, E.; Soubrier, F.; Janssen, F.; Ronco, P. Role of truncating mutations in MME gene in fetomaternal alloimmunisation and antenatal glomerulopathies. Lancet. 2004, 364, 1252–1259. [Google Scholar] [CrossRef]
- Du, Y.; Li, J.; He, F.; Lv, Y.; Liu, W.; Wu, P.; Huang, J.; Wei, S.; Gao, H. The Diagnosis Accuracy of PLA2R-AB in the Diagnosis of Idiopathic Membranous Nephropathy: A Meta-Analysis. PLoS ONE 2014, 9, e104936. [Google Scholar] [CrossRef]
- Noel, L.H.; Zanetti, M.; Droz, D.; Barbanel, C. Long-term prognosis of idiopathic membranous glomerulonephritis. Am. J. Med. 1979, 66, 82–90. [Google Scholar] [CrossRef]
- Poggio, E.D.; McClelland, R.L.; Blank, K.N.; Hansen, S.; Bansal, S.; Bomback, A.S.; Canetta, P.A.; Khairallah, P.; Kiryluk, K.; Lecker, S.H.; et al. Systematic Review and Meta-Analysis of Native Kidney Biopsy Complications. Clin. J. Am. Soc. Nephrol. 2020, 15, 1595–1602. [Google Scholar] [CrossRef]
- Pombas, B.; Rodríguez, E.; Sánchez, J.; Radosevic, A.; Gimeno, J.; Busto, M.; Barrios, C.; Sans, L.; Pascual, J.; Soler, M.J. Risk Factors Associated with Major Complications after Ultrasound-Guided Percutaneous Renal Biopsy of Native Kidneys. Kidney Blood Press. Res. 2020, 45, 122–130. [Google Scholar] [CrossRef]
- Palsson, R.; Short, S.A.P.; Kibbelaar, Z.A.; Amodu, A.; Stillman, I.E.; Rennke, H.G.; McMahon, G.M.; Waikar, S.S. Bleeding Complications After Percutaneous Native Kidney Biopsy: Results From the Boston Kidney Biopsy Cohort. Kidney Int. Rep. 2020, 5, 511–518. [Google Scholar] [CrossRef]
- Ragy, O.; Rautemaa, V.; Smith, A.; Brenchley, P.; Kanigicherla, D.; Hamilton, P. Can use of the serum anti-PLA2R antibody negate the need for a renal biopsy in primary membranous nephropathy? PLoS ONE 2023, 18, e0281726. [Google Scholar] [CrossRef]
- Bobart, S.A.; Fervenza, F.C. Kidney Biopsy Is Required for Nephrotic Syndrome with PLA2R+ and Normal Kidney Function: The Con View. Kidney360 2020, 1, 890–893. [Google Scholar] [CrossRef]
- Svobodova, B.; Honsova, E.; Ronco, P.; Tesar, V.; Debiec, H. Kidney biopsy is a sensitive tool for retrospective diagnosis of PLA2R-related membranous nephropathy. Nephrol. Dial. Transplant. 2013, 28, 1839–1844. [Google Scholar] [CrossRef]
- Bobart, S.A.; De Vriese, A.S.; Pawar, A.S.; Zand, L.; Sethi, S.; Giesen, C.; Lieske, J.C.; Fervenza, F.C. Noninvasive diagnosis of primary membranous nephropathy using phospholipase A2 receptor antibodies. Kidney Int. 2019, 95, 429–438. [Google Scholar] [CrossRef]
- Troyanov, S.; Roasio, L.; Pandes, M.; Herzenberg, A.M.; Cattran, D.C. Renal pathology in idiopathic membranous nephropathy: A new perspective. Kidney Int. 2006, 69, 1641–1648. [Google Scholar] [CrossRef]
- Wiech, T.; Stahl, R.A.K.; Hoxha, E. Diagnostic role of renal biopsy in PLA2R1-antibody-positive patients with nephrotic syndrome. Modern Pathol. 2019, 32, 1320–1328. [Google Scholar] [CrossRef]
- Bobart, S.A.; Han, H.; Tehranian, S.; De Vriese, A.S.; Roman, J.C.L.; Sethi, S.; Zand, L.; Gomez, C.A.; Giesen, C.D.; Soler, M.J.; et al. Noninvasive Diagnosis of PLA2R-Associated Membranous Nephropathy A Validation Study. Clin. J. Am. Soc. Nephrol. 2021, 16, 1833–1839. [Google Scholar] [CrossRef]
- Sharma, S.G.; Bomback, A.S.; Radhakrishnan, J.; Herlitz, L.C.; Stokes, M.B.; Markowitz, G.S.; D’Agati, V.D. The Modern Spectrum of Renal Biopsy Findings in Patients with Diabetes. Clin. J. Am. Soc. Nephrol. 2013, 8, 1718–1724. [Google Scholar] [CrossRef]
- van de Logt, A.E.; Hofstra, J.M.; Wetzels, J.F. Serum anti-PLA2R antibodies can be initially absent in idiopathic membranous nephropathy: Seroconversion after prolonged follow-up. Kidney Int. 2015, 87, 1263–1264. [Google Scholar] [CrossRef]
- Hoxha, E.; Reinhard, L.; Castedello, T.; Becker, J.U. False positivity for PLA2R1 antibody measured by ELISA in a nephrotic patient with no membranous nephropathy. Kidney Int. 2023, 103, 411–415. [Google Scholar] [CrossRef]
- Caza, T.N.; Larsen, C.P. False-positive anti-PLA2R ELISA testing in patients with diabetes mellitus. Kidney Int. 2023, 103, 425. [Google Scholar] [CrossRef]
- Xie, Q.; Li, Y.; Xue, J.; Xiong, Z.; Wang, L.; Sun, Z.; Ren, Y.; Zhu, X.; Hao, C.-M. Renal Phospholipase A2 Receptor in Hepatitis B Virus-Associated Membranous Nephropathy. Am. J. Nephrol. 2015, 41, 345–353. [Google Scholar] [CrossRef]
- Hamilton, P.; Wilson, F.; Chinnadurai, R.; Sinha, S.; Singh, M.; Ponnusamy, A.; Hall, P.; Dhaygude, A.; Kanigicherla, D.; Brenchley, P. The investigative burden of membranous nephropathy in the UK. Clin. Kidney J. 2020, 13, 27–34. [Google Scholar] [CrossRef]
- Mok, I.; Choo, J. P0382 “False” Positivity of Serum Anti-Phospholipase A2 Receptor Antibody (Anti-Pla2r Ab) in Patients with Non Membranous Glomerular Disease. Nephrol. Dial. Transplant. 2020, 35, 689. [Google Scholar] [CrossRef]
- Plaisier, E.; Ronco, P. Screening for Cancer in Patients with Glomerular Diseases. Clin. J. Am. Soc. Nephrol. 2020, 15, 886–888. [Google Scholar] [CrossRef]
| Antigen | Association to MN |
|---|---|
| Neural epidermal growth factor-like 1 (NELL1) | Reported in approximately 16% of primary MN [19]. Reports of associated malignancy [20]. |
| Thrombospondin type-1 domain-containing 7A (THSD7A) | Reported in approximately 3% of patients with primary MN, some double positivity with PLA2R [21]. Increased rates of associated malignancy 20–50% [22,23]. |
| Exostosin 1 and 2 (EXT1/EXT2) | Commonly seen in autoimmune/lupus-associated MN [24] |
| Protocadherin 7 (PCDH7) | Potential association with autoimmune conditions (SLE and Sjögren’s syndrome) and malignancy [25] |
| Semaphorin 3B (Sema3B) | More commonly seen in children/young adults, with possibly an inheritable component [26]. |
| Serine protease high-temperature requirement A1 (HTRA1) | Potential association with malignancy and ANCA vasculitis [27] |
| Neutral endopeptidase (NEP) | Rare antenatal MN which can develop in the fetus of NEP-deficient mothers [7,28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McDonnell, T.; Wu, H.H.L.; Sinha, S.; Chinnadurai, R. The Role of PLA2R in Primary Membranous Nephropathy: Do We Still Need a Kidney Biopsy? Genes 2023, 14, 1343. https://doi.org/10.3390/genes14071343
McDonnell T, Wu HHL, Sinha S, Chinnadurai R. The Role of PLA2R in Primary Membranous Nephropathy: Do We Still Need a Kidney Biopsy? Genes. 2023; 14(7):1343. https://doi.org/10.3390/genes14071343
Chicago/Turabian StyleMcDonnell, Thomas, Henry H. L. Wu, Smeeta Sinha, and Rajkumar Chinnadurai. 2023. "The Role of PLA2R in Primary Membranous Nephropathy: Do We Still Need a Kidney Biopsy?" Genes 14, no. 7: 1343. https://doi.org/10.3390/genes14071343
APA StyleMcDonnell, T., Wu, H. H. L., Sinha, S., & Chinnadurai, R. (2023). The Role of PLA2R in Primary Membranous Nephropathy: Do We Still Need a Kidney Biopsy? Genes, 14(7), 1343. https://doi.org/10.3390/genes14071343





