Selection and Validation of Reference Genes in Different Tissues of Okra (Abelmoschus esculentus L.) under Different Abiotic Stresses
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials, Treatments, and Sample Collecting of Okra
2.2. Total RNA Extraction and cDNA Synthesis of Okra Samples
2.3. The Screening of Potential Reference Genes and Primer Design for RT-qPCR
2.4. PCR and RT-qPCR Analysis
2.5. Ranking of Reference Gene Expression Stability Based on Four Algorithms
2.6. Accuracy Assessment of Reference Genes by the Transcription Factor AeMYB1R1
3. Results
3.1. Amplification Specificity and Efficiency of Reference Genes in Okra
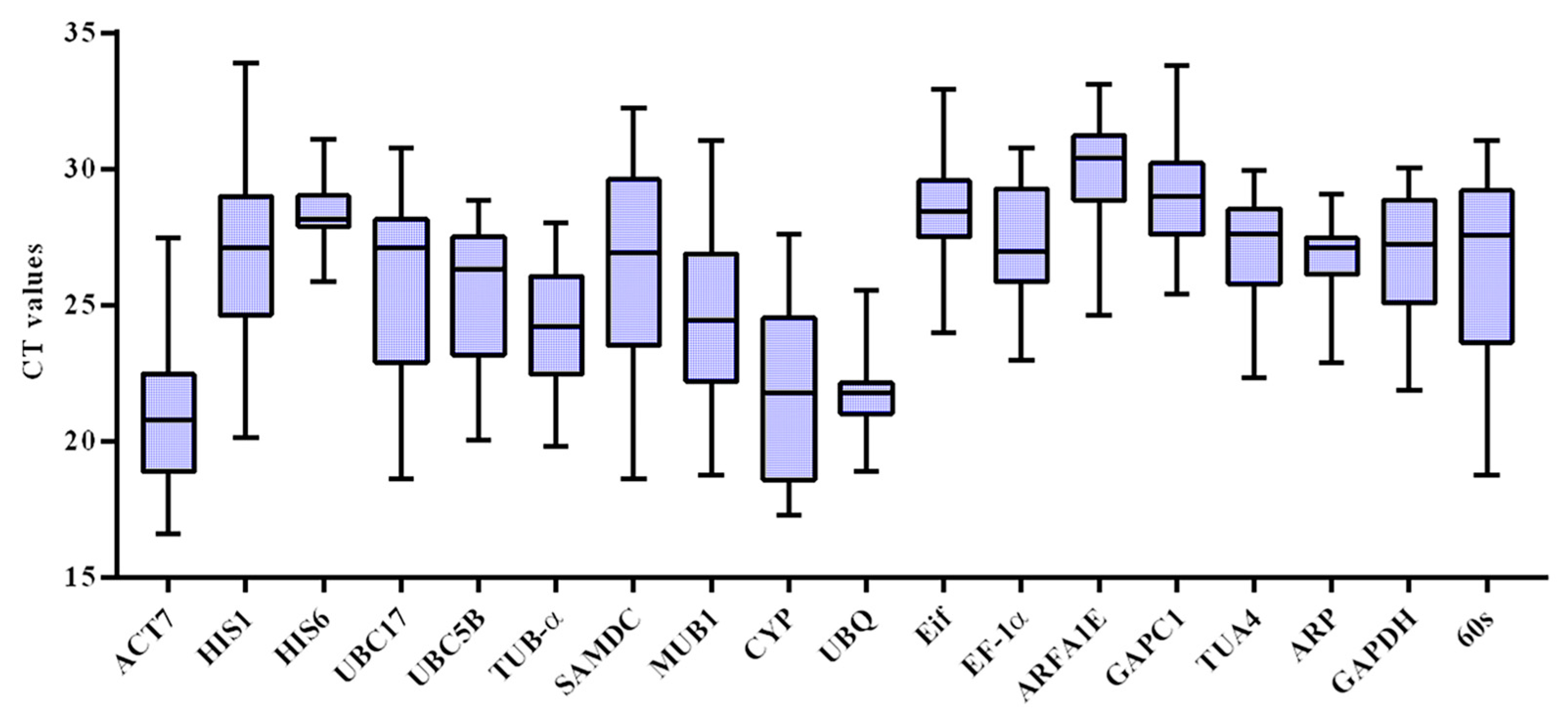
3.2. The Expression Profile Analysis of Reference Genes in Okra
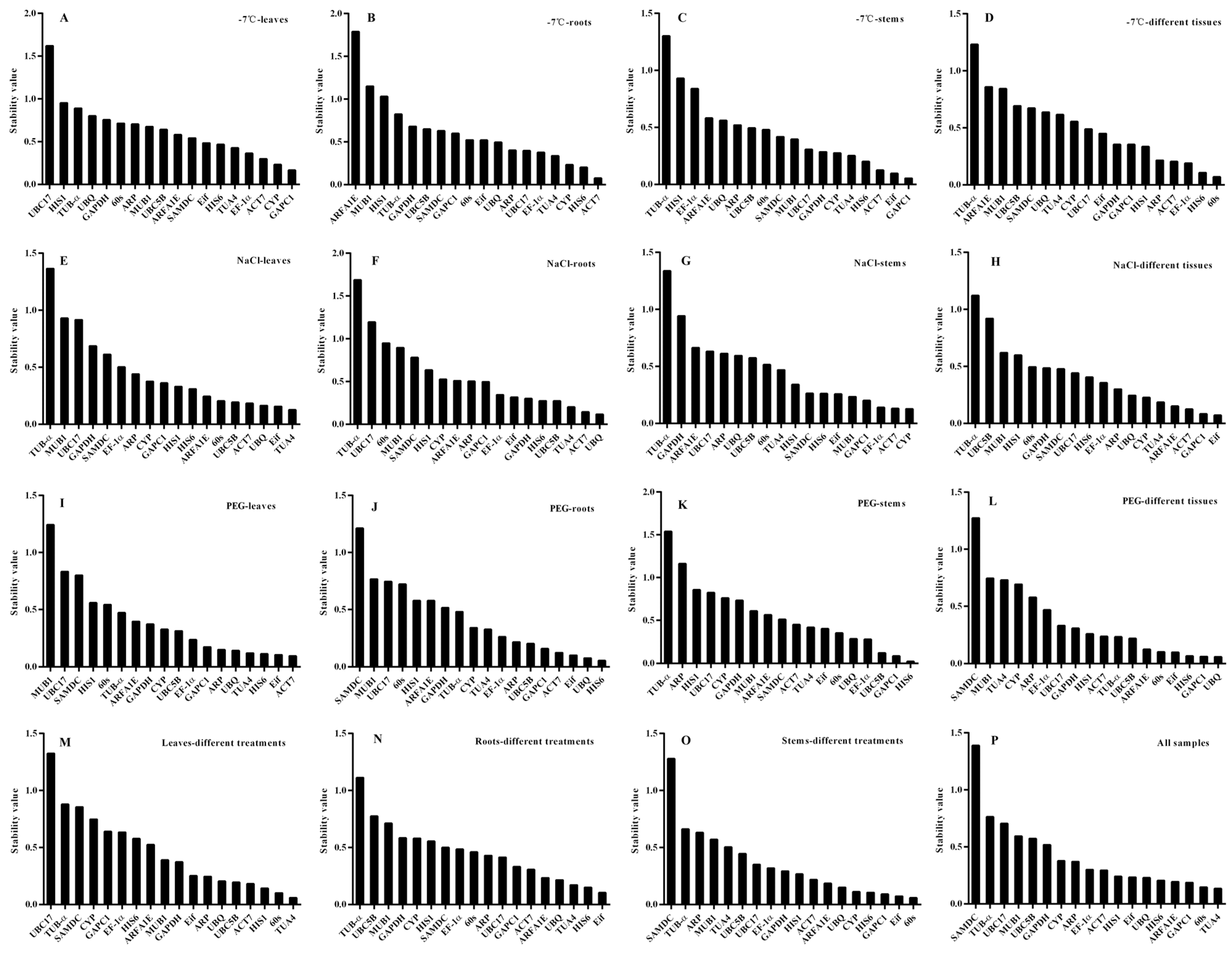
3.3. geNorm Analysis of Reference Genes in Okra
3.4. NormFinder Analysis of Reference Genes in Okra
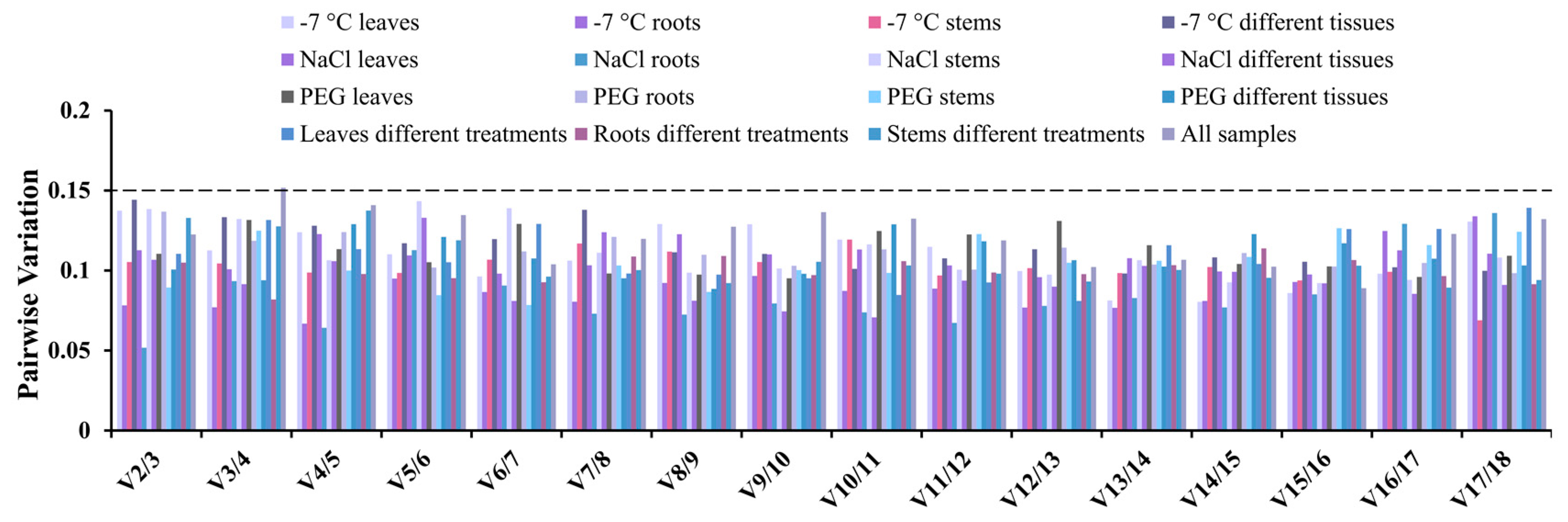
3.5. BestKeeper Analysis of Reference Genes in Okra
3.6. RefFinder Analysis of Reference Genes in Okra
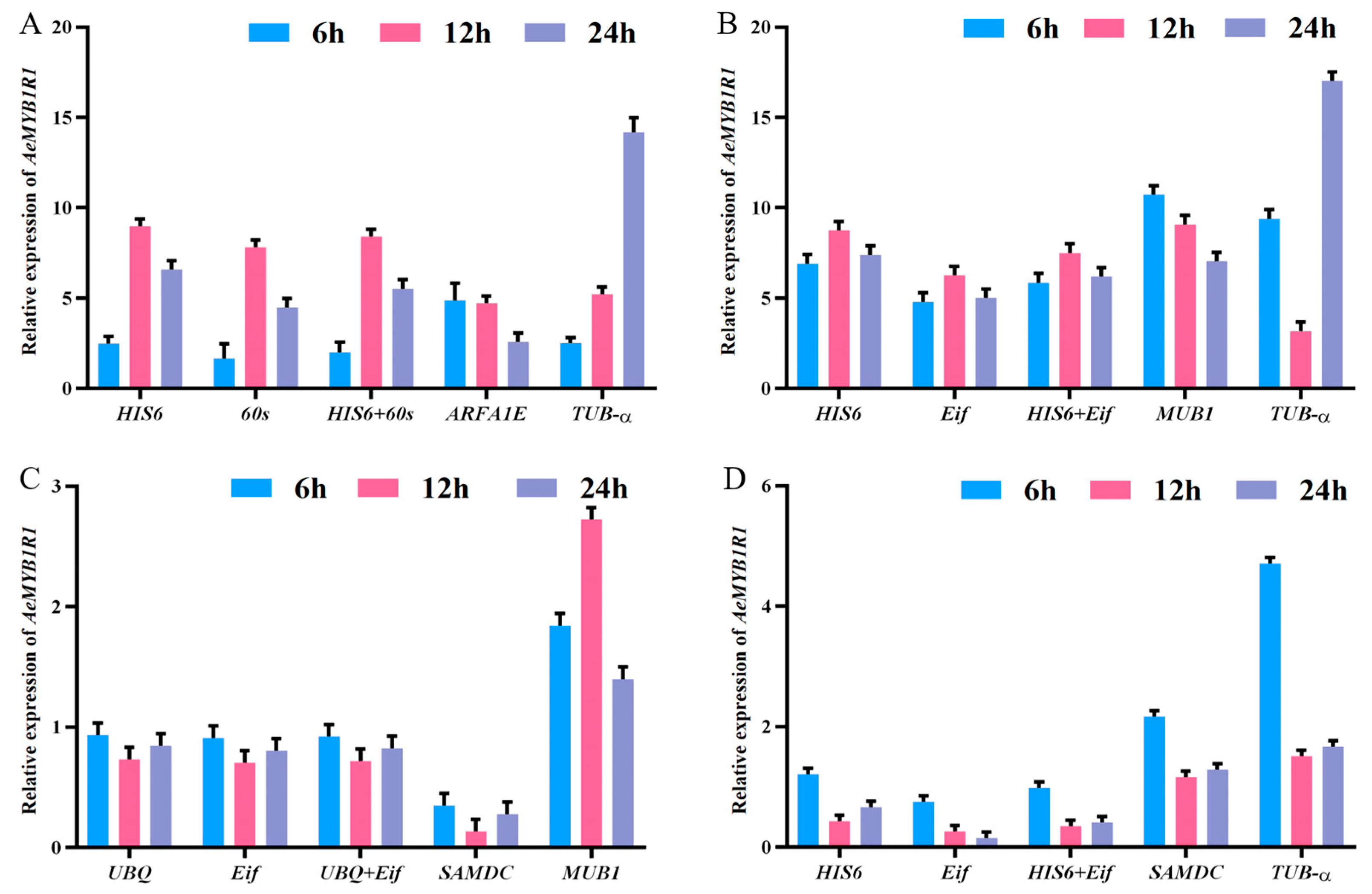
3.7. The Validation of Selected Reference Genes Based on AeMYB1R1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, M.; Yang, X.L.; Zhu, Z.P.; Xu, Q.Y.; Wu, K.X.; Kang, Y.J.; Wang, H.; Xiong, A.S. Comparative transcriptome analysis provides insight into nitric oxide suppressing lignin accumulation of postharvest okra (Abelmoschus esculentus L.) during cold storage. Plant Physiol. Biochem. 2021, 167, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Deng, Y.; Li, Z.; Liu, Z.; Piao, M.; Cui, X. Composition, physicochemical properties, and anti-fatigue activity of water-soluble okra (Abelmoschus esculentus) stem pectins. Int. J. Biol. Macromol. 2020, 165 Pt B, 2630–2639. [Google Scholar] [CrossRef]
- Elkhalifa, A.E.O.; Alshammari, E.; Adnan, M.; Alcantara, J.C.; Awadelkareem, A.M.; Eltoum, N.E.; Mehmood, K.; Panda, B.P.; Ashraf, S.A. Okra (Abelmoschus esculentus) as a Potential Dietary Medicine with Nutraceutical Importance for Sustainable Health Applications. Molecules 2021, 26, 696. [Google Scholar] [CrossRef] [PubMed]
- Romdhane, M.H.; Chahdoura, H.; Barros, L.; Dias, M.I.; Carvalho Gomes Corrêa, R.; Morales, P.; Ciudad-Mulero, M.F.H.; Flamini, G.; Majdoub, H.; Ferreira, I. Chemical Composition, Nutritional Value, and Biological Evaluation of Tunisian Okra Pods (Abelmoschus esculentus L. Moench). Molecules 2020, 25, 4739. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Nian, T.; Liao, Z.; Xiao, F.; Wu, B.; Bi, K.; He, B.; Jia, Y. Antidepressant effects of a polysaccharide from okra (Abelmoschus esculentus (L.) Moench) by anti-inflammation and rebalancing the gut microbiota. Int. J. Biol. Macromol. 2020, 144, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Zhang, J.; Liu, B.; Yan, T.; Xu, F.; Xiao, F.; Wu, B.; Bi, K.; Jia, Y. Polysaccharide from Okra (Abelmoschus esculentus (L.) Moench) Improves Antioxidant Capacity via PI3K/AKT Pathways and Nrf2 Translocation in a Type 2 Diabetes Model. Molecules 2019, 24, 1906. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Novellino, E.; Souto, E.B.; Daliu, P.; Santini, A. Abelmoschus esculentus (L.): Bioactive Components’ Beneficial Properties-Focused on Antidiabetic Role-For Sustainable Health Applications. Molecules 2018, 24, 38. [Google Scholar] [CrossRef]
- Ai, G.; Liu, Q.; Hua, W.; Huang, Z.; Wang, D. Hepatoprotective evaluation of the total flavonoids extracted from flowers of Abelmoschus manihot (L.) Medic: In vitro and in vivo studies. J. Ethnopharmacol. 2013, 146, 794–802. [Google Scholar] [CrossRef]
- Zhan, Y.; Wu, Q.; Chen, Y.; Tang, M.; Sun, C.; Sun, J.; Yu, C. Comparative proteomic analysis of okra (Abelmoschus esculentus L.) seedlings under salt stress. BMC Genom. 2019, 20, 381. [Google Scholar] [CrossRef]
- Wang, W.X.; Vinocur, B.; Shoseyov, O.; Altman, A. Biotechnology Of Plant Osmotic Stress Tolerance Physiological And Molecular Considerations. Acta Hortic. 2001, 560, 285–292. [Google Scholar] [CrossRef]
- Fedoroff, N.V.; Battisti, D.S.; Beachy, R.N.; Cooper, P.J.M.; Fischhoff, D.A.; Hodges, C.N.; Knauf, V.C.; Lobell, D.; Mazur, B.J.; Molden, D.; et al. Radically Rethinking Agriculture for the 21st Century. Science 2010, 327, 833–834. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; James, R.A.; Läuchli, A. Approaches to increasing the salt tolerance of wheat and other cereals. J. Exp. Bot. 2006, 57, 1025–1043. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.A.; Xiang, C.; Farooq, M.; Muhammad, N.; Yan, Z.; Hui, X.; Yuanyuan, K.; Bruno, A.K.; Lele, Z.; Jincai, L. Cold Stress in Wheat: Plant Acclimation Responses and Management Strategies. Front. Plant Sci. 2021, 12, 676884. [Google Scholar] [CrossRef] [PubMed]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Khan, N.; Tang, Y. Epigenetic marks for mitigating abiotic stresses in plants. J. Plant Physiol. 2022, 275, 153740. [Google Scholar] [CrossRef]
- Bustin, S.A.; Pfaffl, A.U.T.A.; Prgomet, C.; Neuvians, T.P. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): Trends and problems. J. Mol. Endocrinol. 2002, 29, 23–39. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Q.; Jiang, Y.; Li, Y.; Zhang, H.; Li, R. Reference genes identification for normalization of qPCR under multiple stresses in Hordeum brevisubulatum. Plant Methods 2018, 14, 110. [Google Scholar] [CrossRef]
- Qu, R.; Miao, Y.; Cui, Y.; Cao, Y.; Zhou, Y.; Tang, X.; Yang, J.; Wang, F. Selection of reference genes for the quantitative real-time PCR normalization of gene expression in Isatis indigotica fortune. BMC Mol. Biol. 2019, 20, 9. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, C.; Lan, H.; Gao, S.; Liu, H.; Liu, J.; Cao, M.; Pan, G.; Rong, T.; Zhang, S. Validation of potential reference genes for qPCR in maize across abiotic stresses, hormone treatments, and tissue types. PLoS ONE 2014, 9, e95445. [Google Scholar] [CrossRef]
- Sudhakar Reddy, P.; Srinivas Reddy, D.; Sivasakthi, K.; Bhatnagar-Mathur, P.; Vadez, V.; Sharma, K.K. Evaluation of Sorghum [Sorghum bicolor (L.)] Reference Genes in Various Tissues and under Abiotic Stress Conditions for Quantitative Real-Time PCR Data Normalization. Front. Plant Sci. 2016, 7, 529. [Google Scholar] [CrossRef]
- Mascia, T.; Santovito, E.; Gallitelli, D.; Cillo, F. Evaluation of reference genes for quantitative reverse-transcription polymerase chain reaction normalization in infected tomato plants. Mol. Plant Pathol. 2010, 11, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cui, H.; Ji, X.; Xue, J.; Jia, X.; Li, R. Selection of the optimal reference genes for transcript expression analysis of lipid biosynthesis-related genes in Okra (Abelmoschus esculentus). Sci. Hortic. 2021, 282, 110044. [Google Scholar] [CrossRef]
- Song, H.; Mao, W.; Duan, Z.; Que, Q.; Zhou, W.; Chen, X.; Li, P. Selection and validation of reference genes for measuring gene expression in Toona ciliata under different experimental conditions by quantitative real-time PCR analysis. BMC Plant Biol. 2020, 20, 450. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, G.; Chen, Y.; Bai, Q.; Gao, C.; Zeng, L.; Li, Z.; Cheng, Y.; Chen, J.; Sun, X.; et al. Selection of Reference Genes for qPCR Analyses of Gene Expression in Ramie Leaves and Roots across Eleven Abiotic/Biotic Treatments. Sci. Rep. 2019, 9, 20004. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, Research0034. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper–Excel-based tool using pair-wise correlations. Biotech. Let. 2004, 26, 509–515. [Google Scholar] [CrossRef]
- Xie, F.; Xiao, P.; Chen, D.; Xu, L.; Zhang, B.; Ai, G.; Liu, Q.; Hua, W.; Huang, Z.; Wang, D. miRDeepFinder: A miRNA analysis tool for deep sequencing of plant small RNAs Hepatoprotective evaluation of the total flavonoids extracted from flowers of Abelmoschus manihot (L.). Plant Mol. Biol. 2012, 146, 794–802. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Y.; Guo, D.; Jing, L. Selection of reference genes for quantitative real-time PCR normalization in the plant pathogen Puccinia helianthi Schw. BMC Plant Biol. 2019, 19, 20. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Z.; Bao, W.; Hu, H.; Chen, M.; Chai, T.; Wang, H. Identification and evaluation of reference genes for quantitative real-time PCR analysis in Polygonum cuspidatum based on transcriptome data. BMC Plant Biol. 2019, 19, 498. [Google Scholar] [CrossRef]
- Zhang, J.R.; Feng, Y.Y.; Yang, M.J.; Xiao, Y.; Liu, Y.S.; Yuan, Y.; Li, Z.; Zhang, Y.; Zhuo, M.; Zhang, J.; et al. Systematic screening and validation of reliable reference genes for qRT-PCR analysis in Okra (Abelmoschus esculentus L.). Sci. Rep. 2022, 12, 12913. [Google Scholar] [CrossRef]
- Gao, M.; Liu, Y.; Ma, X.; Shuai, Q.; Gai, J.; Li, Y. Evaluation of Reference Genes for Normalization of Gene Expression Using Quantitative RT-PCR under Aluminum, Cadmium, and Heat Stresses in Soybean. PLoS ONE 2017, 12, e0168965. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yan, H.; Hua, W.; Huang, Y.; Wang, Z. Selection and Validation of Reference Genes for Quantitative Real-time PCR in Gentiana macrophylla. Front. Plant Sci. 2016, 7, 945. [Google Scholar] [CrossRef]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010, 15, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Yang, L.; Liu, L.; Dong, X.; Chen, S.; Chen, Z.; Liu, G.; Jia, Y.; Yuan, W.; Liu, L. Genome-Wide Analysis of the MYB Transcription Factor Superfamily in Physcomitrella patens. Int. J. Mol. Sci. 2020, 21, 975. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.; Hao, Y.; Kapoor, A.; Dong, C.H.; Fujii, H.; Zheng, X.; Zhu, J.K. A R2R3 type MYB transcription factor is involved in the cold regulation of CBF genes and in acquired freezing tolerance. J. Biol. Chem. 2006, 281, 37636–37645. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Jiang, Y.; Liang, Y.; Chen, L.; Chen, W.; Cheng, B. Expression of the maize MYB transcription factor ZmMYB3R enhances drought and salt stress tolerance in transgenic plants. Plant Physiol. Biochem. 2019, 137, 179–188. [Google Scholar] [CrossRef]
- Shen, X.J.; Wang, Y.Y.; Zhang, Y.X.; Guo, W.; Jiao, Y.Q.; Zhou, X.A. Overexpression of the Wild Soybean R2R3-MYB Transcription Factor GsMYB15 Enhances Resistance to Salt Stress and Helicoverpa Armigera in Transgenic Arabidopsis. Int. J. Mol. Sci. 2018, 19, 3958. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Moon, S.J.; Han, S.; Kim, B.G.; Park, S.R.; Lee, S.K.; Yoon, H.J.; Lee, H.E.; Kwon, H.B.; Baek, D.; et al. Expression of StMYB1R-1, a novel potato single MYB-like domain transcription factor, increases drought tolerance. Plant Physiol. 2011, 155, 421–432. [Google Scholar] [CrossRef]
- Wang, Y.; Liao, P.; Zhao, J.f.; Zhang, X.k.; Liu, C.; Xiao, P.a.; Zhou, C.y.; Zhou, Y. Comparative transcriptome analysis of the Eureka lemon in response to Citrus yellow vein virus infection at different temperatures. Physiol. Mol. Plant Pathol. 2022, 119, 101832. [Google Scholar] [CrossRef]
- Kong, Q.; Yuan, J.; Gao, L.; Zhao, S.; Jiang, W.; Huang, Y.; Bie, Z. Identification of suitable reference genes for gene expression normalization in qRT-PCR analysis in watermelon. PLoS ONE 2014, 9, e90612. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007, 8, R19. [Google Scholar] [CrossRef] [PubMed]
- Boava, L.P.; Laia, M.L.; Jacob, T.R.; Dabbas, K.M.; Gonçalves, J.F.; Ferro, J.A.; Ferro, M.I.; Furtado, E.L. Selection of endogenous genes for gene expression studies in Eucalyptus under biotic (Puccinia psidii) and abiotic (acibenzolar-S-methyl) stresses using RT-qPCR. BMC Res. Notes 2010, 3, 43. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, L.M.; Dos Santos Brito, M.; Favero Peixoto Junior, R.; Marchiori, P.E.R.; Nobile, P.M.; Martins, A.P.B.; Ribeiro, R.V.; Creste, S. Reference genes for normalization of qPCR assays in sugarcane plants under water deficit. Plant Methods 2017, 13, 28. [Google Scholar] [CrossRef]
- Kozera, B.; Rapacz, M. Reference genes in real-time PCR. J. Appl. Genet. 2013, 54, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Huang, X.; Lin, Y.; Wang, X.; Yan, Z.; Wang, Q.; Ding, J.; Gu, T.; Li, Y. Identification of reference genes for transcript normalization in various tissue types and seedlings subjected to different abiotic stresses of woodland strawberry Fragaria vesca. Sci. Hortic. 2020, 261, 108840. [Google Scholar] [CrossRef]
- Kumar, G.; Singh, A.K. Reference gene validation for qRT-PCR based gene expression studies in different developmental stages and under biotic stress in apple. Sci. Hortic. 2015, 197, 597–606. [Google Scholar] [CrossRef]
- Zhao, Y.; Luo, J.; Xu, S.; Wang, W.; Liu, T.; Han, C.; Chen, Y.; Kong, L. Selection of Reference Genes for Gene Expression Normalization in Peucedanum praeruptorum Dunn under Abiotic Stresses, Hormone Treatments and Different Tissues. PLoS ONE 2016, 11, e0152356. [Google Scholar] [CrossRef]
- Li, M.Y.; Song, X.; Wang, F.; Xiong, A.S. Suitable Reference Genes for Accurate Gene Expression Analysis in Parsley (Petroselinum crispum) for Abiotic Stresses and Hormone Stimuli. Front. Plant Sci. 2016, 7, 1481. [Google Scholar] [CrossRef]
- Chen, X.; Ding, A.B.; Zhong, X. Functions and mechanisms of plant histone deacetylases. Sci. China Life Sci. 2020, 63, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kong, X.; Liu, S.; Huang, H.; Chen, Z.; Xu, Y. Selection of Reference Genes for Quantitative Real-Time PCR in Chrysoperla nipponensis (Neuroptera: Chrysopidae) Under Tissues in Reproduction and Diapause. J. Insect Sci. 2020, 20, 20. [Google Scholar] [CrossRef] [PubMed]
- Gururani, M.; Li, Z.; Lu, H.; He, Z.; Wang, C.; Wang, Y.; Ji, X. Selection of appropriate reference genes for quantitative real-time reverse transcription PCR in Betula platyphylla under salt and osmotic stress conditions. PLoS ONE 2019, 14, e0225926. [Google Scholar] [CrossRef]
- Yin, J.; Sun, L.; Zhang, Q.; Cao, C. Screening and evaluation of the stability of expression of reference genes in Lymantria dispar (Lepidoptera: Erebidae) using qRT-PCR. Gene 2020, 749, 144712. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Liu, H.; Dong, Y.; Zhang, Y.; Wong, S.M.; Wang, C.; Zhou, Y.J.; Xu, Q. Determination of Suitable RT-qPCR Reference Genes for Studies of Gene Functions in Laodelphax striatellus (Fallén). Genes 2019, 10, 887. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, X.; Liu, Y.; Li, Y.; Luo, Y.; Wang, X.; Tang, H. Evaluation of suitable reference genes for qRT-PCR normalization in strawberry (Fragaria × ananassa) under different experimental conditions. BMC Mol. Biol. 2018, 19, 8. [Google Scholar] [CrossRef]
- Du, W.; Hu, F.; Yuan, S.; Liu, C.; Li, Z.; Lu, H.; He, Z.; Wang, C.; Wang, Y.; Ji, X. Selection of reference genes for quantitative real-time PCR analysis of photosynthesis-related genes expression in Lilium regale. Physiol. Mol. Biol. Plants 2019, 25, 1497–1506. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, X.; Chen, J.; Huang, Z.; Huo, H.; Jiang, C.; Huang, H.; Zhang, C.; Wei, S. Selection of reference genes for qPCR normalization in buffalobur (Solanum rostratum Dunal). Sci. Rep. 2019, 9, 6948. [Google Scholar] [CrossRef]
- Abe, H.; Urao, T.; Ito, T.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 2003, 15, 63–78. [Google Scholar] [CrossRef]
| Experimental Design | Treatment | Tissue | Biological Repetition | Sampling Points | Number of Samples |
|---|---|---|---|---|---|
| Cold stress | −7 °C | leaves, stems, roots | 3 | 4 | 36 |
| Salt stress | 200 mM NaCl | leaves, stems, roots | 3 | 4 | 36 |
| Drought stress | 20% PEG6000 | leaves, stems, roots | 3 | 4 | 36 |
| Gene Symbol | Gene Name | Accession Number | Primer: Forward/Reverse | Amplification Product Size (bp) | Standard Curve | E (%) | R2 |
|---|---|---|---|---|---|---|---|
| ACT7 | Actin-7 | OP448607 | F:TCGCAGACCGTATGAGCAAG R:GGTGCTGAGTGATGCCAAGA | 127 | y = −3.5111x + 29.777 | 92.67% | 0.9937 |
| HIS1 | Histone acetyltransferase MCC1 | OP448608 | F:GCTTCGGCACTTATCCACGA R:CCTCCGCACACACTTGAATG | 138 | y = −3.5239x + 30.469 | 92.21% | 0.9974 |
| HIS6 | Histone deacetylase 6 | OP448609 | F:TGAGGCTTCTGGGTTTTGCT R:TTTGCCATTACCCACCCCAA | 226 | y = −3.4937x + 29.442 | 93.30% | 0.9976 |
| UBC17 | Ubiquitin-conjugating enzyme E2–17 kDa | OP448610 | F:TGCCGAGCTATACCCGATTG R:GGCCAACACCTTGCTTTCAC | 190 | y = −3.4411x + 26.954 | 95.26% | 0.9971 |
| TUB-α | Tubulin α-3 chain | OP448611 | F:CCGAGAGCTGTGTTTGTGGA R:CGCCGACAGCATTAAACACC | 241 | y = −3.3538x + 29.262 | 98.69% | 0.9967 |
| UBC5B | Ubiquitin-conjugating enzyme E2 5B | OP448612 | F:AGTGTTCCTTCCGCAACTTC R:CGCTTAGCTCTTCGTCGCT | 188 | y = −3.3125x + 25.576 | 100.39% | 0.9929 |
| SAMDC | S-adenosylmethionine decarboxylase proenzyme | OP448613 | F:TGATGCCCTTGAGCCATGTT R:ACTTGAGTCTTGCCATCGGG | 106 | y = −3.3157x + 29.643 | 100.26% | 0.9986 |
| MUB1 | Membrane-anchoredubiquitin-foldprotein 1 | OP448614 | F:CTTTCCGGCGGCTACAAGT R:ACATCACAAAGAGGGCTCTGG | 173 | y = −3.3915x + 28.576 | 97.18% | 0.9979 |
| CYP | cyclophilin | OP448615 | F:CCGGAGGCGAATCCATCTAC R:AGACCCAACTTTCTCGACGG | 224 | y = −3.4893x + 30.282 | 93.46% | 0.9968 |
| UBQ | polyubiquitin | OP448616 | F:CTGCACTTGGTCCTTCGTTTG R:GGAGCACCAAATGAAGAGTGG | 244 | y = −3.5635x + 29.459 | 90.82% | 0.9998 |
| Eif | eukaryotic translation initiation factor | OP448617 | F:TGCTTGGCAATGGTCGATGT R:CCAGCAGCAATCCAAACCTTC | 100 | y = −3.5559x + 26.519 | 91.08% | 0.9992 |
| EF-1α | Elongation factor 1-α | OP448618 | F:CCCGGTGACAATGTGGGATT R:GGCATAGCCGTTTCCGATCT | 165 | y = −3.4148x + 29.239 | 96.27% | 0.9922 |
| ARFA1E | ADP-ribosylation factor A1E | OP448619 | F:CCCGGTGACAATGTGGGATT R:GGCATAGCCGTTTCCGATCT | 165 | y = −3.3776x + 25.795 | 97.73% | 0.9997 |
| GAPC1 | Glyceraldehyde-3-phosphatedehydrogenase C subunit 1 | OP448620 | F:CGTGTCCCTACACCCAATGT R:TCGGTTGCTGTAACCCCATT | 270 | y = −3.4221x + 29.247 | 95.98% | 0.9932 |
| TUA4 | Tubulin α-4 chain | OP448621 | F:ATCTCAACCGCCTTGTCTCG R:CTCGGTACATGAGGCAGCAA | 285 | y = −3.2557x + 28.359 | 102.84% | 0.9946 |
| ARP | actin-related protein 2 | OP448622 | F:GGAGACGCATGTGCTGATTTG R:GTGACACCATCACCAGAGTCA | 317 | y = −3.3273x + 25.988 | 99.78% | 0.9913 |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase | OP448623 | F:TGGAAGGATCGGTCGTTTGG R:CACCCCACGGAATTTCCTCA | 239 | y = −3.4732x + 29.391 | 94.05% | 0.9964 |
| 60s | 60s ribosomal protein l36 | OP448624 | F:CAACAAGGGCCACAAAACCA R:TTGACGATCTCGCGAACGAA | 102 | y = −3.5047x + 28.514 | 92.90% | 0.9937 |
| MYB1R1 | Transcription factor MYB1R1 | OM963000 | F:CGCACCCATAACAACTCCCA R:TCTTTCACTTACTCCCTCTTCAGC | 178 | y = −3.4389x + 32.574 | 95.34% | 0.9926 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Yu, J.; Tang, X.; Xiong, A.; Sun, M. Selection and Validation of Reference Genes in Different Tissues of Okra (Abelmoschus esculentus L.) under Different Abiotic Stresses. Genes 2023, 14, 603. https://doi.org/10.3390/genes14030603
Zhu Z, Yu J, Tang X, Xiong A, Sun M. Selection and Validation of Reference Genes in Different Tissues of Okra (Abelmoschus esculentus L.) under Different Abiotic Stresses. Genes. 2023; 14(3):603. https://doi.org/10.3390/genes14030603
Chicago/Turabian StyleZhu, Zhipeng, Jianxiang Yu, Xinhui Tang, Aisheng Xiong, and Miao Sun. 2023. "Selection and Validation of Reference Genes in Different Tissues of Okra (Abelmoschus esculentus L.) under Different Abiotic Stresses" Genes 14, no. 3: 603. https://doi.org/10.3390/genes14030603
APA StyleZhu, Z., Yu, J., Tang, X., Xiong, A., & Sun, M. (2023). Selection and Validation of Reference Genes in Different Tissues of Okra (Abelmoschus esculentus L.) under Different Abiotic Stresses. Genes, 14(3), 603. https://doi.org/10.3390/genes14030603







