Integration of GWAS and RNA-Seq Analysis to Identify SNPs and Candidate Genes Associated with Alkali Stress Tolerance at the Germination Stage in Mung Bean
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Phenotyping for Alkali Stress
2.2. Whole-Genome Re-Sequencing and GWAS Analysis
2.3. RNA Sequencing and Data Analysis
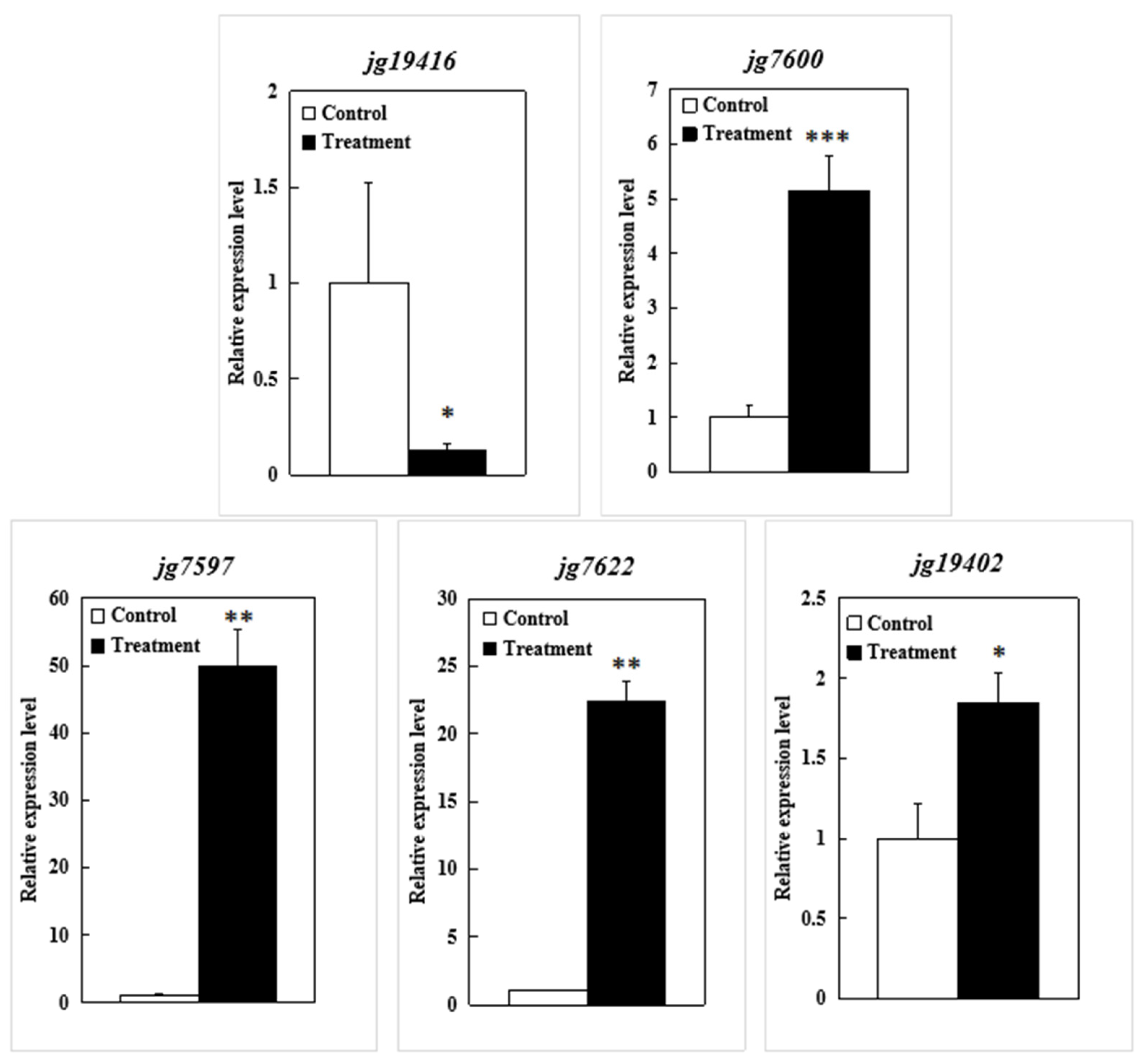
2.4. Quantitative Real-Time PCR (qRT-PCR) Analysis
3. Results
3.1. Phenotypic Trait Analysis
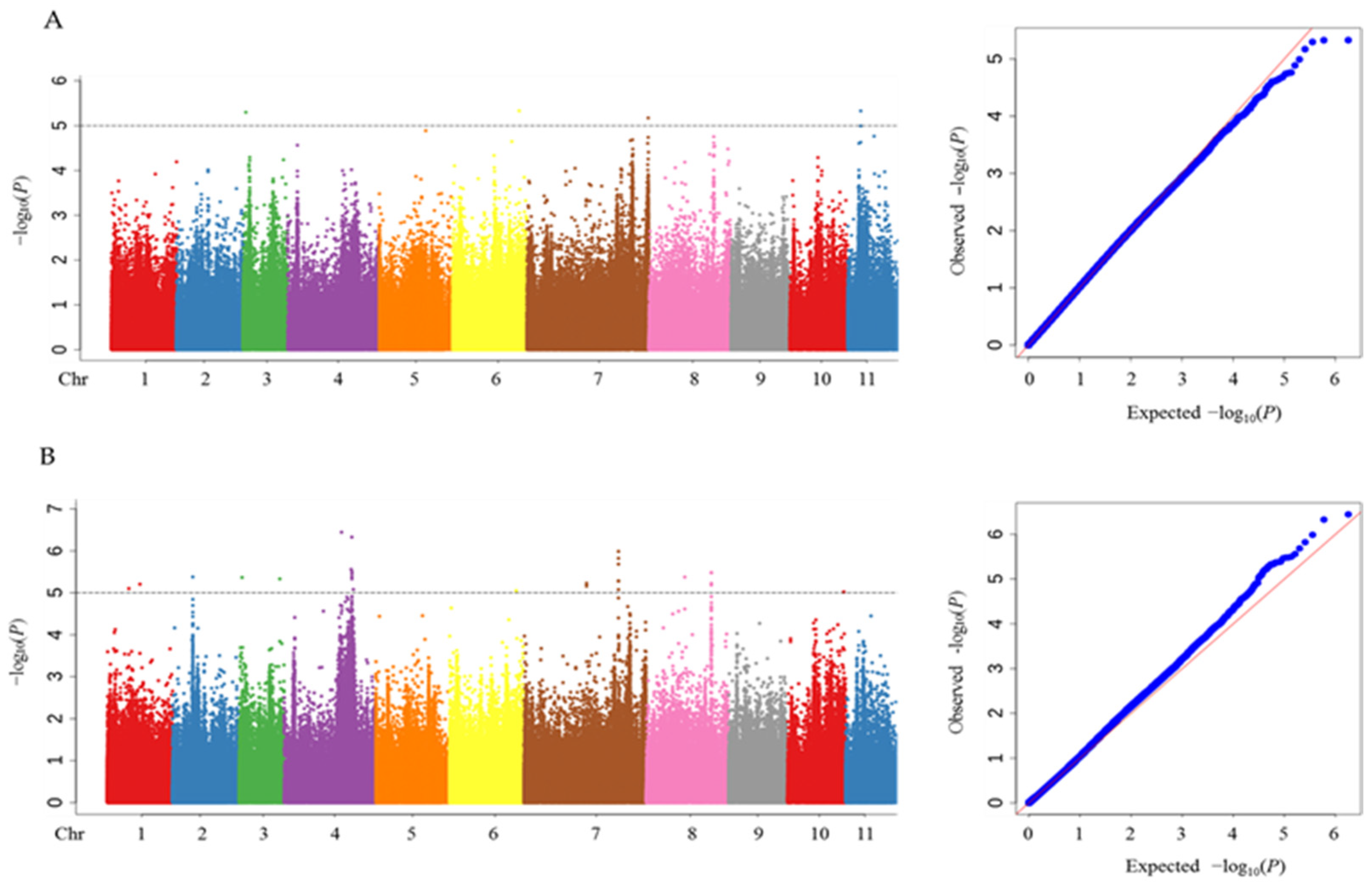
3.2. Genome-Wide Association Study
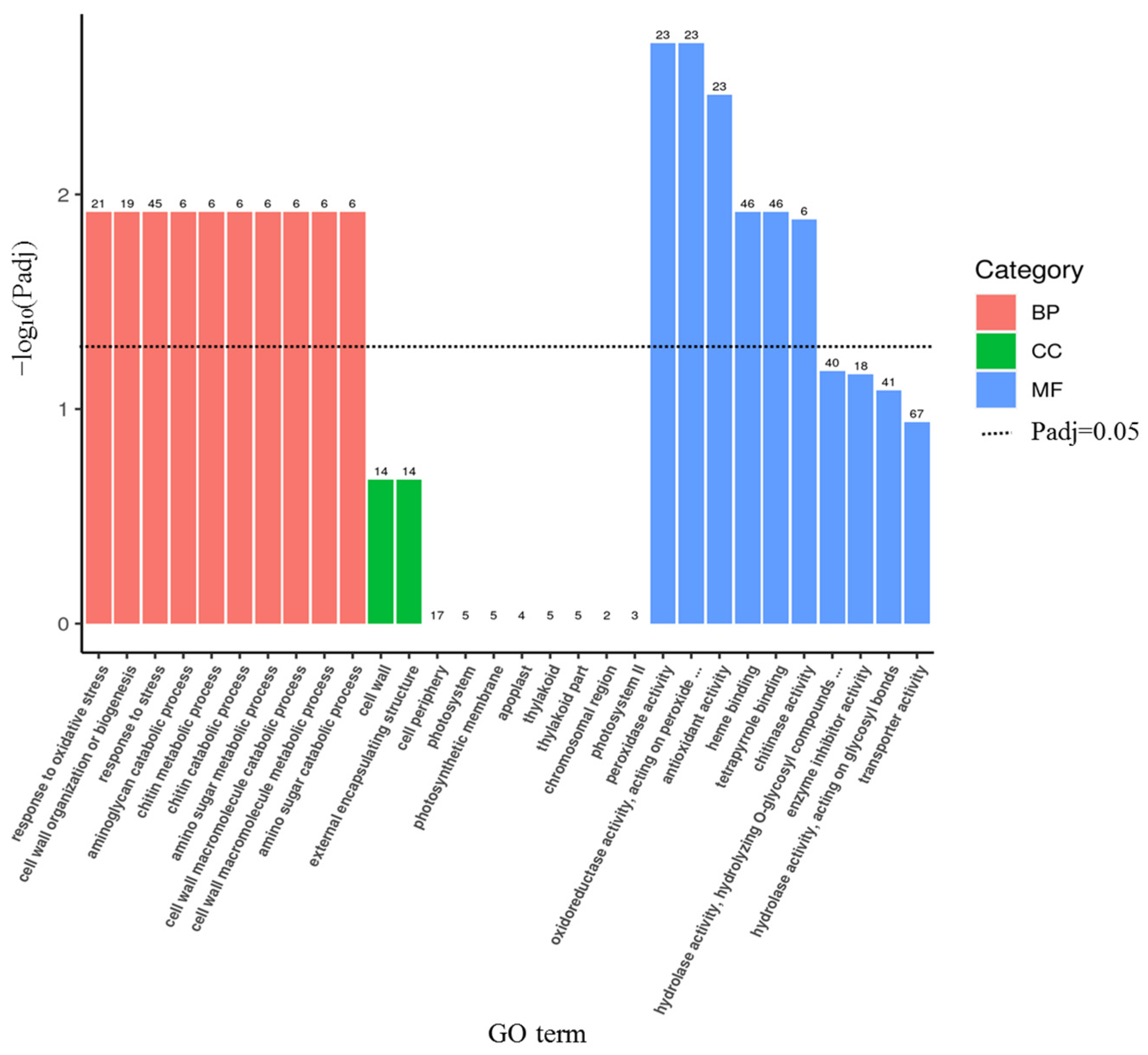
3.3. Transcriptome Sequencing Analysis
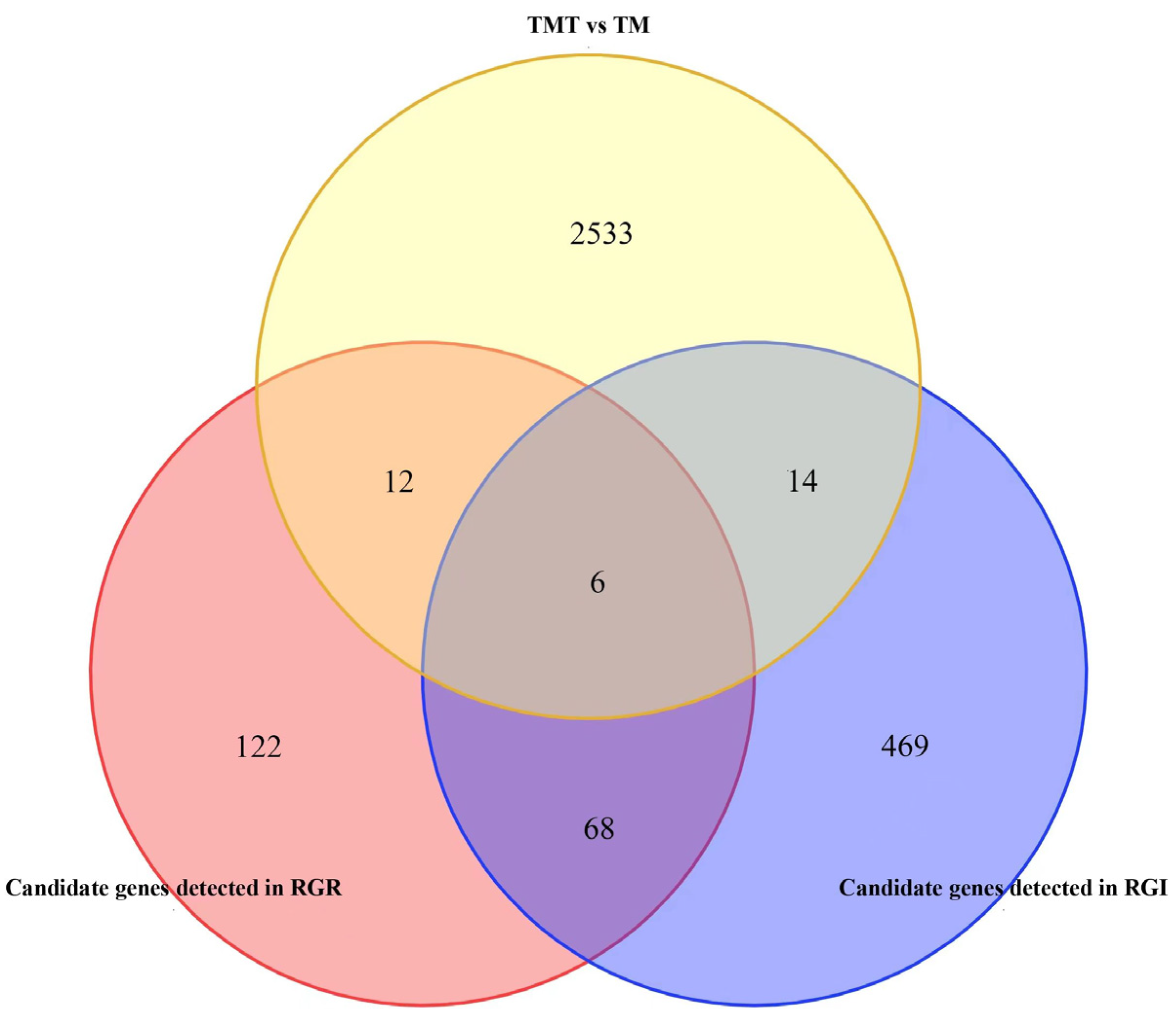
3.4. Screening Candidate Genes by Combining Analyses of GWAS and RNA-Seq
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Zelm, E.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef]
- Cheeseman, J.M. The evolution of halophytes, glycophytes and crops, and its implications for food security under saline conditions. New Phytol. 2015, 206, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.H.; Kumar, V.; Khare, T.; Guddimalli, R.; Parveda, M.; Solymosi, K.; Suprasanna, P.; Kavi Kishor, P.B. Engineering salinity tolerance in plants: Progress and prospects. Planta 2020, 251, 76. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.; Zhang, G.; Jiang, J.; Lu, J.; Zhang, F. Combining genome-wide association study and gene-based haplotype analysis to identify candidate genes for alkali tolerance at the germination stage in rice. Front. Plant Sci. 2022, 13, 887239. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Wang, R.; Wang, J.; Hua, K.; Wang, Y.; Liu, X.; Yao, S. ALT1, a Snf2 family chromatin remodeling ATPase, negatively regulates alkaline tolerance through enhanced defense against oxidative stress in rice. PLoS ONE 2014, 9, e112515. [Google Scholar] [CrossRef]
- HanumanthaRao, B.; Nair, R.M.; Nayyar, H. Salinity and high temperature tolerance in mungbean [Vigna radiata (L.) Wilczek] from a physiological perspective. Front. Plant Sci. 2016, 7, 957. [Google Scholar] [CrossRef]
- Nair, R.M.; Pandey, A.K.; War, A.R.; Hanumantharao, B.; Shwe, T.; Alam, A.; Pratap, A.; Malik, S.R.; Karimi, R.; Mbeyagala, E.K.; et al. Biotic and abiotic constraints in mungbean production-progress in genetic improvement. Front. Plant Sci. 2019, 10, 1340. [Google Scholar] [CrossRef]
- Wang, L.; Wang, S.; Luo, G.; Zhang, J.; Chen, Y.; Chen, H.; Cheng, X. Evaluation of the production potential of mung bean cultivar “Zhonglv 5”. Agronomy 2022, 12, 707. [Google Scholar] [CrossRef]
- Nair, R.M.; Schafleitner, R.; Kenyon, L.; Ramasamy, S.; Easdown, W.; Ebert, A.; Hanson, P. Genetic improvement of mungbean. SABRAO J. Breed. Genet. 2012, 44, 177–190. [Google Scholar]
- Ge, Y.; Li, Y.; Zhu, Y.M.; Bai, X.; Lv, D.K.; Guo, D.; Ji, W.; Cai, H. Global transcriptome profiling of wild soybean (Glycine soja) roots under NaHCO3 treatment. BMC Plant Biol. 2010, 10, 153. [Google Scholar] [CrossRef]
- Chankaew, S.; Isemura, T.; Naito, K.; Ogiso-Tanaka, E.; Tomooka, N.; Somta, P.; Kaga, A.; Vaughan, D.A.; Srinives, P. QTL mapping for salt tolerance and domestication-related traits in Vigna marina subsp. Oblonga, a halophytic species. Theor. Appl. Genet. 2014, 127, 691–702. [Google Scholar] [CrossRef]
- Monika; Priyanka; Wati, L. Screening of rhizobial isolates from Vigna radiata for plant growth promoting traits. Res. Crops 2017, 18, 190–195. [Google Scholar] [CrossRef]
- Liang, J.; Qu, Y.; Yang, C.; Ma, X.; Cao, G.; Zhao, Z.; Zhang, S.; Zhang, T.; Han, L. Identification of QTLs associated with salt or alkaline tolerance at the seedling stage in rice under salt or alkaline stress. Euphytica 2015, 201, 441–452. [Google Scholar] [CrossRef]
- Quan, X.; Liu, J.; Zhang, N.; Xie, C.; Li, H.; Xia, X.; He, W.; Qin, Y. Genome-wide association study uncover the genetic architecture of salt tolerance-related traits in common wheat (Triticum aestivum L.). Front. Genet. 2021, 12, 663941. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, Z.; Gong, Z.; Liang, Y.; Sang, Z.; Xu, Y.; Li, X.; Wang, J. Genome-wide association analysis of salt-tolerant traits in terrestrial cotton at seedling stage. Plants 2021, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- Akram, S.; Arif, M.A.R.; Hameed, A. A GBS-based GWAS analysis of adaptability and yield traits in bread wheat (Triticum aestivum L.). J. Appl. Genet. 2021, 62, 27–41. [Google Scholar] [CrossRef]
- Xu, P.; Guo, Q.; Meng, S.; Zhang, X.; Xu, Z.; Guo, W.; Shen, X. Genome-wide association analysis reveals genetic variations and candidate genes associated with salt tolerance related traits in Gossypium hirsutum. BMC Genom. 2021, 22, 26. [Google Scholar] [CrossRef]
- Flint-Garcia, S.A.; Thornsberry, J.M.; Buckler, E.S. 4th. Structure of linkage disequilibrium in plants. Annu. Rev. Plant Biol. 2003, 54, 357–374. [Google Scholar] [CrossRef] [PubMed]
- Breria, C.M.; Hsieh, C.H.; Yen, T.B.; Yen, J.Y.; Noble, T.J.; Schafleitner, R. A SNP-based genome-wide association study to mine genetic loci associated to salinity tolerance in mungbean (Vigna radiata L.). Genes 2020, 11, 759. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xue, C.; Lin, Y.; Yan, Q.; Chen, J.; Wu, R.; Zhang, X.; Chen, X.; Yuan, X. Genetic analysis and identification of VrFRO8; a salt tolerance-related gene in mungbean. Gene 2022, 836, 146658. [Google Scholar] [CrossRef]
- Li, N.; Zheng, H.; Cui, J.; Wang, J.; Liu, H.; Sun, J.; Liu, T.; Zhao, H.; Lai, Y.; Zou, D. Genome-wide association study and candidate gene analysis of alkalinity tolerance in japonica rice germplasm at the seedling stage. Rice 2019, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, H.; Wu, W.; Liu, H.; Wang, J.; Jia, Y.; Li, J.; Yang, L.; Lei, L.; Zou, D.; et al. QTL mapping and candidate gene analysis for alkali tolerance in japonica rice at the bud stage based on linkage mapping and genome-wide association study. Rice 2020, 13, 48. [Google Scholar] [CrossRef]
- Li, C.; Jia, Y.; Zhou, R.; Liu, L.; Cao, M.; Zhou, Y.; Wang, Z.; Di, H. GWAS and RNA-seq analysis uncover candidate genes associated with alkaline stress tolerance in maize (Zea mays L.) seedlings. Front. Plant Sci. 2022, 13, 963874. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Singh, C.K.; Taunk, J.; Gaikwad, K.; Singh, V.; Sanwal, S.K.; Karwa, S.; Singh, D.; Sharma, P.C.; Yadav, R.K.; et al. Linking genome wide RNA sequencing with physio-biochemical and cytological responses to catalogue key genes and metabolic pathways for alkalinity stress tolerance in lentil (Lens culinaris Medikus). BMC Plant Biol. 2022, 22, 99. [Google Scholar] [CrossRef]
- Xu, Y.; Tao, S.; Zhu, Y.; Zhang, Q.; Li, P.; Wang, H.; Zhang, Y.; Bakirov, A.; Cao, H.; Qin, M.; et al. Identification of alkaline salt tolerance genes in Brassica napus L. by transcriptome analysis. Genes 2022, 13, 1493. [Google Scholar] [CrossRef]
- Yuan, Y.; Xing, H.; Zeng, W.; Xu, J.; Mao, L.; Wang, L.; Feng, W.; Tao, J.; Wang, H.; Zhang, H.; et al. Genome-wide association and differential expression analysis of salt tolerance in Gossypium hirsutum L at the germination stage. BMC Plant Biol. 2019, 19, 394. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Su, B.; Ibrahim, S.; Kuang, L.; Tian, Z.; Wang, X.; Wang, H.; Dun, X. Deciphering the genetic basis of root and biomass traits in rapeseed (Brassica napus L.) through the integration of GWAS and RNA-Seq under nitrogen stress. Int. J. Mol. Sci. 2022, 23, 7958. [Google Scholar] [CrossRef]
- Zhou, H.; Xiao, X.; Asjad, A.; Han, D.; Zheng, W.; Xiao, G.; Huang, Y.; Zhou, Q. Integration of GWAS and transcriptome analyses to identify SNPs and candidate genes for aluminum tolerance in rapeseed (Brassica napus L.). BMC Plant Biol. 2022, 22, 130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, J.; Xu, Q.; Wang, D.; Di, H.; Huang, J.; Yang, X.; Wang, Z.; Zhang, L.; Dong, L.; et al. Identification of candidate tolerance genes to low-temperature during maize germination by GWAS and RNA-seqapproaches. BMC Plant Biol. 2020, 20, 333. [Google Scholar] [CrossRef]
- Guo, J.; Li, C.; Zhang, X.; Li, Y.; Zhang, D.; Shi, Y.; Song, Y.; Li, Y.; Yang, D.; Wang, T. Transcriptome and GWAS analyses reveal candidate gene for seminal root length of maize seedlings under drought stress. Plant Sci. 2020, 292, 110380. [Google Scholar] [CrossRef]
- Xu, N.; Chen, B.; Wang, M.; Bao, S.; Wang, G.; Guo, Z. Identification of alkali to tolerance of mungbean germplasm resources during germination. Acta Agron. Sin. 2017, 43, 112–121. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Bao, Y.; Wu, Y.; Su, X.; Zhang, H. Inheritance of rice seed germination ability under salt stress. Rice Sci. 2010, 17, 105–110. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Y.; Peng, J.; Fan, B.; Xu, D.; Wu, J.; Cao, Z.; Gao, Y.; Wang, X.; Li, S.; et al. High-quality genome assembly and pan-genome studies facilitate genetic discovery in mung bean and its improvement. Plant Commun. 2022, 3, 100352. [Google Scholar] [CrossRef]
- Alexander, D.H.; Novembre, J.; Lange, K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009, 19, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Dong, S.; Xu, J.; He, W.; Yang, T. PopLDdecay: A fast and effective tool for linkage disequilibrium decay analysis based on variant call format files. Bioinformatics 2019, 35, 1786–1788. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Stephens, M. Genome-wide efficient mixed-model analysis for association studies. Nat. Genet. 2012, 44, 821–824. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Pressoir, G.; Briggs, W.H.; Vroh Bi, I.; Yamasaki, M.; Doebley, J.F.; McMullen, M.D.; Gaut, B.S.; Nielsen, D.M.; Holland, J.B.; et al. A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nat. Genet. 2006, 38, 203–208. [Google Scholar] [CrossRef]
- Gómez-Rubio, V. ggplot2-elegant graphics for data analysis (2nd edition). J. Stat. Softw. 2017, 77, 3–5. [Google Scholar] [CrossRef]
- Turner, S.D. qqman: An R package for visualizing GWAS results using Q-Q and manhattan plots. J. Open Source Softw. 2018, 3, 731. [Google Scholar] [CrossRef]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT. StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39 (Suppl. S2), W316–W322. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ibrahim, E.A. Seed priming to alleviate salinity stress in germinating seeds. J. Plant Physiol. 2016, 192, 38–46. [Google Scholar] [CrossRef]
- Mudgal, V.; Madaan, N.; Mudgal, A. Biochemical mechanisms of salt tolerance in plants: A review. Int. J. Bot. 2010, 6, 136–143. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Zhang, R.; Yuan, H.; Wang, M.; Yang, H.; Ma, H.; Liu, D.; Jiang, C.; Liang, Z. Root damage under alkaline stress is associated with reactive oxygen species accumulation in rice (Oryza sativa L.). Front. Plant Sci. 2017, 8, 1580. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, P.; Wang, C.; Zhang, N.; Zhu, Y.; Zou, C.; Yuan, G.; Yang, C.; Gao, S.; Pan, G.; et al. Genome-wide association study uncovers new genetic loci and candidate genes underlying seed chilling-germination in maize. PeerJ 2021, 9, e11707. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Zhang, Y.; Zhang, C.; Nelson, M.N.; Yuan, J.; Guo, L.; Xu, Z. Genome-wide association mapping unravels the genetic control of seed vigor under low-temperature conditions in rapeseed (Brassica napus L.). Plants 2021, 10, 426. [Google Scholar] [CrossRef]
- Wu, L.; Chang, Y.; Wang, L.; Wang, S.; Wu, J. Genome-wide association analysis of drought resistance based on seed germination vigor and germination rate at the bud stage in common bean. Agron. J. 2021, 113, 2980–2990. [Google Scholar] [CrossRef]
- Si, A.; Sun, Z.; Li, Z.; Chen, B.; Gu, Q.; Zhang, Y.; Wu, L.; Zhang, G.; Wang, X.; Ma, Z. A genome wide association study revealed key single nucleotide polymorphisms/genes associated with seed germination in Gossypium hirsutum L. Front. Plant Sci. 2022, 13, 844946. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Guo, X.; Liang, H.; Fu, L.; Shi, J.; Zhang, Y.; Chuang, L. Analysis of saline-alkaline tolerance and screening of identification indicators at the germination stage among different mung bean genotypes. Plant Physiol. J. 2017, 53, 1629–1639. [Google Scholar] [CrossRef]
- Kang, Y.J.; Kim, S.K.; Kim, M.Y.; Lestari, P.; Kim, K.H.; Ha, B.K.; Jun, T.H.; Hwang, W.J.; Lee, T.; Lee, J.; et al. Genome sequence of mungbean and insights into evolution within Vigna species. Nat. Commun. 2014, 5, 5443. [Google Scholar] [CrossRef]
- ResearchGate. Available online: https://www.researchgate.net/publication/347076195_High-quality_genome_assembly_annotation_and_evolutionary_analysis_of_the_mungbean_Vigna_radiata_genome (accessed on 10 March 2023).
- Xie, D.; Dai, Z.; Yang, Z.; Tang, Q.; Deng, C.; Xu, Y.; Wang, J.; Chen, J.; Zhao, D.; Zhang, S.; et al. Combined genome-wide association analysis and transcriptome sequencing to identify candidate genes for flax seed fatty acid metabolism. Plant Sci. 2019, 286, 98–107. [Google Scholar] [CrossRef]
- Tomar, S.; Subba, A.; Bala, M.; Singh, A.K.; Pareek, A.; Singla-Pareek, S.L. Genetic conservation of CBS domain containing protein family in Oryza species and their association with abiotic stress responses. Int. J. Mol. Sci. 2022, 23, 1687. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, H.R.; Singh, A.K.; Sopory, S.K.; Singla-Pareek, S.L.; Pareek, A. Genome wide expression analysis of CBS domain containing proteins in Arabidopsis thaliana (L.) Heynh and Oryza sativa L. reveals their developmental and stress regulation. BMC Genom. 2009, 28, 200. [Google Scholar] [CrossRef]
- Yoo, K.S.; Ok, S.H.; Jeong, B.C.; Jung, K.W.; Cui, M.H.; Hyoung, S.; Lee, M.R.; Song, H.K.; Shin, J.S. Single cystathionine β-synthase domain-containing proteins modulate development by regulating the thioredoxin system in Arabidopsis. Plant Cell 2011, 23, 3577–3594. [Google Scholar] [CrossRef]
- Shin, J.S.; So, W.M.; Kim, S.Y.; Noh, M.; Hyoung, S.; Yoo, K.S.; Shin, J.S. CBSX3-Trxo-2 regulates ROS generation of mitochondrial complex II (succinate dehydrogenase) in Arabidopsis. Plant Sci. 2020, 294, 110458. [Google Scholar] [CrossRef]
- Singh, A.K.; Kumar, R.; Pareek, A.; Sopory, S.K.; Singla-Pareek, S.L. Overexpression of rice CBS domain containing protein improves salinity, oxidative, and heavy metal tolerance in transgenic tobacco. Mol. Biotechnol. 2012, 52, 205–216. [Google Scholar] [CrossRef]
- Ishibashi, N.; Yamauchi, D.; Minamikawa, T. Stored mRNA in cotyledons of Vigna unguiculata seeds: Nucleotide sequence of cloned cDNA for a stored mRNA and induction of its synthesis by precocious germination. Plant Mol. Biol. 1990, 15, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Odintsova, T.I.; Slezina, M.P.; Istomina, E.A. Defensins of grasses: A systematic review. Biomolecules 2020, 10, 1029. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.R.; Lin, Y.C.; Chuang, H.W. Laminarin modulates the chloroplast antioxidant system to enhance abiotic stress tolerance partially through the regulation of the defensin-like gene expression. Plant Sci. 2016, 247, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.S.; Zhang, Z. Proteomic changes in the xylem sap of Brassica napus under cadmium stress and functional validation. BMC Plant Biol. 2019, 19, 280. [Google Scholar] [CrossRef]
- Luo, J.S.; Huang, J.; Zeng, D.; Peng, J.; Zhang, G.; Ma, H.; Guan, Y.; Yi, H.; Fu, Y.; Han, B.; et al. A defensin-like protein drives cadmium efflux and allocation in rice. Nat. Commun. 2018, 9, 645. [Google Scholar] [CrossRef]
- Luo, J.S.; Xiao, Y.; Yao, J.; Wu, Z.; Yang, Y.; Ismail, A.M.; Zhang, Z. Overexpression of a defensin-like gene CAL2 enhances cadmium accumulation in plants. Front. Plant Sci. 2020, 11, 217. [Google Scholar] [CrossRef]
- Gu, T.; Qi, Z.; Chen, S.; Yan, J.; Fang, Z.; Wang, J.; Gong, J. Dual-function DEFENSIN 8 mediates phloem cadmium unloading and accumulation in rice grains. Plant Physiol. 2023, 191, 515–527. [Google Scholar] [CrossRef]
- Luo, J.S.; Yang, Y.; Gu, T.; Wu, Z.; Zhang, Z. The Arabidopsis defensin gene AtPDF2.5 mediates cadmium tolerance and accumulation. Plant Cell Environ. 2019, 42, 2681–2695. [Google Scholar] [CrossRef]
- Luo, J.S.; Gu, T.; Yang, Y.; Zhang, Z. A non-secreted plant defensin AtPDF2.6 conferred cadmium tolerance via its chelation in Arabidopsis. Plant Mol. Biol. 2019, 100, 561–569. [Google Scholar] [CrossRef]
- Coll-Garcia, D.; Mazuch, J.; Altmann, T.; Müssig, C. EXORDIUM regulates brassinosteroid-responsive genes. FEBS Lett. 2004, 563, 82–86. [Google Scholar] [CrossRef]
- Schröder, F.; Lisso, J.; Lange, P.; Müssig, C. The extracellular EXO protein mediates cell expansion in Arabidopsis leaves. BMC Plant Biol. 2009, 9, 20. [Google Scholar] [CrossRef]
- Schröder, F.; Lisso, J.; Müssig, C. EXORDIUM-LIKE1 promotes growth during low carbon availability in Arabidopsis. Plant Physiol. 2011, 156, 1620–1630. [Google Scholar] [CrossRef] [PubMed]
- Schröder, F.; Lisso, J.; Müssig, C. Expression pattern and putative function of EXL1 and homologous genes in Arabidopsis. Plant Signal. Behav. 2012, 7, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.G.; Wang, B.; Jin, S.H.; Qu, X.X.; Li, Y.J.; Hou, B.K. Ectopic expression of Arabidopsis glycosyltransferase UGT85A5 enhances salt stress tolerance in tobacco. PLoS ONE 2013, 8, e59924. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhang, Y.; Qu, X.; Wu, F.; Li, X.; Ren, M.; Tong, Y.; Wu, X.; Yang, A.; Chen, Y.; et al. Genome-wide analysis of UDP-glycosyltransferases family and identification of UGT genes involved in abiotic stress and flavonol biosynthesis in Nicotiana tabacum. BMC Plant Biol. 2023, 23, 204. [Google Scholar] [CrossRef]
- Dong, T.; Xu, Z.Y.; Park, Y.; Kim, D.H.; Lee, Y.; Hwang, I. Abscisic acid uridine diphosphate glucosyltransferases play a crucial role in abscisic acid homeostasis in Arabidopsis. Plant Physiol. 2014, 165, 277–289. [Google Scholar] [CrossRef]
- Behr, M.; Neutelings, G.; El Jaziri, M.; Baucher, M. You want it sweeter: How glycosylation affects plant response to oxidative stress. Front. Plant Sci. 2020, 11, 571399. [Google Scholar] [CrossRef]
- Rehman, H.M.; Nawaz, M.A.; Shah, Z.H.; Ludwig-Müller, J.; Chung, G.; Ahmad, M.Q.; Yang, S.H.; Lee, S.I. Comparative genomic and transcriptomic analyses of Family-1 UDP glycosyltransferase in three Brassica species and Arabidopsis indicates stress-responsive regulation. Sci. Rep. 2018, 8, 1875. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, Y.; Li, M.; Long, R. CrUGT87A1, a UDP-sugar glycosyltransferases (UGTs) gene from Carex rigescens, increases salt tolerance by accumulating flavonoids for antioxidation in Arabidopsis thaliana. Plant Physiol. Biochem. 2021, 159, 28–36. [Google Scholar] [CrossRef]
- Dong, L.; Tang, Z.; Yang, T.; Hao, F.; Deng, X. Genome-wide analysis of UGT genes in petunia and identification of PhUGT51 involved in the regulation of salt resistance. Plants 2022, 11, 2434. [Google Scholar] [CrossRef]
- He, X.; Zhao, X.; Gao, L.; Shi, X.; Dai, X.; Liu, Y.; Xia, T.; Wang, Y. Isolation and characterization of key genes that promote flavonoid accumulation in purple-leaf tea (Camellia sinensis L.). Sci. Rep. 2018, 8, 130. [Google Scholar] [CrossRef] [PubMed]
- Gollery, M.; Harper, J.; Cushman, J.; Mittler, T.; Girke, T.; Zhu, J.K.; Bailey-Serres, J.; Mittler, R. What makes species unique? The contribution of proteins with obscure features. Genome Biol. 2006, 7, R57. [Google Scholar] [CrossRef] [PubMed]
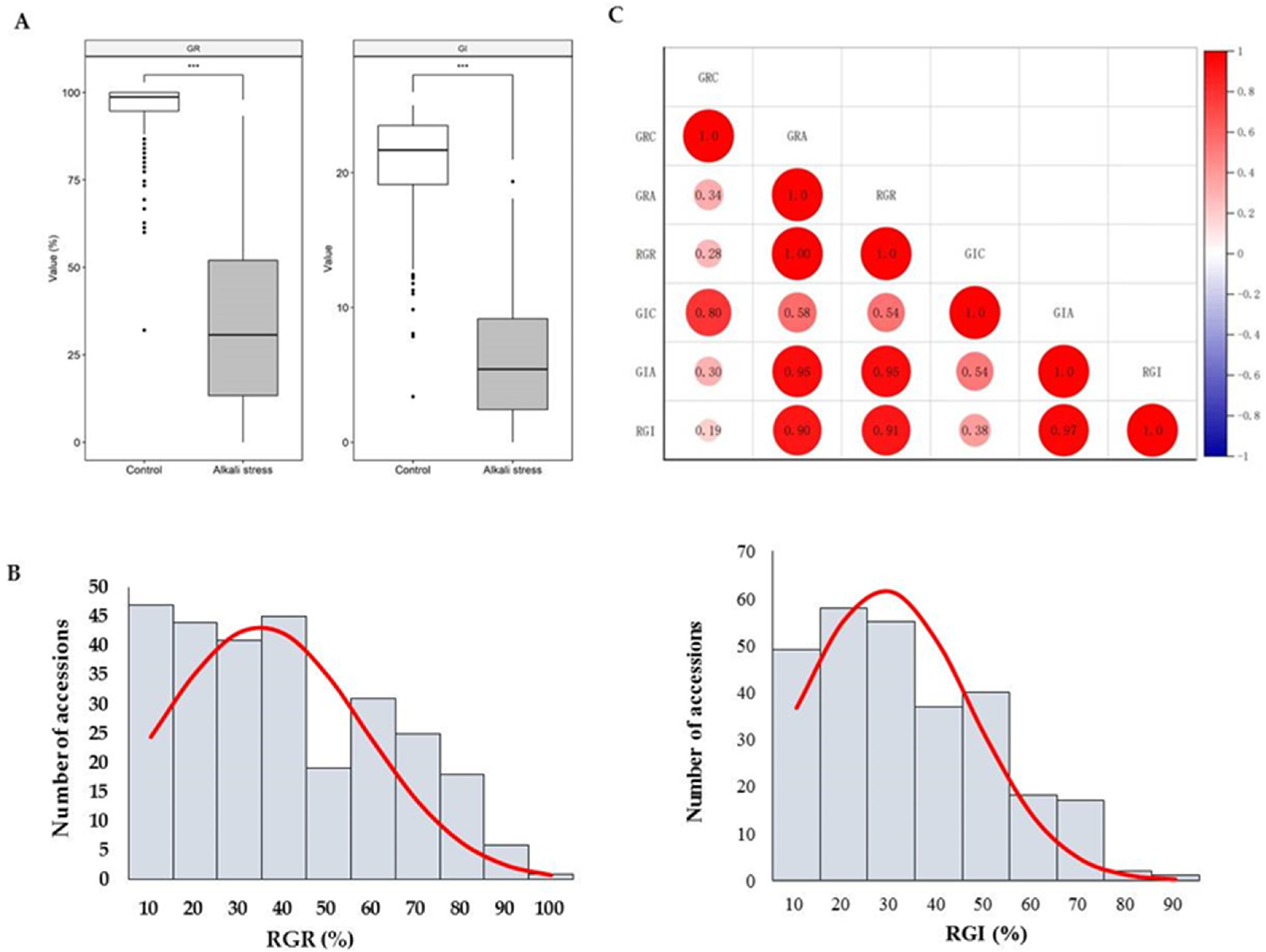


| Traits | Min (%) | Median (%) | Mean (%) | SD | Skewness | Kurtosis | CV (%) |
|---|---|---|---|---|---|---|---|
| RGR | 0.00 | 32.00 | 34.67 | 23.09 | 0.43 | −0.85 | 66.59 |
| RGI | 0.00 | 26.35 | 28.46 | 18.12 | 0.52 | −0.57 | 63.68 |
| Traits | QTLs | No. of SNPs | Peak SNP | Chr. | Position | Alleles | −log10P | R2 (%) |
|---|---|---|---|---|---|---|---|---|
| RGR | qRGR3 | 1 | Chr3_1599285 | 3 | 1599285 | T/C | 5.30 | 11.3 |
| qRGR6 | 1 | Chr6_39567121 | 6 | 39567121 | G/A | 5.33 | 14.6 | |
| qRGR7 | 1 | Chr7_72278080 | 7 | 72278080 | G/A | 5.17 | 4.0 | |
| qRGR11 | 1 | Chr11_7640538 | 11 | 7640538 | G/A | 5.33 | 3.6 | |
| RGI | qRGI1-1 | 1 | Chr1_12682868 | 1 | 12682868 | C/A | 5.10 | 13.4 |
| qRGI1-2 | 1 | Chr1_19279047 | 1 | 19279047 | C/T | 5.20 | 8.9 | |
| qRGI2 | 1 | Chr2_11936562 | 2 | 11936562 | T/C | 5.38 | 5.0 | |
| qRGI3-1 | 1 | Chr3_1599285 | 3 | 1599285 | T/C | 5.37 | 11.3 | |
| qRGI3-2 | 1 | Chr3_24016456 | 3 | 24016456 | A/C | 5.33 | 10.1 | |
| qRGI4-1 | 1 | Chr4_34059117 | 4 | 34059117 | T/A | 6.44 | 5.1 | |
| qRGI4-2 | 1 | Chr4_39812897 | 4 | 39812897 | G/A | 5.56 | 13.6 | |
| qRGI4-3 | 5 | Chr4_40188515 | 4 | 40188515 | C/G | 6.33 | 4.8 | |
| qRGI4-4 | 1 | Chr4_41058948 | 4 | 41058948 | A/G | 5.08 | 4.3 | |
| qRGI6 | 1 | Chr6_39567121 | 6 | 39567121 | G/A | 5.05 | 13.7 | |
| qRGI7-1 | 2 | Chr7_37173579 | 7 | 37173579 | G/A | 5.23 | 11.9 | |
| qRGI7-2 | 6 | Chr7_56140780 | 7 | 56140780 | C/G | 5.99 | 8.4 | |
| qRGI8-1 | 1 | Chr8_22721042 | 8 | 22721042 | A/G | 5.37 | 12.3 | |
| qRGI8-2 | 4 | Chr8_38508969 | 8 | 38508969 | G/A | 5.48 | 4.2 | |
| qRGI10 | 1 | Chr10_33340353 | 10 | 33340353 | C/T | 5.02 | 12.5 |
| Gene ID | Gene Name | TMT vs. TM | Description | GO in UniProtKB |
|---|---|---|---|---|
| jg7597 | NA | up | NA | NA |
| jg7600 | CBSX5 | up | CBS domain-containing protein | AMP binding, protein kinase binding, protein kinase regulator activity, cellular response to glucose starvation, cellular response to hypoxia, protein phosphorylation, and regulation of catalytic activity |
| jg7622 | NA | up | Defensin-like protein | Defense response to fungi and killing of cells of another organism |
| jg19402 | EXO | up | Protein EXORDIUM | Response to brassinosteroid |
| jg19404 | EXO | up | Protein EXORDIUM | Response to brassinosteroid |
| jg19416 | UGT85A24 | down | 7-Deoxyloganetin glucosyltransferase | UDP glucosyltransferase activity |
| jg25742 | MFT | up | Protein MOTHER of FT and TFL1 | Abscisic-acid-activated signaling pathway, positive regulation of seed germination, and response to abscisic acid |
| jg25753 | FKBP17-2 | up | Peptidyl-prolyl cis-trans isomerase | Peptidyl-prolyl cis-trans isomerase activity |
| jg25764 | At2g01390/At2g01380 | down | Pentatricopeptide repeat-containing protein | NA |
| jg25771 | NMT1 | down | Phosphoethanolamine N-methyltransferase 1 | Methyltransferase activity, phosphoethanolamine N-methyltransferase activity, choline biosynthetic process, and post-embryonic root development |
| jg25787 | NFXL1 | up | NF-X1-type zinc finger protein | Regulation of hydrogen peroxide metabolism, response to salt stress, salicylic acid biosynthetic process, DNA-binding transcription factor activity, RNA polymerase II-specific activity, and protein ubiquitination |
| jg25790 | NA | down | Retrovirus-related Pol polyprotein from transposon TNT 1-94 | Aspartic-type endopeptidase activity, endonuclease activity, nucleic acid binding, RNA-directed DNA polymerase activity, zinc ion binding, DNA integration, and proteolysis |
| jg25802 | NA | down | NA | NA |
| jg25807 | AGD15 | down | Probable ADP-ribosylation factor GTPase-activating protein | GTPase activator activity and metal ion binding |
| jg34113 | AVT3C | up | Amino acid transporter | L-amino acid transmembrane transporter activity and neutral amino acid transmembrane transporter activity |
| jg34122 | MLO8 | down | MLO-like protein 8 | Calmodulin binding and defense response |
| jg34124 | ATL8 | up | RING-H2 finger protein | Metal ion binding, transferase activity, and protein ubiquitination |
| jg34162 | At4g19185 | up | WAT1-related protein | Transmembrane transporter activity |
| jg14901 | RPL9 | down | Ribosomal protein L9 | Structural constituent of ribosomes, rRNA binding, and translation |
| jg14094 | ACA12 | up | Calcium-transporting ATPase 12; plasma membrane-type | ATPase-coupled cation transmembrane transporter activity, ATP binding, ATP hydrolysis activity, calmodulin binding, metal ion binding, and P-type calcium transporter activity |
| jg14117 | AVT6A | up | Amino acid transporter | L-amino acid transmembrane transporter activity |
| jg10665 | PSKR2 | up | Phytosulfokine receptor 2 | ATP binding, peptide receptor activity, protein serine/threonine kinase activity, protein serine kinase activity, and protein phosphorylation |
| jg5709 | KTI3 | up | Trypsin inhibitor A | Serine-type endopeptidase inhibitor activity |
| jg5712 | NA | up | NA | NA |
| jg6099 | NA | up | NA | NA |
| jg37480 | NA | down | NA | NA |
| jg37481 | SMC1 | down | Structural maintenance of chromosome protein 1 | ATP binding, ATP hydrolysis activity, DNA binding, cell division, chromosome segregation, and DNA repair |
| jg38807 | ERF1B | up | Ethylene-responsive transcription factor 1B | DNA-binding transcription factor activity, defense response, ethylene-activated signaling pathway, and jasmonic-acid-mediated signaling pathway |
| jg38809 | ERF096 | up | Ethylene-responsive transcription factor | DNA-binding transcription factor activity, ethylene-activated signaling pathway, positive regulation of abscisic-acid-activated signaling pathway, and positive regulation of cellular defense response |
| jg38828 | TRAF1B | up | TNF-receptor-associated factor homolog 1b | Autophagosome organization and innate immune response |
| jg38837 | NA | down | NA | NA |
| jg32966 | RZF1 | up | E3 ubiquitin protein ligase | Metal ion binding, ubiquitin protein ligase activity, ubiquitin protein transferase activity, negative regulation of proline biosynthetic process, regulation of response to osmotic stress, regulation of response to water deprivation, response to osmotic stress, response to water deprivation, and water homeostasis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, N.; Chen, B.; Cheng, Y.; Su, Y.; Song, M.; Guo, R.; Wang, M.; Deng, K.; Lan, T.; Bao, S.; et al. Integration of GWAS and RNA-Seq Analysis to Identify SNPs and Candidate Genes Associated with Alkali Stress Tolerance at the Germination Stage in Mung Bean. Genes 2023, 14, 1294. https://doi.org/10.3390/genes14061294
Xu N, Chen B, Cheng Y, Su Y, Song M, Guo R, Wang M, Deng K, Lan T, Bao S, et al. Integration of GWAS and RNA-Seq Analysis to Identify SNPs and Candidate Genes Associated with Alkali Stress Tolerance at the Germination Stage in Mung Bean. Genes. 2023; 14(6):1294. https://doi.org/10.3390/genes14061294
Chicago/Turabian StyleXu, Ning, Bingru Chen, Yuxin Cheng, Yufei Su, Mengyuan Song, Rongqiu Guo, Minghai Wang, Kunpeng Deng, Tianjiao Lan, Shuying Bao, and et al. 2023. "Integration of GWAS and RNA-Seq Analysis to Identify SNPs and Candidate Genes Associated with Alkali Stress Tolerance at the Germination Stage in Mung Bean" Genes 14, no. 6: 1294. https://doi.org/10.3390/genes14061294
APA StyleXu, N., Chen, B., Cheng, Y., Su, Y., Song, M., Guo, R., Wang, M., Deng, K., Lan, T., Bao, S., Wang, G., Guo, Z., & Yu, L. (2023). Integration of GWAS and RNA-Seq Analysis to Identify SNPs and Candidate Genes Associated with Alkali Stress Tolerance at the Germination Stage in Mung Bean. Genes, 14(6), 1294. https://doi.org/10.3390/genes14061294




