Molecular Evolution and Genetic Variation of G2-Like Transcription Factor Genes in Wheat (Triticum aestivum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Gene Identification
2.2. Sequence Analysis
2.3. Phylogenetic Investigation and Categorization of Wheat G2-Like Genes
2.4. Analysis of TaG2-Like Gene Expression
2.5. Chromosomal Distribution and Gene Replication
2.6. Plant Materials and Treatments
2.7. RNA Isolation and Gene Expression Profiling
2.8. Determination of Subcellular Localization
3. Results
3.1. Identification of the G2-Like Proteins in Wheat
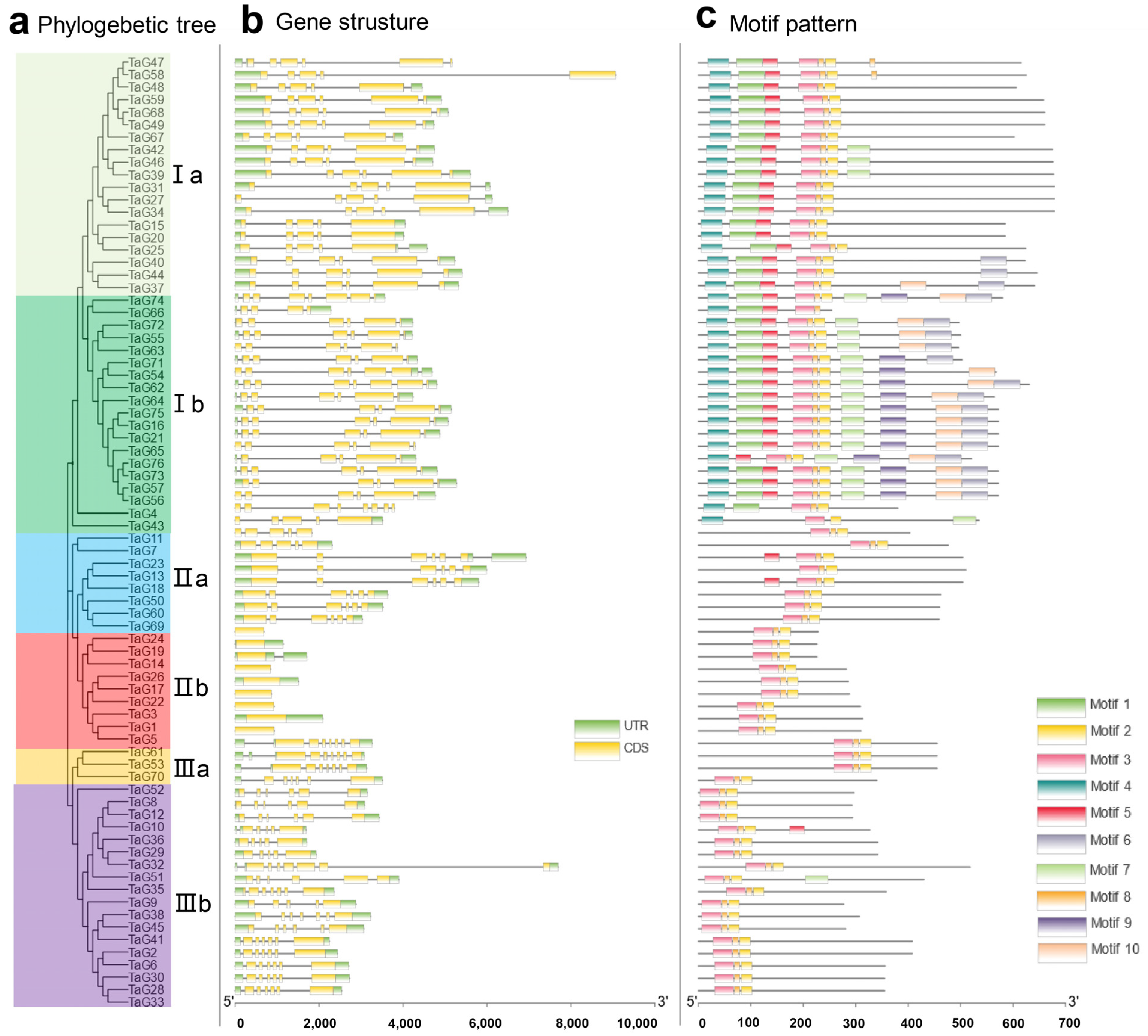
3.2. Gene Structure and Motif Composition of TaG2-Like Gene Family
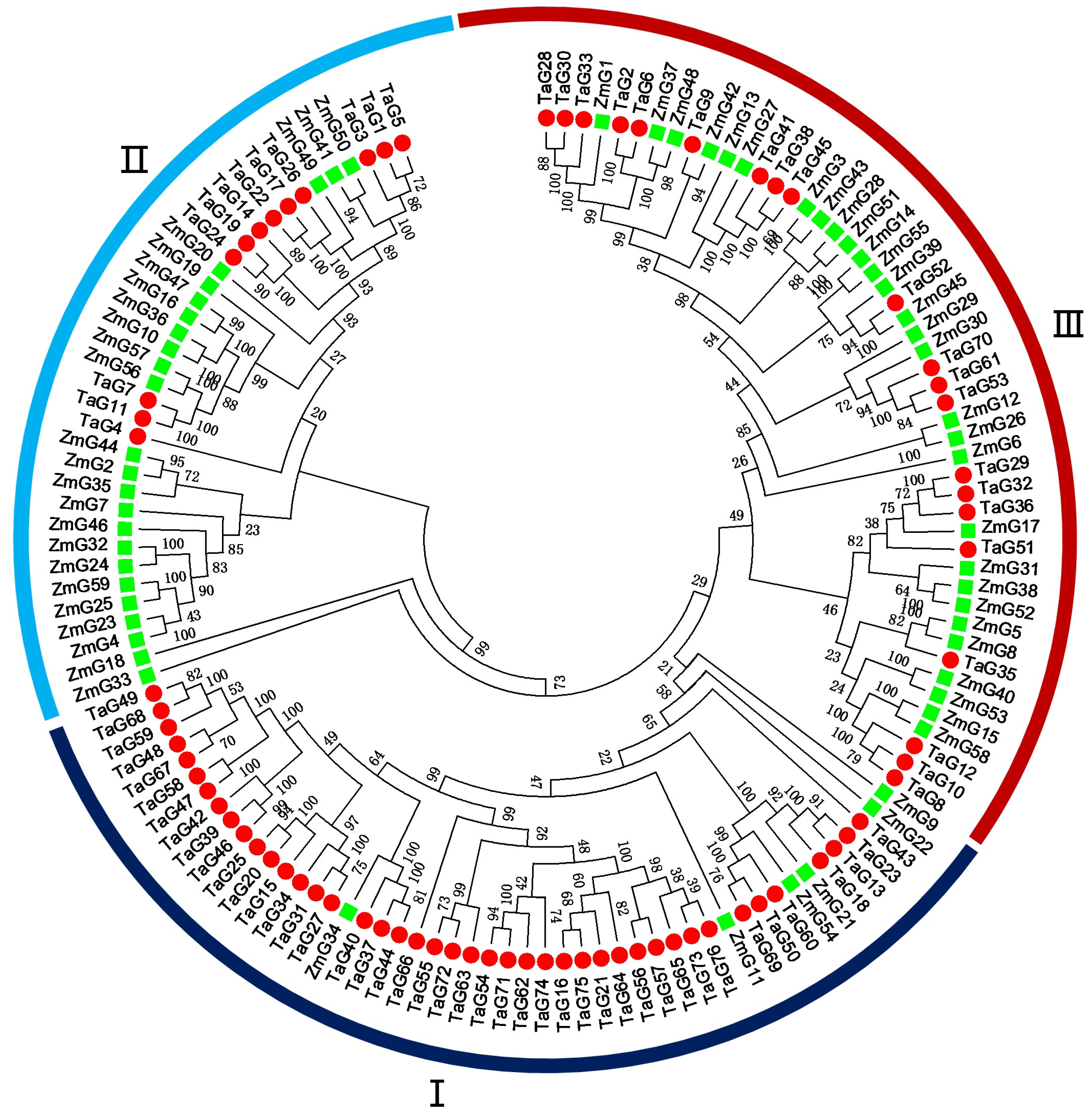
3.3. Phylogenetic Analysis of the G2-Like Genes between Wheat and Maize
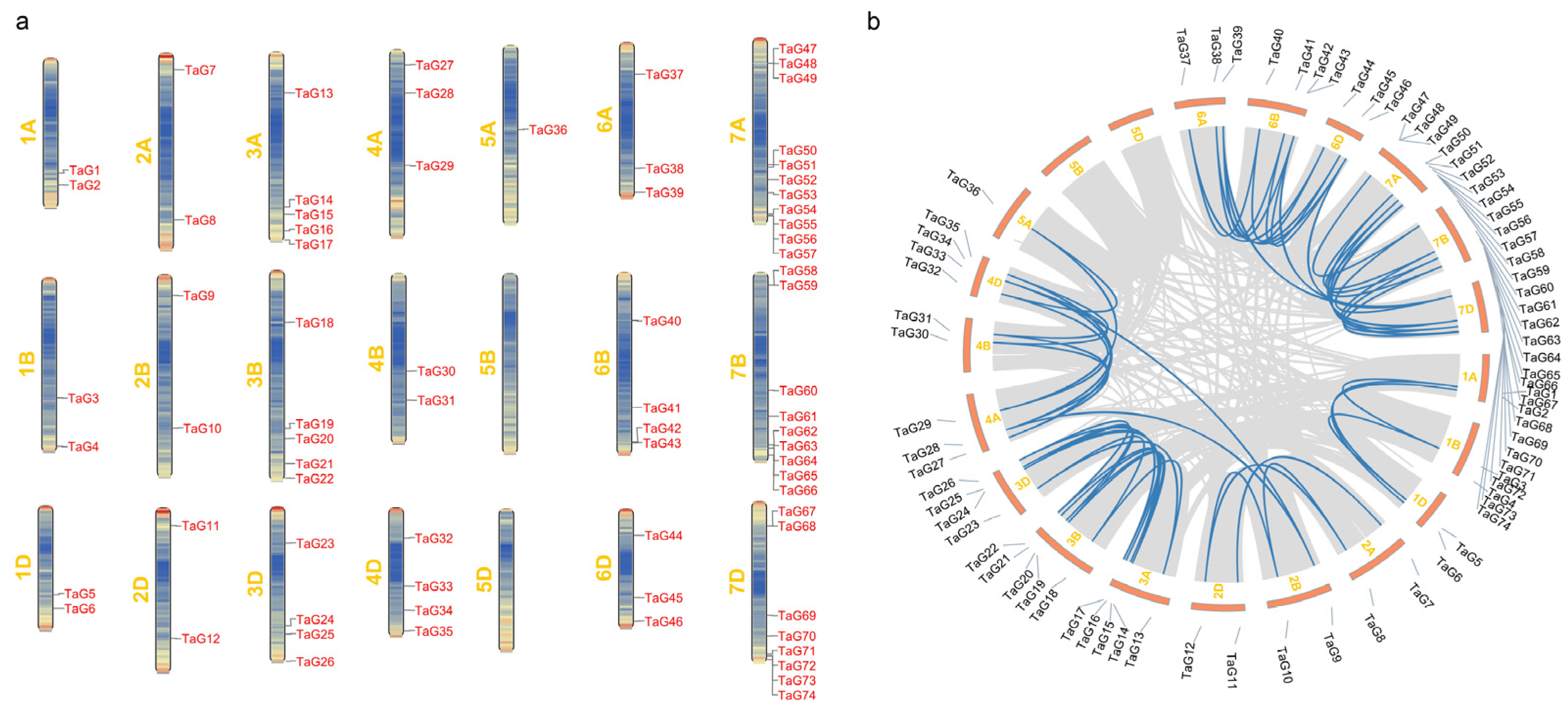
3.4. Chromosomal Localization and Synteny Analysis of TaG2-Like Genes
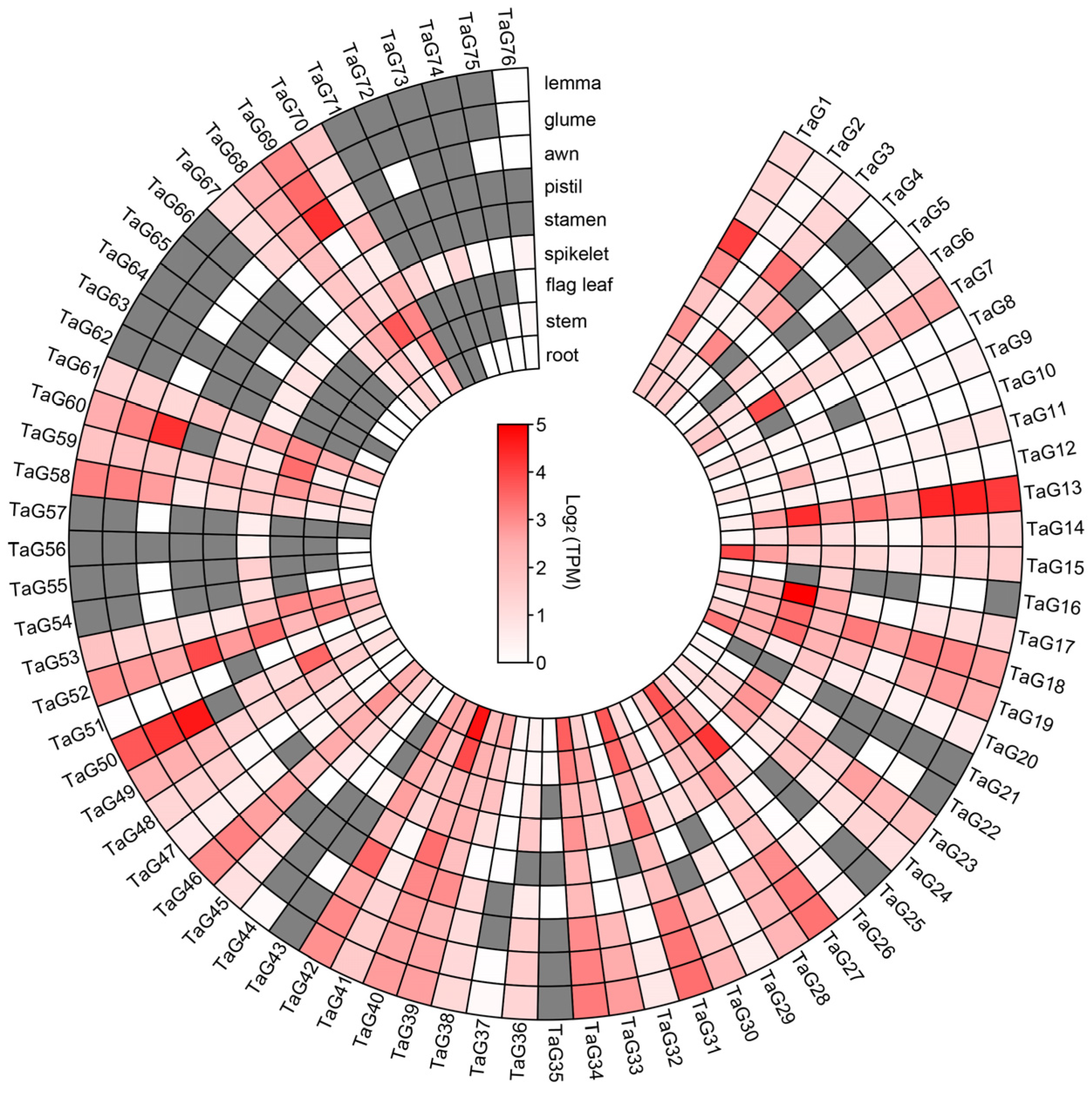
3.5. Expression Profiling of Wheat TaG2-Like Genes in Different Tissues or Organs
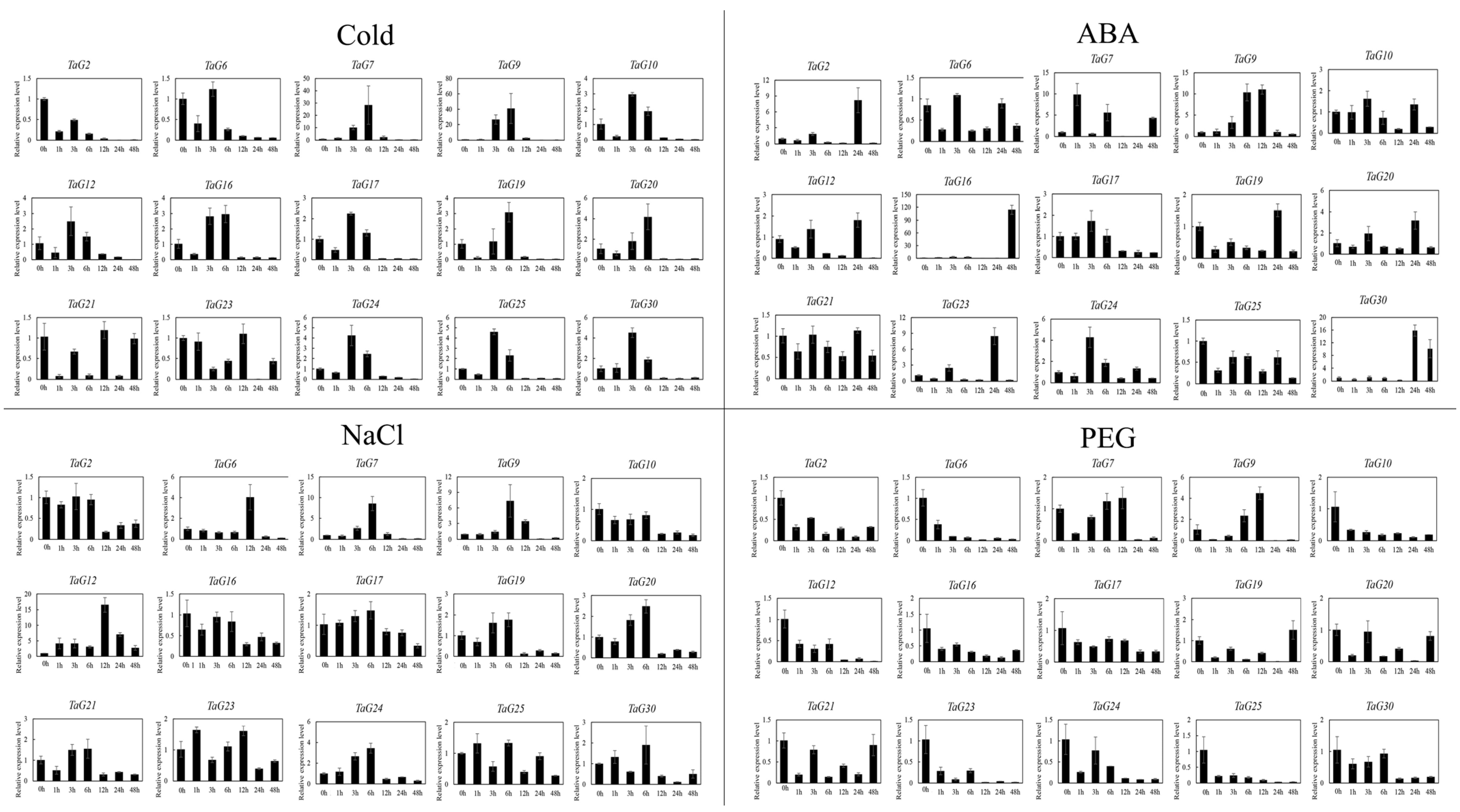
3.6. Expression Patterns of Wheat TaG2-Like Genes in Abiotic Stress
3.7. Subcellular Localization of TaG2-Like Proteins
4. Discussion
4.1. Characteristics and Evolution of Wheat G2-Like Gene Family Members
4.2. Various Functions of the Wheat G2-Like Gene Family Members
4.3. Abiotic Stresses Affect the Expressions of Wheat G2-Like Genes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, J.F.; Zhang, J.; Li, H.; Zhao, T.T.; Li, J.F. Research progress of plant GOLDEN2-Like transcription factor. Mol. Plant Breed. 2017, 15, 3949–3956. [Google Scholar]
- Hall, L.N.; Rossini, L.; Cribb, L.; Langdale, J.A. GOLDEN 2: A novel transcriptional regulator of cellular differentiation in the maize leaf. Plant Cell 1998, 10, 925–936. [Google Scholar] [CrossRef]
- Liu, F.; Xu, Y.; Han, G.; Zhou, L.; Ali, A.; Zhu, S.; Li, X. Molecular Evolution and Genetic Variation of G2-Like Transcription Factor Genes in Maize. PLoS ONE 2016, 11, e0161763. [Google Scholar] [CrossRef]
- Rossini, L.; Cribb, L.; Martin, D.J.; Langdale, J.A. The maize golden2 gene defines a novel class of transcriptional regulators in plants. Plant Cell 2001, 13, 1231–1244. [Google Scholar] [CrossRef]
- Wang, P.; Fouracre, J.; Kelly, S.; Karki, S.; Gowik, U.; Aubry, S.; Shaw, M.K.; Westhoff, P.; Slamet-Loedin, I.H.; Quick, W.P. Evolution of GOLDEN2-LIKE gene function in C3 and C4 plants. Planta 2013, 237, 481–495. [Google Scholar] [CrossRef]
- Waters, M.T.; Wang, P.; Korkaric, M.; Capper, R.G.; Saunders, N.J.; Langdale, J.A. GLK transcription factors coordinate expression of the photosynthetic apparatus in Arabidopsis. Plant Cell 2009, 21, 1109–1128. [Google Scholar] [CrossRef]
- Fitter, D.W.; Martin, D.J.; Copley, M.J.; Scotland, R.W.; Langdale, J.A. GLK gene pairs regulate chloroplast development in diverse plant species. Plant J. 2002, 31, 713–727. [Google Scholar] [CrossRef]
- Petridis, A.; Doll, S.; Nichelmann, L.; Bilger, W.; Mock, H.P. Arabidopsis thaliana G2-LIKE FLAVONOID REGULATOR and BRASSINOSTEROID ENHANCED EXPRESSION1 are low-temperature regulators of flavonoid accumulation. New Phytol. 2016, 211, 912–925. [Google Scholar] [CrossRef]
- Powell, A.L.; Nguyen, C.V.; Hill, T.; Cheng, K.L.; Figueroa-Balderas, R.; Aktas, H.; Ashrafi, H.; Pons, C.; Fernandez-Munoz, R.; Vicente, A.; et al. Uniform ripening encodes a Golden 2-like transcription factor regulating tomato fruit chloroplast development. Science 2012, 336, 1711–1715. [Google Scholar] [CrossRef]
- Zeng, L.; Liu, X.; Zhou, Z.; Li, D.; Zhao, X.; Zhu, L.; Luo, Y.; Hu, S. Identification of a G2-like transcription factor, OsPHL3, functions as a negative regulator of flowering in rice by co-expression and reverse genetic analysis. BMC Plant Biol. 2018, 18, 157. [Google Scholar] [CrossRef]
- Liu, J. Bioinformatics Analysis of Tomato G2-Like Transcription Factor Family and Identification of Resistance-Related Genes. Master’s Thesis, Northeast Agricultural University, Harbin, China, 2018. [Google Scholar]
- Qin, M.; Zhang, B.; Gu, G.; Yuan, J.; Yang, X.; Yang, J.; Xie, X. Genome-wide analysis of the G2-like transcription factor genes and their expression in different senescence stages of tobacco (Nicotiana tabacum L.). Front. Genet. 2021, 787, 626352. [Google Scholar] [CrossRef]
- Finn, R.D.; Clements, J.; Eddy, S.R. HMMER web server: Interactive sequence similarity searching. Nucleic Acids Res. 2011, 39, W29–W37. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Tocquin, P.; Corbesier, L.; Havelange, A.; Pieltain, A.; Kurtem, E.; Bernier, G.; Perilleux, C. A novel high efficiency, low maintenance, hydroponic system for synchronous growth and flowering of Arabidopsis thaliana. BMC Plant Biol. 2003, 3, 2. [Google Scholar] [CrossRef]
- Ma, J.; He, Y.H.; Wu, C.H.; Liu, H.P.; Hu, Z.Y.; Sun, G.M. Cloning and Molecular Characterization of a SERK Gene Transcriptionally Induced During Somatic Embryogenesis in Ananas comosus cv. Shenwan. Plant Mol. Biol. Rep. 2012, 30, 195–203. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yang, X.; Lu, Y.W.; Zhao, X.; Jiang, L.L.; Xu, S.C.; Peng, J.J.; Zheng, H.Y.; Lin, L.; Wu, Y.H.; MacFarlane, S.; et al. Downregulation of Nuclear Protein H2B Induces Salicylic Acid Mediated Defense Against PVX Infection in Nicotiana benthamiana. Front. Microbiol. 2019, 10, 1000. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Wu, L.; Zhang, L.; Liu, G.; Xia, C.; Liu, X.; Kong, X. Transcriptome profiling of developing leaf and shoot apices to reveal the molecular mechanism and co-expression genes responsible for the wheat heading date. BMC Genom. 2021, 22, 468. [Google Scholar] [CrossRef]
- Pont, C.; Murat, F.; Guizard, S.; Flores, R.; Foucrier, S.; Bidet, Y.; Quraishi, U.M.; Alaux, M.; Doležel, J.; Fahima, T. Wheat syntenome unveils new evidences of contrasted evolutionary plasticity between paleo-and neoduplicated subgenomes. Plant J. 2013, 76, 1030–1044. [Google Scholar] [CrossRef]
- Chen, Z.J. Genetic and epigenetic mechanisms for gene expression and phenotypic variation in plant polyploids. Annu. Rev. Plant Biol. 2007, 58, 377–406. [Google Scholar] [CrossRef]
- Otto, S.P. The evolutionary consequences of polyploidy. Cell 2007, 131, 452–462. [Google Scholar] [CrossRef]
- Hu, L.; Liu, S. Genome-wide identification and phylogenetic analysis of the ERF gene family in cucumbers. Genet. Mol. Biol. 2011, 34, 624–633. [Google Scholar] [CrossRef]
- Zhang, J. Evolution by gene duplication: An update. Trends Ecol. Evol. 2003, 18, 292–298. [Google Scholar] [CrossRef]
- Langdale, J.A.; Kidner, C.A. bundle sheath defective, a mutation that disrupts cellular differentiation in maize leaves. Development 1994, 120, 673–681. [Google Scholar] [CrossRef]
- Rauf, M.; Arif, M.; Dortay, H.; Matallana-Ramirez, L.P.; Waters, M.T.; Gil Nam, H.; Lim, P.O.; Mueller-Roeber, B.; Balazadeh, S. ORE1 balances leaf senescence against maintenance by antagonizing G2-like-mediated transcription. EMBO Rep. 2013, 14, 382–388. [Google Scholar] [CrossRef]
- Tang, X.; Miao, M.; Niu, X.; Zhang, D.; Cao, X.; Jin, X.; Zhu, Y.; Fan, Y.; Wang, H.; Liu, Y. Ubiquitin-conjugated degradation of golden 2-like transcription factor is mediated by CUL 4-DDB 1-based E 3 ligase complex in tomato. New Phytol. 2016, 209, 1028–1039. [Google Scholar] [CrossRef]
- Nagatoshi, Y.; Mitsuda, N.; Hayashi, M.; Inoue, S.-I.; Okuma, E.; Kubo, A.; Murata, Y.; Seo, M.; Saji, H.; Kinoshita, T. GOLDEN 2-LIKE transcription factors for chloroplast development affect ozone tolerance through the regulation of stomatal movement. Proc. Natl. Acad. Sci. USA 2016, 113, 4218–4223. [Google Scholar] [CrossRef]
- Savitch, L.V.; Allard, G.; Seki, M.; Robert, L.S.; Tinker, N.A.; Huner, N.P.; Shinozaki, K.; Singh, J. The effect of overexpression of two Brassica CBF/DREB1-like transcription factors on photosynthetic capacity and freezing tolerance in Brassica napus. Plant Cell Physiol. 2005, 46, 1525–1539. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, G.; Li, J.; Wang, X.; Kang, Y.; Li, Y.; Niu, J.; Yin, J. Molecular Evolution and Genetic Variation of G2-Like Transcription Factor Genes in Wheat (Triticum aestivum L.). Genes 2023, 14, 1341. https://doi.org/10.3390/genes14071341
Hu G, Li J, Wang X, Kang Y, Li Y, Niu J, Yin J. Molecular Evolution and Genetic Variation of G2-Like Transcription Factor Genes in Wheat (Triticum aestivum L.). Genes. 2023; 14(7):1341. https://doi.org/10.3390/genes14071341
Chicago/Turabian StyleHu, Ge, Junchang Li, Xiang Wang, Yunfei Kang, Yongchun Li, Jishan Niu, and Jun Yin. 2023. "Molecular Evolution and Genetic Variation of G2-Like Transcription Factor Genes in Wheat (Triticum aestivum L.)" Genes 14, no. 7: 1341. https://doi.org/10.3390/genes14071341
APA StyleHu, G., Li, J., Wang, X., Kang, Y., Li, Y., Niu, J., & Yin, J. (2023). Molecular Evolution and Genetic Variation of G2-Like Transcription Factor Genes in Wheat (Triticum aestivum L.). Genes, 14(7), 1341. https://doi.org/10.3390/genes14071341




