Nuclear Receptor Subfamily 2 Group E Member 3 (NR2E3): Role in Retinal Development and Disease
Abstract
1. Introduction
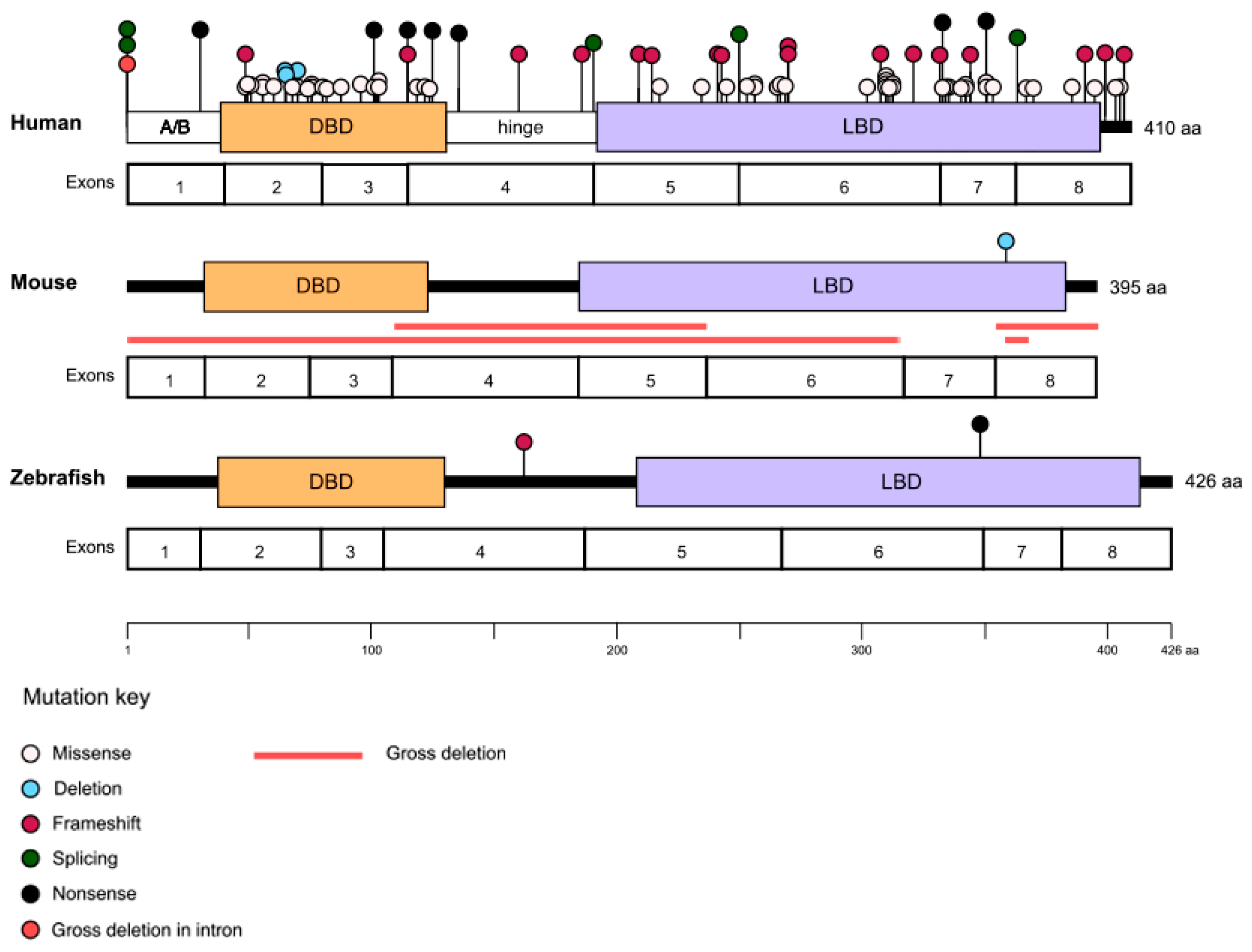
2. NR2E3 Structure
3. NR2E3 Function
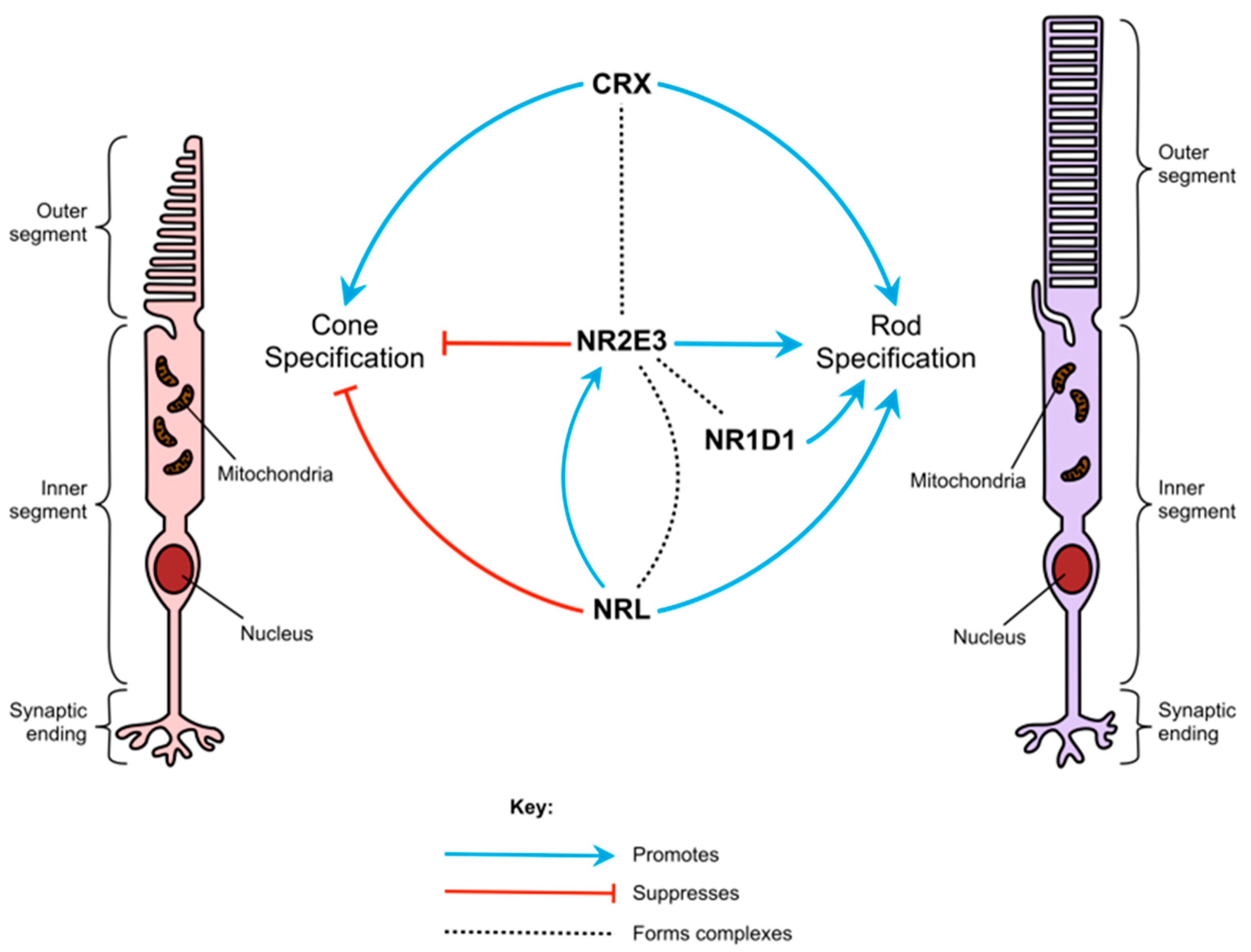
3.1. Rod and Cone Photoreceptor Differentiation
3.2. Role in the Adult Retina and Other Tissues
4. Clinical Phenotype
| Region | Variant | Mutation Type | Amino Acid Change | Protein Domain | Reported Phenotypes | References |
|---|---|---|---|---|---|---|
| Intron 1 | c.119-2A>C | Splicing | ESCS, GFS, CPRD, RP | [30,33,41] | ||
| c.119-3C>G | Splicing | ESCS | [46] | |||
| c.119-57_166 | Gross deletion | RP | [47] | |||
| Exon 1 | c.95G>A | Nonsense | p.Trp32 * | A/B | RP | [48] |
| Exon 2 | c.142C>T | Missense | p.Arg48Cys | DBD | ESCS | [40] |
| c.143_144delGCins25 | Indel/ Frameshift | p.Arg48Glufs*66 | DBD | RP | [49] | |
| c.145G>A | Missense | p.Val49Met | DBD | ESCS | [46] | |
| c.151G>A | Missense | p.Gly51Arg | DBD | ESCS | [40] | |
| c.166G>A | Missense | p.Gly56Arg | DBD | RP | [44] | |
| c.166G>C | Missense | p.Gly56Arg | DBD | RP | [50] | |
| c.188C>A | Missense | p.Ala63Asp | DBD | Cone–rod dystrophy | [51] | |
| c.194_202del | Deletion | p.Asn65_Cys67del | DBD | RP, ESCS | [30,52] | |
| c.196_201delGGCTGC | Deletion | p.Gly66_Cys67del | DBD | ESCS | [53] | |
| c.202A>G | Missense | p.Ser68Gly | DBD | ESCS | [54] | |
| c.211T>C | Missense | p.Phe71Leu | DBD | ESCS | [31] | |
| c.211_213delTTC | Deletion | p.Phe71del | DBD | ESCS | [42] | |
| c.223G>A | Missense | p.Val75Ile | DBD | RP | [55] | |
| c.226C>T | Missense | p.Arg76Trp | DBD | ESCS | [30] | |
| c.227G>A | Missense | p.Arg76Gln | DBD | ESCS | [30] | |
| c.242A>G | Missense | p.Tyr81Cys | DBD | ESCS | [46] | |
| Exon 3 | c.248G>A | Missense | p.Cys83Tyr | DBD | ESCS | [56] |
| c.263G>T | Missense | p.Gly88Val | DBD | ESCS | [57] | |
| c.290G>A | Missense | p.Arg97His | DBD | ESCS, RD | [30,58] | |
| c.305C>A | Missense | p.Ala102Asp | DBD | ESCS, RP, RD | [59,60,61] | |
| c.309C>A | Nonsense | p.Cys103 * | DBD | RP | [62] | |
| c.310C>T | Missense | p.Arg104Trp | DBD | ESCS | [30] | |
| c.311G>A | Missense | p.Arg104Gln | DBD | ESCS, RP, RD | [63,64,65] | |
| c.328C>T | Nonsense | p.Gln110 * | DBD | RD, RP | [16,66] | |
| c.328dupC | Insertion/ Frameshift | p.Gln110Profs*31 | DBD | GFS | [67] | |
| Exon 4 | c.352G>A | Missense | p.Val118Met | DBD | RP | [68] |
| c.364C>T | Missense | p.Arg122Cys | DBD | RP, RD | [16,69] | |
| c.371C>T | Missense | p.Pro124Leu | DBD | RP | [70] | |
| c.373C>T | Nonsense | p.Arg125 * | DBD | ESCS, RP | [71,72] | |
| c.406G>T | Nonsense | p.Glu136 * | DBD | RP | [66] | |
| c.481delA | Deletion/ Frameshift | p.Thr161Hisfs*18 | Hinge | RP, ESCS | [57,73] | |
| c.554delA | Deletion/ Frameshift | p.Lys185Serfs*66 | Hinge | RP | [74] | |
| Intron 4 | c.571+2T>C | Splicing | RP | [75] | ||
| Exon 5 | c.626dupA | Insertion/ Frameshift | p.Tyr209 * | LBD | RP | [74] |
| c.639_640insT | Insertion/ Frameshift | p.Pro214Serfs*9 | LBD | RD | [76] | |
| c.646G>A | Missense | p.Gly216Ser | LBD | GFS, RP | [72,77] | |
| c.701G>C | Missense | p.Trp234Ser | LBD | ESCS | [30] | |
| c.724_725delTC | Deletion/ Frameshift | p.Ser242Glnfs*17 | LBD | ESCS, RP, cone–rod dystrophy | [78,79,80] | |
| c.731delT | Deletion/ Frameshift | p.Leu244Argfs*7 | LBD | RP | [81] | |
| c.739C>T | Missense | p.Arg247Trp | LBD | ESCS | [31] | |
| Intron 5 | c.747+1G>C | Splicing | ESCS | [39] | ||
| Exon 6 | c.755T>C | Missense | p.Leu252Pro | LBD | ESCS | [82] |
| c.767C>A | Missense | p.Ala256Glu | LBD | ESCS, RD | [33,65] | |
| c.767C>T | Missense | p.Ala256Val | LBD | ESCS | [83] | |
| c.788T>C | Missense | p.Leu263Pro | LBD | ESCS | [57] | |
| c.790G>A | Missense | p.Gly264Arg | LBD | ESCS | [84] | |
| c.797T>A | Missense | p.Ile266Asn | LBD | RD | [61] | |
| c.808_809delCT | Deletion/ Frameshift | p.Leu270Alafs*70 | LBD | ESCS | [85] | |
| c.827_843del17 | Deletion/ Frameshift | p.Leu270Alafs*70 | LBD | ESCS, GFS, CPRD | [33] | |
| c.908T>C | Missense | p.Leu303Pro | LBD | ESCS | [31] | |
| c.919_920delAT | Deletion/ Frameshift | p.Ile307Leufs*33 | LBD | ESCS | [86] | |
| c.925C>G | Missense | p.Arg309Gly | LBD | ESCS | [30] | |
| c.925C>T | Missense | p.Arg309Trp | LBD | GFS | [87] | |
| c.926G>T | Missense | p.Arg309Leu | LBD | GFS | [72] | |
| c.926G>A | Missense | p.Arg309Gln | LBD | ESCS | [31] | |
| c.930C>G | Missense | p.Phe310Leu | LBD | ESCS | [88] | |
| c.931C>T | Missense | p.Arg311Trp | LBD | RD, RP | [66,89] | |
| c.932G>A | Missense | p.Arg311Gln | LBD | RP, ESCS, GFS, CPRD | [30,33,41,43] | |
| c.951delC | Deletion/ Frameshift | p.Thr318Argfs*6 | LBD | RP | [90] | |
| c.967dupA | Insertion/ Frameshift | p.Met323Asnfs*18 | LBD | RP | [52] | |
| Exon 7 | c.994G>A | Missense | p.Glu332Lys | LBD | ESCS | [59] |
| c.994G>T | Nonsense | p.Glu332 * | LBD | RD | [91] | |
| c.1000C>G | Missense | p.Arg334Gly | LBD | ESCS | [63] | |
| c.1007T>C | Missense | p.Leu336Pro | LBD | ESCS | [57] | |
| c.1018G>A | Missense | p.Glu340Lys | LBD | ESCS | [92] | |
| c.1025T>C | Missense | p.Val342Ala | LBD | ESCS | [59] | |
| c.1025T>G | Missense | p.Val342Gly | LBD | RP | [93] | |
| c.1034_1038delTGCAG | Deletion/ Frameshift | p.Leu345 * | LBD | RP | [41] | |
| c.1048C>G | Missense | p.Gln350Glu | LBD | RD | [76] | |
| c.1048C>T | Nonsense | p.Gln350 * | LBD | ESCS | [94] | |
| c.1049A>G | Missense | p.Gln350Arg | LBD | ESCS, RP | [42,95] | |
| c.1057C>G | Missense | p.Leu353Val | LBD | ESCS | [57] | |
| Intron 7 | c.1101-1G>A | Splicing | ESCS | [46] | ||
| Exon 8 | c.1112T>G | Missense | p.Leu371Trp | LBD | ESCS | [92] |
| c.1118T>C | Missense | p.Leu373Pro | LBD | ESCS, RD | [16,96] | |
| c.1154G>C | Missense | p.Arg385Pro | LBD | ESCS | [30] | |
| c.1171_1172delTT | Deletion/ Frameshift | p.Phe391Profs*15 | LBD | RP | [97] | |
| c.1184T>C | Missense | p.Ile395Thr | LBD | RP | [98] | |
| c.1194delT | Deletion/ Frameshift | p.Pro399Glnfs*79 | ESCS | [59] | ||
| c.1217A>G | Missense | p.Asp406Gly | GFS | [99] | ||
| c.1220T>A | Missense | p.Met407Lys | ESCS | [30] | ||
| c.1223delT | Deletion/ Frameshift | p.Phe408Serfs*7 | RD | [16] |
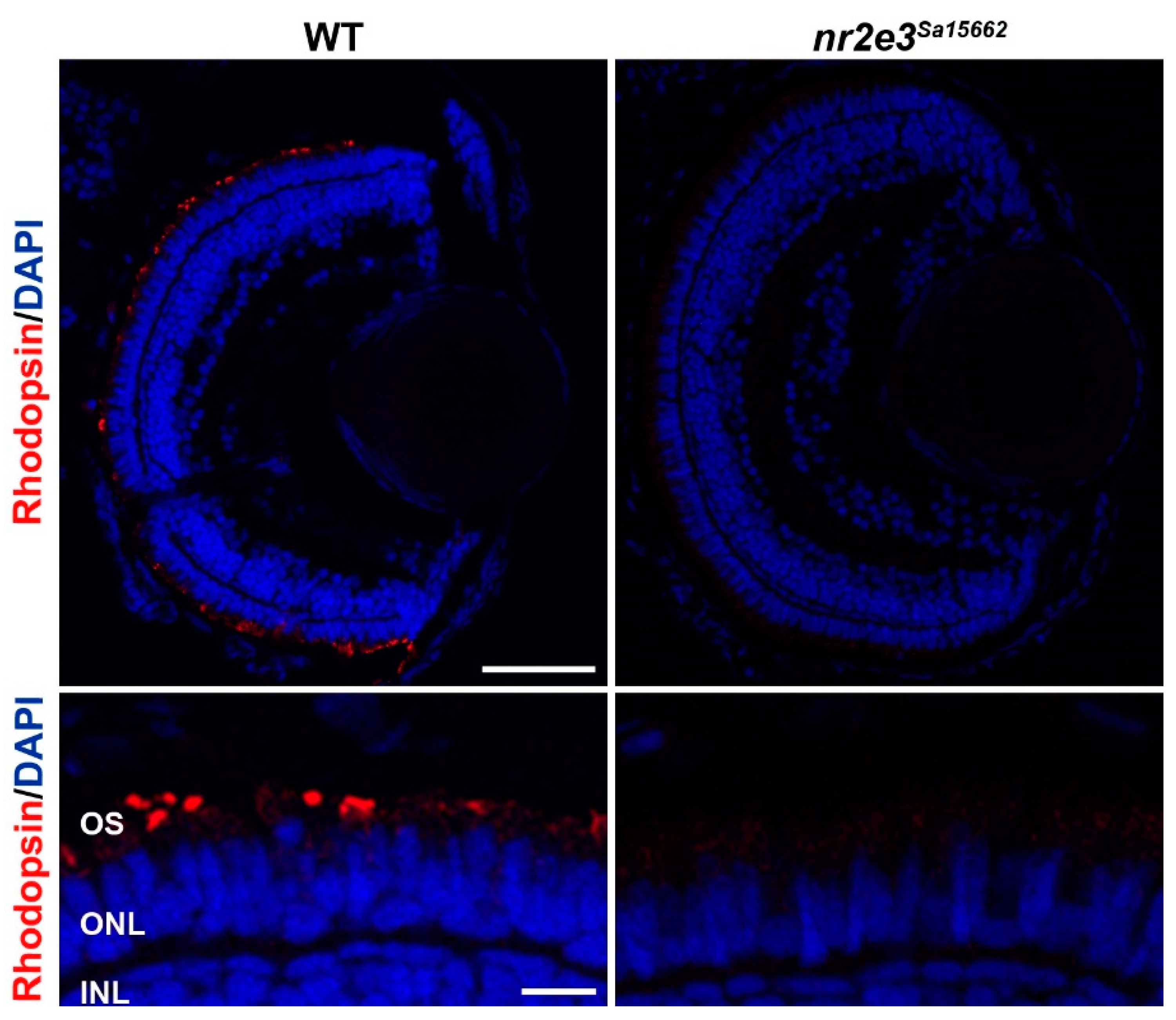
5. Animal Models
6. Treatments
7. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, J.; Rattner, A.; Nathans, J. The rod photoreceptor-specific nuclear receptor Nr2e3 represses transcription of multiple cone-specific genes. J. Neurosci. 2005, 25, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Aísa-Marín, I.; López-Iniesta, M.J.; Milla, S.; Lillo, J.; Navarro, G.; de la Villa, P.; Marfany, G. Nr2e3 functional domain ablation by CRISPR-Cas9D10A identifies a new isoform and generates retinitis pigmentosa and enhanced S-cone syndrome models. Neurobiol. Dis. 2020, 146, 105122. [Google Scholar] [CrossRef] [PubMed]
- Greenstein, V.C.; Zaidi, Q.; Hood, D.C.; Spehar, B.; Cideciyan, A.V.; Jacobson, S.G. The Enhanced S Cone Syndrome: An Analysis of Receptoral and Post-receptoral Changes. Vis. Res. 1996, 36, 3711–3722. [Google Scholar] [CrossRef]
- Hood, D.C.; Cideciyan, A.V.; Roman, A.J.; Jacobson, S.G. Enhanced S cone syndrome: Evidence for an abnormally large number of S cones. Vis. Res. 1995, 35, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Milam, A.H.; Rose, L.; Cideciyan, A.V.; Barakat, M.R.; Tang, W.-X.; Gupta, N.; Aleman, T.S.; Wright, A.F.; Stone, E.M.; Sheffield, V.C.; et al. The nuclear receptor NR2E3 plays a role in human retinal photoreceptor differentiation and degeneration. Proc. Natl. Acad. Sci. USA 2002, 99, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Haider, N.B.; Naggert, J.K.; Nishina, P.M. Excess cone cell proliferation due to lack of a functional NR2E3 causes retinal dysplasia and degeneration in rd7/rd7 mice. Hum. Mol. Genet. 2001, 10, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.E.; Zhou, X.E.; Soon, F.F.; Li, X.; Li, J.; Yong, E.L.; Melcher, K.; Xu, H.E. The crystal structure of the orphan nuclear receptor NR2E3/PNR ligand binding domain reveals a dimeric auto-repressed conformation. PLoS ONE 2013, 8, e74359. [Google Scholar] [CrossRef]
- Schorderet, D.F.; Escher, P. NR2E3 mutations in enhanced S-cone sensitivity syndrome (ESCS), Goldmann-Favre syndrome (GFS), clumped pigmentary retinal degeneration (CPRD), and retinitis pigmentosa (RP). Hum. Mutat. 2009, 30, 1475–1485. [Google Scholar] [CrossRef]
- Mollema, N.; Haider, N.B. Focus on Molecules: Nuclear hormone receptor Nr2e3: Impact on retinal development and disease. Exp. Eye Res. 2010, 91, 116–117. [Google Scholar] [CrossRef]
- Von Alpen, D.; Tran, H.V.; Guex, N.; Venturini, G.; Munier, F.L.; Schorderet, D.F.; Haider, N.B.; Escher, P. Differential Dimerization of Variants Linked to Enhanced S-Cone Sensitivity Syndrome (ESCS) Located in the NR2E3 Ligand-Binding Domain. Hum. Mutat. 2015, 36, 599–610. [Google Scholar] [CrossRef]
- Bowmaker, J.K. Evolution of colour vision in vertebrates. Eye 1998, 12, 541–547. [Google Scholar] [CrossRef]
- Mustafi, D.; Engel, A.H.; Palczewski, K. Structure of cone photoreceptors. Prog. Retin. Eye Res. 2009, 28, 289–302. [Google Scholar] [CrossRef]
- Lamb, T.D. Why rods and cones? Eye 2016, 30, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Calkins, D.J. Seeing with S cones. Prog. Retin. Eye Res. 2001, 20, 255–287. [Google Scholar] [CrossRef] [PubMed]
- Curcio, C.A.; Allen, K.A.; Sloan, K.R.; Lerea, C.L.; Hurley, J.B.; Klock, I.B.; Milam, A.H. Distribution and morphology of human cone photoreceptors stained with anti-blue opsin. J. Comp. Neurol. 1991, 312, 610–624. [Google Scholar] [CrossRef]
- Murro, V.; Mucciolo, D.P.; Sodi, A.; Passerini, I.; Giorgio, D.; Virgili, G.; Rizzo, S. Novel clinical findings in autosomal recessive NR2E3-related retinal dystrophy. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Haider, N.B.; Mollema, N.; Gaule, M.; Yuan, Y.; Sachs, A.J.; Nystuen, A.M.; Naggert, J.K.; Nishina, P.M. Nr2e3-directed transcriptional regulation of genes involved in photoreceptor development and cell-type specific phototransduction. Exp. Eye Res. 2009, 89, 365–372. [Google Scholar] [CrossRef]
- Cheng, H.; Khan, N.W.; Roger, J.E.; Swaroop, A. Excess cones in the retinal degeneration rd7 mouse, caused by the loss of function of orphan nuclear receptor Nr2e3, originate from early-born photoreceptor precursors. Hum. Mol. Genet. 2011, 20, 4102–4115. [Google Scholar] [CrossRef] [PubMed]
- Daniele, L.L.; Lillo, C.; Lyubarsky, A.L.; Nikonov, S.S.; Philp, N.; Mears, A.J.; Swaroop, A.; Williams, D.S.; Pugh, E.N. Cone-like morphological, molecular, and electrophysiological features of the photoreceptors of the Nrl knockout mouse. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2156–2167. [Google Scholar] [CrossRef]
- O’Brien, K.M.B.; Schulte, D.; Hendrickson, A.E. Expression of photoreceptor-associated molecules during human fetal eye development. Mol. Vis. 2003, 9, 401–409. [Google Scholar]
- Cheng, H.; Khanna, H.; Oh, E.C.; Hicks, D.; Mitton, K.; Swaroop, A. Photoreceptor-specific nuclear receptor NR2E3 functions as a transcriptional activator in rod photoreceptors. Hum. Mol. Genet. 2004, 13, 1563–1575. [Google Scholar] [CrossRef]
- Freund, C.L.; Wang, Q.-L.; Chen, S.; Muskat, B.L.; Wiles, C.D.; Sheffield, V.C.; Jacobson, S.G.; Mclnnes, R.R.; Zack, D.J.; Stone, E.M. De novo mutations in the CRX homeobox gene associated with Leber congenital amaurosis. Nat. Genet. 1998, 18, 311–312. [Google Scholar] [CrossRef] [PubMed]
- Freund, C.L.; Gregory-Evans, C.Y.; Furukawa, T.; Papaioannou, M.; Looser, J.; Ploder, L.; Bellingham, J.; Ng, D.; Herbrick, J.A.S.; Duncan, A.; et al. Cone-rod dystrophy due to mutations in a novel photoreceptor-specific homeobox gene (CRX) essential for maintenance of the photoreceptor. Cell 1997, 91, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.-H.; Ahmad, O.; Ahmad, F.; Liu, J.; Chen, S. The photoreceptor-specific nuclear receptor Nr2e3 interacts with Crx and exerts opposing effects on the transcription of rod versus cone genes. Hum. Mol. Genet. 2005, 14, 747–764. [Google Scholar] [CrossRef]
- Olivares, A.M.; Jelcick, A.S.; Reinecke, J.; Leehy, B.; Haider, A.; Morrison, M.A.; Cheng, L.; Chen, D.F.; DeAngelis, M.M.; Haider, N.B. Multimodal Regulation Orchestrates Normal and Complex Disease States in the Retina. Sci. Rep. 2017, 7, 690. [Google Scholar] [CrossRef]
- Li, S.; Datta, S.; Brabbit, E.; Love, Z.; Woytowicz, V.; Flattery, K.; Capri, J.; Yao, K.; Wu, S.; Imboden, M.; et al. Nr2e3 is a genetic modifier that rescues retinal degeneration and promotes homeostasis in multiple models of retinitis pigmentosa. Gene Ther. 2021, 28, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Khanal, T.; Leung, Y.-K.; Jiang, W.; Timchenko, N.; Ho, S.-M.; Kim, K. NR2E3 is a key component in p53 activation by regulating a long noncoding RNA DINO in acute liver injuries. FASEB J. 2019, 33, 8335–8348. [Google Scholar] [CrossRef] [PubMed]
- Khanal, T.; Choi, K.; Leung, Y.-K.; Wang, J.; Kim, D.; Janakiram, V.; Cho, S.-G.; Puga, A.; Ho, S.-M.; Kim, K. Loss of NR2E3 represses AHR by LSD1 reprogramming, is associated with poor prognosis in liver cancer. Sci. Rep. 2017, 7, 10662. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Kim, K.; Kim, S.; Hennessy, B.T.; Kim, S.M.; Park, E.S.; Lim, J.Y.; Li, J.; Lu, Y.; Gonzalez-Angulo, A.M.; et al. Reconstruction of nuclear receptor network reveals that NR2E3 is a novel upstream regulator of ESR1 in breast cancer. EMBO Mol. Med. 2012, 4, 52–67. [Google Scholar] [CrossRef]
- Haider, N.B.; Jacobson, S.G.; Cideciyan, A.V.; Swiderski, R.; Streb, L.M.; Searby, C.; Beck, G.; Hockey, R.; Hanna, D.B.; Gorman, S.; et al. Mutation of a nuclear receptor gene, NR2E3, causes enhanced S cone syndrome, a disorder of retinal cell fate. Nat. Genet. 2000, 24, 127–131. [Google Scholar] [CrossRef]
- de Carvalho, E.R.; Robson, A.G.; Arno, G.; Boon, C.J.; Webster, A.A.; Michaelides, M. Enhanced S-Cone Syndrome: Spectrum of Clinical, Imaging, Electrophysiologic, and Genetic Findings in a Retrospective Case Series of 56 Patients. Ophthalmol. Retina 2021, 5, 195–214. [Google Scholar] [CrossRef] [PubMed]
- Marmor, M.F.; Jacobson, S.G.; Foerster, M.H.; Kellner, U.; Weleber, R.G. Diagnostic Clinical Findings of a New Syndrome with Night Blindness, Maculopathy, and Enhanced S Cone Sensitivity. Am. J. Ophthalmol. 1990, 110, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Sharon, D.; Sandberg, M.A.; Caruso, R.C.; Berson, E.L.; Dryja, T.P. Shared mutations in NR2E3 in enhanced S-cone syndrome, Goldmann-Favre syndrome, and many cases of clumped pigmentary retinal degeneration. Arch. Ophthalmol. 2003, 121, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, S.G.; Román, A.J.; Román, M.I.; Gass, J.D.M.; Parker, J.A. Relatively Enhanced S Cone Function in the Goldmann-Favre Syndrome. Am. J. Ophthalmol. 1991, 111, 446–453. [Google Scholar] [CrossRef]
- Fishman, G.A.; Jampol, L.M.; Goldberg, M.F. Diagnostic features of the Favre-Goldmann syndrome. Br. J. Ophthalmol. 1976, 60, 345–353. [Google Scholar] [CrossRef]
- To, K.W.; Adamian, M.; Jakobiec, F.A.; Berson, E.L. Clinical and Histopathologic Findings in Clumped Pigmentary Retinal Degeneration. Arch. Ophthalmol. 1996, 114, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Kelly, F.; Hoyos, M.G.; Martinez, M.A.L.; Lopez-Molina, M.I.; Riveiro-Alvarez, R.; Jose, P.F.-S.; Avila-Fernandez, A.; Corton, M.; Millan, J.M.; Sandoval, B.G.; et al. Dominant Retinitis Pigmentosa, p.Gly56Arg Mutation in NR2E3: Phenotype in a Large Cohort of 24 Cases. PLoS ONE 2016, 11, e0149473. [Google Scholar] [CrossRef]
- Garafalo, A.V.; Calzetti, G.; Cideciyan, A.V.; Roman, A.J.; Saxena, S.; Sumaroka, A.; Choi, W.; Wright, A.F.; Jacobson, S.G. Cone Vision Changes in the Enhanced S-Cone Syndrome Caused by NR2E3Gene Mutations. Investig. Opthalmol. Vis. Sci. 2018, 59, 3209–3219. [Google Scholar] [CrossRef]
- Bandah, D.; Merin, S.; Ashhab, M.; Banin, E.; Sharon, D. The Spectrum of Retinal Diseases Caused by NR2E3 Mutations in Israeli and Palestinian Patients. Arch. Ophthalmol. 2009, 127, 297–302. [Google Scholar] [CrossRef]
- Kuniyoshi, K.; Hayashi, T.; Sakuramoto, H.; Nakao, A.; Sato, T.; Utsumi, T.; Tsuneoka, H.; Shimomura, Y. Novel Mutations in Enhanced S-cone Syndrome. Ophthalmology 2013, 120, 431.e1-6. [Google Scholar] [CrossRef]
- Bernal, S.; Solans, T.; Gamundi, M.; Hernan, I.; De Jorge, L.; Carballo, M.; Navarro, R.; Tizzano, E.; Ayuso, C.; Baiget, M. Analysis of the involvement of the NR2E3 gene in autosomal recessive retinal dystrophies. Clin. Genet. 2008, 73, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Pachydaki, S.I.; Klaver, C.; Barbazetto, I.A.; Roy, M.S.; Gouras, P.; Allikmets, R.; Yannuzzi, L.A. Phenotypic features of patients with NR2E3 mutations. Arch Ophthalmol. 2009, 127, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Gerber, S.; Rozet, J.M.; Takezawa, S.I.; Coutinho dos Santos, L.; Lopes, L.; Gribouval, O.; Penet, C.; Perrault, I.; Ducroq, D.; Souied, E.; et al. The photoreceptor cell-specific nuclear receptor gene (PNR) accounts for retinitis pigmentosa in the Crypto-Jews from Portugal (Marranos), survivors from the Spanish Inquisition. Hum. Genet. 2000, 107, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Coppieters, F.; Leroy, B.P.; Beysen, D.; Hellemans, J.; De Bosscher, K.; Haegeman, G.; Robberecht, K.; Wuyts, W.; Coucke, P.J.; De Baere, E. Recurrent Mutation in the First Zinc Finger of the Orphan Nuclear Receptor NR2E3 Causes Autosomal Dominant Retinitis Pigmentosa. Am. J. Hum. Genet. 2007, 81, 147–157. [Google Scholar] [CrossRef]
- Escher, P.; Gouras, P.; Tiab, L.; Bolay, S.; Delarive, T.; Chen, S.; Tsai, C.-C.; Hayashi, M.; Zernant, J.; Merriam, J.E.; et al. Mutations in NR2E3 can cause dominant or recessive retinal degenerations in the same family. Hum. Mutat. 2009, 30, 342–351. [Google Scholar] [CrossRef]
- Audo, I.; Michaelides, M.; Robson, A.G.; Hawlina, M.; Vaclavik, V.; Sandbach, J.M.; Neveu, M.M.; Hogg, C.R.; Hunt, D.M.; Moore, A.T.; et al. Phenotypic Variation in Enhanced S-cone Syndrome. Investig. Opthalmol. Vis. Sci. 2008, 49, 2082–2093. [Google Scholar] [CrossRef]
- van Huet, R.A.; Pierrache, L.H.; Meester-Smoor, M.A.; Klaver, C.C.; van den Born, L.I.; Hoyng, C.B.; de Wijs, I.J.; Collin, R.W.; Hoefsloot, L.H.; Klevering, B.J. The efficacy of microarray screening for autosomal recessive retinitis pigmentosa in routine clinical practice. Mol. Vis. 2015, 21, 461–476. [Google Scholar]
- Neveling, K.; Collin, R.W.; Gilissen, C.; van Huet, R.A.; Visser, L.; Kwint, M.P.; Gijsen, S.J.; Zonneveld, M.N.; Wieskamp, N.; de Ligt, J.; et al. Next-generation genetic testing for retinitis pigmentosa. Hum. Mutat. 2012, 33, 963–972. [Google Scholar] [CrossRef]
- Kannabiran, C.; Singh, H.; Sahini, N.; Jalali, S.; Mohan, G. Mutations in TULP1, NR2E3, and MFRP genes in Indian families with autosomal recessive retinitis pigmentosa. Mol. Vis. 2012, 18, 1165–1174. [Google Scholar]
- Xu, Y.; Guan, L.; Shen, T.; Zhang, J.; Xiao, X.; Jiang, H.; Li, S.; Yang, J.; Jia, X.; Yin, Y.; et al. Mutations of 60 known causative genes in 157 families with retinitis pigmentosa based on exome sequencing. Hum. Genet. 2014, 133, 1255–1271. [Google Scholar] [CrossRef]
- I Gire, A.; Sullivan, L.S.; Bowne, S.J.; Birch, D.G.; Hughbanks-Wheaton, D.; Heckenlively, J.R.; Daiger, S.P. The Gly56Arg mutation in NR2E3 accounts for 1–2% of autosomal dominant retinitis pigmentosa. Mol. Vis. 2007, 13, 1970–1975. [Google Scholar] [PubMed]
- Bravo-Gil, N.; Méndez-Vidal, C.; Romero-Pérez, L.; Pozo, M.G.-D.; la Rúa, E.R.-D.; Dopazo, J.; Borrego, S.; Antiñolo, G. Improving the management of Inherited Retinal Dystrophies by targeted sequencing of a population-specific gene panel. Sci. Rep. 2016, 6, 23910. [Google Scholar] [CrossRef] [PubMed]
- Udar, N.; Small, K.; Chalukya, M.; Silva-Garcia, R.; Marmor, M. Developmental or degenerative—NR2E3 gene mutations in two patients with enhanced S cone syndrome. Mol. Vis. 2011, 17, 519–525. [Google Scholar]
- Park, S.P.; Hong, I.H.; Tsang, S.H.; Lee, W.; Horowitz, J.; Yzer, S.; Allikmets, R.; Chang, S. Disruption of the human cone photoreceptor mosaic from a defect in NR2E3 transcription factor function in young adults. Graefe’s Arch. Clin. Exp. Ophthalmol. 2013, 251, 2299–2309. [Google Scholar] [CrossRef]
- Maeda, A.; Yoshida, A.; Kawai, K.; Arai, Y.; Akiba, R.; Inaba, A.; Takagi, S.; Fujiki, R.; Hirami, Y.; Kurimoto, Y.; et al. Development of a molecular diagnostic test for Retinitis Pigmentosa in the Japanese population. Jpn. J. Ophthalmol. 2018, 62, 451–457. [Google Scholar] [CrossRef]
- Rocha-Sousa, A.; Hayashi, T.; Gomes, N.L.; Penas, S.; Brandão, E.; Rocha, P.; Urashima, M.; Yamada, H.; Tsuneoka, H.; Falcão-Reis, F. A novel mutation (Cys83Tyr) in the second zinc finger of NR2E3 in enhanced S-cone syndrome. Graefes Arch. Clin. Exp. Ophthalmol. 2011, 249, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.F.; Reddick, A.C.; Schwartz, S.B.; Ferguson, J.S.; Aleman, T.S.; Kellner, U.; Jurklies, B.; Schuster, A.; Zrenner, E.; Wissinger, B.; et al. Mutation analysis ofNR2E3 andNRL genes in Enhanced S Cone Syndrome. Hum. Mutat. 2004, 24, 439. [Google Scholar] [CrossRef]
- Huang, X.-F.; Huang, F.; Wu, K.-C.; Wu, J.; Chen, J.; Pang, C.-P.; Lu, F.; Qu, J.; Jin, Z.-B. Genotype–phenotype correlation and mutation spectrum in a large cohort of patients with inherited retinal dystrophy revealed by next-generation sequencing. Genet. Med. 2015, 17, 271–278. [Google Scholar] [CrossRef]
- Hull, S.; Arno, G.; Sergouniotis, P.I.; Tiffin, P.; Borman, A.D.; Chandra, A.; Robson, A.; Holder, G.E.; Webster, A.R.; Moore, A.T. Clinical and Molecular Characterization of Enhanced S-Cone Syndrome in Children. JAMA Ophthalmol. 2014, 132, 1341–1349. [Google Scholar] [CrossRef]
- Stone, E.M.; Andorf, J.L.; Whitmore, S.S.; DeLuca, A.P.; Giacalone, J.C.; Streb, L.M.; Braun, T.A.; Mullins, R.F.; Scheetz, T.E.; Sheffield, V.C.; et al. Clinically Focused Molecular Investigation of 1000 Consecutive Families with Inherited Retinal Disease. Ophthalmology 2017, 124, 1314–1331. [Google Scholar] [CrossRef]
- Lingao, M.D.; Ganesh, A.; Karthikeyan, A.S.; Al Zuhaibi, S.; Al-Hosni, A.; Al Khayat, A.; Capasso, J.; Trumler, A.A.; Stroh, E.; Al Shekaili, H.; et al. Macular cystoid spaces in patients with retinal dystrophy. Ophthalmic Genet. 2016, 37, 377–383. [Google Scholar] [CrossRef]
- Birtel, J.; Gliem, M.; Mangold, E.; Müller, P.L.; Holz, F.G.; Neuhaus, C.; Lenzner, S.; Zahnleiter, D.; Betz, C.; Eisenberger, T.; et al. Next-generation sequencing identifies unexpected genotype-phenotype correlations in patients with retinitis pigmentosa. PLoS ONE 2018, 13, e0207958. [Google Scholar] [CrossRef]
- Hayashi, T.; Gekka, T.; Goto-Omoto, S.; Takeuchi, T.; Kubo, A.; Kitahara, K. Novel NR2E3 mutations (R104Q, R334G) associated with a mild form of enhanced S-cone syndrome demonstrate compound heterozygosity. Ophthalmology 2005, 112, 2115. [Google Scholar] [CrossRef]
- Kimchi, A.; Khateb, S.; Wen, R.; Guan, Z.; Obolensky, A.; Beryozkin, A.; Kurtzman, S.; Blumenfeld, A.; Pras, E.; Jacobson, S.G.; et al. Nonsyndromic Retinitis Pigmentosa in the Ashkenazi Jewish Population: Genetic and Clinical Aspects. Ophthalmology 2018, 125, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Carss, K.J.; Arno, G.; Erwood, M.; Stephens, J.; Sanchis-Juan, A.; Hull, S.; Megy, K.; Grozeva, D.; Dewhurst, E.; Malka, S.; et al. Comprehensive Rare Variant Analysis via Whole-Genome Sequencing to Determine the Molecular Pathology of Inherited Retinal Disease. Am. J. Hum. Genet. 2017, 100, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Weisschuh, N.; Obermaier, C.D.; Battke, F.; Bernd, A.; Kuehlewein, L.; Nasser, F.; Zobor, D.; Zrenner, E.; Weber, E.; Wissinger, B.; et al. Genetic architecture of inherited retinal degeneration in Germany: A large cohort study from a single diagnostic center over a 9-year period. Hum. Mutat. 2020, 41, 1514–1527. [Google Scholar] [CrossRef] [PubMed]
- Carrigan, M.; Duignan, E.; Malone, C.P.G.; Stephenson, K.; Saad, T.; McDermott, C.; Green, A.; Keegan, D.; Humphries, P.; Kenna, P.F.; et al. Panel-Based Population Next-Generation Sequencing for Inherited Retinal Degenerations. Sci. Rep. 2016, 6, 33248. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Chen, L.J.; Chiang, S.W.; Tam, P.O.; Lai, T.Y.; Chan, C.K.; Wang, N.; Lam, D.S.; Pang, C.P. Association of NR2E3 but not NRL mutations with retinitis pigmentosa in the Chinese population. Investigative Ophthalmol. Vis. Sci. 2010, 51, 2229–2235. [Google Scholar] [CrossRef]
- Bocquet, B.; Marzouka, N.A.D.; Hebrard, M.; Manes, G.; Sénéchal, A.; Meunier, I.; Hamel, C.P. Homozygosity mapping in autosomal recessive retinitis pigmentosa families detects novel mutations. Mol. Vis. 2013, 19, 2487–2500. [Google Scholar]
- Takahashi, V.K.L.; Xu, C.L.; Takiuti, J.T.; Apatoff, M.B.L.; Duong, J.K.; Mahajan, V.B.; Tsang, S.H. Comparison of structural progression between ciliopathy and non-ciliopathy associated with autosomal recessive retinitis pigmentosa. Orphanet J. Rare Dis. 2019, 14, 187. [Google Scholar] [CrossRef]
- Cassiman, C.; Spileers, W.; De Baere, E.; De Ravel, T.; Casteels, I. Peculiar fundus abnormalities and pathognomonic electrophysiological findings in a 14-month-old boy with NR2E3 mutations. Ophthalmic Genet. 2013, 34, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Aldahmesh, M.A.; Alkuraya, H.; Anazi, S.; Alsharif, H.; Khan, A.O.; Sunker, A.; Al-Mohsen, S.; Abboud, E.B.; Nowilaty, S.R.; et al. Expanding the clinical, allelic, and locus heterogeneity of retinal dystrophies. Genet. Med. 2016, 18, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.-C.C.; Yu, H.-C.; Martin, R.; Cirulli, E.T.; Schenker-Ahmed, N.M.; Hicks, M.; Cohen, I.V.; Jönsson, T.J.; Heister, R.; Napier, L.; et al. Precision medicine integrating whole-genome sequencing, comprehensive metabolomics, and advanced imaging. Proc. Natl. Acad. Sci. USA 2020, 117, 3053–3062. [Google Scholar] [CrossRef] [PubMed]
- Jespersgaard, C.; Fang, M.; Bertelsen, M.; Dang, X.; Jensen, H.; Chen, Y.; Bech, N.; Dai, L.; Rosenberg, T.; Zhang, J.; et al. Molecular genetic analysis using targeted NGS analysis of 677 individuals with retinal dystrophy. Sci. Rep. 2019, 9, 1219. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, D.; Votruba, M. A novel NR2E3 gene mutation in autosomal recessive retinitis pigmentosa with cystic maculopathy. Acta Ophthalmol. 2018, 96, e535–e536. [Google Scholar] [CrossRef] [PubMed]
- Al-Khuzaei, S.; Broadgate, S.; Halford, S.; Jolly, J.K.; Shanks, M.; Clouston, P.; Downes, S.M. Novel Pathogenic Sequence Variants in NR2E3 and Clinical Findings in Three Patients. Genes 2020, 11, 1288. [Google Scholar] [CrossRef]
- Seo, G.H.; Kim, T.; Choi, I.H.; Park, J.Y.; Lee, J.; Kim, S.; Won, D.G.; Oh, A.; Lee, Y.; Choi, J.; et al. Diagnostic yield and clinical utility of whole exome sequencing using an automated variant prioritization system, EVIDENCE. Clin. Genet. 2020, 98, 562–570. [Google Scholar] [CrossRef]
- Collin, R.W.J.; Born, L.I.V.D.; Klevering, B.J.; de Castro-Miró, M.; Littink, K.W.; Arimadyo, K.; Azam, M.; Yazar, V.; Zonneveld, M.N.; Paun, C.C.; et al. High-Resolution Homozygosity Mapping Is a Powerful Tool to Detect Novel Mutations Causative of Autosomal Recessive RP in the Dutch Population. Investig. Opthalmol. Vis. Sci. 2011, 52, 2227–2239. [Google Scholar] [CrossRef]
- Glöckle, N.; Kohl, S.; Mohr, J.; Scheurenbrand, T.; Sprecher, A.; Weisschuh, N.; Bernd, A.; Rudolph, G.; Schubach, M.; Poloschek, C.; et al. Panel-based next generation sequencing as a reliable and efficient technique to detect mutations in unselected patients with retinal dystrophies. Eur. J. Hum. Genet. 2014, 22, 99–104. [Google Scholar] [CrossRef]
- Haer-Wigman, L.; Van Zelst-Stams, W.A.G.; Pfundt, R.; Born, L.I.V.D.; Klaver, C.; Verheij, J.B.G.M.; Hoyng, C.B.; Breuning, M.H.; Boon, C.; Kievit, A.J.; et al. Diagnostic exome sequencing in 266 Dutch patients with visual impairment. Eur. J. Hum. Genet. 2017, 25, 591–599. [Google Scholar] [CrossRef]
- Martin-Merida, I.; Avila-Fernandez, A.; Del Pozo-Valero, M.; Blanco-Kelly, F.; Zurita, O.; Perez-Carro, R.; Aguilera-Garcia, D.; Riveiro-Alvarez, R.; Arteche, A.; Trujillo-Tiebas, M.J.; et al. Genomic Landscape of Sporadic Retinitis Pigmentosa: Findings from 877 Spanish Cases. Ophthalmology 2019, 126, 1181–1188. [Google Scholar] [CrossRef]
- Cehajic-Kapetanovic, J.; Cottriall, C.L.; Jolly, J.K.; Shanks, M.; Clouston, P.; Issa, P.C.; MacLaren, R.E. Electrophysiological verification of enhanced S-cone syndrome caused by a novel c.755T>C NR2E3 missense variant. Ophthalmic Genet. 2019, 40, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Lam, B.L.; Goldberg, J.L.; Hartley, K.L.; Stone, E.M.; Liu, M. Atypical Mild Enhanced S-Cone Syndrome with Novel Compound Heterozygosity of the NR2E3 Gene. Am. J. Ophthalmol. 2007, 144, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Termühlen, J.; Alex, A.F.; Glöckle, N.; Kellner, U.; Fiedler, B.; Eter, N.; Uhlig, C.E. A new mutation in enhanced S-cone syndrome. Acta Ophthalmol. 2018, 96, e539–e540. [Google Scholar] [CrossRef] [PubMed]
- Collison, F.T.; Park, J.C.; Fishman, G.A.; Stone, E.M.; McAnany, J.J. Two-color pupillometry in enhanced S-cone syndrome caused by NR2E3 mutations. Doc. Ophthalmol. 2016, 132, 157–166. [Google Scholar] [CrossRef]
- Kuniyoshi, K.; Hayashi, T.; Sakuramoto, H.; Mishima, H.; Tsuneoka, H.; Tsunoda, K.; Iwata, T.; Shimomura, Y. New truncation mutation of the NR2E3 gene in a Japanese patient with enhanced S-cone syndrome. Jpn. J. Ophthalmol. 2016, 60, 476–485. [Google Scholar] [CrossRef]
- Bai, Z.; Xie, Y.; Liu, L.; Shao, J.; Liu, Y.; Kong, X. Genetic investigation of 211 Chinese families expands the mutational and phenotypical spectra of hereditary retinopathy genes through targeted sequencing technology. BMC Med. Genom. 2021, 14, 92. [Google Scholar] [CrossRef]
- Bechet, L.; Atia, R.; Zeitz, C.; Mohand-Saïd, S.; Sahel, J.-A.; Barale, P.-O.; Audo, I. Management of a case of Enhanced S-cone syndrome with massive foveoschisis treated with pars plana vitrectomy with silicone oil tamponade. Ophthalmic Genet. 2021, 42, 615–618. [Google Scholar] [CrossRef]
- Patel, N.; Alkuraya, H.; Alzahrani, S.; Nowailaty, S.; Seidahmed, M.; Alhemidan, A.; Ben-Omran, T.; Ghazi, N.; Al-Aqeel, A.; Al-Owain, M.; et al. Mutations in known disease genes account for the majority of autosomal recessive retinal dystrophies. Clin. Genet. 2018, 94, 554–563. [Google Scholar] [CrossRef]
- Abu-Safieh, L.; Alrashed, M.; Anazi, S.; Alkuraya, H.; Khan, A.O.; Al-Owain, M.; Al-Zahrani, J.; Al-Abdi, L.; Hashem, M.; Al-Tarimi, S.; et al. Autozygome-guided exome sequencing in retinal dystrophy patients reveals pathogenetic mutations and novel candidate disease genes. Genome Res. 2013, 23, 236–247. [Google Scholar] [CrossRef]
- Dockery, A.; Stephenson, K.; Keegan, D.; Wynne, N.; Silvestri, G.; Humphries, P.; Kenna, P.F.; Carrigan, M.; Farrar, G.J. Target 5000: Target Capture Sequencing for Inherited Retinal Degenerations. Genes 2017, 8, 304. [Google Scholar] [CrossRef]
- Ripamonti, C.; Aboshiha, J.; Henning, G.B.; Sergouniotis, P.I.; Michaelides, M.; Moore, A.T.; Webster, A.R.; Stockman, A. Vision in Observers with Enhanced S-Cone Syndrome: An Excess of S-Cones but Connected Mainly to Conventional S-Cone Pathways. Investig. Opthalmol. Vis. Sci. 2014, 55, 963–976. [Google Scholar] [CrossRef] [PubMed]
- Siemiatkowska, A.M.; Arimadyo, K.; Moruz, L.M.; Astuti, G.D.; De Castro-Miro, M.; Zonneveld, M.N.; Strom, T.M.; De Wijs, I.J.; Hoefsloot, L.H.; Faradz, S.M.; et al. Molecular genetic analysis of retinitis pigmentosa in Indonesia using genome-wide homozygosity mapping. Mol. Vis. 2011, 17, 3013–3024. [Google Scholar] [PubMed]
- Nakamura, Y.; Hayashi, T.; Kozaki, K.; Kubo, A.; Omoto, S.; Watanabe, A.; Toda, K.; Takeuchi, T.; Gekka, T.; Kitahara, K. Enhanced S-cone syndrome in a Japanese family with a nonsense NR2E3 mutation (Q350X). Acta Ophthalmol. Scand. 2004, 82, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Maltese, P.E.; Colombo, L.; Martella, S.; Rossetti, L.; El Shamieh, S.; Sinibaldi, L.; Passarelli, C.; Coppè, A.M.; Buzzonetti, L.; Falsini, B.; et al. Genetics of Inherited Retinal Diseases in Understudied Ethnic Groups in Italian Hospitals. Front. Genet. 2022, 13, 914345. [Google Scholar] [CrossRef]
- Minnella, A.M.; Pagliei, V.; Savastano, M.C.; Federici, M.; Bertelli, M.; Maltese, P.E.; Placidi, G.; Corbo, G.; Falsini, B.; Caporossi, A. Swept source optical coherence tomography and optical coherence tomography angiography in pediatric enhanced S-cone syndrome: A case report. J. Med. Case Rep. 2018, 12, 287. [Google Scholar] [CrossRef]
- Song, F.; Owczarek-Lipska, M.; Ahmels, T.; Book, M.; Aisenbrey, S.; Menghini, M.; Barthelmes, D.; Schrader, S.; Spital, G.; Neidhardt, J. High-Throughput Sequencing to Identify Mutations Associated with Retinal Dystrophies. Genes 2021, 12, 1269. [Google Scholar] [CrossRef]
- Gao, F.-J.; Li, J.-K.; Chen, H.; Hu, F.-Y.; Zhang, S.-H.; Qi, Y.-H.; Xu, P.; Wang, D.-D.; Wang, L.-S.; Chang, Q.; et al. Genetic and Clinical Findings in a Large Cohort of Chinese Patients with Suspected Retinitis Pigmentosa. Ophthalmology 2019, 126, 1549–1556. [Google Scholar] [CrossRef]
- Manayath, G.J.; Namburi, P.; Periasamy, S.; Kale, J.A.; Narendran, V.; Ganesh, A. A novel mutation in the NR2E3 gene associated with Goldmann-Favre syndrome and vasoproliferative tumor of the retina. Mol. Vis. 2014, 20, 724–731. [Google Scholar]
- Roduit, R.; Escher, P.; Schorderet, D.F. Mutations in the DNA-binding domain of NR2E3 affect in vivo dimerization and interaction with CRX. PLoS ONE 2009, 4, e7379. [Google Scholar] [CrossRef]
- Akhmedov, N.B.; Piriev, N.I.; Chang, B.; Rapoport, A.L.; Hawes, N.L.; Nishina, P.M.; Nusinowitz, S.; Heckenlively, J.R.; Roderick, T.H.; Kozak, C.A.; et al. A deletion in a photoreceptor-specific nuclear receptor mRNA causes retinal degeneration in the rd7 mouse. Proc. Natl. Acad. Sci. USA 2000, 97, 5551–5556. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Rattner, A.; Nathans, J. Effects of L1 retrotransposon insertion on transcript processing, localization and accumulation: Lessons from the retinal degeneration 7 mouse and implications for the genomic ecology of L1 elements. Hum. Mol. Genet. 2006, 15, 2146–2156. [Google Scholar] [CrossRef]
- Iannaccone, A.; Brabbit, E.; Lopez-Miro, C.; Love, Z.; Griffiths, V.; Kedrov, M.; Haider, N.B. Interspecies Correlations between Human and Mouse NR2E3-Associated Recessive Disease. J. Clin. Med. 2021, 10, 475. [Google Scholar] [CrossRef] [PubMed]
- Webber, A.L.; Hodor, P.; Thut, C.J.; Vogt, T.F.; Zhang, T.; Holder, D.J.; Petrukhin, K. Dual role of Nr2e3 in photoreceptor development and maintenance. Exp. Eye Res. 2008, 87, 35–48. [Google Scholar] [CrossRef]
- Xie, S.; Han, S.; Qu, Z.; Liu, F.; Li, J.; Yu, S.; Reilly, J.; Tu, J.; Liu, X.; Lu, Z.; et al. Knockout of Nr2e3 prevents rod photoreceptor differentiation and leads to selective L-/M-cone photoreceptor degeneration in zebrafish. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Cruz, N.M.; Yuan, Y.; Leehy, B.D.; Baid, R.; Kompella, U.; DeAngelis, M.M.; Escher, P.; Haider, N.B. Modifier genes as therapeutics: The nuclear hormone receptor Rev Erb Alpha (Nr1d1) rescues Nr2e3 associated retinal disease. PLoS ONE 2014, 9, e87942. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, P.A.; Tang, S.; Shimchuk, A.A.; Ding, S.; Reh, T.A. Potential of Small Molecule–Mediated Reprogramming of Rod Photoreceptors to Treat Retinitis Pigmentosa. Investig. Opthalmol. Vis. Sci. 2016, 57, 6407–6415. [Google Scholar] [CrossRef]
- Montana, C.L.; Kolesnikov, A.V.; Shen, S.Q.; Myers, C.A.; Kefalov, V.J.; Corbo, J.C. Reprogramming of adult rod photoreceptors prevents retinal degeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 1732–1737. [Google Scholar] [CrossRef] [PubMed]
- Simunovic, M.; Shen, W.; Lin, J.; Protti, D.; Lisowski, L.; Gillies, M. Optogenetic approaches to vision restoration. Exp. Eye Res. 2019, 178, 15–26. [Google Scholar] [CrossRef]
- Tochitsky, I.; Kienzler, M.A.; Isacoff, E.; Kramer, R.H. Restoring Vision to the Blind with Chemical Photoswitches. Chem. Rev. 2018, 118, 10748–10773. [Google Scholar] [CrossRef]
- Bohrer, L.R.; Wiley, L.A.; Burnight, E.R.; Cooke, J.A.; Giacalone, J.C.; Anfinson, K.R.; Andorf, J.L.; Mullins, R.F.; Stone, E.M.; Tucker, B.A. Correction of NR2E3 Associated Enhanced S-cone Syndrome Patient-specific iPSCs using CRISPR-Cas9. Genes 2019, 10, 278. [Google Scholar] [CrossRef] [PubMed]
- Naessens, S.; Ruysschaert, L.; Lefever, S.; Coppieters, F.; De Baere, E. Antisense Oligonucleotide-Based Downregulation of the G56R Pathogenic Variant Causing NR2E3-Associated Autosomal Dominant Retinitis Pigmentosa. Genes 2019, 10, 363. [Google Scholar] [CrossRef] [PubMed]
- Terrell, D.; Comander, J. Current Stem-Cell Approaches for the Treatment of Inherited Retinal Degenerations. Semin. Ophthalmol. 2019, 34, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Gasparini, S.J.; Llonch, S.; Borsch, O.; Ader, M. Transplantation of photoreceptors into the degenerative retina: Current state and future perspectives. Prog. Retin. Eye Res. 2019, 69, 1–37. [Google Scholar] [CrossRef]
- Iannaccone, A.; Fung, K.H.; Eyestone, M.E.; Stone, E.M. Treatment of Adult-Onset Acute Macular Retinoschisis in Enhanced S-cone Syndrome with Oral Acetazolamide. Am. J. Ophthalmol. 2009, 147, 307–312.e2. [Google Scholar] [CrossRef]
- Chatzistergiou, V.; Papasavvas, I.; Escher, P.; Durig, J.; Vaudaux, J.; Pournaras, J.-A.; Ambresin, A. Optical Coherence Tomography Analysis of Cystoid Macular Edema in Retinal Dystrophy Treated with Oral Acetazolamide: Two Cases. Klin. Mon. Für Augenheilkd. 2020, 237, 484–486. [Google Scholar] [CrossRef]
- Wiley, L.A.; Burnight, E.R.; DeLuca, A.P.; Anfinson, K.R.; Cranston, C.M.; Kaalberg, E.E.; Penticoff, J.A.; Affatigato, L.M.; Mullins, R.F.; Stone, E.M.; et al. cGMP production of patient-specific iPSCs and photoreceptor precursor cells to treat retinal degenerative blindness. Sci. Rep. 2016, 6, 30742. [Google Scholar] [CrossRef]
- Terray, A.; Slembrouck, A.; Nanteau, C.; Chondroyer, C.; Zeitz, C.; Sahel, J.-A.; Audo, I.; Reichman, S.; Goureau, O. Generation of an induced pluripotent stem cell (iPSC) line from a patient with autosomal dominant retinitis pigmentosa due to a mutation in the NR2E3 gene. Stem Cell Res. 2017, 24, 1–4. [Google Scholar] [CrossRef]
- Diakatou, M.; Dubois, G.; Erkilic, N.; Sanjurjo-Soriano, C.; Meunier, I.; Kalatzis, V. Allele-Specific Knockout by CRISPR/Cas to Treat Autosomal Dominant Retinitis Pigmentosa Caused by the G56R Mutation in NR2E3. Int. J. Mol. Sci. 2021, 22, 2607. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toms, M.; Ward, N.; Moosajee, M. Nuclear Receptor Subfamily 2 Group E Member 3 (NR2E3): Role in Retinal Development and Disease. Genes 2023, 14, 1325. https://doi.org/10.3390/genes14071325
Toms M, Ward N, Moosajee M. Nuclear Receptor Subfamily 2 Group E Member 3 (NR2E3): Role in Retinal Development and Disease. Genes. 2023; 14(7):1325. https://doi.org/10.3390/genes14071325
Chicago/Turabian StyleToms, Maria, Natasha Ward, and Mariya Moosajee. 2023. "Nuclear Receptor Subfamily 2 Group E Member 3 (NR2E3): Role in Retinal Development and Disease" Genes 14, no. 7: 1325. https://doi.org/10.3390/genes14071325
APA StyleToms, M., Ward, N., & Moosajee, M. (2023). Nuclear Receptor Subfamily 2 Group E Member 3 (NR2E3): Role in Retinal Development and Disease. Genes, 14(7), 1325. https://doi.org/10.3390/genes14071325







