Female Pattern Hair Loss: An Overview with Focus on the Genetics
Abstract
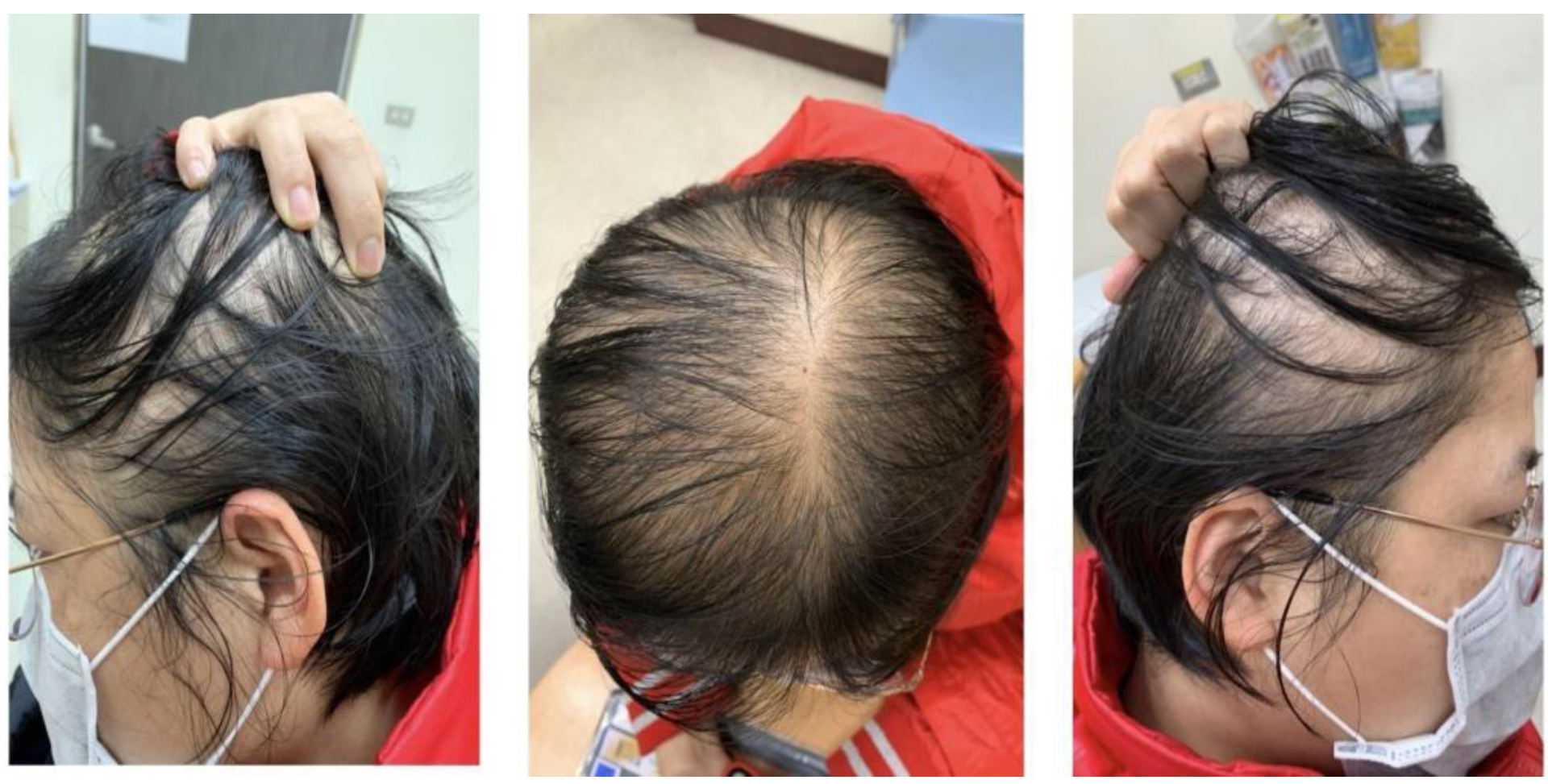
1. Introduction
2. Epidemiology
3. Etiology and Pathogenesis
4. Genetics of FPHL
4.1. Mode of Inheritance and Genome-Wide Association Studies (GWAS)
4.2. Correlation between Nutrition and FPHL
4.3. Polycystic Ovary Syndrome (PCOS) with FPHL
4.4. Dickkopf WNT Signaling Pathway Inhibitor 1
4.5. Prediction of Dermal Sheath Cup Cell Therapy
4.6. Sex Steroid Hormones Gene Polymorphism
4.7. Inconsistency of Association with M-AGA Genetics
5. Therapies of FPHL
5.1. Standard Management
5.2. Miscellaneous
6. Summary of Genetic Data on FPHL, M-AGA, and Other Non-Scarring Alopecia
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bertoli, M.J.; Sadoughifar, R.; Schwartz, R.A.; Lotti, T.M.; Janniger, C.K. Female pattern hair loss: A comprehensive review. Dermatology 2020, 33, e14055. [Google Scholar] [CrossRef]
- Lolli, F.; Pallotti, F.; Rossi, A.; Fortuna, M.C.; Caro, G.; Lenzi, A.; Sansone, A.; Lombardo, F. Androgenetic alopecia: A review. Endocrine 2017, 57, 9–17. [Google Scholar] [CrossRef]
- Ludwig, E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br. J. Dermatol. 1977, 97, 247–254. [Google Scholar] [CrossRef]
- Olsen, E.A. The midline part: An important physical clue to the clinical diagnosis of androgenetic alopecia in women. J. Am. Acad. Dermatol. 1999, 40, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Biondo, S.; Goble, D.; Sinclair, R. Women who present with female pattern hair loss tend to underestimate the severity of their hair loss. Br. J. Dermatol. 2004, 150, 750–752. [Google Scholar] [CrossRef] [PubMed]
- Starace, M.; Orlando, G.; Alessandrini, A.; Piraccini, B.M. Female Androgenetic Alopecia: An Update on Diagnosis and Management. Am. J. Clin. Dermatol. 2020, 21, 69–84. [Google Scholar] [CrossRef]
- Davis, D.S.; Callender, V.D. Review of quality of life studies in women with alopecia. Int. J. Womens Dermatol. 2018, 4, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Fischer, T.W.; Chren, M.M.; Strauss, B.M.; Elsner, P. Strategies of coping and quality of life in women with alopecia. Br. J. Dermatol. 2001, 144, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- Chaikittisilpa, S.; Rattanasirisin, N.; Panchaprateep, R.; Orprayoon, N.; Phutrakul, P.; Suwan, A.; Jaisamrarn, U. Prevalence of female pattern hair loss in postmenopausal women: A cross-sectional study. Menopause 2022, 29, 415–420. [Google Scholar] [CrossRef]
- Youssef, S.M.E.; Atallah, R.B.; Zaky, M.S.; Eldeek, B.S.; Elsaie, M.L. Urban-rural differences in the prevalence of female pattern hair loss among secondary school girls: A cross-sectional study. J. Cosmet. Dermatol. 2022, 21, 2229–2235. [Google Scholar] [CrossRef]
- Ramos, P.M.; Miot, H.A. Female Pattern Hair Loss: A clinical and pathophysiological review. Bras. Dermatol. 2015, 90, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Redler, S.; Messenger, A.G.; Betz, R.C. Genetics and other factors in the aetiology of female pattern hair loss. Exp. Dermatol. 2017, 26, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Knopp, E. The scalp biopsy for hair loss and its interpretation. Semin. Cutan. Med. Surg. 2015, 34, 57–66. [Google Scholar] [CrossRef]
- Ieremia, E.; Stefanato, C.M. The role of hair follicle counts and ratios in the histopathological assessment of androgenic alopecia, alopecia areata and telogen effluvium: Does counting count? Hum. Pathol. 2023. [Google Scholar] [CrossRef]
- Peyravian, N.; Deo, S.; Daunert, S.; Jimenez, J.J. The Inflammatory Aspect of Male and Female Pattern Hair Loss. J. Inflamm. Res. 2020, 13, 879–881. [Google Scholar] [CrossRef]
- Heilmann-Heimbach, S.; Hochfeld, L.M.; Paus, R.; Nöthen, M.M. Hunting the genes in male-pattern alopecia: How important are they, how close are we and what will they tell us? Exp. Dermatol. 2016, 25, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Birch, M.P.; Messenger, A.G. Genetic factors predispose to balding and non-balding in men. Eur. J. Dermatol. 2001, 11, 309–314. [Google Scholar]
- Ohn, J.; Son, H.Y.; Yu, D.A.; Kim, M.S.; Kwon, S.; Park, W.S.; Kim, J.I.; Kwon, O. Early onset female pattern hair loss: A case-control study for analyzing clinical features and genetic variants. J. Dermatol. Sci. 2022, 106, 21–28. [Google Scholar] [CrossRef]
- Piccini, I.; Sousa, M.; Altendorf, S.; Jimenez, F.; Rossi, A.; Funk, W.; Bíró, T.; Paus, R.; Seibel, J.; Jakobs, M.; et al. Intermediate Hair Follicles from Patients with Female Pattern Hair Loss Are Associated with Nutrient Insufficiency and a Quiescent Metabolic Phenotype. Nutrients 2022, 14, 3357. [Google Scholar] [CrossRef]
- Fawzi, M.M.; Mahmoud, S.B.; Ahmed, S.F.; Shaker, O.G. Assessment of vitamin D receptors in alopecia areata and androgenetic alopecia. J. Cosmet. Dermatol. 2016, 15, 318–323. [Google Scholar] [CrossRef]
- Seleit, I.; Bakry, O.A.; Badr, E.; Mabrouk, M. Vitamin D Receptor Gene Polymorphisms Taq-1 and Cdx-1 in Female Pattern Hair Loss. Indian J. Dermatol. 2020, 65, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Banihashemi, M.; Nahidi, Y.; Meibodi, N.T.; Jarahi, L.; Dolatkhah, M. Serum Vitamin D3 Level in Patients with Female Pattern Hair Loss. Int. J. Trichol. 2016, 8, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Jiang, V.S.; Hawkins, S.D.; McMichael, A. Female pattern hair loss and polycystic ovarian syndrome: More than just hirsutism. Curr. Opin. Endocrinol. Diabetes Obes. 2022, 29, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.A.; Lin, S.J.; Chen, P.L.; Chou, C.H.; Huang, C.C.; Ho, H.N.; Chen, M.J. HSD3B1 gene polymorphism and female pattern hair loss in women with polycystic ovary syndrome. J. Med. Assoc. 2019, 118, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, E.A.; Elgarhy, L.H.; Hasby, E.A.; Mohammad, L. Dickkopf-1 Expression in Androgenetic Alopecia and Alopecia Areata in Male Patients. Am. J. Dermatol. 2019, 41, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Kwack, M.H.; Jun, M.S.; Sung, Y.K.; Kim, J.C.; Kim, M.K. Ectodysplasin-A2 induces dickkopf 1 expression in human balding dermal papilla cells overexpressing the ectodysplasin A2 receptor. Biochem. Biophys. Res. Commun. 2020, 529, 766–772. [Google Scholar] [CrossRef]
- Hashimoto, M.; Kawai, Y.; Masutani, T.; Tanaka, K.; Ito, K.; Iddamalgoda, A. Effects of watercress extract fraction on R-spondin 1-mediated growth of human hair. Int. J. Cosmet. Sci. 2022, 44, 154–165. [Google Scholar] [CrossRef]
- Yoshida, Y.; Takahashi, M.; Yamanishi, H.; Nakazawa, Y.; Kishimoto, J.; Ohyama, M. Changes in the Expression of Smooth Muscle Cell-Related Genes in Human Dermal Sheath Cup Cells Associated with the Treatment Outcome of Autologous Cell-Based Therapy for Male and Female Pattern Hair Loss. Int. J. Mol. Sci. 2022, 23, 7125. [Google Scholar] [CrossRef]
- Tsuboi, R.; Niiyama, S.; Irisawa, R.; Harada, K.; Nakazawa, Y.; Kishimoto, J. Autologous cell-based therapy for male and female pattern hair loss using dermal sheath cup cells: A randomized placebo-controlled double-blinded dose-finding clinical study. J. Am. Acad. Dermatol. 2020, 83, 109–116. [Google Scholar] [CrossRef]
- Martinez-Jacobo, L.; Villarreal-Villarreal, C.D.; Ortiz-López, R.; Ocampo-Candiani, J.; Rojas-Martínez, A. Genetic and molecular aspects of androgenetic alopecia. Indian J. Dermatol. Venereol. Leprol. 2018, 84, 263–268. [Google Scholar] [CrossRef]
- Bhat, Y.J.; Saqib, N.U.; Latif, I.; Hassan, I. Female Pattern Hair Loss-An Update. Indian Dermatol. Online J. 2020, 11, 493–501. [Google Scholar] [CrossRef]
- Sánchez, P.; Serrano-Falcón, C.; Torres, J.M.; Serrano, S.; Ortega, E. 5α-Reductase isozymes and aromatase mRNA levels in plucked hair from young women with female pattern hair loss. Arch. Dermatol. Res. 2018, 310, 77–83. [Google Scholar] [CrossRef]
- Łukasik, A.; Kozicka, K.; Pisarek, A.; Wojas-Pelc, A. The role of CYP19A1 and ESR2 gene polymorphisms in female androgenetic alopecia in the Polish population. Postep. Dermatol. Alergol. 2022, 39, 708–713. [Google Scholar] [CrossRef]
- Yip, L.; Zaloumis, S.; Irwin, D.; Severi, G.; Hopper, J.; Giles, G.; Harrap, S.; Sinclair, R.; Ellis, J. Association analysis of oestrogen receptor beta gene (ESR2) polymorphisms with female pattern hair loss. Br. J. Dermatol. 2012, 166, 1131–1134. [Google Scholar] [CrossRef] [PubMed]
- Redler, S.; Birch, P.; Drichel, D.; Hofmann, P.; Dobson, K.; Böhmer, A.C.; Becker, J.; Giehl, K.A.; Tazi-Ahnini, R.; Kruse, R.; et al. The oestrogen receptor 2 (ESR2) gene in female-pattern hair loss: Replication of association with rs10137185 in German patients. Br. J. Dermatol. 2014, 170, 982–985. [Google Scholar] [CrossRef]
- Yip, L.; Zaloumis, S.; Irwin, D.; Severi, G.; Hopper, J.; Giles, G.; Harrap, S.; Sinclair, R.; Ellis, J. Gene-wide association study between the aromatase gene (CYP19A1) and female pattern hair loss. Br. J. Dermatol. 2009, 161, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Redler, S.; Birch, M.P.; Drichel, D.; Dobson, K.; Brockschmidt, F.F.; Tazi-Ahnini, R.; Giehl, K.A.; Kluck, N.; Kruse, R.; Lutz, G.; et al. Investigation of variants of the aromatase gene (CYP19A1) in female pattern hair loss. Br. J. Dermatol. 2011, 165, 703–705. [Google Scholar] [CrossRef]
- Rui, W.; Sheng, Y.; Hu, R.; Miao, Y.; Han, Y.; Guo, X.; Qi, S.; Xu, F.; Xu, J.; Yang, Q. Association of Single Nucleotide Polymorphisms in the CYP19A1 Gene with Female Pattern Hair Loss in a Chinese Population. Dermatology 2015, 231, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Takahashi, M.; Obayashi, Y.; Serizawa, T.; Murakoshi, M.; Ohyama, M. The ovariectomized mouse simulates the pathophysiology of postmenopausal female pattern hair loss. J. Dermatol. Sci. 2017, 87, 79–82. [Google Scholar] [CrossRef]
- Endo, Y.; Obayashi, Y.; Ono, T.; Serizawa, T.; Murakoshi, M.; Ohyama, M. Reversal of the hair loss phenotype by modulating the estradiol-ANGPT2 axis in the mouse model of female pattern hair loss. J. Dermatol. Sci. 2018, 91, 43–51. [Google Scholar] [CrossRef]
- Pirastu, N.; Joshi, P.K.; de Vries, P.S.; Cornelis, M.C.; McKeigue, P.M.; Keum, N.; Franceschini, N.; Colombo, M.; Giovannucci, E.L.; Spiliopoulou, A.; et al. GWAS for male-pattern baldness identifies 71 susceptibility loci explaining 38% of the risk. Nat. Commun. 2017, 8, 1584. [Google Scholar] [CrossRef]
- Kanti, V.; Messenger, A.; Dobos, G.; Reygagne, P.; Finner, A.; Blumeyer, A.; Trakatelli, M.; Tosti, A.; Del Marmol, V.; Piraccini, B.M.; et al. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men - short version. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 11–22. [Google Scholar] [CrossRef]
- Liang, B.; Ding, Y.; Zhou, Y.; Yang, C.; Cheng, Z. Evaluation of Susceptibility Genes/Loci Associated with Male Androgenetic Alopecia (MAGA) for Female-Pattern Hair Loss in a Chinese Han Population and a Brief Literature Review. Med. Sci. Monit. 2021, 27, e933424. [Google Scholar] [CrossRef]
- Redler, S.; Brockschmidt, F.F.; Tazi-Ahnini, R.; Drichel, D.; Birch, M.P.; Dobson, K.; Giehl, K.A.; Herms, S.; Refke, M.; Kluck, N.; et al. Investigation of the male pattern baldness major genetic susceptibility loci AR/EDA2R and 20p11 in female pattern hair loss. Br. J. Dermatol. 2012, 166, 1314–1318. [Google Scholar] [CrossRef]
- Redler, S.; Dobson, K.; Drichel, D.; Heilmann, S.; Wolf, S.; Brockschmidt, F.F.; Tazi-Ahnini, R.; Birch, P.; Teßmann, P.; Giehl, K.A.; et al. Investigation of six novel susceptibility loci for male androgenetic alopecia in women with female pattern hair loss. J. Dermatol. Sci. 2013, 72, 186–188. [Google Scholar] [CrossRef]
- Nuwaihyd, R.; Redler, S.; Heilmann, S.; Drichel, D.; Wolf, S.; Birch, P.; Dobson, K.; Lutz, G.; Giehl, K.A.; Kruse, R.; et al. Investigation of four novel male androgenetic alopecia susceptibility loci: No association with female pattern hair loss. Arch. Dermatol. Res. 2014, 306, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, H.; Redler, S.; Birch, P.; Drichel, D.; Dobson, K.; Tazi-Ahnini, R.; Teßmann, P.; Giehl, K.A.; Kruse, R.; Lutz, G.; et al. Selected variants of the melanocortin 4 receptor gene (MC4R) do not confer susceptibility to female pattern hair loss. Arch. Dermatol. Res. 2013, 305, 249–253. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, Y.; Huang, Y.; Wang, J.; Yang, K.; Zhang, Y.; Pu, W.; Liu, J.; Shi, X.; Ma, Y.; et al. Insights into male androgenetic alopecia using comparative transcriptome profiling: Hypoxia-inducible factor-1 and Wnt/β-catenin signalling pathways. Br. J. Dermatol. 2022, 187, 936–947. [Google Scholar] [CrossRef] [PubMed]
- van Zuuren, E.J.; Fedorowicz, Z.; Schoones, J. Interventions for female pattern hair loss. Cochrane Database Syst. Rev. 2016, 2016, Cd007628. [Google Scholar] [CrossRef] [PubMed]
- Valente Duarte de Sousa, I.C.; Tosti, A. New investigational drugs for androgenetic alopecia. Expert Opin. Investig. Drugs 2013, 22, 573–589. [Google Scholar] [CrossRef]
- Ocampo-Garza, J.; Griggs, J.; Tosti, A. New drugs under investigation for the treatment of alopecias. Expert Opin. Investig. Drugs 2019, 28, 275–284. [Google Scholar] [CrossRef]
- Paichitrojjana, A.; Paichitrojjana, A. Platelet Rich Plasma and Its Use in Hair Regrowth: A Review. Drug Des. Devel. 2022, 16, 635–645. [Google Scholar] [CrossRef]
- Torabi, P.; Behrangi, E.; Goodarzi, A.; Rohaninasab, M. A systematic review of the effect of platelet-rich plasma on androgenetic alopecia of women. Dermatology 2020, 33, e13835. [Google Scholar] [CrossRef]
- Egger, A.; Resnik, S.R.; Aickara, D.; Maranda, E.; Kaiser, M.; Wikramanayake, T.C.; Jimenez, J.J. Examining the Safety and Efficacy of Low-Level Laser Therapy for Male and Female Pattern Hair Loss: A Review of the Literature. Skin Appendage Disord. 2020, 6, 259–267. [Google Scholar] [CrossRef]
- Iamsumang, W.; Leerunyakul, K.; Suchonwanit, P. Finasteride and Its Potential for the Treatment of Female Pattern Hair Loss: Evidence to Date. Drug Des. Devel. 2020, 14, 951–959. [Google Scholar] [CrossRef]
- Gupta, A.K.; Talukder, M. Topical finasteride for male and female pattern hair loss: Is it a safe and effective alternative? J. Cosmet. Dermatol. 2022, 21, 1841–1848. [Google Scholar] [CrossRef]
- Suchonwanit, P.; Iamsumang, W.; Leerunyakul, K. Topical finasteride for the treatment of male androgenetic alopecia and female pattern hair loss: A review of the current literature. J. Dermatol. Treat. 2022, 33, 643–648. [Google Scholar] [CrossRef]
- Olszewska, M.; Rudnicka, L. Effective treatment of female androgenic alopecia with dutasteride. J. Drugs Dermatol. 2005, 4, 637–640. [Google Scholar]
- Burns, L.J.; De Souza, B.; Flynn, E.; Hagigeorges, D.; Senna, M.M. Spironolactone for treatment of female pattern hair loss. J. Am. Acad. Dermatol. 2020, 83, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.M.; Sinclair, R.D.; Kasprzak, M.; Miot, H.A. Minoxidil 1 mg oral versus minoxidil 5% topical solution for the treatment of female-pattern hair loss: A randomized clinical trial. J. Am. Acad. Dermatol. 2020, 82, 252–253. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, E.S.M.; Ramos, P.M.; Silva, M.R.; Nascimento, E.S.R.; Barbosa Raposo, N.R. Randomized clinical trial of low-dose oral minoxidil for the treatment of female pattern hair loss: 0.25 mg versus 1 mg. J. Am. Acad. Dermatol. 2022, 87, 396–399. [Google Scholar] [CrossRef]
- Saini, K.; Mysore, V. Role of vitamin D in hair loss: A short review. J. Cosmet. Dermatol. 2021, 20, 3407–3414. [Google Scholar] [CrossRef]
- Zempleni, J.; Hassan, Y.I.; Wijeratne, S.S. Biotin and biotinidase deficiency. Expert Rev. Endocrinol. Metab. 2008, 3, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.W.; Hipler, U.C.; Elsner, P. Effect of caffeine and testosterone on the proliferation of human hair follicles in vitro. Int. J. Dermatol. 2007, 46, 27–35. [Google Scholar] [CrossRef]
- Fischer, T.W.; Slominski, A.; Tobin, D.J.; Paus, R. Melatonin and the hair follicle. J. Pineal. Res. 2008, 44, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Plonka, P.M.; Handjiski, B.; Popik, M.; Michalczyk, D.; Paus, R. Zinc as an ambivalent but potent modulator of murine hair growth in vivo- preliminary observations. Exp. Dermatol. 2005, 14, 844–853. [Google Scholar] [CrossRef]
- Ibrahim, I.M.; Hasan, M.S.; Elsabaa, K.I.; Elsaie, M.L. Pumpkin seed oil vs. minoxidil 5% topical foam for the treatment of female pattern hair loss: A randomized comparative trial. J. Cosmet. Dermatol. 2021, 20, 2867–2873. [Google Scholar] [CrossRef] [PubMed]
- Campiche, R.; Le Riche, A.; Edelkamp, J.; Botello, A.F.; Martin, E.; Gempeler, M.; Bertolini, M. An extract of Leontopodium alpinum inhibits catagen development ex vivo and increases hair density in vivo. Int. J. Cosmet. Sci. 2022, 44, 363–376. [Google Scholar] [CrossRef]
- Nakamura, T.; Yamamura, H.; Park, K.; Pereira, C.; Uchida, Y.; Horie, N.; Kim, M.; Itami, S. Naturally Occurring Hair Growth Peptide: Water-Soluble Chicken Egg Yolk Peptides Stimulate Hair Growth Through Induction of Vascular Endothelial Growth Factor Production. J. Med. Food 2018, 21, 701–708. [Google Scholar] [CrossRef]
- Moon, I.J.; Yoon, H.K.; Kim, D.; Choi, M.E.; Han, S.H.; Park, J.H.; Hong, S.W.; Cho, H.; Lee, D.K.; Won, C.H. Efficacy of Asymmetric siRNA Targeting Androgen Receptors for the Treatment of Androgenetic Alopecia. Mol. Pharm. 2023, 20, 128–135. [Google Scholar] [CrossRef]
- Simakou, T.; Butcher, J.P.; Reid, S.; Henriquez, F.L. Alopecia areata: A multifactorial autoimmune condition. J. Autoimmun. 2019, 98, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Tafazzoli, A.; Forstner, A.J.; Broadley, D.; Hofmann, A.; Redler, S.; Petukhova, L.; Giehl, K.A.; Kruse, R.; Blaumeiser, B.; Böhm, M.; et al. Genome-Wide MicroRNA Analysis Implicates miR-30b/d in the Etiology of Alopecia Areata. J. Invest. Dermatol. 2018, 138, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Seleit, I.; Bakry, O.A.; Badr, E.; Hassan, E.H. Vitamin D Receptor Gene Polymorphism In Chronic Telogen Effluvium; A Case-Control Study. Clin. Cosmet. Investig. Dermatol. 2019, 12, 745–750. [Google Scholar] [CrossRef] [PubMed]



| Highly Admixed Population | |||||||
| Study | Region | Age Groups | |||||
| 20–29 * | 30–39 * | 40–49 * | 50–59 * | 60–69 * | >70 * | ||
| 2022, Tsutsui | Brazil | 9 | 22 | 50 | 69 | - | - |
| Caucasian | |||||||
| Study | Region | Age groups | |||||
| 20–29 * | 30–39 * | 40–49 * | 50–59 * | 60–69 * | >70 * | ||
| 2005, Gan | Australia | 12 | 17 | 25 | 28 | 41 | 54 |
| 2001, Norwood | USA | 3 | 17 | 16 | 23 | 25 | 29 |
| 2001, Birch | England | 3 | 10 | 5 | 14 | 33 | 38 |
| Asian | |||||||
| Study | Region | Age groups | |||||
| 20–29 * | 30–39 * | 40–49 * | 50–59 * | 60–69 * | >70 * | ||
| 2012, Su | Taiwan | - | 6 | 10 | 12 | 13 | 15 |
| 2010, Wang | China | 1 | 2 | 5 | 8 | 11 | 12 |
| 2008, Xu | China (Shanghai) | - | - | 1 | 2 | 3 | 15 |
| 2001, Paik | South Korea | - | 2 | 4 | 7 | 12 | 35 |
| Han Chinese * | ||
|---|---|---|
| Locus | SNP | Association with FPHL |
| 1p36.22 | rs12565727 | No significant association |
| 2q35 | rs10193725 | No significant association |
| rs7349332 | ||
| 2q37.3 | rs9752491 | No significant association |
| rs9711321 | ||
| rs12613833 | ||
| 3q25.1 | rs4679955 | No significant association |
| 5q33.3 | rs929626 | No significant association |
| rs1081073 | ||
| 7p21.1 | rs2249817 | No significant association |
| rs12056282 | ||
| rs756853 | ||
| rs13230142 | ||
| rs17350355 | ||
| 12p12.1 | rs7975017 | No significant association |
| rs9668810 | ||
| 18q12.3 | rs10502861 | No significant association |
| 20p11 | rs6137444 | No significant association |
| rs2180439 | ||
| rs1998076 | ||
| rs6113491 | ||
| rs201571 | ||
| UK/Germany # | ||
| AR/EDA2R | rs4827379 | Significant association in a subgroup with an early age of onset |
| rs1397631 | ||
| rs2497938 | ||
| rs962458 | ||
| rs6152 | ||
| rs7885198 | ||
| rs5918801 | ||
| 1p36.22 | rs2003046 | No significant association |
| rs12565727 | ||
| rs11576658 | ||
| 2q35 | rs10193725 | No significant association |
| rs7349332 | ||
| 2q37.3 | rs9711321 | No significant association |
| rs11683401 | ||
| rs9287638 | ||
| 3q25.1 | rs7648585 | No significant association |
| rs4679955 | ||
| 5q33.3 | rs929626 | No significant association |
| rs1081073 | ||
| 7p21.1 | rs957958 | No significant association |
| rs2073963 | ||
| rs2073964 | ||
| 7q11.22 | rs6947344 | No significant association |
| rs6945541 | ||
| rs4718865 | ||
| 12p12.1 | rs9668810 | No significant association |
| rs7975017 | ||
| 17q21.31 | rs12373124 | No significant association |
| rs17769552 | ||
| rs17650991 | ||
| 18q21.1 | rs8083006 | No significant association |
| rs10502861 | ||
| rs12959797 | ||
| 20p11 | rs6137444 | No significant association |
| rs2180439 | ||
| rs1998076 | ||
| rs201571 | ||
| rs6113491 | ||
| CYP19A1 | rs16964189 | No significant association |
| rs4646 | ||
| rs28757184 | ||
| rs2470158 | ||
| ESR1 | [44] | No significant association |
| PGR | ||
| SRD5A1 | ||
| SRD5A2 | ||
| ESR2 | rs10137185 | Significant association in the overall German sample |
| MC4R | [47] | No significant association |
| Australia $ | ||
| CYP19A1 | rs4646 | CC genotype is significantly more frequent in some cases |
| ESR2 | rs10137185 | Nominal significance |
| rs17101774 | ||
| rs2022748 | ||
| Clinical Presentation | Histopathological Findings | Related Genes | |
|---|---|---|---|
| FPHL |
|
| CYP19A1 ESR2 |
| M-AGA |
| AR MC4R EDA2R SRD5A1 SRD5A2 [2,47] | |
| Alopecia areata |
|
| NOTCH4 C6orf10 BTNL2 HLA-DRA HLA-A IL-2 IL2RA STX17 TNXB [71,72] |
| Chronic Telogen effluvium |
|
| Cdx1 VDR Taq1 VDR [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, C.-Y.; Chen, J.Y.-F.; Hsu, W.-L.; Yu, S.; Chen, W.-C.; Chiu, S.-H.; Yang, H.-R.; Lin, S.-Y.; Wu, C.-Y. Female Pattern Hair Loss: An Overview with Focus on the Genetics. Genes 2023, 14, 1326. https://doi.org/10.3390/genes14071326
Ho C-Y, Chen JY-F, Hsu W-L, Yu S, Chen W-C, Chiu S-H, Yang H-R, Lin S-Y, Wu C-Y. Female Pattern Hair Loss: An Overview with Focus on the Genetics. Genes. 2023; 14(7):1326. https://doi.org/10.3390/genes14071326
Chicago/Turabian StyleHo, Chih-Yi, Jeff Yi-Fu Chen, Wen-Li Hsu, Sebastian Yu, Wei-Chiao Chen, Szu-Hao Chiu, Hui-Ru Yang, Sheng-Yao Lin, and Ching-Ying Wu. 2023. "Female Pattern Hair Loss: An Overview with Focus on the Genetics" Genes 14, no. 7: 1326. https://doi.org/10.3390/genes14071326
APA StyleHo, C.-Y., Chen, J. Y.-F., Hsu, W.-L., Yu, S., Chen, W.-C., Chiu, S.-H., Yang, H.-R., Lin, S.-Y., & Wu, C.-Y. (2023). Female Pattern Hair Loss: An Overview with Focus on the Genetics. Genes, 14(7), 1326. https://doi.org/10.3390/genes14071326







