Abstract
Ammonium transporters (AMTs) are plasma membrane proteins mediating ammonium uptake and transport. As such, AMTs play vital roles in ammonium acquisition and mobilization, plant growth and development, and stress and pathogen defense responses. Identification of favorable AMT genotypes is a prime target for crop improvement. However, to date, systematic identification and expression analysis of AMT gene family members has not yet been reported for rapeseed (Brassica napus L.). In this study, 20 AMT genes were identified in a comprehensive search of the B. napus genome, 14 members of AMT1 and 6 members of AMT2. Tissue expression analyses revealed that the 14 AMT genes were primarily expressed in vegetative organs, suggesting that different BnaAMT genes might function in specific tissues at the different development stages. Meanwhile, qRT-PCR analysis found that several BnaAMTs strongly respond to the exogenous N conditions, implying the functional roles of AMT genes in ammonium absorption in rapeseed. Moreover, the rapeseed AMT genes were found to be differentially regulated by N, P, and K deficiency, indicating that crosstalk might exist in response to different stresses. Additionally, the subcellular localization of several BnaAMT proteins was confirmed in Arabidopsis protoplasts, and their functions were studied in detail by heterologous expression in yeast. In summary, our studies revealed the potential roles of BnaAMT genes in N acquisition or transportation and abiotic stress response and could provide valuable resources for revealing the functionality of AMTs in rapeseed.
1. Introduction
Nitrogen (N) is an essential macronutrient for plant growth and development that can be acquired as nitrate (NO3−), ammonium (NH4+), amino acids, and other N-containing substances [1]. In plants, NH4+ ions accumulating in cells, either through uptake from the soil via ammonium transporters (AMTs) or through reduction of NO3−, may be directed into the glutamine synthetase/glutamate synthase (GS/GOGAT) cycle [2]. Due to lower energy requirements for uptake and assimilation of NH4+ than NO3−, NH4+ is the preferred N source for uptake through roots, particularly in N-deficient plants [3,4]. On the other hand, excessive NH4+ concentrations are toxic and inhibit plant growth [5]. Thus, well-regulated homeostasis of internal NH4+ concentrations is essential for plant health and productivity.
Physiological studies of higher plants have revealed two transport systems for NH4+ on root cell membranes: a high-affinity ammonium transport system (HATS) and a low-affinity ammonium transport system (LATS). Under low external NH4+ concentrations, the HATS is upregulated for efficient absorption, while LATS products are more highly expressed at higher external NH4+ concentrations [6]. Coincidently, it is well known that plant AMTs are encoded by two distinct gene subfamilies: the AMT1 subfamily (AMT1 cluster) and the AMT2 subfamily (AMT2/3/4 clusters) [7]. AMT1s and AMT2s each typically contain 11–12 putative transmembrane regions, with two signature sequences located at transmembrane regions 5 and 10 [6,8].
To date, numerous AMT genes have been characterized in many plant species and are well documented in a published review [9], including Arabidopsis and rice. Previous studies found that AMTs have different expression characteristics within a plant species. For example, in Arabidopsis thaliana, AtAMT1;1 is mainly expressed in roots and leaves, while AtAMT1;2, AtAMT1;3, AtAMT1;5, and AtAMT2;1 are predominantly expressed in roots [10]. It was demonstrated that AtAMT1;1 and AtAMT1;3 account for 30–35% of NH4+ uptake in N-deficient roots [11], and AtAMT1;2 accounts for 18–26% [12]. Additionally, AtAMT1;4 is a pollen-specific ammonium transporter, mediating ammonium uptake across the plasma membrane of pollen, which contributes to N nutrition of pollen [13].
Ammonium is a major and preferential N form for rice grown in paddy fields due to poor aeration. It has been well documented that rice contains at least 12 AMTs [14], with these sequences being divisible into four subfamilies (OsAMT1, OsAMT2, OsAMT3, and OsAMT4), each of which is expressed in roots. Among these rice AMT subfamilies, OsAMT1 members have been characterized as HATS transporters, while the other subfamilies contain only LATS transporters [15,16]. As well as AtAMT1;1, OsAMT1;1 also exhibited ammonium uptake ability, with knockout of OsAMT1;1 reducing the ammonium uptake capacity of rice by about 25–30% in vivo. Furthermore, this gene was mainly expressed in root stele, root and shoot vascular bundles, and leaf mesophyll cells. Knockout of OsAMT1;1 resulted in a higher distribution of N in the root under low-NH4+ conditions [17], indicating that OsAMT1;1 contributes to root-to-shoot ammonium translocation. Recently, research has found that knockout of OsAMT1;1, OsAMT1;2, and OsAMT1;3 resulted in a 95% reduction in ammonium uptake, suggesting these three genes were cooperatively responsible for ammonium uptake under low-NH4+ conditions [18].
Besides the physiological roles of AMTs in mediating NH4+ acquisition from soil, root-to-shoot translocation of NH4+, and NH4+ uptake in leaves and the reproductive organs, AMTs also revealed roles in abiotic stress defense. It was reported that overexpression of PutAMT1;1 promoted early root growth after seed germination in transgenic Arabidopsis under salt stress, suggesting that AMT could alleviate NH4+ toxicity caused by salt stress [19]. In addition, several AMTs were also related to drought stress, such as AMT1;2 and AMT1;6 upregulated in Populus simonii [20]. It is also found in Malus prunifolia. Two ammonium transporters (AMT1;2 and AMT4;2) were notably upregulated together with the net influx of NH4+ at the surface of the roots under drought stress [21].
Rapeseed (B. napus L.) is one of the most essential and widely cultured oilseed crops worldwide for food and non-food purposes. In agriculture, rapeseed growth and yield require abundant N supplies [22,23]. Improving understanding of how uptake and transport of NH4+ and NO3− are regulated in this genus might facilitate improved nutrient management in rapeseed crops, especially under N deficiency conditions. In this study, we isolated and characterized 20 AMT genes from a rapeseed genomic sequence. Subsequently, we comprehensively analyzed rapeseed AMT genes’ transcription profiles in various plant tissues subjected to NH4+ deficiency or sufficiency treatments. The distinct expression patterns of BnaAMTs might indicate the diverse physiological roles played by ammonium transporters in rapeseed. Overall, this genome-wide analysis of rapeseed AMT genes provides a basis for further investigation of these genes to identify specific valuable functions that can be selected to improve rapeseed productivity.
2. Materials and Methods
2.1. Plant Materials and Stress Treatments
A widely grown Chinese rapeseed cultivar, Zhongshuang 11 (B. napus cv. ZS11), was used in this study. This variety was bred by the Oil Crops Research Institute at the Chinese Academy of Agricultural Sciences (CAAS).
For tissue-specific expression analysis of BnaAMT genes, rapeseed plants were harvested at different developmental stages for RNAseq assays. Details regarding sample harvesting and RNAseq analysis were described by previous studies [24,25]. Rapeseed seedlings were cultured in normal N conditions for 10 days in hydroponics to analyze BnaAMTs’ responses to different forms of N supply. Seedlings were then transferred into N starvation conditions for 5 days, after which young rapeseed leaves, old leaves, and roots were separately collected at 1, 4, 8, 12, and 24 h after resupplying N-deficient rapeseed plants with NO3− or NH4+. Samples were collected for each date and immediately stored at −80 °C for RNA extraction and subsequent qRT-PCR analysis.
To analyze the potential functions of BnaAMT genes in response to different nutrient deficiencies and drought stress, the experiments were conducted through simulation of these different stresses in hydroponics or a pot experiment as described before [26], respectively.
2.2. Identification and Bioinformatics Analyses of AMT Genes in Rapeseed
The amino acid sequences of all reported AMT members in Arabidopsis, rice, and wheat were used as query sequences to identify the AMT genes in rapeseed based on the B. napus genome database (http://www.genoscope.cns.fr/brassicanapus/ (accessed on 13 February 2023)). All potential proteins from the BLAST search were further filtered based on the presence of the conserved domain of AMT proteins (Pfam: PF00909) through an HMMER (3.1) search with the threshold value set at 0.001. The nucleotide and amino sequences of confirmed BnaAMT genes and their chromosomal locations were obtained from the Brassica database website. AMT Genes were then named according to their homologous genes in Arabidopsis. The distribution of BnaAMT genes on rapeseed chromosomes was plotted using the R package RIdeogram (https://github.com/TickingClock1992/RIdeogram (accessed on 13 February 2023)). Protein molecular weights and theoretical pI values were computed in the ProtParam tool (http://web.expasy.org/protparam/ (accessed on 13 February 2023)). Subcellular localization predictions for rapeseed AMT proteins were performed in ProtComp 9.0 (http://linux1.softberry.com/berry.phtml?group=programs&subgroup=proloc&topic=protcomppl (accessed on 13 February 2023)). Protein sequence alignment was performed using ClustalW and subsequently visualized in Genedoc. The logos of consensus transport residues were generated in WebLogo 3 online (http://weblogo.threeplusone.com/ (accessed on 13 February 2023)). The phylogenetic tree was constructed based on protein sequence alignment of AMT family sequences through the neighbor-joining method with 1000 bootstrap replicates in the MEGA 7.0 program (http://www.megasoftware.net/download_form (accessed on 13 February 2023)). The CDS and genomic sequences of rapeseed AMT genes downloaded from the database were used to paint gene structures through the use of Gene Structure Display Server 2.0 (http://gsds.cbi.pku.edu.cn (accessed on 13 February 2023)).
2.3. Expression Analysis
Total RNA from different rapeseed samples was extracted using RNAisoTM Plus reagent (Takara Bio, Otsu, Shiga, Japan) according to the manufacturer’s manual. RNA samples were then purified with RNase-free DNaseI (Invitrogen, Grand Island, NY, USA) to remove any contaminating genomic DNA. Total RNA quality assessment was checked via NanoDrop® spectrophotometry (TGem Plus, Tiangen, Beijing, China) and agarose gel electrophoresis to confirm the 28S:18S rRNA ratio. Next, first-strand cDNA sequences were synthesized using the PrimeScript™ RT Master Mix (Takara, Tokyo, Japan) according to the manufacturer’s protocols. The synthesized cDNA was used for forqRT-PCR reactions in a CFX connect Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA) using the SYBR® Premix Ex Taq™ II (TaKaRa, Tokyo, Japan). The related primers for qRT-PCR are listed in Table S1 and were designed with Primer-NCBI (https://www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi?LINK_LOC=BlastHome (accessed on 13 February 2023)). The PCR reactions were performed according to the manual of the SYBR® Premix Ex Taq™ II and with a total volume of 20 μL under the following conditions: 95 °C for 1 min, followed by 40 cycles of 95 °C for 15 s, 60 °C for 15 s, and 72 °C for 30 s. The expression of each AMT gene was calculated by the method described before and normalized to actin 7 [27,28]. Four biological replicates were used for each measurement.
2.4. Yeast Mutant Complementation Analysis
Full-length cDNA sequences of BnaAMT1;1b, BnaAMT1;1c, BnaAMT1;4a, BnaAMT1;5a, and BnaAMT2;2a were amplified by PCR using specific primers containing the HindIII, XbaI, or KpnI sites, as listed in Table S2. Returned open reading frames were ligated into the yeast expression vector pYES2 after the vector was linearized by HindIII, XbaI, or KpnI digestion. Yeast Δmep1, 2, 3 mutant 31019b, which cannot grow with <5 mM NH4+ provided as the sole N source [29], was transformed with pYES2-BnaAMT1;1b, pYES2-BnaAMT1;1c, pYES2-BnaAMT1;4a, pYES2-BnaAMT1;5a, or pYES2-BnaAMT2;2a, with the empty vector pYES2 also included as a negative control. All transformants were first selected on a solid yeast nitrogen base medium (2% agar) supplemented with 2% D-galactose and 2 mM L-arginine as the N source. A single colony was then picked, suspended in 100 mL of water, serially diluted, and dropped (2 µL) onto solid SD medium supplemented with 2% D-galactose and 0.02, 0.2, 2, or 5 mM NH4Cl provided as the sole N source, with pH values adjusted to 5.8 as described before with slight modification [30].
2.5. Subcellular Localization of Rapeseed AMT Proteins
Five representative BnaAMT proteins were cloned to generate constructs for subcellular localization analysis in Arabidopsis protoplasts. The ORF of each BnaAMT gene was amplified for insertion into the pMDC43 vector with HindIII/KpnI and XbaI to generate BnaAMT-GFP fusion proteins driven by the CaMV 35S promoter. The gene-specific primers are listed in Table S2. The vectors were respectively transformed into Arabidopsis protoplasts, which were isolated from 4-week-old leaves according to previous work [31]. After transfection and incubation in a plate under weak light for 12–16 h, fluorescent cells were imaged using a laser scanning confocal microscope (OLYMPUS FV10-ASW, Olympus, Tokyo, Japan).
2.6. Statistical Analyses
All data were analyzed in Microsoft Excel 2010. The comparisons were performed between the means of control and stress treatments using the one-way analysis of variance (ANOVA) method at the 5% and 1% probability level in SPSS statistics 25.
3. Results
3.1. Identification of AMT Genes in Rapeseed
Potential AMT genes were first identified by BLAST searching the B. napus genome with AMT sequences from Arabidopsis, rice, and wheat. The potential B. napus AMTs were checked for the ammonium transporter family Pfam domain (‘PF00909′) to further narrow down rapeseed AMT genes. Ultimately, 20 putative AMT proteins and their encoding genes were identified from the B. napus genome. These genes were named based on the order of homologies from Arabidopsis. The length of encoded proteins ranged from 447 amino acids (a.a.) to 512 a.a., with 7 to 11 transmembrane regions included in each protein sequence (Table 1 and Figure 1A) and two signature sequences located at transmembrane domains 5 and 10 (Figure 1B). All the putative proteins identified were predicted to localize to the plasma membrane (Table 1). The conserved motifs in the AMT protein sequences were predicted by MEME in rapeseed (Figure 1C). The BnaAMT proteins in the same subgroup showed identical motif components. Motifs 1–10 were commonly identified in all members of the AMT1 subgroup, except for BnaAMT1;3c. However, Motif 12 seemed to be distributed explicitly in the AMT2 subgroup.

Table 1.
Information about AMT family genes in rapeseed.
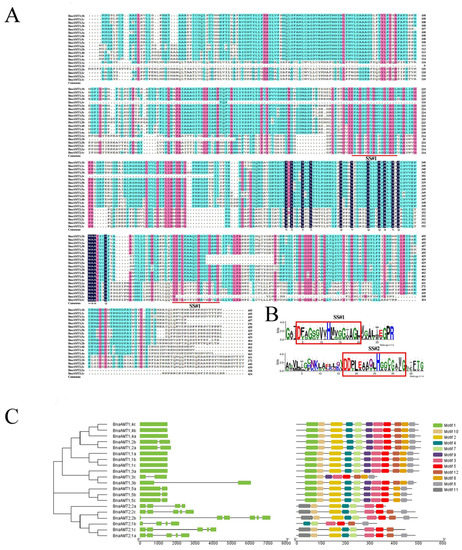
Figure 1.
Multiple alignment of rapeseed AMT family proteins and gene structure/conserved motifs characteristic of rapeseed AMT family members. (A) Multiple alignment of amino sequences of BnaAMTs. Red lines underneath alignments indicate two reported signature sequences within the AMT family. (B) The logo of these signature sequences. (C) The gene structure map of BnaAMTs. Green boxes indicate exons while black lines represent introns. The conserved motifs of rapeseed AMT proteins were analyzed through MEME.
3.2. Phylogenetic Analyses and Chromosomal Locations of BnaAMT Genes
To evaluate evolutionary relationships among orthologous AMT genes, a phylogenetic tree of AMTs from rapeseed, Arabidopsis, soybean, rice, corn, wheat, and three Brassica relatives of rapeseed was constructed using the neighbor-joining method in MEGA 7.0. As shown in Figure 2A, two major clades and four clusters are distinguishable in this phylogenetic tree. Among the 20 AMT genes in rapeseed, 14 fell into the AMT1 cluster and 6 were found in the AMT2 cluster. No rapeseed or other brassica AMT genes were placed into either of the two remaining clusters (AMT3 and AMT4). The chromosomal locations of rapeseed AMT genes are also shown in Figure 1. These BnaAMT genes are scattered across the B. napus genome, with the only cluster found containing BnaAMT2;1b and BnaAMT2;1c on chromosome C04 (Figure 2B).
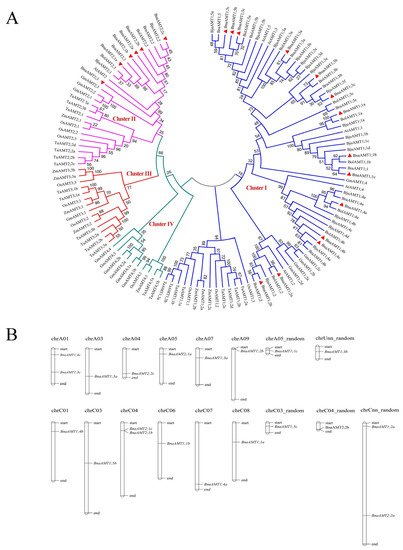
Figure 2.
Phylogenetic relationships of AMT family proteins in diverse species and the chromosomal locations of AMT family genes in rapeseed. (A) Phylogenetic tree of AMT family proteins. A total of 125 proteins from nine species were used to construct a phylogenetic tree based on the neighbor-joining method in MEGA 7.0 (1000 replicates). From previous reports, 39 AMT family proteins from A. thaliana (At), Oryza sativa (Os), and Triticum aestivum (Ta) were identified. The AMT family proteins from B. napus (Bna), Glycine max (Gm), Zea mays (Zm), B. rape (Bra), B. oleracea (Bol), and B. juncea (Bju) were predicted from these 39 known AMT proteins. Different clusters are labeled by different colors. The 20 aligned BnaAMTs are marked by red triangles. (B) Positions of AMT family genes on rapeseed chromosomes.
3.3. Expression Patterns of BnaAMT Genes in Various Rapeseed Tissues
For indications of the biological functions filled by BnaAMT genes, the expression patterns of these genes were determined in different tissues through RNA-seq analysis. Among the 20 AMT genes examined, two (BnaAMT1;5b and BnaAMT1;5c) were not detected in any tissue under the applied growth conditions, and eight exhibited relatively low expression levels in a majority of tissues (Figure 3). Expression of the remaining half of BnaAMT genes, though variable, was relatively high in most tissues. BnaAMT1;1b, BnaAMT1;1c, BnaAMT2;1a, and BnaAMT2;1c were widely expressed across most tissues, both aboveground and belowground, including vegetative and reproductive organs. BnaAMT1;4a, BnaAMT1;4b, and BnaAMT1;4c were most highly expressed in new pistil and bud tissues. In addition, BnaAMT2;2a was also interesting for being expressed at high levels in sepals and blossomy pistils (Figure 3). Finally, under the conditions of this experiment, no BnaAMT genes were found to be specifically expressed in roots.
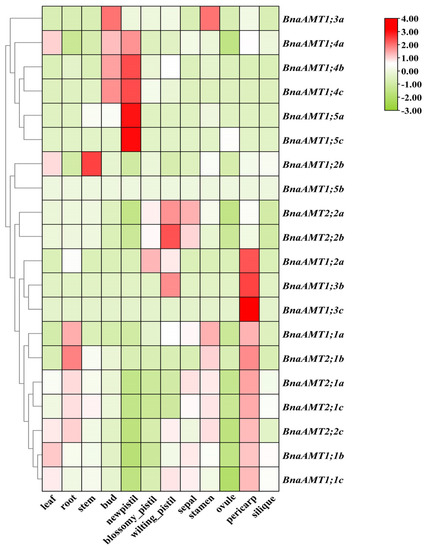
Figure 3.
Tissue-specific expression profiles of BnaAMT family genes. Heat maps of BnaAMT expression were generated from RNA-seq analysis in twelve different tissues, including leaves, roots, stems, buds, new pistils, blossomy pistils, wilting pistils, sepals, stamens, ovules, pericarps, and siliques. The red color indicates upregulation, while the green color indicates downregulation.
3.4. Expression of BnaAMT Genes in Response to N Deficiency and Resupply
To test whether any BnaAMT responses to N deficiency were mediated by NH4+ or NO3−, expression of BnaAMTs was examined in 10-day-old rapeseed seedlings grown under N deficiency conditions for 5 days and then resupplied with 1 mM NH4+ or 1 mM NO3− for 0, 1, 4, 8, 12, and 24 h. Within 1 h of initiating ammonium resupply, BnaAMT1;1a and BnaAMT2;2a mRNA levels increased in old leaves and then quickly peaked before receding to previous levels or even lower (Figure 4). Interestingly, while expression of BnaAMT1;1b and BnaAMT1;1c was similar to expression of BnaAMT2;1a and BnaAMT2;1c across tissues (Figure 3), their responses to NH4+ and NO3− resupply treatments contrasted according to their subfamily membership (Figure 4). BnaAMT1;1b and BnaAMT1;1c mRNA levels increased in the old leaves of N-deficient rapeseed resupplied with either NH4+ or NO3−, though responses peaked temporarily in hours 4–12 of NH4+ resupply treatments and continued increasing for the full 24 h of observations when NO3− was resupplied (Figure 4). In contrast, both BnaAMT2;1a and BnaAMT2;1c were downregulated in response to NH4+ or NO3− resupply treatments in young leaves and old leaves, but not in roots. Notably, BnaAMT2;1b expression strongly declined in old leaves with ammonium or nitrate resupplies, but its expressions in roots increased significantly, especially after 8 h of nitrate resupply (Figure 4).
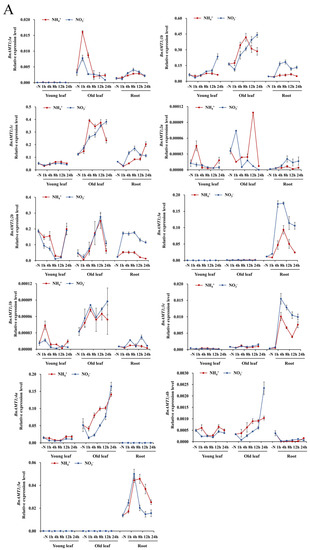
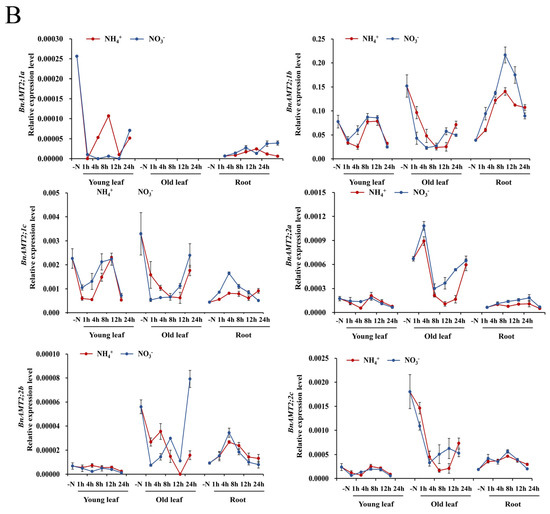
Figure 4.
The expression profiles of BnaAMTs in different tissues of rapeseed with ammonium and nitrate supply. (A) BnaAMT1s; (B) BnaAMT2s. The red lines indicate ammonium source, while blue lines indicate nitrate. Rapeseed young leaves, old leaves, and roots were separately collected at 1 h, 4 h, 8 h, 12 h, and 24 h after resupplying N-deficient rapeseed plants with NH4+ or NO3−. Samples were stored at −80 °C for RNA extraction and qRT-PCR analysis. The relative expression levels of BnaAMTs were relative to the control (actin 7). Four biological replicates were performed. Error bars represent standard deviation.
Among the remaining BnaAMT genes, the expression of several, including BnaAMT1;2a, BnaAMT1;2b, BnaAMT1;4a, and BnaAMT1;4b, peaked 12 h to 24 h after being resupplied with NH4+ or NO3−. Three BnaAMT genes, BnaAMT1;3a, BnaAMT1;3c, and BnaAMT1;5a, were detected almost exclusively in roots, where they all displayed transient peaks in expression 4–8 h after the onset of NH4+ or NO3− resupply treatments (Figure 4).
3.5. Quantitative RT-PCR Analysis of BnaAMT Genes in Nutrient-Deficient Rapeseed Plants
Beyond potential functions in acquiring transient supplies of N, BnaAMTs might also be involved in responses to nutrient deficiency. To test this, expression of BnaAMT members was quantified in the roots and leaves of rapeseed plants subjected to deficiency in N, phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), sulfur (S), and boron (B). Among the 20 identified BnaAMT genes, the expression of 7 BnaAMTs was either not detected or was detected with very low levels of expression in the roots or leaves under any of the control or nutrient deficiency conditions. Rapeseed leaves responded to N deficiency by upregulating eight BnaAMT genes and downregulating one, while roots responded to N deficiency by upregulating five BnaAMT genes. Under P deficiency stress, only three BnaAMT genes were upregulated in leaves or roots, and five were remarkably depressed. In K-deprived rapeseed, nine BnaAMT genes were upregulated in leaves or roots, while only two exhibited significant decreases in abundance in K-deficient leaves. Interestingly, expression of BnaAMT1;1a, BnaAMT1;2b, and BnaAMT1;3c increased in the leaves of rapeseed in nearly all of the nutrient deficiency treatments, while only BnaAMT1;3c, being significantly upregulated in the roots of six of the nutrient deficiency treatments, responded consistently in roots across nutrient deficiency treatments (Figure 5).
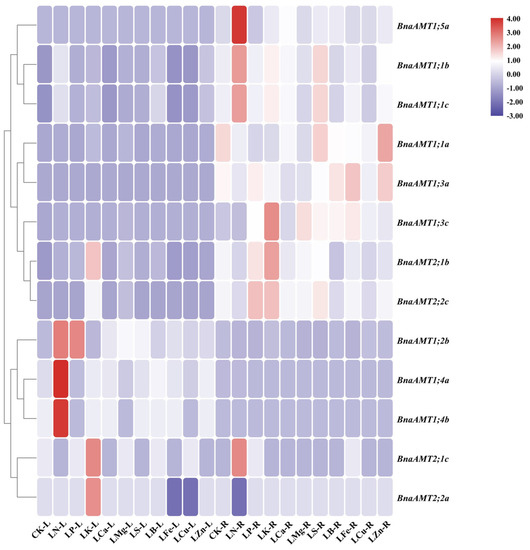
Figure 5.
The relative expression profiles of BnaAMT family gene responses to different nutrient deficiencies. LN, N deficiency; LP, P deficiency; LK, K deficiency; LCa, Ca deficiency; LMg, Mg deficiency; LS, S deficiency; LB, B deficiency; LFe, Fe deficiency; LCu, Cu deficiency; LZn, Zn deficiency; CK, control. L, leaf; R, root. The red color indicates upregulation, while the blue color indicates downregulation. The relative expression levels of BnaAMTs were relative to the control (actin 7). Four biological replicates were performed.
3.6. Expression of BnaAMT Genes in Drought- or Waterlogging-Stressed Rapeseed
Drought and waterlogging are two common stresses in rapeseed production systems. To date, little is known about the expression profiles of BnaAMT genes in response to these two stresses. Therefore, the expression of 17 detectable BnaAMT transcripts was examined by qRT-PCR analysis under drought and waterlogging stress conditions. As in the nutrient deficiency experiment described above, BnaAMT1;4c, BnaAMT1;5b, and BnaAMT1;5c showed no expression. In addition, BnaAMT1;3b and BnaAMT1;5a exhibited only shallow expression in this drought and waterlogging experiment, so they were also excluded from this analysis. Therefore, results from waterlogging and drought stress trials are presented for the remaining 15 BnaAMT genes.
Under drought stress conditions, BnaAMT gene expression could be divided into four categories, as shown in Figure 6. Expressions of BnaAMT1;2a, BnaAMT1;2b, and BnaAMT1;3a were inhibited by drought stress in older leaves and roots. Several BnaAMT genes were inhibited by drought stress upon rehydration (rather than demonstrating a recovery in expression levels), including BnaAMT1;1a, BnaAMT1;1b, BnaAMT1;1c, BnaAMT1;3c, and BnaAMT2;2a. Transcription levels of BnaAMT2;1a, BnaAMT2;1c, BnaAMT2;2b, and BnaAMT2;1b increased dramatically with rehydration, especially in older leaves of rapeseed. In contrast to those downregulated BnaAMT genes, the expression of BnaAMT1;2a, BnaAMT1;2b, BnaAMT1;4a, and BnaAMT1;4b was significantly enhanced in young leaves after 7 days of drought stress.
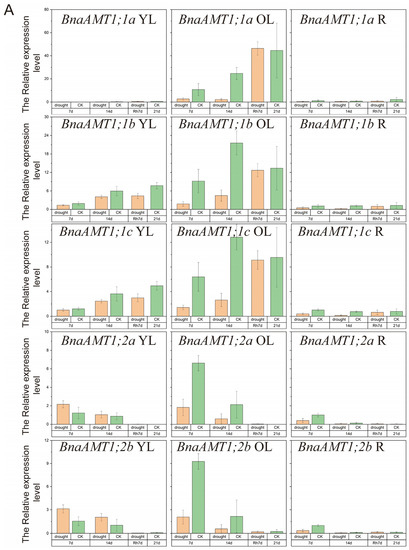
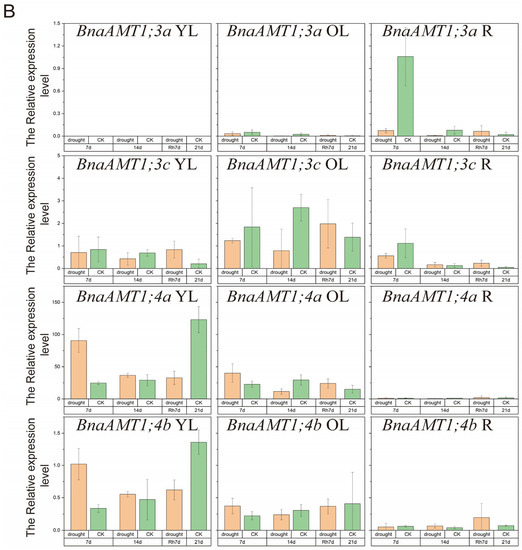
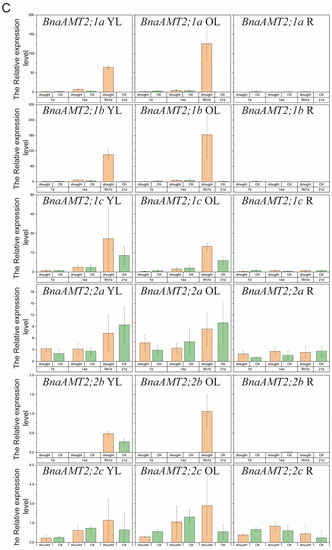
Figure 6.
Expression profiles of BnaAMT family genes responding to drought stress. (A) The relative expression level of BnaAMT1;1a-BnaAMT1;2b; (B) the relative expression level of BnaAMT1;3a-BnaAMT1;4b; (C) the relative expression level of BnaAMT2;1a-BnaAMT2;2c. YL, young leaf; OL, older leaf; R, root; CK, control; drought, drought stress treatment; 7d, 7th day of drought stress; 14d, 14th day of drought stress; Rh7d, 7th day after rehydration; 21d, 21st day of drought stress. Rapeseed seedlings at the five-leaf growth stage were subjected to drought stress for 14 days before rehydration. The relative expression levels of BnaAMTs were relative to the control (actin 7). Four biological replicates were performed. Error bars represent standard deviation.
Under waterlogging stress conditions, the expression of several BnaAMT genes was inhibited by waterlogging stress, especially in early stress-treated leaves, such as three BnaAMT2;1 members (Figure 7). Additionally, the expression of a number of BnaAMTs was remarkably enhanced in response to waterlogging stress in older leaves, namely the transcript of BnaAMT1;1c and BnaAMT1;2b, which were strongly upregulated after 7 days treatment, as well as the transcript of BnaAMT1;1a and BnaAMT2;2c, which dramatically increased with treatment after 14 days (Figure 7). These results implied that waterlogging stress might disrupt N metabolism in plants, and AMT genes might participate in translocating NH4+ to regulate N status in stressed plants.
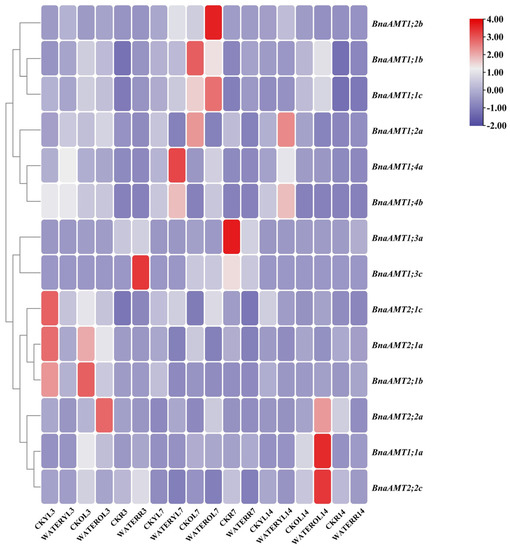
Figure 7.
The relative expression profiles of BnaAMT family gene responses to waterlogging stress. CK, control treatment; WATER, waterlogging stress treatment; YL, young leaf; OL, old leaf; R, root; 3, after 3 days of treatment; 7, after 7 days of treatment; 14, after 14 days of treatment. The red color indicates upregulation, while the blue color indicates downregulation. The relative expression levels of BnaAMTs were relative to the control (actin 7). Four biological replicates were performed.
3.7. Functional Complementation Analysis of Selected BnaAMT Genes in a Yeast Mutant Strain
To rapidly test the putative NH4+ transport roles filled by BnaAMTs, the ORFs of BnaAMT1;1b, BnaAMT1;1c, BnaAMT1;4a, BnaAMT1;5a, and BnaAMT2;2a were separately cloned into lines of the yeast expression vector pYES2 and then transformed into yeast Δmep1, 2, 3 mutant 31019b, which cannot grow on media containing less than 5 mM NH4+ as the sole N source. Yeast 31019b cells carrying BnaAMT1;1b, BnaAMT1;1c, BnaAMT1;4a, BnaAMT1;5a, BnaAMT2;2a, or the empty pYES2 vector as control were all able to proliferate on yeast growth medium with 2 mM L-arginine provided as the sole N source (Figure 8A). Transformation with the empty vector pYES2 or pYES2 harboring BnaAMT2;2a did not stimulate growth on the medium containing up to 5 mM NH4+ (supplied as NH4Cl) as the sole source of N, while the transformation of 31019b with pYES2 harboring BnaAMT1;1b, BnaAMT1;1c, BnaAMT1;4a, and BnaAMT1;5a allowed yeast growth on media containing as little as 0.02 mM NH4Cl as a sole N source, with increasing NH4+ leading to more growth (Figure 8B). On the whole, results of yeast transformation indicate that BnaAMT1;1b, BnaAMT1;1c, BnaAMT1;4a, and BnaAMT1;5a facilitate NH4+ permeation across the plasma membrane, while BnaAMT2;2a may not function in this capacity.
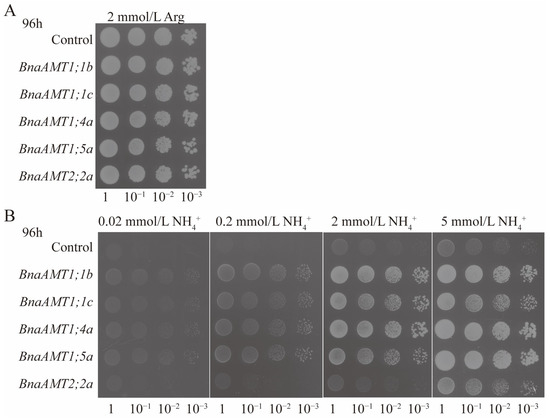
Figure 8.
Functional complementation of the yeast mutant 31019b defective in NH4+ uptake by heterologous expression of BnaAMTs (BnaAMT1;1b, BnaAMT1;1c, BnaAMT1;4a, BnaAMT1;5a, BnaAMT2;2a). (A) The 31019b cells carrying the empty vector pYES2 (control), BnaAMT1;1b, BnaAMT1;1c, BnaAMT1;4a, BnaAMT1;5a, and BnaAMT2;2a were allowed yeast growth on 2 mM L-arginine as a sole N source. (B) The 31019b cells carrying the empty vector pYES2 (control), BnaAMT1;1b, BnaAMT1;1c, BnaAMT1;4a, BnaAMT1;5a, and BnaAMT2;2a were allowed yeast growth on 0.02, 0.2, 2, and 5 mM NH4Cl as a sole N source.
3.8. Subcellular Localization of BnaAMT Proteins
To explore the subcellular localization of the BnaAMT proteins, we first explored subcellular localization as predicted by ProtComp analysis. As shown in Table 1, all of the identified BnaAMT proteins were predicted to target plasma membranes. This was followed by experimental observations of the subcellular localizations of five selected BnaAMT proteins (BnaAMT1;1b, BnaAMT1;1c, BnaAMT1;4a, BnaAMT1;5a, and BnaAMT2;2a) through the transient expression of GFP::BnaAMT fusions in Arabidopsis protoplast cultures expressing the membrane marker OsMCA1. Microscopic observation revealed that each of the 35GFP::BnaAMT fusion constructs localized to plasma membranes along with OsMCA1 (Figure 9). These results strongly suggest that BnaAMT proteins consistently localize to plasma membranes, where they fulfill specific biological functions in rapeseed cells.
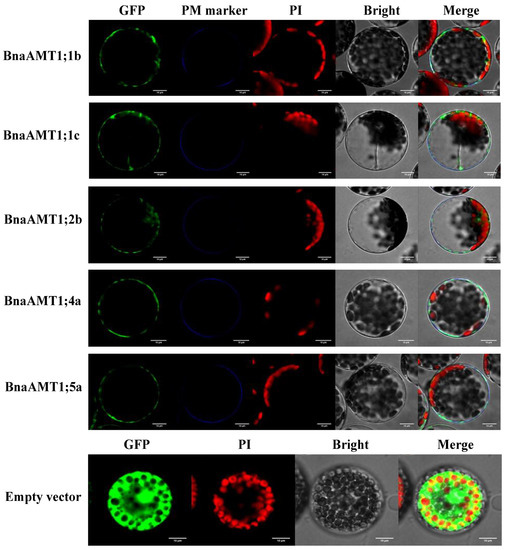
Figure 9.
Subcellular localization of five representative BnaAMTs in A. thaliana protoplasts. OsMCA1, as the plasma membrane marker with the blue signals (PM Marker), was co-introduced into A. thaliana protoplasts with BnaAMT ORFs fused with GFP. An empty vector was included as a control. GFP, green fluorescence protein (green signals); PI, propidium iodide (red signals); Bright, bright field; Merge, overlay of all signals. White bars equal 10 μ0.
4. Discussion
Ammonium transporters play vital roles in ammonium uptake and translocation [1,4]. The AMT gene family has been investigated and characterized in various plant species, including rice, wheat, maize, cassava, and poplar [6,32,33,34,35]. Nevertheless, information on the AMT gene family remains lacking for rapeseed, a widely cultivated oil crop. Moreover, rapeseed is sensitive to N deficiency as an allotetraploid crop resulting from hybridization between B. rapa and B. oleracea [36]. Maintenance of productivity in rapeseed crops requires relatively large inputs of N fertilizer. Therefore, characterization of AMT members in rapeseed is a promising avenue to explore for improvements in the nutrient management of rapeseed or the targeting of traits in breeding programs aiming to produce rapeseed varieties with stronger tolerance to low N availability.
In this study, we comprehensively identified and characterized the AMT gene family in rapeseed, i.e., B. napus, which included analyzing the information of AMT homologous sequences in the B. napus genome, such as phylogenetic relationships, chromosome locations, gene structures, conserved motifs, and cis-acting promoter elements. Furthermore, the expression profiles were quantified across organs under different nutrient stresses. Comprehensive characterization of BnaAMT genes will provide a foundation for building programs to improve N management and maintain productivity in N-deficient soil for this critical oil crop species.
The 20 rapeseed BnaAMT genes distributed over twelve chromosomes and five random chromosomes are at least twice as many as the six in Arabidopsis [4,12,13], eight in maize [37], or twelve in rice [32]. Phylogenetic analysis clustered all of the AMT proteins from multiple species into four distinct subfamilies: AMT1, AMT2, AMT3, and AMT4. However, the 20 BnaAMTs in rapeseed only fell into the AMT1 (14 AMT proteins) and AMT2 (6 AMT proteins) subfamily clusters, which is similar to Arabidopsis AMT family proteins (Figure 2). Interestingly, each Arabidopsis AMT gene exists as a single copy, whereas each BnaAMT gene fell into homologous clusters of 2–3 copies (Figure 2). These homologous genes further clustered into single phylogenetic branches without exception (Figure 2). The results herein indicate that duplications of rapeseed AMT gene family members resulted primarily from a whole-genome duplication. Thus, polyploidization is likely the main force driving the expansion of the AMT gene family in B. napus, an allotetraploid plant species [38].
Structural analysis of BnaAMT genes revealed that the two subfamilies exhibit divergent exon–intron patterns (Figure 1C). In general, AMT2 family genes contain more introns than AMT1 family genes. The AMT genes with less intronic sequences, except BnaAMT1;3a, were the most highly expressed genes in each of the tested tissues under the applied nutrition treatments. Motif analysis in the MEME application revealed conserved AMT structures across the rapeseed genome (Figure 1C). Specifically, rapeseed AMT1 family genes have 10–11 nearly uniform motifs. The lone exception is BnaAMT1;3c, which lacks Motif2, Motif4, Motif7, and Motif11. In contrast, all the AMT2 family members in rapeseed lacked Motif 1, Motif 8, and Motif 9, and also contained the AMT2-specific Motif 11.
Gene expression profiles may provide essential clues for predicting gene functions. To this end, RNA-seq data were used to investigate BnaAMT gene expression levels in diverse tissues of B. napus [24,25]. In these observations, 4 of the 20 identified BnaAMT genes were minimally expressed across the 12 tested tissues (Figure 3), indicating that these genes fill few functional roles in the tested tissues. On the other hand, BnaAMT1.1, BnaAMT2.1, and BnaAMT2;2 family members were expressed across tissues, including in leaves, pericarps, and roots (Figure 3). This did not coincide with previous studies in Arabidopsis, which have demonstrated that the members of the AMT1 clade might preferentially express in roots [11]. In contrast, few rapeseed AMT genes exhibited tissue or organ specificity. It is worth mentioning that BnaAMT1;4b and BnaAMT1;4c were most highly expressed in the young tissues of rapeseed, such as buds and new pistils (Figure 3), indicating that these genes might play roles in rapeseed flower and bud development.
It is generally considered that AMT products mediate ammonium uptake, which has been verified for AtAMT1;1 by expression under low-NH4+ conditions in the yeast mutant 31019b, which lacks three ammonium transporter homologous genes known as Meps [29]. Under low-ammonium supply conditions, 31019b mutants harboring AtAMT1;1 can sufficiently restore ammonium uptake for cellular proliferation. Beyond AtAMT1;1, the other Arabidopsis AMTs have also restored 31019b growth under low-ammonium conditions [4]. In recent years, the 31019b yeast mutant has been widely applied for functional complementation studies of homologous AMT genes in several plant species [39,40,41,42]. For example, in the present study, four of the five rapeseed AMT proteins selected for testing proved capable of restoring 31019b growth under low-ammonium conditions, with BnaAMT2;2a being the lone exception (Figure 8). Therefore, it is reasonable to conclude that rapeseed AMT genes also function primarily in ammonium uptake.
In plants, AMT expression levels are often regulated by the status of multiple nitrogen compounds [4,34,43,44,45]. In the present study, relative to transcription under N deficiency conditions, all rapeseed AMT2 family members (except BnaAMT2;2a) were repressed in young or old leaves subjected to NH4+ or NO3− addition, while expression in roots was generally induced by either NH4+ and NO3− application (Figure 5). Meanwhile, expression of AMT1 family genes was also enhanced to varying degrees by resupply of either NH4+ or NO3−, which is consistent with a previous report that AMT transcript levels are subjected to control by NO3− availability [34]. Several AMT genes, such as BnaAMT1;1a and BnaAMT1;2a, responded more to resupply with NH4+ than with NO3−, especially in old leaves (Figure 5). This result is inconsistent with reports that transcription of AMT1.1 in Arabidopsis is downregulated when NH4+ is resupplied to N-deficient plants [12]. However, the transient responses observed herein for rapeseed genes such as BnaAMT1;1a, BnaAMT1;2a, BnaAMT1;3a, and BnaAMT2;2a indicate that NH4+ signaling might spike during early phases of N resupply.
With NH4+ being the preferred N source for plants suffering from N starvation, N deficiency has been noted to strongly induce AMT1.1 and AMT1.3 expression in Arabidopsis. In comparison, the transcription level of AMT1.2 has largely been unaffected by N deficiency [12]. In our study, the expression of 11 rapeseed AMT genes was significantly enhanced by low-N conditions in leaves or roots, with BnaAMT1;1b and BnaAMT1;1c being most notable (Figure 5). In realistic conditions, rapeseed planted in fields may suffer from various nutrient deficiencies. In the present study, a portion of the identified BnaAMTs exhibited transcriptional responses to several nutrient deficiencies. Particularly noteworthy was the observation that the expression of BnaAMT1;3c in the roots increased across all nutrient-deficient conditions, except for N deficiency (Figure 5). This is consistent with previous reports concerning Pht family genes in rapeseed [46], which also demonstrated that the expression of rapeseed AMTs appears to be involved in mineral nutrient homeostasis and crosstalk among ion signals in response to multiple nutrient stresses.
Both waterlogging and drought stress are also common limiting factors for rapeseed production. Previous studies have found that interactions between waterlogging and fertilizer applications affect rapeseed productivity. In detail, applying N fertilizer has been noted to alleviate the effects of waterlogging stress on rapeseed growth and development, while waterlogging stress can also influence N metabolism [47]. Among our studies, several plant AMT genes are affected by drought or waterlogging stress. For example, AMT2 family members have been significantly enhanced by rehydration after 14 days of drought stress (Figure 6C). In addition, several rapeseed AMT genes have also been reported as being influenced by waterlogging stress in both young and old leaves (Figure 7). Previous studies have found that plant AMTs could be involved in responses to stress. For example, overexpression of the Puccinellia tenuiflora gene PutAMT1;1 in Arabidopsis significantly improves salt tolerance during the early root growth stage after seed germination. This suggests that ammonium transport might alleviate ammonia toxicity caused by salt stress [19]. Overall, upregulation of AMTs in response to waterlogging or drought stress suggests that these genes might alleviate stress by improving N uptake and metabolism under stress conditions or by avoiding ammonia toxicity possibly caused by stress through transport across plant tissues.
5. Conclusions
In summary, this study includes a comprehensive identification and analysis of AMT gene family members in rapeseed. A total of 20 BnaAMT genes were identified in the rapeseed genome, each of which was then subjected to bioinformatic and expression profile analyses to reveal their potential functions. The results indicate that BnaAMT genes are actively involved in regulating rapeseed plant growth, development, and responses to nutrient deficiency and stresses brought on by drought or waterlogging stress. These results provide a solid foundation for additional functional studies of BnaAMT genes and their contributions to stress tolerance in rapeseed, which can be applied to improving crop performance under diverse conditions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes14030658/s1, Table S1: Specific primers of the rapeseed AMT genes used in qRT-PCR assays; Table S2: Primers used in the yeast complementation experiments; Table S3: Primers used in the subcellular localization experiments.
Author Contributions
L.Q., J.D., P.H. and X.L. designed the study and wrote the manuscript. J.D., T.C.W. and L.Y. carried out bioinformatic analyses. P.H., Y.L. and C.G. collected plant materials and carried out the qRT-PCR analyses. L.C. carried out the subcellular localization experiment. P.H. carried out yeast mutant complementation analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the China Agriculture Research System of MOF and MARA (CARS-22), as well as the Agricultural Science and Technology Innovation Program of the Chinese Academy of Agricultural Sciences (CAAS-ASTIP-2013-OCRI).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The AMT protein sequences of B. rape, B. oleracea, and B. juncea were collected from BRAD (http://brassicadb.org/brad/ (accessed on 13 February 2023)) and the genome and protein sequences of B. napus were downloaded from Genoscope (http://www.genoscope.cns.fr/brassicanapus/ (accessed on 13 February 2023)). All data generated or analyzed in this study were included in this published article and its additional files. The materials are available upon request by contacting the corresponding author.
Acknowledgments
We greatly thank Su Yanhua and Yang Shunying from the Institute of Soil Science at the Chinese Academy of Sciences for kindly providing support to the yeast mutant complementation experiment. We thank Huawei’s group at the Oil Crops Research Institute at the Chinese Academy of Agricultural Sciences for providing RNA-seq data of rapeseed for analysis, and would also like to offer special thanks to Xie Lihua, Hu Wenshi, Yang Lu, and Li Xiaoyong for their help in each experiment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- von Wittgenstein, N.J.; Le, C.H.; Hawkins, B.J.; Ehlting, J. Evolutionary classification of ammonium, nitrate, and peptide transporters in land plants. BMC Evol. Biol. 2014, 14, 11. [Google Scholar] [CrossRef]
- Nunes-Nesi, A.; Fernie, A.R.; Stitt, M. Metabolic and signaling aspects underpinning the regulation of plant carbon nitrogen interactions. Mol. Plant 2010, 3, 973–996. [Google Scholar] [CrossRef] [PubMed]
- Bloom, A.J.; Sukrapanna, S.S.; Warner, R.L. Root Respiration Associated with Ammonium and Nitrate Absorption and Assimilation by Barley. Plant Physiol. 1992, 99, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Gazzarrini, S.; Lejay, L.; Gojon, A.; Ninnemann, O.; Frommer, W.B.; von Wirén, N. Three functional transporters for constitutive, diurnally regulated, and starvation-induced uptake of ammonium into Arabidopsis roots. Plant Cell 1999, 11, 937–948. [Google Scholar] [CrossRef]
- Britto, D.T.; Kronzucker, H.J. NH4+ toxicity in higher plants: A critical review. J. Plant Physiol. 2002, 159, 567–584. [Google Scholar] [CrossRef]
- Couturier, J.; Montanini, B.; Martin, F.; Brun, A.; Blaudez, D.; Chalot, M. The expanded family of ammonium transporters in the perennial poplar plant. New Phytol. 2007, 174, 137–150. [Google Scholar] [CrossRef]
- Ludewig, U.; Neuhäuser, B.; Dynowski, M. Molecular mechanisms of ammonium transport and accumulation in plants. FEBS Lett. 2007, 581, 2301–2308. [Google Scholar] [CrossRef]
- Wirén, N.V.; Merrick, M. Regulation and function of ammonium carriers in bacteria, fungi, and plants. In Molecular Mechanisms Controlling Transmembrane Transport; Springer: Berlin/Heidelberg, Germany, 2004; pp. 95–120. [Google Scholar]
- Hao, D.L.; Zhou, J.Y.; Yang, S.Y.; Qi, W.; Yang, K.J.; Su, Y.H. Function and regulation of ammonium transporters in plants. Int. J. Mol. Sci. 2020, 21, 3557. [Google Scholar] [CrossRef]
- Loqué, D.; von Wirén, N. Regulatory levels for the transport of ammonium in plant roots. J. Exp. Bot. 2004, 55, 1293–1305. [Google Scholar] [CrossRef] [PubMed]
- Loqué, D.; Yuan, L.X.; Kojima, S.; Gojon, A.; Wirth, J.; Gazzarrini, S.; Ishiyama, K.; Takahashi, H.; von Wirén, N. Additive contribution of AMT1;1 and AMT1;3 to high-affinity ammonium uptake across the plasma membrane of nitrogen-deficient Arabidopsis roots. Plant J. 2006, 48, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.X.; Loqué, D.; Kojima, S.; Rauch, S.; Ishiyama, K.; Inoue, E.; Takahashi, H.; von Wirén, N. The organization of high-affinity ammonium uptake in Arabidopsis roots depends on the spatial arrangement and biochemical properties of AMT1-type transporters. Plant Cell 2007, 19, 2636–2652. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.X.; Graff, L.; Loqué, D.; Kojima, S.; Tsuchiya, Y.N.; Takahashi, H.; von Wirén, N. AtAMT1;4, a pollen-specific high-affinity ammonium transporter of the plasma membrane in Arabidopsis. Plant Cell Physiol. 2009, 50, 13–25. [Google Scholar] [CrossRef]
- Kumar, A.; Silim, S.N.; Okamoto, M.; Siddiqi, M.Y.; Glass, A.D. Differential expression of three members of the AMT1 gene family encoding putative high-affinity NH4+ transporters in roots of Oryza sativa subspecies indica. Plant Cell Environ. 2003, 26, 907–914. [Google Scholar] [CrossRef]
- Sonoda, Y.; Ikeda, A.; Saiki, S.; von Wirén, N.; Yamaya, T.; Yamaguchi, J. Distinct expression and function of three ammonium transporter genes (OsAMT1;1-1;3) in rice. Plant Cell Physiol. 2003, 44, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.F.; Yang, W.Z.; Wei, J.H.; Yoon, H.; An, G. Transcription factor OsDOF18 controls ammonium uptake by inducing ammonium transporters in rice roots. Mol. Cells 2017, 40, 178–185. [Google Scholar]
- Li, C.; Tang, Z.; Wei, J.; Qu, H.Y.; Xie, Y.J.; Xu, G.H. The OsAMT1.1 gene functions in ammonium uptake and ammonium-potassium homeostasis over low and high ammonium concentration ranges. J. Genet. Genom. 2016, 43, 639–649. [Google Scholar] [CrossRef]
- Konishi, N.; Ma, J.F. Three polarly localized ammonium transporter 1 members are cooperatively responsible for ammonium uptake in rice under low ammonium condition. New Phytol. 2021, 232, 1778–1792. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.Y.; Takano, T.; Liu, S.K. The role of ammonium transporter (AMT) against salt stress in plants. Plant Signal. Behav. 2019, 14, 1625696. [Google Scholar] [CrossRef]
- Meng, S.; Zhang, C.X.; Su, L.; Li, Y.M.; Zhao, Z. Nitrogen uptake and metabolism of Populus simonii in response to PEG-induced drought stress. Environ. Exp. Bot. 2016, 123, 78–87. [Google Scholar] [CrossRef]
- Huang, L.L.; Li, M.J.; Zhou, K.; Sun, T.T.; Hu, L.Y.; Li, C.Y.; Ma, F.W. Uptake and metabolism of ammonium and nitrate in response to drought stress in Malus prunifolia. Plant Physiol. Biochem. 2018, 127, 185–193. [Google Scholar] [CrossRef]
- Williams, S.T.; Vail, S.; Arcand, M.M. Nitrogen use efficiency in parent vs. hybrid canola under varying nitrogen availabilities. Plants 2021, 10, 2364. [Google Scholar] [CrossRef]
- Hua, Y.P.; Zhou, T.; Huang, J.Y.; Yue, C.P.; Song, H.X.; Guan, C.Y.; Zhang, Z.H. Genome-wide differential DNA methylation and miRNA expression profiling reveals epigenetic regulatory mechanisms underlying nitrogen-limitation-triggered adaptation and use efficiency enhancement in allotetraploid rapeseed. Int. J. Mol. Sci. 2020, 21, 8453. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.M.; Fan, G.Y.; Hu, Q.; Zhou, Y.M.; Guan, M.; Tong, C.B.; Li, J.N.; Du, D.Z.; Qi, C.K.; Jiang, L.C.; et al. The high-quality genome of Brassica napus cultivar ‘ZS11’ reveals the introgression history in semi-winter morphotype. Plant J. 2017, 92, 452–468. [Google Scholar] [CrossRef]
- Li, Y.; Dong, C.H.; Hu, M.; Bai, Z.T.; Tong, C.B.; Zuo, R.; Liu, Y.Y.; Cheng, X.H.; Cheng, M.X.; Huang, J.Y.; et al. Identification of flower-specific promoters through comparative transcriptome analysis in Brassica napus. Int. J. Mol. Sci. 2019, 20, 5949. [Google Scholar] [CrossRef]
- Tong, J.F.; Walk, T.C.; Han, P.P.; Chen, L.Y.; Shen, X.J.; Li, Y.S.; Gu, C.M.; Xie, L.H.; Hu, X.J.; Liao, X.; et al. Genome-wide identification and analysis of high-affinity nitrate transporter 2 (NRT2) family genes in rapeseed (Brassica napus L.) and their responses to various stresses. BMC Plant Biol. 2020, 20, 1–16. [Google Scholar] [CrossRef]
- Han, P.P.; Qin, L.; Li, Y.S.; Liao, X.S.; Xu, Z.X.; Hu, X.J.; Xie, L.H.; Yu, C.B.; Wu, Y.F.; Liao, X. Identification of suitable reference genes in leaves and roots of rapeseed (Brassica napus L.) under different nutrient deficiencies. J. Integr. Agr. 2017, 16, 809–819. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Marini, A.M.; Soussi-Boudekou, S.; Vissers, S.; Andre, B. A family of ammonium transporters in Saccharomyces cerevisiae. Mol. Cell Biol. 1997, 17, 4282–4293. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.T.; Sheng, Y.T.; Yang, S.Y.; Han, L.; Gao, Y.C.; Zhang, K.; Cheng, J.S.; Zhang, H.X.; Song, Z.Z.; Su, Y.H. Isolation and characterization of a high-affinity ammonium transporter ApAMT1;1 in alligatorweed. Plant Growth Regul. 2019, 89, 321–330. [Google Scholar] [CrossRef]
- Yoo, S.D.; Cho, Y.H.; Sheen, J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2007, 2, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Li, B.Z.; Merrick, M.; Li, S.M.; Li, H.Y.; Zhu, S.W.; Shi, W.M.; Su, Y.H. Molecular basis and regulation of ammonium transporter in rice. Rice Sci. 2009, 16, 314–322. [Google Scholar] [CrossRef]
- Li, T.Y.; Liao, K.; Xu, X.F.; Gao, Y.; Wang, Z.Y.; Zhu, X.F.; Jia, B.L.; Xuan, Y.H. Wheat ammonium transporter (AMT) gene family: Diversity and possible role in host-pathogen interaction with stem rust. Front. Plant Sci. 2017, 8, 1637. [Google Scholar] [CrossRef]
- Gu, R.L.; Duan, F.Y.; An, X.; Zhang, F.S.; von Wirén, N.; Yuan, L.X. Characterization of AMT-mediated high-affinity ammonium uptake in roots of maize (Zea mays L.). Plant Cell Physiol. 2013, 54, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.Q.; Liu, Y.D.; Zhang, T.T.; Wang, Y.; Jiang, X.Y.; Zhou, Y. Genome-wide identification and expression analysis of ammonium transporter 1 (AMT1) gene family in cassava (Manihot esculenta Crantz) and functional analysis of MeAMT1;1 in transgenic Arabidopsis. 3 Biotech 2022, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rathke, G.W.; Christen, O.; Diepenbrock, W. Effects of nitrogen source and rate on productivity and quality of winter oilseed rape (Brassica napus L.) grown in different crop rotations. Field Crop. Res. 2005, 94, 103–113. [Google Scholar] [CrossRef]
- Dechorgnat, J.; Francis, K.L.; Dhugga, K.S.; Rafalski, J.A.; Tyerman, S.D.; Kaiser, B.N. Tissue and nitrogen-linked expression profiles of ammonium and nitrate transporters in maize. BMC Plant Biol. 2019, 19, 206. [Google Scholar] [CrossRef]
- Chalhoub, B.; Denoeud, F.; Liu, S.Y.; Parkin, I.A.; Tang, H.B.; Wang, X.Y.; Chiquet, J.; Belcram, H.; Tong, C.B.; Samans, B.; et al. Plant genetics. Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science 2014, 345, 950–953. [Google Scholar] [CrossRef]
- Hui, J.; An, X.; Li, Z.B.; Neuhäuser, B.; Ludewig, U.; Wu, X.N.; Schulze, W.X.; Chen, F.J.; Feng, G.; Lambers, H.; et al. The mycorrhiza-specific ammonium transporter ZmAMT3;1 mediates mycorrhiza-dependent nitrogen uptake in maize roots. Plant Cell 2022, 34, 4067–4087. [Google Scholar] [CrossRef]
- Cheng, K.; Wei, M.; Jin, X.X.; Tang, M.; Zhang, H.Q. LbAMT3-1, an ammonium transporter induced by arbuscular mycorrhizal in Lycium barbarum, confers tobacco with higher mycorrhizal levels and nutrient uptake. Plant Cell Rep. 2022, 41, 1477–1480. [Google Scholar] [CrossRef]
- Li, W.X.; Feng, Z.M.; Zhang, C.X. Ammonium transporter PsAMT1.2 from Populus simonii functions in nitrogen uptake and salt resistance. Tree Physiol. 2021, 41, 2392–2408. [Google Scholar] [CrossRef]
- Zhu, Y.N.; Huang, X.M.; Hao, Y.W.; Su, W.; Liu, H.C.; Sun, G.W.; Chen, R.Y.; Song, S.W. Ammonium transporter (BcAMT1.2) mediates the interaction of ammonium and nitrate in Brassica campestris. Front. Plant Sci. 2020, 10, 1776. [Google Scholar] [CrossRef]
- Liu, L.H.; Fan, T.F.; Shi, D.X.; Li, C.J.; He, M.J.; Chen, Y.Y.; Zhang, L.; Yang, C.; Cheng, X.Y.; Chen, X.; et al. Coding-sequence identification and transcriptional profiling of nine AMTs and Four NRTs From tobacco revealed their differential regulation by developmental stages, nitrogen nutrition, and photoperiod. Front. Plant Sci. 2018, 9, 210. [Google Scholar] [CrossRef]
- Straub, D.; Ludewig, U.; Neuhäuser, B. A nitrogen-dependent switch in the high affinity ammonium transport in Medicago truncatula. Plant Mol. Biol. 2014, 86, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Camañes, G.; Cerezo, M.; Primo-Millo, E.; Gojon, A.; García-Agustín, P. Ammonium transport and CitAMT1 expression are regulated by N in Citrus plants. Planta 2009, 229, 331–342. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Zhang, H.; Wang, S.L.; Ye, X.S.; Shi, L.; Xu, F.S.; Ding, G.D. Molecular identification of the phosphate transporter family 1 (PHT1) genes and their expression profiles in response to phosphorus deprivation and other abiotic stresses in Brassica napus. PLoS ONE 2019, 14, e0220374. [Google Scholar] [CrossRef] [PubMed]
- Men, S.N.; Chen, H.L.; Chen, S.H.; Zheng, S.H.; Shen, X.S.; Wang, C.T.; Yang, Z.P.; Liu, D.H. Effects of supplemental nitrogen application on physiological characteristics, dry matter and nitrogen accumulation of winter rapeseed (Brassica napus L.) under waterlogging stress. Sci. Rep. 2020, 10, 10201. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).