Abscisic Acid Inhibits Cortical Microtubules Reorganization and Enhances Ultraviolet-B Tolerance in Arabidopsis thaliana
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants and Growth Conditions
2.2. UV-B and Pharmaceutical Treatments
2.3. ABA Extraction and Quantification
2.4. Cortical Microtubules Immunolabeling and Observations
2.5. Phenotype Analysis
2.6. The Morphology of Root Cells Staining by PI and Observations
2.7. Statistical Analysis
3. Results
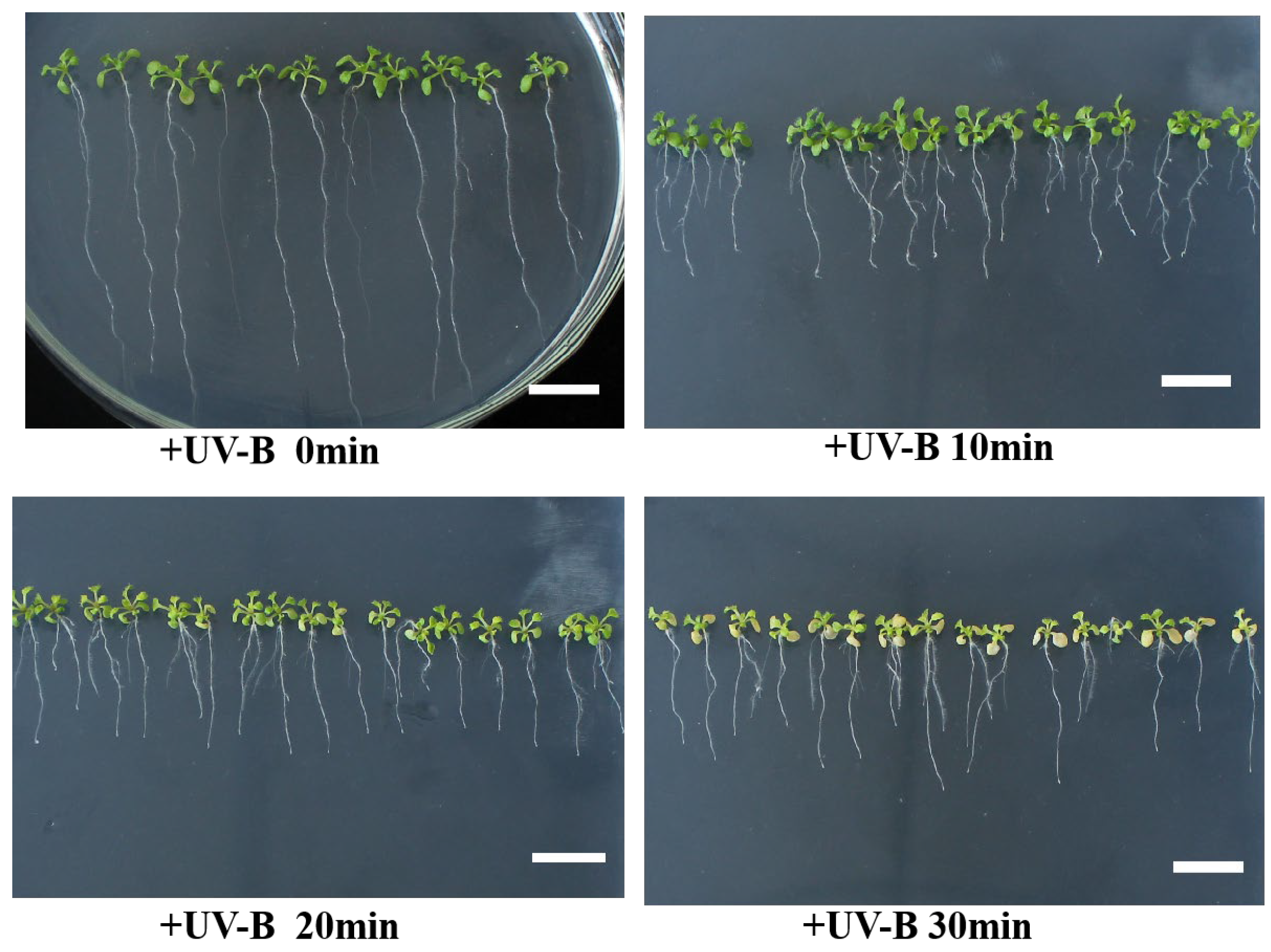
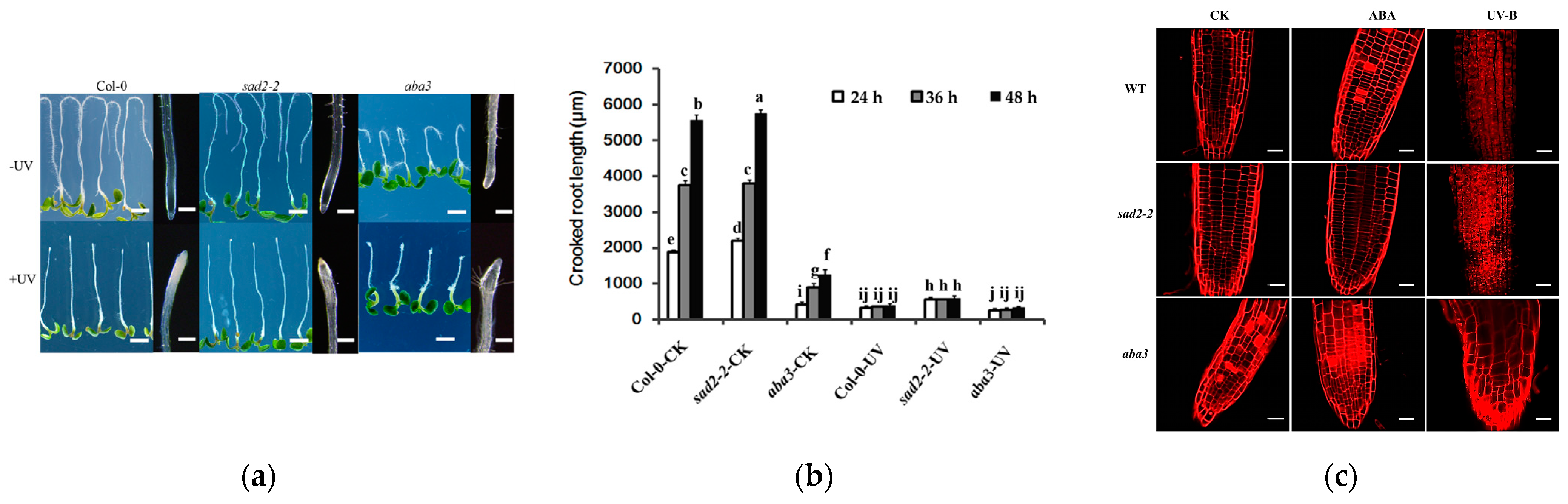
3.1. ABA Strengthens the Adaptive Response to UV-B Stress in A. thaliana
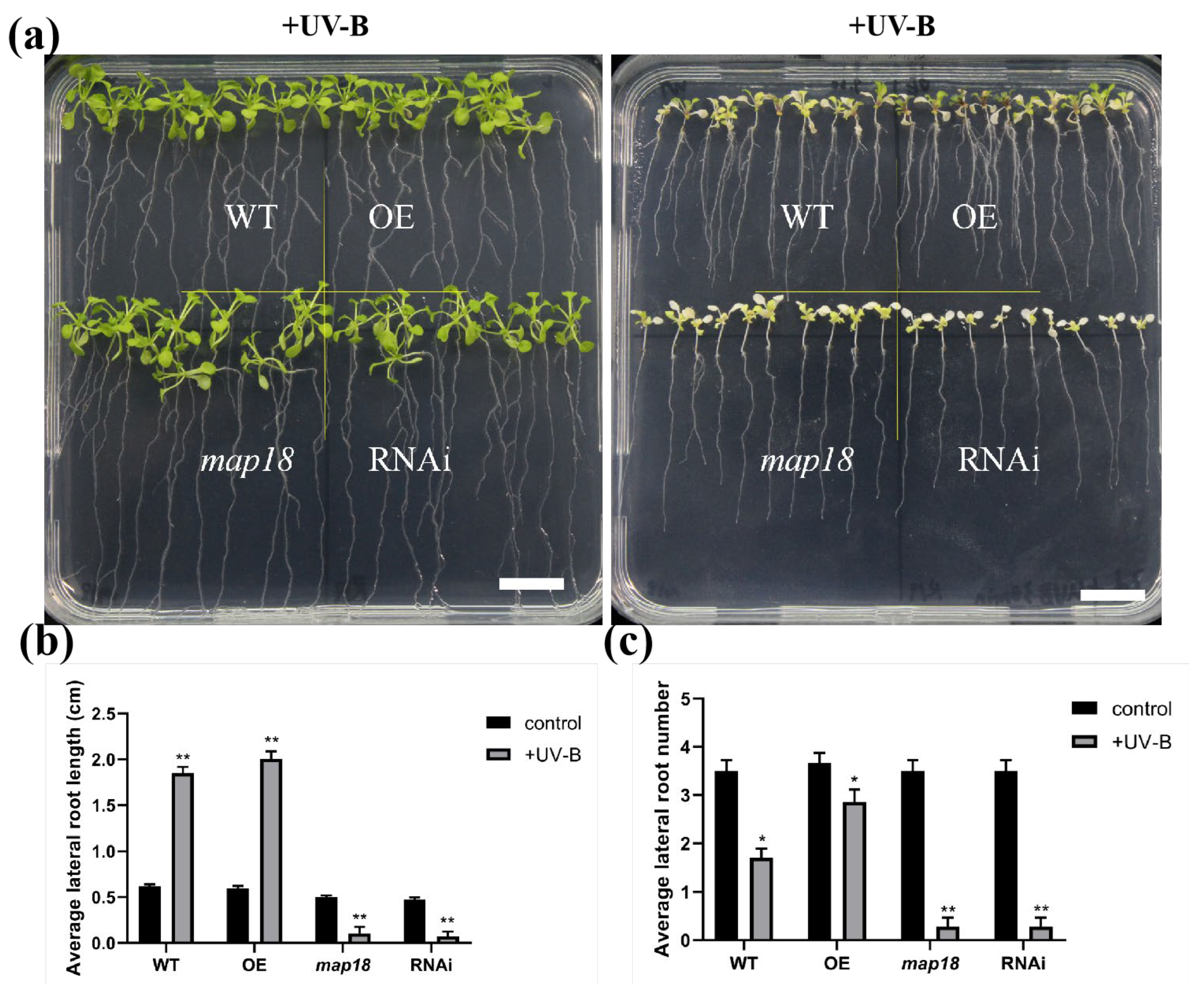
3.2. UV-B Intervenes Root Growth through ABA Signaling
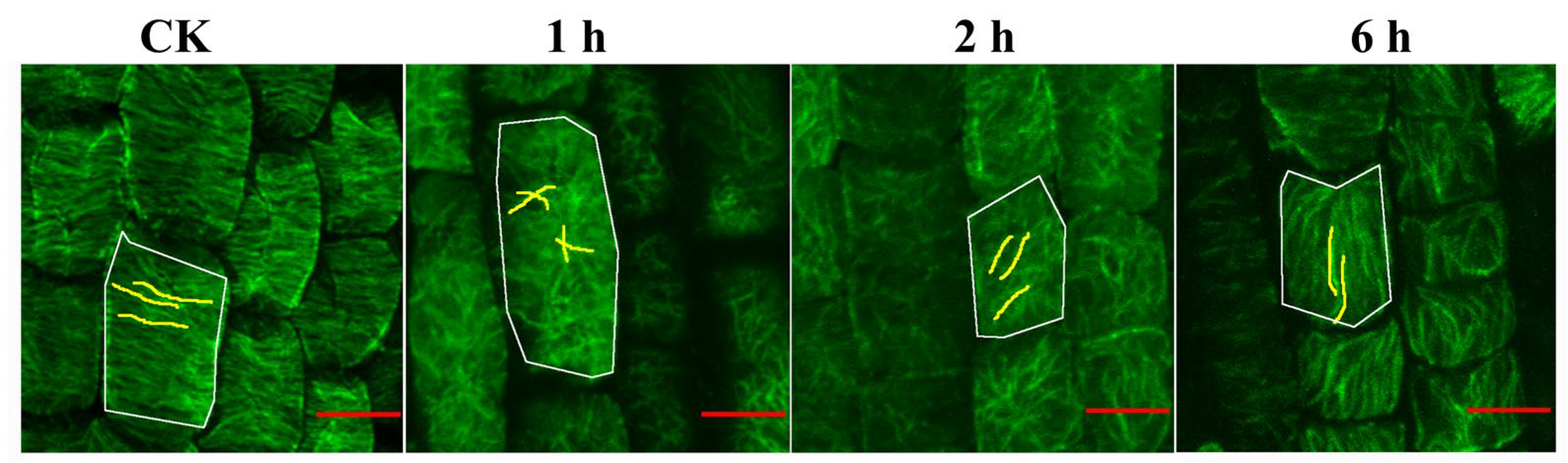
3.3. UV-B Remodels Microtubule Arrangement in Root Tip
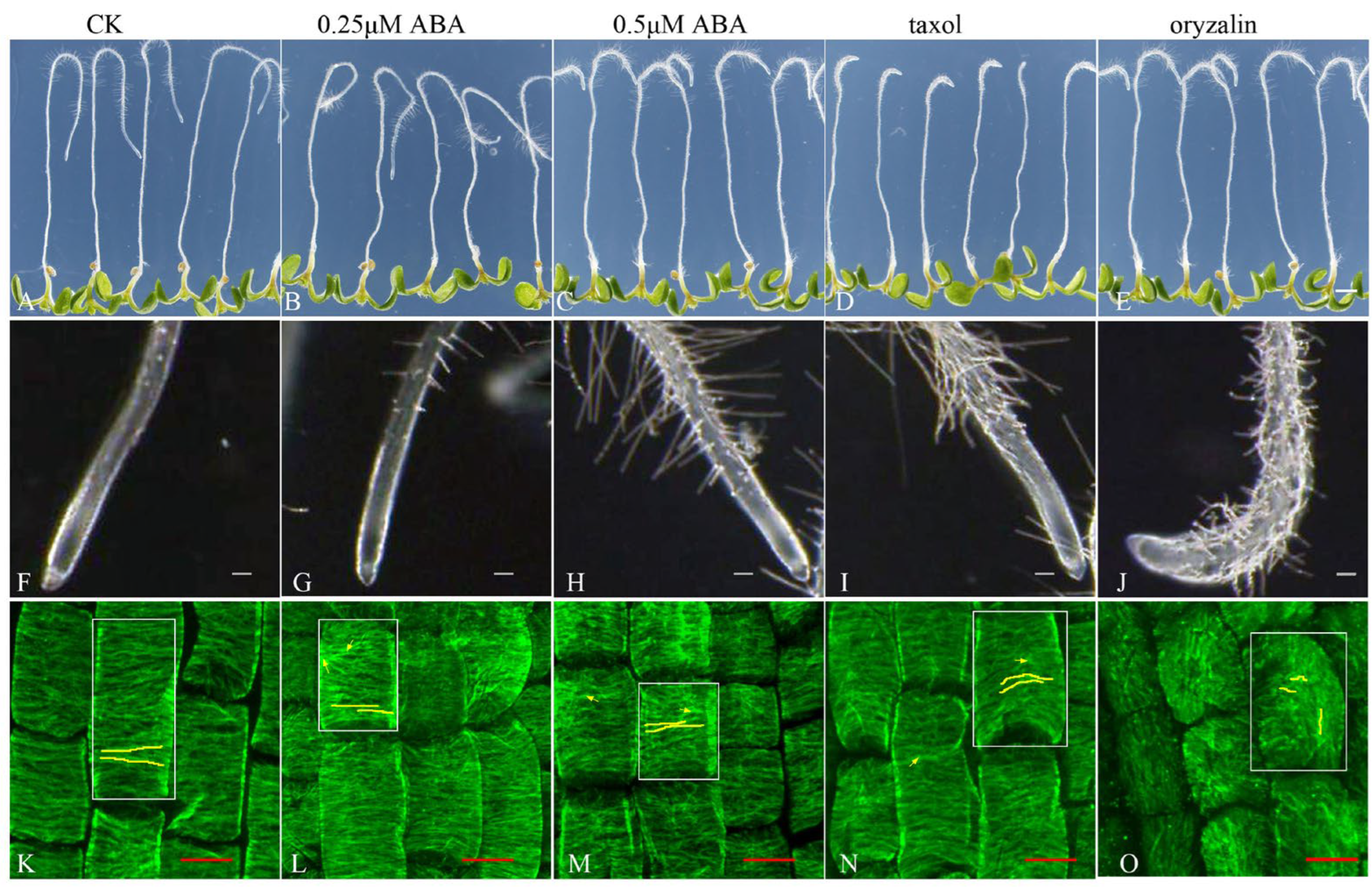
3.4. ABA Stabilizes the Microtubule Array and Reduces UV-B-Induced Reorganization
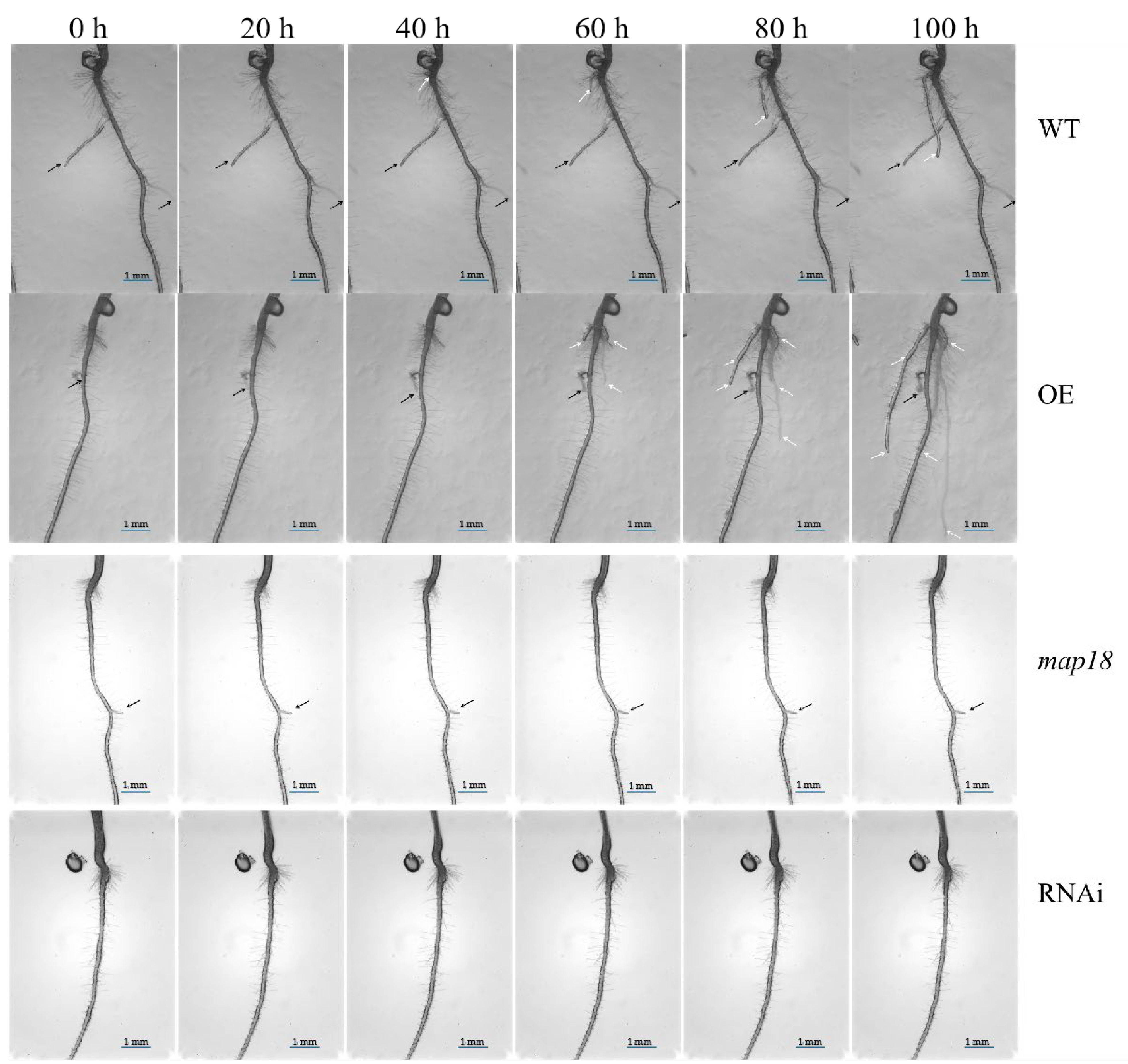
3.5. ABA Regulates Primary Root Tip Growth via Stabilizing Cortical Microtubule Arrays
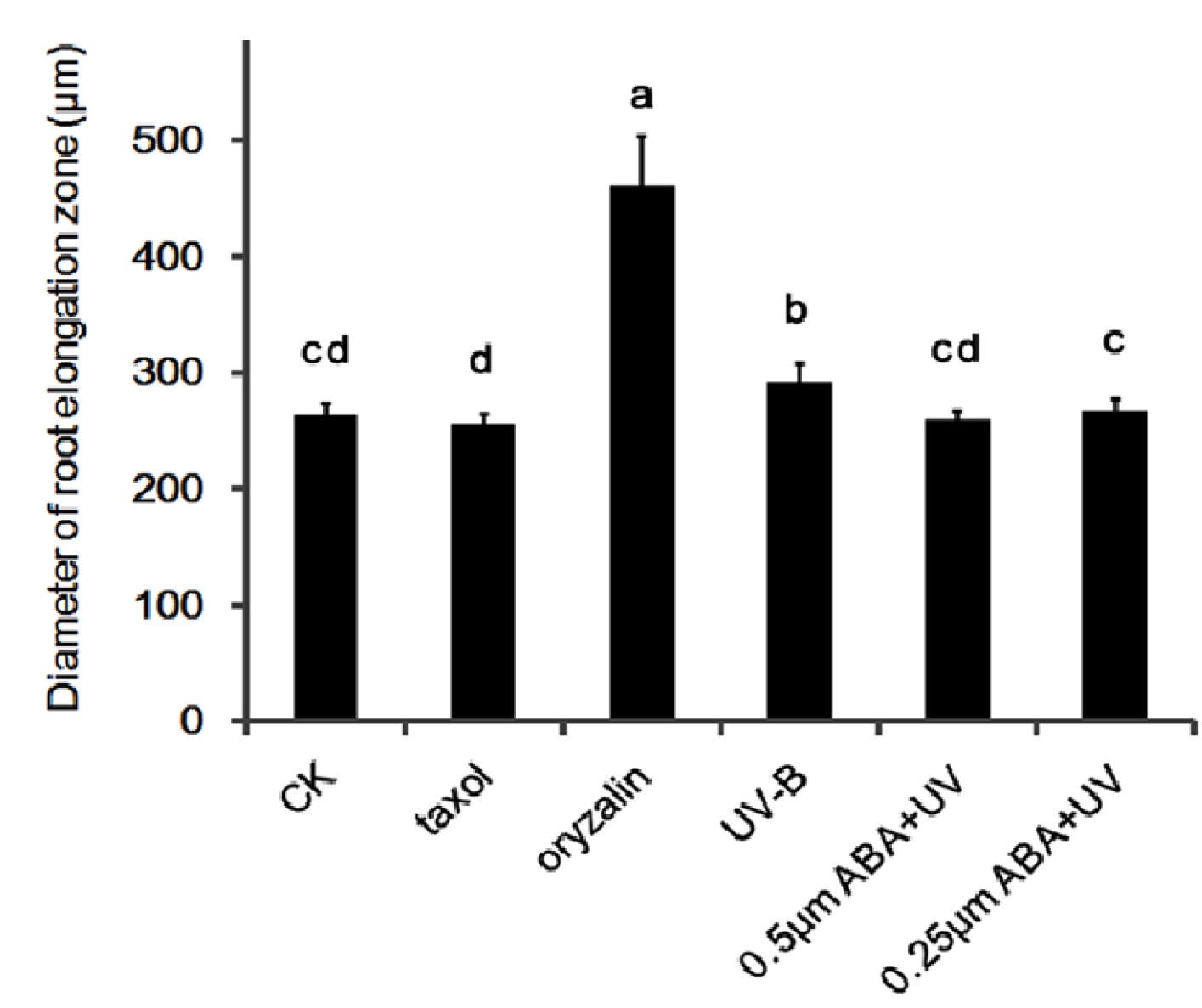
3.6. ABA Reduces UV-B-Induced Root Tip Expansion
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Dotto, M.; Casati, P. Developmental reprogramming by UV-B radiation in plants. Plant Sci. 2017, 264, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Manova, V.; Gruszka, D. DNA damage and repair in plants—From models to crops. Front. Plant Sci. 2015, 6, 885. [Google Scholar] [CrossRef] [PubMed]
- Maulión, E.; Gomez, M.S.; Bustamante, C.A.; Casati, P. AtCAF-1 mutants show different DNA damage responses after ultraviolet-B than those activated by other genotoxic agents in leaves. Plant Cell Environ. 2019, 42, 2730–2745. [Google Scholar] [CrossRef]
- Ramamoorthy, P.; Bheemanahalli, R.; Meyers, S.L.; Shankle, M.W.; Reddy, K.R. Drought, low nitrogen stress, and Ultraviolet-B radiation effects on growth, development, and physiology of sweet potato cultivars during early season. Genes 2022, 13, 156. [Google Scholar] [CrossRef]
- Tossi, V.; Lamattina, L.; Cassia, R. An increase in the concentration of abscisic acid is critical for nitric oxide-mediated plant adaptive responses to UV-B irradiation. New Phytol. 2009, 181, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Krasylenko, Y.A.; Yemets, A.I.; Sheremet, Y.A.; Blume, Y.B. Nitric oxide as a critical factor for perception of UV-B irradiation by microtubules in Arabidopsis. Physiol. Plant. 2012, 145, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Sato, M.; Sawada, Y.; Tanaka, M.; Matsui, A.; Kanno, Y.; Hirai, M.Y.; Seki, M.; Sakamoto, A.; Seo, M. Arabidopsis molybdenum cofactor sulfurase ABA3 contributes to anthocyanin accumulation and oxidative stress tolerance in ABA dependent and independent ways. Sci. Rep. 2018, 8, 16592. [Google Scholar] [CrossRef]
- Ktitorova, N.I.; Demchenko, P.N.; Kalimova, B.I.; Demchenko, N.K.; Skobeleva, V.O. Cellular analysis of UV-B-induced barley root subapical swelling. Russ. J. Plant Physiol. 2006, 53, 824–836. [Google Scholar] [CrossRef]
- Leasure, C.D.; Tong, H.; Yuen, G.; Hou, X.W.; Sun, X.F.; He, Z.H. ROOT UV-B SENSITIVE2 acts with ROOT UV-B SENSITIVE1 in a root ultraviolet B-sensing pathway. Plant Physiol. 2009, 150, 1902–1915. [Google Scholar] [CrossRef]
- Rizzini, L.; Favory, J.J.; Cloix, C.; Faggionato, D.; O’Hara, A.; Kaiserli, E.; Baumeister, R.; Schafer, E.; Nagy, F.; Jenkins, G.I.; et al. Perception of UV-B by the Arabidopsis UVR8 protein. Science 2011, 332, 103–106. [Google Scholar] [CrossRef]
- Podolec, R.; Demarsy, E.; Ulm, R. Perception and signaling of Ultraviolet-B radiation in plants. Annu. Rev. Plant Biol. 2021, 72, 793–822. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, L.; Chen, P. UV-B photoreceptor UVR8 interacts with MYB73/MYB77 to regulate auxin responses and lateral root development. EMBO J. 2020, 39, 101928. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, M.L.; Simonelli, L.; Giustozzi, M.; Casati, P. Ultraviolet-B radiation represses primary root elongation by inhibiting cell proliferation in the meristematic zone of Arabidopsis seedlings. Front. Plant Sci. 2022, 13, 829336. [Google Scholar] [CrossRef] [PubMed]
- Sean, R.C.; Pedro, L.R.; Ruth, R.F.; Suzanne, R.A. Abscisic Acid: Emergence of a core signaling network. Annu. Rev. Plant Biol. 2010, 61, 651–679. [Google Scholar]
- López-Ruiz, B.A.; Zluhan-Martínez, E.; Sánchez, M.d.l.P.; Álvarez-Buylla, E.R.; Garay-Arroyo, A. Interplay between hormones and several abiotic stress conditions on Arabidopsis thaliana primary root development. Cells 2020, 9, 2576. [Google Scholar] [CrossRef]
- Li, X.Q.; Chen, L.; Forde, B.G.; Davies, W.J. The biphasic root growth response to abscisic acid in Arabidopsis involves interaction with ethylene and auxin signalling pathways. Front. Plant Sci. 2017, 8, 1493. [Google Scholar] [CrossRef]
- Sun, L.R.; Wang, Y.B.; He, S.B.; Hao, F.S. Mechanisms for abscisic acid inhibition of primary root growth. Plant Signal. Behav. 2018, 13, 1500069. [Google Scholar] [CrossRef]
- Xiong, L.; Ishitani, M.; Lee, H.; Zhu, J.K. The Arabidopsis LOS5/ABA3 locus encodes a molybdenum cofactor sulfurase and modulates cold stress- and osmotic stress-responsive gene expression. Plant Cell 2001, 13, 2063–2083. [Google Scholar]
- Verslues, P.E.; Guo, Y.; Dong, C.H.; Ma, W.J.; Zhu, J.K. Mutation of SAD2, an importin beta-domain protein in Arabidopsis alters abscisic acid sensitivity. Plant J. 2006, 47, 776–787. [Google Scholar] [CrossRef]
- Zhao, J.F.; Zhang, W.H.; Zhao, Y.; Gong, X.M.; Guo, L.; Zhu, G.L.; Wang, X.C.; Gong, Z.Z.; Schumaker, K.S.; Guo, Y. SAD2, an importin β-like protein, is required for UV-B response in Arabidopsis by mediating MYB4 nuclear trafficking. Plant Cell 2007, 19, 3805–3818. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, Q.H.; Li, X.Y.; Li, Y.Z. MAP65-1 Is Required for the depolymerization and reorganization of cortical microtubules in the response to salt stress in Arabidopsis. Plant Sci. 2017, 264, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M. Microtubule nucleating and severing enzymes for modifying microtubule array organization and cell morphogenesis in response to environmental cues. New Phytol. 2015, 205, 1022–1027. [Google Scholar] [CrossRef] [PubMed]
- Vineyard, L.; Elliott, A.; Dhingra, S.; Lucas, J.R.; Shawa, S.L. Progressive transverse microtubule array organization in hormone-induced Arabidopsis hypocotyl cells. Plant Cell 2013, 25, 662–676. [Google Scholar] [CrossRef]
- Shi, F.M.; Yao, L.L.; Pei, B.L.; Zhou, Q.; Li, X.L.; Li, Y.; Li, Y.Z. Cortical microtubule as a sensor and target of nitric oxide signal during the defence responses to Verticillium dahliae toxins in Arabidopsis. Plant Cell Environ. 2009, 32, 428–438. [Google Scholar] [CrossRef]
- Zhao, S.S.; Zhang, Q.K.; Liu, M.Y.; Zhou, H.P.; Ma, C.L.; Wang, P.P. Regulation of plant responses to salt stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef] [PubMed]
- Krasylenko, Y.; Yemets, A.; Blume, Y. Plant microtubules reorganization under the indirect UV-B exposure and during UV-B-induced programmed cell death. Plant Signal. Behav. 2013, 8, 24031. [Google Scholar] [CrossRef] [PubMed]
- Lipka, E.; Müller, S. Nitrosative stress triggers microtubule reorganization in Arabidopsis thaliana. J. Exp. Bot. 2014, 65, 4177–4189. [Google Scholar] [CrossRef]
- Halat, L.S.; Bali, B.; Wasteneys, G. Cytoplasmic linker protein-associating protein at the nexus of hormone signaling, microtubule organization, and the transition from division to differentiation in primary roots. Front. Plant Sci. 2022, 13, 883363. [Google Scholar] [CrossRef]
- Angelini, J.; Klassen, R.; Široká, J.; Novák, O.; Záruba, K.; Siegel, J.; Novotná, Z.; Valentová, O. Silver nanoparticles alter microtubule arrangement, dynamics and stress phytohormone levels. Plants 2022, 11, 313. [Google Scholar] [CrossRef]
- Miao, R.; Siao, W.; Zhang, N.; Lei, Z.L.; Lin, D.S.; Bhalerao, R.P.; Lu, C.M.; Xu, W.F. Katanin-dependent microtubule ordering in association with ABA is important for root hydrotropism. Int. J. Mol. Sci. 2022, 23, 3846. [Google Scholar] [CrossRef]
- Tong, H.Y.; Leasure, C.D.; Hou, X.W.; Yuen, G.; Briggs, W.; He, Z.H. Role of root UV-B sensing in Arabidopsis early seedling development. Proc. Natl. Acad. Sci. USA 2008, 105, 21039–21044. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Chen, P.; Fu, Z.D.; Luo, K.; Du, Q.; Gao, C.; Ren, J.B.; Yang, X.L.; Liu, S.S.; Yang, L.D.; et al. Research progress on regulation of root nodule formation and development of legume by light signals and photosynthetic products. Chin. J. Eco-Agric. 2023, 31, 21–30. [Google Scholar]
- Lee, H.J.; Ha, J.H.; Kim, S.G.; Choi, H.K.; Kim, Z.H.; Han, Y.J.; Kim, J.I.; Oh, Y.J.; Variluska, F.; Shin, K.S.; et al. Stem-piped light activates phytochrome B to trigger light responses in Arabidopsis thaliana roots. Sci. Signal. 2016, 9, 106. [Google Scholar] [CrossRef]
- Gao, Y.Q.; Bu, L.H.; Han, M.L.; Wang, Y.L.; Li, Z.Y.; Liu, H.T.; Chao, D.Y. Long-distance blue light signalling regulates phosphate deficiency-induced primary root growth inhibition. Molecular Plant. 2022, 15, 1636–1637. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, H.T. Coordinated Shoot and Root Responses to Light Signaling in Arabidopsis. Plant Comm. 2020, 1, 100026. [Google Scholar] [CrossRef]
- Suzuki, N.; Bassil, E.; Hamilton, J.S.; Inupakutika, M.A.; Zandalinas, S.I.; Tripathy, D.; Luo, Y.T.; Dion, E.; Fukui, G.; Kumazaki, A.; et al. ABA is required for plant acclimation to a combination of salt and heat stress. PLoS ONE 2016, 11, 0147625. [Google Scholar] [CrossRef]
- Ma, Y.L.; Cao, J.; He, J.H.; Chen, Q.Q.; Li, X.F.; Yang, Y. Molecular mechanism for the regulation of ABA homeostasis during plant development and stress responses. Int. J. Mol. Sci. 2018, 19, 3643. [Google Scholar] [CrossRef]
- Mathan, J.; Singh, A.; Ranjan, A. Sucrose transport in response to drought and salt stress involves ABA-mediated induction of OsSWEET13 and OsSWEET15 in rice. Physiol. Plant. 2020, 171, 620–637. [Google Scholar] [CrossRef]
- Tossi, V.; Cassia, R.; Bruzzone, S.; Zocchi, E.; Lamattina, L. ABA says NO to UV-B: A universal response? Trends Plant Sci. 2012, 17, 510–517. [Google Scholar] [CrossRef]
- Yan, H.F.; Chaumont, N.; Gilles, J.F.; Bolte, S.; Hamant, O.; Bailly, C. Microtubule self-organisation during seed germination in Arabidopsis. BMC Biol. 2020, 18, 44. [Google Scholar] [CrossRef]
- Fu, Y.; Xu, T.D.; Zhu, L.; Wen, M.Z.; Yang, Z.B. A ROP GTPase signaling pathway controls cortical microtubule ordering and cell expansion in Arabidopsis. Curr. Biol. 2009, 19, 1827–1832. [Google Scholar] [CrossRef] [PubMed]
- Takatani, S.; Hirayama, T.; Hashimoto, T.; Takahashi, T.; Motose, H. Abscisic acid induces ectopic outgrowth in epidermal cells through cortical microtubule reorganization in Arabidopsis thaliana. Sci. Rep. 2015, 5, 11364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, W.H. Regulation of developmental and environmental signaling by interaction between microtubules and membranes in plant cells. Protein Cell 2015, 7, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Ehrhardt, W.D.; Shaw, L.S. Microtubule dynamics and organization in the plant cortical array. Annu. Rev. Plant Biol. 2006, 57, 859–875. [Google Scholar] [CrossRef]
- Paredez, A.R.; Persson, S.; Ehrhardt, D.W.; Somerville, C.R. Genetic evidence that cellulose synthase activity influences microtubule cortical array organization. Plant Physiol. 2008, 147, 1723–1734. [Google Scholar] [CrossRef]
- Khokhlova, P.L.; Olinevich, V.O. Rearrangement of the cytoskeleton in triticum aestivum cells during cold hardening and after treatment with abscisic acid. Russ. J. Plant Physiol. 2003, 50, 470–481. [Google Scholar] [CrossRef]
- Da, S.A.; Toorop, P.E.; Van, L.A.; Hilhorst, H.M. ABA inhibits embryo cell expansion and early cell division events during coffee (Coffea arabica ‘Rubi’) seed germination. Ann. Bot. 2008, 102, 425–433. [Google Scholar]
- Jacques, E.; Hectors, K.; Guisez, Y.; Prinsen, E.; Jansen, M.A.K.; Verbelen, J.P.; Vissenberg, K. UV radiation reduces epidermal cell expansion in Arabidopsis thaliana leaves without altering cellular microtubule organization. Plant Signal. Behav. 2011, 6, 83–85. [Google Scholar] [CrossRef]


 . Repress
. Repress  .
.
 . Repress
. Repress  .
.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, L.; Lin, K.; Su, T.; Shi, F. Abscisic Acid Inhibits Cortical Microtubules Reorganization and Enhances Ultraviolet-B Tolerance in Arabidopsis thaliana. Genes 2023, 14, 892. https://doi.org/10.3390/genes14040892
Shi L, Lin K, Su T, Shi F. Abscisic Acid Inhibits Cortical Microtubules Reorganization and Enhances Ultraviolet-B Tolerance in Arabidopsis thaliana. Genes. 2023; 14(4):892. https://doi.org/10.3390/genes14040892
Chicago/Turabian StyleShi, Lichun, Kun Lin, Tongbing Su, and Fumei Shi. 2023. "Abscisic Acid Inhibits Cortical Microtubules Reorganization and Enhances Ultraviolet-B Tolerance in Arabidopsis thaliana" Genes 14, no. 4: 892. https://doi.org/10.3390/genes14040892
APA StyleShi, L., Lin, K., Su, T., & Shi, F. (2023). Abscisic Acid Inhibits Cortical Microtubules Reorganization and Enhances Ultraviolet-B Tolerance in Arabidopsis thaliana. Genes, 14(4), 892. https://doi.org/10.3390/genes14040892




