Scoping Review on Epigenetic Mechanisms in Primary Immune Thrombocytopenia
Abstract
1. Introduction
2. Materials and Methods
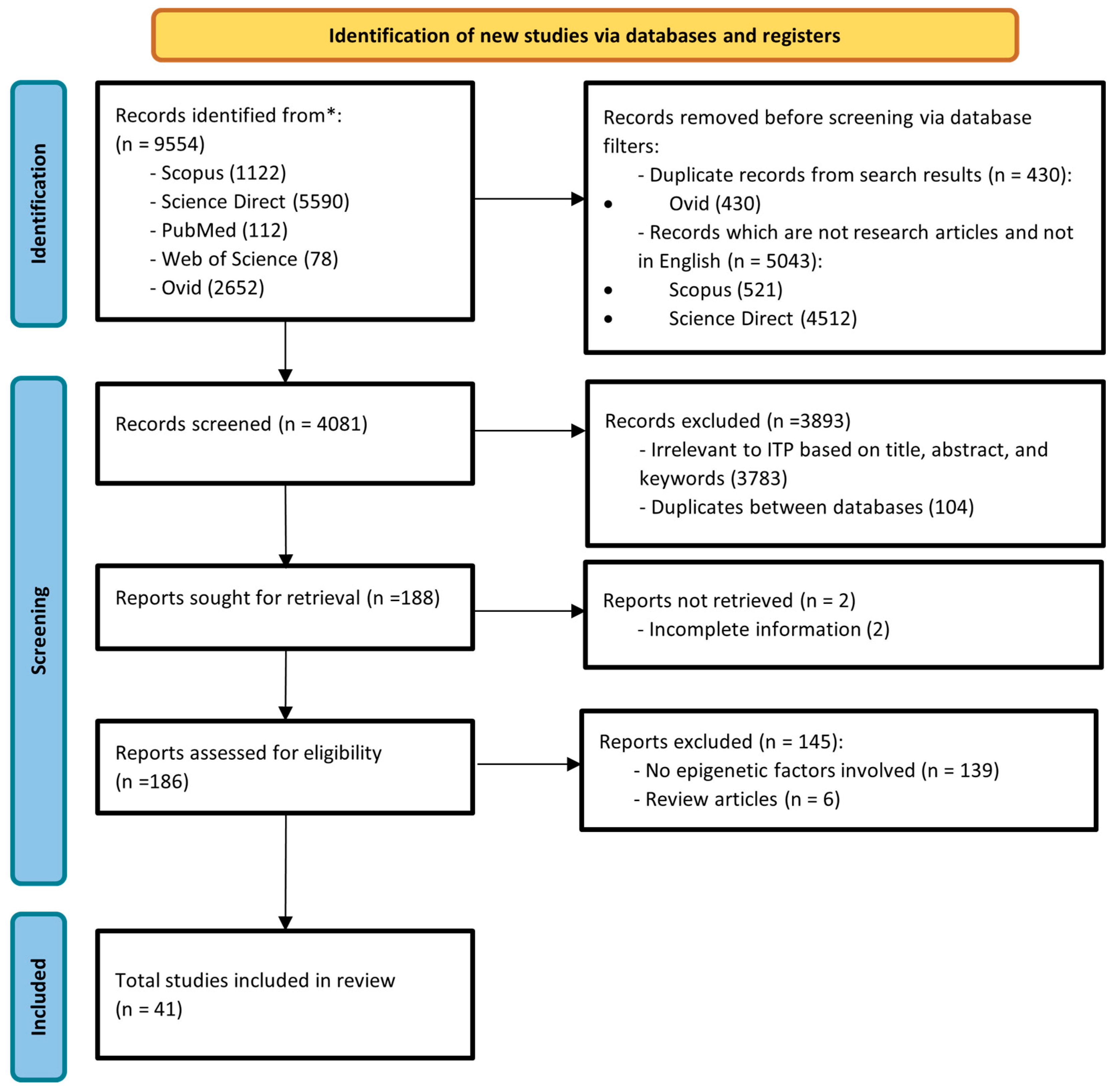
2.1. Search Strategy and Selection Criteria
2.2. Data Collection
3. Results
3.1. DNA Methylation
| Epigenetic Mechanisms | Type of Study | Methodology | Major Findings | Pathway Affected/Functions | References |
|---|---|---|---|---|---|
| DNA Methylation | |||||
| FOXP3 hypermethylation | In vitro and in silico | PBMCs extraction, DNA extraction, DNA methylation analysis (MALDI-TOF, MassCLEAVE) | The Foxp3 promoter has CpG sites with higher levels of methylation. The CpG-6 promoter of Foxp3 is methylated at various amounts in different ITP patients. | Maintenance and function of Treg cells | [31] |
| CD70 (TNFSF7) promoter hypomethylation | In vitro | PBMCs and CD4+ cells isolation, flow cytometric analysis, DNA extraction and bisulfate modification, methylation-sensitive high-resolution melting analysis, RNA isolation, quantitative real-time PCR | Overexpression of CD70, DNMTs and MBD2. Hypomethylation of CD70 promoter in CD4+ T cells of ITP patients. CD70 transcription levels negatively correlated with methylation indices but positively correlated with DNMT1, DNMT3A, DNMT3B. | CD70-CD27 costimulatory pathway, CD28-CD80/86 pathway, ERK signal pathway | [34] |
| DNMT3A mRNA expression decreased as a result of aberrant DNA methylation | In vitro | PBMCs extraction, RNA isolation, reverse transcription, quantitative real-time PCR, reverse-phase HPLC | Reduced DNMT3A mRNA expression, and increased SAH levels. | Not mentioned | [35] |
| Increased methylation on promoter of perforin and IFN-γ but not associated with ITP | In vitro | RNA isolation, reverse transcription, and quantitative real-time PCR; DNA extraction and bisulfate sequencing | Expression of IFN-γ, IL-4, Foxp3, and perforin is negatively correlated with methylation of respective promoters | Th1 polarization response | [33] |
| Decreased MBD2 and MBD4 gene expression, hypomethylation of genome | In vitro | PBMCs and CD4+ cells isolation, RNA and DNA extraction, ELISA for dmC, quantitative real-time PCR | Reduced global DNA methylation in CD4+ cells, reduced MBD2 and MBD4 mRNA expression, methylation index negatively correlated with MBD2 and MBD4 expression. | Global methylation and demethylation activity | [37] |
| Hypermethylated NOTCH1 in Th1 and Th2 cell differentiation pathways | In vitro | PBMCs and bone marrow mononuclear cells (BMMCs) isolation, reduced representation bisulfite sequencing (RRBS), BSMAP software, wANNOVAR, GO analysis, KEGG analysis, Luminex assay, quantitative real-time PCR, and ELISA | NOTCH1 and TYK2 | Th1 and Th2 pathways | [32] |
| Decreased DNA methyltransferase 3A and 3B mRNA expression in peripheral blood mononuclear cells and increased plasma SAH concentration | PBMC, isolation of total RNA, reverse transcription, real-time quantitative PCR, reversed phase HPLC | 3A and 3B mRNA | SAH pathway | [36] | |
| Histone modification | |||||
| Global hypomethylation of H3K9 in CD4+ T cells | In vitro | RNA isolation, reverse transcription, real-time PCR, global histone H3K4/H3K9 methylation assay | Global histone H3K9 hypomethylation with reduced expression level of EZH2 and SUV39H2, while SUV39H1 has no changes. | H3K9 trimethylation and H3K27 methylation | [38] |
| Increased citrullinated histone and histone-DNA complexes. | In vitro | Plasma extraction, specific ELISA bioassays for S100A8/A9, histone/DNA complexes, citrullinated histone H3, cfDNA, ADAMTS13, and Anti-ADAMTS13 IgG | Plasma inflammatory mediators, NETosis markers, cfDNA play a role in pathogenesis of ITP | NETosis and inflammatory process | [39] |
| LncRNA | |||||
| Decreased lncRNA PVT1 expression in ITP patients | In vitro | CD4+ T cell isolation, Th17 cell differentiation, Flow cytometry, quantitative real-time PCR, T cell transfection, ELISA, Western blot, ubiquitination analysis | In ITP patients, PVT1 expression was downregulated whereas Th17 cell expression was elevated. PVT1 overexpression reduced IL-17, RORt, and NOTCH1 levels as well as the quantity of Th17 cells. | Notch signaling pathway | [40] |
| Eight lncRNAs and eleven miRNAs associated with ITP | In silico | Affymetrix array, GO analysis, KEGG analysis, miRNA prediction: (starBase), Protein-protein and coexpression network mapping (Cytoscape) | 7 genes, 8 lncRNA, 11 miRNAs are found to be associated with ITP pathogenesis | Around 30 pathways related to autoimmunity | [41] |
| Increased MALAT1 and THRIL expression | In vitro | RNA extraction from blood, reverse transcription | MALAT1 and THRIL were upregulated, and positive correlation found between the expression level of lncRNA. | TLR2 signaling pathway; Alternative splicing regulation | [42] |
| Aberrant lncRNA of ENST00000440492, ENST00000528366, NR_038920, and ENST00000552576 expression | In vitro and in silico | RNA isolation from blood, microarray, KEGG Pathway analysis, GO analysis, quantitative real-time PCR, CNC analysis | A total of 4 immune-related lncRNAs were found associated with genes and proteins related to autoimmune disease | TNF signaling pathway, granulocyte macrophage colony-stimulating factor production, coreceptor activity, cytokine–cytokine receptor interaction, chemokine signaling pathway | [43] |
| Decreased TMEVPG1 expression | In vitro | Plasma extraction, peripheral blood mononuclear cell culture, real-time PCR, ELISA for cytokine | TMEVPG1 may increase IFN-γ transcription, and IFN-γ overexpression negative feedback controlled by TMEVPG1 expression | IFN-γ mediated signaling pathway | [44] |
| GAS5 regulates Th17 and STAT3 ubiquitination | In vitro and in vivo | PBMC extraction, CD4+ T cell transfection and induction of Th17 differentiation, quantitative real-time PCR, flow cytometry, Western blot analysis, ELISA, RNA pull-down assay, RNA binding protein immunoprecipitation (RIP), ubiquitination assay | In both humans and mice with ITP, GAS5 expression was downregulated. GAS5 regulates differentiation of Th17 through TRAF6-mediated ubiquitination of STAT3 | MAPK pathway and Th17 differentiation | [45] |
| MEG3 regulates miR-125a-5p, CXCL3, and ratio of Treg/Th17 | In vitro | CD4+ T cell extraction, flow cytometry analysis, RNA isolation, real-time PCR, Western blot analysis, ELISA, RNA pull-down assay, luciferase reporter gene assay | Elevated MEG3 expression in ITP patients. Treg/Th17 imbalance brought on by miR-125a-5p. MEG3 inhibits miR-125a-5p. | Th17/Treg balance ratio | [46] |
| The Ifng antisense RNA 1 (IFNG-AS1) and growth arrest-specific transcript 5 (GAS5) | In vitro | RNA extraction from venous blood, reverse transcription, quantitative real-time PCR (qPCR) for detection of long non-coding RNAs | lncRNAs IFNG-AS1 and GAS5 is overexpressed in childhood ITP. | Not mentioned | [47] |
| MicroRNA | |||||
| MicroRNA increased gene expression of CXCL13 and IL-21 in patients with ITP. | In vitro and in silico | T cell extraction from blood, RNA extraction, miRNA labeling and hybridization, DNA microarray, GO analysis (TargetScan, Miranda, Cytoscape, ClueGO). | Identified 17 microRNAs that are linked to the expression of 57 immune system-related target genes. | T-cell activation, regulation of immunoglobulin production | [48] |
| Increased miR-302c-3p, miR-483-5p, miR-223-3p, and miR-597 expression. Decreased miR-544a and miR-302a-3p expression. | In vitro | miRNA isolation from plasma with quantitative real-time PCR. | Found 7 miRNA as biomarkers for further research of ITP pathogenesis. | Not Mentioned | [49] |
| miR-98-5p downregulates IGF2BP1 and upregulates p53 | In vitro and in vivo | Human mesenchymal stem cells extraction, murine model with ITP, miRNA microarray, Western blot analysis, and ELISA Assay. | MSC apoptosis is correlated with miR-98-5p overexpression. MiR-98-5p downregulates IGF2BP1 and upregulates p53. | MSC apoptosis; IGF-2/PI3K/Akt Pathway | [50] |
| Aberrant expression of microRNA in CD4 + cells contributes to Th17/Treg imbalance | In vivo | Cohort of 52 ITP patients and 56 healthy controls. RT-PCR, flowcytometry, Western blot analysis. | The expression of miRNAs related to helper T or Treg cells was found to regulate the Th17/Treg ratio in CD4+ T cells. Lower levels of miR-99a were observed in ITP patients compared with healthy controls, while higher levels of miR-182-5p and miR183–5p were seen among ITP patients. Positive correlation between increased percentage of Treg and decreased levels of miRNA-99a was also noted in ITP patients. | Th17/Treg pathway | [51] |
| Bone marrow mesenchymal stem cell-derived exosomes induce the Th17/Treg imbalance in immune thrombocytopenia through miR-146a-5p/IRAK1 axis. | In vitro and In vivo | Density-gradient centrifugation to separate BMSCs, culturing the separated BMSC, identification of BMSCs by culturing them up to third generation. | BMSCs-exosomes’ treatment significantly reduced the Th17/Treg ratio in CD4 + T cells | Th17/Treg pathway | [52] |
| Differential expression of miR-106b-5p and miR-200c-3p in newly diagnosed versus chronic ITP patients. | In vivo and in silico | Microarray, RT-PCR, bioinformatic analysis. | Three specific microRNA molecules -miR-106b–5p, miR200c–3p and mir92a-3p showed significantly different expressions among all groups studied. MiR 106b 5 p and 200 c-3p had higher levels of expression in patients with ITP compared to normal controls; and chronic ITP vs. newly diagnosed cases. | Not mentioned | [53] |
| Downregulation of microRNA-155-5p prevents immune thrombocytopenia. | In vitro | Transfection of macrophages and PBMC’s with treated plasmids, ELISA. | miR-155-5p was upregulated and SOCS1 down regulated in PBMCs and macrophages from ITP. Inhibition of miR-155-5p or upregulation of SOCS1 facilitated the M2 polarization, increased M2/M1 ratio. Silencing SOCS1 blocks PD1/PDL1 pathway and upregulates miR-155-5p. | PDL/PDI pathway through Socs1 | [54] |
| miR-21 and miR-150 expression. | RT-PCR. | A significant relationship between the expression of miR-21 with hemoglobin, hematocrit and red blood cell hemoglobin concentration, but not miR-150. | Not mentioned | [55] | |
| Increased miR-155 expression correlated with serum cytokine profiles. | In vitro | RT-PCR, ELISA. | Increased plasma IL-17A and decreased IL-4, IL-10 and TGF-β1 levels in ITP patients. miR-155 levels were negatively correlated with platelet counts, SOCS1 mRNA levels, and the plasma levels of IL-4, IL-10 and TGF-β1, but positively correlated with plasma IL-17A levels. | SOCS1 pathway | [56] |
| Integrated mRNA and miRNA with deregulation of the cellular stress response in bone marrow mesenchymal stem cells derived ITP. | In vitro | Bone marrow samples of ITP patients taken, isolation expansion and characterization of MSC’s, RNA extraction, microarray study. | A total of 740 genes and 32 miRNAs were differentially expressed between ITP patients and controls. A compromised unfolded protein response (UPR) and decreased DNA transcription were shown to be significantly related to MSC-ITP. | Not mentioned | [57] |
| MicroRNA expression profile in Treg cells in ITP. | In vitro | Platelet collection of ITP patients, miRNA microarray analysis. | miR-155–5p, miR-146b–5p, and miR-142–3p significantly decreased in Tregs from patients with ITP. | Tregs pathway | [58] |
| MicroRNA profiling of platelets from immune thrombocytopenia and target gene prediction. | In vitro | Platelet collection of ITP patients and healthy controls, RT-qPCR, microarray analysis. | A total of 115 miRNAs are differentially expressed in ITP patients compared to healthy controls. There were 6 specific miRNA targets which may be involved in processes such as apoptosis (cell death involving programmed cell self-destruction) and adhesion related to the pathogenesis of ITP. | Not mentioned | [59] |
| MicroRNA-21-5p regulates CD3+ T lymphocytes through VCL and LTF. | In silico | Bioinformatic analysis. | S100A8 regulates CD3+ T lymphocytes in ITP patients. MiR-21-5p regulates the differentially expressed gene LTF by inhibiting the core downstream target gene VCL and participates in the immune mechanism of T lymphocytes in ITP patients. miR-155-5p involved in the immunoregulatory mechanism of T lymphocytes in ITP patients. | Not mentioned | [60] |
| miR-106b-5p induces immune imbalance of Treg/Th17 in immune thrombocytopenic purpura through NR4A3/Foxp3 pathway. | In vitro and in vivo | miRNA expression levels, cell proliferation rates, cytokine production profiles, gene sequencing, qRT-PCR, Western blot, ELISA. | miR-106b-5p was elevated in peripheral blood of patients with ITP, and NR4A3 expression was decreased. sh-NR4A3 significantly decreased Foxp3 and TGF-β expressions, indicating that NR4A3 may regulate Treg differentiation via Foxp3. NR4A3 was identified as a target of miR-106b-5p, and miR-106b-5p was able to negatively modulate NR4A3 expression. miR-106b-5p induced immune imbalance of Treg/Th17 through NR4A3. Silencing miR-106b-5p promoted Treg differentiation and increased the number of platelets. | NR4A3/Foxp3 pathway | [61] |
| MiRNA-148b-3p targeting SOCS3 inhibits macrophage M2 polarization by JAK2/STAT3 pathway. | In vitro and in vivo | Platelet collection of ITP patients, real-Time PCR, Western blot, dual-luciferase reporter gene assay | A significant correlation between miR-148b-3p expression and platelet count. Suppression of miR-148b-3p or up-regulation of SOCS3 promoted macrophage M2 polarization by inhibiting JAK2/STAT3 pathway. | JAK2/STAT3 pathway | [62] |
| Correlation between different expression of miRNA levels between the ITP patients and healthy children. | In vitro | Platelet collection of ITP patients, qRT-PCR. | Seven miRNAs (miR-302c-3p, miR-483-5p, miR-410, miRNA 302a 3P, miRNA 223 3P and miRNA 597) had significantly different expression levels between the ITP patients and healthy children. | Not mentioned | [63] |
| Characterization of miRNA in ITP patients. | In vitro | Plasma collection of ITP patients, microRNA microarray analysis, qRT-PCR. | Upregulated miRNAs (miR-320c, miR-642b-3p, miR-1275, miR-3141, miR-4270, miR-4499, miR-4739 and miR-6126) and three down-regulated miRNAs (miR-144-3p, miR-1281 and miR-3162-3p) in ITP patients. | Not mentioned | [64] |
| miRNA through exosome trafficking to the cell membrane. | In vitro | Plasma collection of ITP patients, exosome extraction, qRT-PCR, RNA sequencing, Western blot. | Three differentially expressed miRNAs (miR-584-5p, miR-142-5p and miR-29b-3p) were identified in ITP patients. | Not mentioned | [65] |
| Reduced expression of miR409-3p. | In vitro | Total RNA extraction, DNA synthesis, RT PCR assays. | MIR409-3p expression was decreased in PBMCs of active ITP patients but recovered after effective therapy. | Not mentioned | [66] |
| Reduced miR130A is involved in ITP via targeting TGFB1 and IL18. | In vitro | Peripheral blood collected, miRNA array and TaqMan real-time polymerase chain reaction. | MiR130A expression significantly decreased in PBMCs of ITP patients. MiR130A targeted the TGFB1 and IL18 genes. Post-treatment upregulates expression of miR130A and TGFB1, downregulates IL18 expression. | TGFB1 pathway via IL18 | [67] |
| The aberrant expression of microRNAs and correlations with T cell subsets. | In vitro | Peripheral blood collected, miRNA array and TaqMan real-time polymerase chain reaction. | miR-146a positively correlated with the frequencies of Treg cells and platelet counts. miR-146a expression upregulation contributed to the differentiation of Th17 and Treg in ITP patients. miR-146a may be involved in Tregs differentiation and function. | Th17/Treg pathway | [68] |
| Increased let-7b-5p is associated with enhanced BAFF-R expression and B cell survival in immune thrombocytopenia. | In vitro | B cells extraction, RNA extraction and quantitative real-time PCR, Western Blot, flow cytometry. | Overexpression of let-7b-5p in B cells enhanced the expression of surface BAFF-R and promoted B cell survival. let-7b-5p enhanced the phosphorylation of NF-κB2 p100 and upregulated the expression of survival factor Bcl-xL after BAFF induction. | NF-κB2 pathway | [69] |
| The increased expression of miR-146 predicts poor prognosis of ITP. | In vitro | Plasma and megakaryocytes extraction, RNA extraction, (qRT-PCR). | miR-146 is overexpressed in ITP patients. Higher expression of miR-146 is associated with poorer prognosis, lower platelet count, and increased risk of relapse. | Not mentioned | [70] |
| miR-557 inhibits the differentiation and maturation of megakaryocytes. | In vitro and in vivo | MTT assay, CFSE staining, qRT-PCR. | miR-557 inhibitor increased the numbers of platelets and megakaryocytes and improved the symptoms of ITP. miR-557 inhibitor regulates apoptosis-related genes: Caspase-3 and Bax inhibition, and upregulation of bcl-2, p-Akt and p-ERK. | Akt/ERK pathway | [71] |
3.2. Histone Modification
3.3. LncRNA
3.4. MicroRNA
4. Discussion
4.1. Pathogenesis of ITP
4.2. Th1/Th2 and Th17/Treg Imbalance
4.3. Potential Epi-Biomarkers of ITP
4.4. Potential Treatment of ITP
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fogarty, P.F.; Segal, J.B. The epidemiology of immune thrombocytopenic purpura. Curr. Opin. Hematol. 2007, 14, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Provan, D.; Arnold, D.M.; Bussel, J.B.; Chong, B.H.; Cooper, N.; Gernsheimer, T.; Ghanima, W.; Godeau, B.; González-López, T.J.; Grainger, J.; et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019, 3, 3780–3817. [Google Scholar] [CrossRef] [PubMed]
- Stasi, R. Immune Thrombocytopenia: Pathophysiologic and Clinical Update. Semin. Thromb. Hemost. 2012, 38, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Al-Samkari, H.; Kuter, D.J. Immune Thrombocytopenia in Adults: Modern Approaches to Diagnosis and Treatment. Semin. Thromb. Hemost. 2020, 46, 275–288. [Google Scholar] [CrossRef]
- Cooper, N.; Ghanima, W. Immune Thrombocytopenia. N. Engl. J. Med. 2019, 381, 945–955. [Google Scholar] [CrossRef]
- Rodeghiero, F.; Stasi, R.; Gernsheimer, T.; Michel, M.; Provan, D.; Arnold, D.M.; Bussel, J.B.; Cines, D.B.; Chong, B.H.; Cooper, N.; et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: Report from an international working group. Blood 2009, 113, 2386–2393. [Google Scholar] [CrossRef]
- Michel, M. Immune Thrombocytopenia Nomenclature, Consensus Reports, and Guidelines: What Are the Consequences for Daily Practice and Clinical Research? Semin. Hematol. 2013, 50 (Suppl. S1), S50–S54. [Google Scholar] [CrossRef]
- Cines, D.B.; Liebman, H.; Stasi, R. Pathobiology of Secondary Immune Thrombocytopenia. Semin. Hematol. 2009, 46 (Suppl. S2), S2–S14. [Google Scholar] [CrossRef]
- Kohli, R.; Chaturvedi, S. Epidemiology and Clinical Manifestations of Immune Thrombocytopenia. Hämostaseologie 2019, 39, 238–249. [Google Scholar] [CrossRef]
- Hamzah, R.; Yusof, N.; Tumian, N.R.; Aziz, S.A.; Basri, N.S.M.; Shin, L.T.; Wah, H.K.; Selvaratnam, V.; Mui, T.S.; Jamil, S.A.M. Study of clinical epidemiology and bleeding characteristics in newly diagnosed immune thrombocytopenia (ITP) in adult multicentre in Malaysia. Pathology 2022, 54, S74. [Google Scholar] [CrossRef]
- Audia, S.; Mahévas, M.; Samson, M.; Godeau, B.; Bonnotte, B. Pathogenesis of immune thrombocytopenia. Autoimmun. Rev. 2017, 16, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Swinkels, M.; Rijkers, M.; Voorberg, J.; Vidarsson, G.; Leebeek, F.W.G.; Jansen, A.J.G. Emerging Concepts in Immune Thrombocytopenia. Front. Immunol. 2018, 9, 880. [Google Scholar] [CrossRef] [PubMed]
- Cines, D.B.; Cuker, A.; Semple, J.W. Pathogenesis of immune thrombocytopenia. Presse Med. 2014, 43, e49–e59. [Google Scholar] [CrossRef] [PubMed]
- Zufferey, A.; Kapur, R.; Semple, J.W. Pathogenesis and Therapeutic Mechanisms in Immune Thrombocytopenia (ITP). J. Clin. Med. 2017, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, L.; Wang, Y.; Huang, G.; Li, X.; Xie, Z.; Zhou, Z. Insights Into the Role of DNA Methylation in Immune Cell Development and Autoimmune Disease. Front. Cell Dev. Biol. 2021, 9, 3025. [Google Scholar] [CrossRef] [PubMed]
- Provan, D.; Semple, J.W. Recent Advances in the Mechanisms and Treatment of Immune Thrombocytopenia-NC-ND License (Http://Creativecommons.Org/Licenses/by-Nc-Nd/4.0/). EBioMedicine 2022, 76, 103820. [Google Scholar] [CrossRef]
- McKenzie, C.G.J.; Guo, L.; Freedman, J.; Semple, J.W. Cellular immune dysfunction in immune thrombocytopenia (ITP). Br. J. Haematol. 2013, 163, 10–23. [Google Scholar] [CrossRef]
- Vrbensky, J.R.; Nazy, I.; Clare, R.; Larché, M.; Arnold, D.M. T cell–mediated autoimmunity in immune thrombocytopenia. Eur. J. Haematol. 2022, 108, 18–27. [Google Scholar] [CrossRef]
- Ye, Q.-D.; Jiang, H.; Liao, X.-L.; Chen, K.; Li, S.-S. Identification and Validation of Gene Expression Pattern and Signature in Patients with Immune Thrombocytopenia. SLAS Discov. Adv. Sci. Drug Discov. 2017, 22, 187–195. [Google Scholar] [CrossRef]
- Chen, D.-P.; Lin, W.-T.; Wen, Y.-H.; Wang, W.-T. Investigation of the correlation between immune thrombocytopenia and T cell activity-regulated gene polymorphism using functional study. Sci. Rep. 2022, 12, 1–8. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, X.; Leng, S.; Xu, Q.; Sheng, Z.; Zhang, Y.; Yu, J.; Feng, Q.; Hou, M.; Peng, J.; et al. Immune Checkpoint-Related Gene Polymorphisms Are Associated With Primary Immune Thrombocytopenia. Front. Immunol. 2021, 11, 615941. [Google Scholar] [CrossRef]
- Crews, D.; Gillette, R.; Miller-Crews, I.; Gore, A.C.; Skinner, M.K. Nature, nurture and epigenetics. Mol. Cell. Endocrinol. 2014, 398, 42–52. [Google Scholar] [CrossRef]
- Tammen, S.A.; Friso, S.; Choi, S.-W. Epigenetics: The link between nature and nurture. Mol. Asp. Med. 2012, 34, 753–764. [Google Scholar] [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA Methylation and Its Basic Function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef]
- Gusic, M.; Prokisch, H. ncRNAs: New Players in Mitochondrial Health and Disease? Front. Genet. 2020, 11, 95. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zhong, L.; Weng, Y.; Peng, L.; Huang, Y.; Zhao, Y.; Liang, X.-J. Therapeutic siRNA: State of the art. Signal Transduct. Target. Ther. 2020, 5, 101. [Google Scholar] [CrossRef] [PubMed]
- Hung, J.; Miscianinov, V.; Sluimer, J.; Newby, D.E.; Baker, A.H. Targeting Non-coding RNA in Vascular Biology and Disease. Front. Physiol. 2018, 9, 1655. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.K.W.; Chow, M.Y.T.; Zhang, Y.; Leung, S.W.S. siRNA Versus miRNA as Therapeutics for Gene Silencing. Mol. Ther.-Nucleic Acids 2015, 4, e252. [Google Scholar] [CrossRef]
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef]
- Chen, Z.; Guo, Z.; Ma, J.; Ma, J.; Liu, F.; Wu, R. Foxp3 methylation status in children with primary immune thrombocytopenia. Hum. Immunol. 2014, 75, 1115–1119. [Google Scholar] [CrossRef]
- Ma, L.; Zhou, Z.; Wang, H.; Zhou, H.; Zhang, D.; Li, H.; Qi, A.; Yang, R. Increased expressions of DNA methyltransferases contribute to CD70 promoter hypomethylation and over expression of CD70 in ITP. Mol. Immunol. 2011, 48, 1525–1531. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-Y.; Qu, H.-T.; Sun, R.-J.; Yuan, D.; Sui, X.-H.; Shan, N.-N. High-throughput DNA methylation analysis in ITP confirms NOTCH1 hypermethylation through the Th1 and Th2 cell differentiation pathways. Int. Immunopharmacol. 2022, 111, 109105. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-P.; Gu, D.-S.; Zhou, Z.-P.; Chen, X.-L.; Guo, Z.-X.; Du, W.-T.; Ge, J.; Ren, Q.; Yang, R.-C. Decreased expression of MBD2 and MBD4 gene and genomic-wide hypomethylation in patients with primary immune thrombocytopenia. Hum. Immunol. 2011, 72, 486–491. [Google Scholar] [CrossRef] [PubMed]
- El-Shiekh, E.H.; Bessa, S.S.; Abdou, S.M.; El-Refaey, W.A. Role of DNA methyltransferase 3A mRNA expression in Egyptian patients with idiopathic thrombocytopenic purpura. Int. J. Lab. Hematol. 2012, 34, 369–376. [Google Scholar] [CrossRef]
- Zhao, H.; Du, W.; Wang, D.; Gu, D.; Xue, F.; Ge, J.; Sui, T.; Yang, R. The expression of IFN-γ, IL-4, Foxp3 and perforin genes are not correlated with DNA methylation status in patients with immune thrombocytopenic purpura. Platelets 2010, 21, 137–143. [Google Scholar] [CrossRef]
- Tao, J.; Yang, M.; Chen, Z.; Huang, Y.; Zhao, Q.; Xu, J.; Ren, H.; Zhao, H.; Chen, Z.; Ren, Q.; et al. Decreased DNA Methyltransferase 3A and 3B mRNA Expression in Peripheral Blood Mononuclear Cells and Increased Plasma SAH Concentration in Adult Patients with Idiopathic Thrombocytopenic Purpura. J. Clin. Immunol. 2008, 28, 432–439. [Google Scholar] [CrossRef]
- Zhao, H.; Xue, F.; Xu, J.; Fang, Z. Aberrant histone methylation in the patients with immune thrombocytopenia. Platelets 2014, 25, 207–210. [Google Scholar] [CrossRef]
- Sui, J.; Lu, R.; Halkidis, K.; Kocher, N.K.; Cao, W.; Marques, M.B.; Zheng, X.L. Plasma levels of S100A8/A9, histone/DNA complexes, and cell-free DNA predict adverse outcomes of immune thrombotic thrombocytopenic purpura. J. Thromb. Haemost. 2021, 19, 370–379. [Google Scholar] [CrossRef]
- Zou, Y.; Xu, H. Involvement of long noncoding RNAs in the pathogenesis of autoimmune diseases. J. Transl. Autoimmun. 2020, 3, 100044. [Google Scholar] [CrossRef]
- Hur, K.; Kim, S.-H.; Kim, J.-M. Potential Implications of Long Noncoding RNAs in Autoimmune Diseases. Immune Netw. 2019, 19, e4. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, D.; Zhu, X.; Qu, X.; Ji, C.; Hou, M. Elevated profile of Th17, Th1 and Tc1 cells in patients with immune thrombocytopenic purpura. Haematologica 2009, 94, 1326–1329. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhang, L.; Jiang, Z.; Yu, B. Decreasing lncRNA PVT1 causes Treg/Th17 imbalance via NOTCH signaling in immune thrombocytopenia. Hematology 2021, 26, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Wang, X.; Li, P.; Mei, C.; Zhang, M.; Zhao, C.; Song, Y. Systematic Identification of lncRNA-Associated ceRNA Networks in Immune Thrombocytopenia. Comput. Math. Methods Med. 2020, 2020, 6193593. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, S.E.; Hefzy, E.M.; El-Hmid, R.G.A.; Ahmed, N.A.; Khalefa, A.A.; Ali, D.Y.; Ali, M.A. Analysis of the expression profile of long non-coding RNAs MALAT1 and THRIL in children with immune thrombocytopenia. IUBMB Life 2020, 72, 1941–1950. [Google Scholar] [CrossRef]
- Li, T.; Gu, M.; Liu, P.; Liu, Y.; Guo, J.; Zhang, W.; Deng, A.; Qian, C. Abnormal Expression of Long Noncoding RNAs in Primary Immune Thrombocytopenia: A Microarray Related Study. Cell. Physiol. Biochem. 2018, 48, 618–632. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hao, Y.; Zhang, D.; Fu, R.; Liu, W.; Zhang, X.; Xue, F.; Yang, R. Aberrant expression of long noncoding RNA TMEVPG1 in patients with primary immune thrombocytopenia. Autoimmunity 2016, 49, 496–502. [Google Scholar] [CrossRef]
- Ali, M.A.; Hussein, S.K.; Khalifa, A.A.; Ali, A.M.E.A.; Farhan, M.S.; Amin, A.A.I.; Mohamed, E.A. The Ifng antisense RNA 1 (IFNG-AS1) and growth arrest-specific transcript 5 (GAS5) are novel diagnostic and prognostic markers involved in childhood ITP. Front. Mol. Biosci. 2022, 9, 1083. [Google Scholar] [CrossRef]
- Li, J.; Tian, J.; Lu, J.; Wang, Z.; Ling, J.; Wu, X.; Yang, F.; Xia, Y. LncRNA GAS5 inhibits Th17 differentiation and alleviates immune thrombocytopenia via promoting the ubiquitination of STAT3. Int. Immunopharmacol. 2020, 80, 106127. [Google Scholar] [CrossRef]
- Li, J.-Q.; Hu, S.-Y.; Wang, Z.-Y.; Lin, J.; Jian, S.; Dong, Y.-C.; Wu, X.-F.; Lan, D.; Cao, L.-J. Long non-coding RNA MEG3 inhibits microRNA-125a-5p expression and induces immune imbalance of Treg/Th17 in immune thrombocytopenic purpura. Biomed. Pharmacother. 2016, 83, 905–911. [Google Scholar] [CrossRef]
- Pauley, K.M.; Cha, S.; Chan, E.K. MicroRNA in autoimmunity and autoimmune diseases. J. Autoimmun. 2009, 32, 189–194. [Google Scholar] [CrossRef]
- Hua, M.; Li, J.; Wang, C.; Shao, L.; Hou, M.; Peng, J.; Feng, Q. Aberrant expression of microRNA in CD4+ cells contributes to Th17/Treg imbalance in primary immune thrombocytopenia. Thromb. Res. 2019, 177, 70–78. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Ji, D.; Lu, W.; Li, F.; Huang, X.; Huang, R.; Chen, G. Bone marrow mesenchymal stem cell-derived exosomes induce the Th17/Treg imbalance in immune thrombocytopenia through miR-146a-5p/IRAK1 axis. Human Cell 2021, 34, 1360–1374. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Yan, W.; Li, T.; Cui, Q.; Liu, P.; Gu, M.; Guo, J.; Zhang, W.; Ren, C.; Wu, T.; et al. Differential Expression of MiR-106b-5p and MiR-200c-3p in Newly Diagnosed Versus Chronic Primary Immune Thrombocytopenia Patients Based on Systematic Analysis. Cell. Physiol. Biochem. 2018, 45, 301–318. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Chen, X.; Tian, Y.; Gao, X.; Liu, Z.; Dong, X.; Zhou, J. Downregulation of microRNA-155-5p prevents immune thrombocytopenia by promoting macrophage M2 polarization via the SOCS1-dependent PD1/PDL1 pathway. Life Sci. 2020, 257, 118057. [Google Scholar] [CrossRef] [PubMed]
- Khodadi, E.; Asnafi, A.A.; Mohammadi-Asl, J.; Hosseini, S.A.; Malehi, A.S.; Saki, N. Evaluation of miR-21 and miR-150 expression in immune thrombocytopenic purpura pathogenesis: A case-control study. Front. Biol. 2017, 12, 361–369. [Google Scholar] [CrossRef]
- Qian, B.-H.; Ye, X.; Zhang, L.; Sun, Y.; Zhang, J.-R.; Gu, M.-L.; Qin, Q.; Chen, J.; Deng, A.-M. Increased miR-155 Expression in Peripheral Blood Mononuclear Cells of Primary Immune Thrombocytopenia Patients Was Correlated with Serum Cytokine Profiles. Acta Haematol. 2015, 133, 257–263. [Google Scholar] [CrossRef]
- Zhang, J.-M.; Zhu, X.-L.; Xue, J.; Liu, X.; Zheng, X.L.; Chang, Y.-J.; Liu, K.-Y.; Huang, X.-J.; Zhang, X.-H. Integrated mRNA and miRNA profiling revealed deregulation of cellular stress response in bone marrow mesenchymal stem cells derived from patients with immune thrombocytopenia. Funct. Integr. Genom. 2018, 18, 287–299. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, H.; Xie, X.; Zheng, Z.; Ling, Y. MicroRNA expression profile in Treg cells in the course of primary immune thrombocytopenia. J. Investig. Med. 2019, 67, 1118–1124. [Google Scholar] [CrossRef]
- Deng, G.; Yu, S.; He, Y.; Sun, T.; Liang, W.; Yu, L.; Xu, D.; Li, Q.; Zhang, R. MicroRNA profiling of platelets from immune thrombocytopenia and target gene prediction. Mol. Med. Rep. 2017, 16, 2835–2843. [Google Scholar] [CrossRef]
- He, X.; Wang, T.; Ran, N.; Liu, C.; Xing, L.; Shao, Z. MicroRNA-21-5p Regulates CD3+T Lymphocytes Through VCL and LTF in Patients with Immune Thrombocytopenia. Clin. Lab. 2022, 68, 1–10. [Google Scholar] [CrossRef]
- Li, J.Q.; Tian, J.M.; Fan, X.R.; Wang, Z.Y.; Ling, J.; Wu, X.F.; Xia, Y.L. miR-106b-5p induces immune imbalance of Treg/Th17 in immune thrombocytopenic purpura through NR4A3/Foxp3 pathway. Cell Cycle 2020, 19, 1265–1274. [Google Scholar] [CrossRef]
- Yang, Y.; Fu, L.; Chen, C.; Hu, M. miRNA-148b-3p targeting SOCS3 inhibits macrophage M2 polarization by JAK2/STAT3 pathway in immune thrombocytopenia. BIOCELL 2022, 46, 1319–1328. [Google Scholar] [CrossRef]
- Bay, A.; Coskun, E.; Oztuzcu, S.; Ergun, S.; Yilmaz, F.; Aktekin, E. Plasma microRNA profiling of pediatric patients with immune thrombocytopenic purpura. Blood Coagul. Fibrinolysis 2014, 25, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Zuo, B.; Zhai, J.; You, L.; Zhao, Y.; Yang, J.; Weng, Z.; Dai, L.; Wu, Q.; Ruan, C.; He, Y. Plasma microRNAs characterising patients with immune thrombo cytopenic purpura. Thromb. Haemost. 2017, 117, 1420–1431. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hou, Y.; Meng, G.; Han, P.; Zhao, Y.; Wang, H.; Xu, M.; Wang, Y.; Qiu, J.; Peng, J.; et al. Proteomic analysis and microRNA expression profiling of plasma-derived exosomes in primary immune thrombocytopenia. Br. J. Haematol. 2021, 194, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, H.; Xue, F.; Zhang, X.; Zhang, D.; Ge, J.; Yang, R. Reduced expression of MIR 409-3p in primary immune thrombocytopenia. Br. J. Haematol. 2013, 161, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, H.; Du, W.; Zhang, D.; Ge, J.; Xue, F.; Yang, R. Reduced MIR 130 A is involved in primary immune thrombocytopenia via targeting TGFB 1 and IL 18. Br. J. Haematol. 2014, 166, 767–773. [Google Scholar] [CrossRef]
- Liu, L.; Hua, M.; Liu, C.; He, N.; Li, Z.; Ma, D. The aberrant expression of microRNAs and correlations with T cell subsets in patients with immune thrombocytopenia. Oncotarget 2016, 7, 76453–76463. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, X.; Zhou, L.; Yin, J.; Zhao, Y.; Dai, L.; Zuo, B.; He, Y. Increased let-7b-5p is associated with enhanced BAFF-R expression and B cell survival in immune thrombocytopenia. Int. Immunopharmacol. 2021, 93, 107393. [Google Scholar] [CrossRef]
- Deng, L.; Jiang, L.; Tseng, K.F.; Wang, X.; Liu, Y.; Zhang, X.; Lu, Z. The increased expression of miR-146 predicts poor prognosis of immune thrombocytopenia. Int. J. Clin. Exp. 2016, 9, 9461–9466. [Google Scholar]
- Wang, Y.; Guo, Y.; Zhang, X.; Zhao, H.; Zhang, B.; Wu, Y.; Zhang, J. The role and mechanism of miR-557 in inhibiting the differentiation and maturation of megakaryocytes in immune thrombocytopenia. RNA Biol. 2021, 18, 1953–1968. [Google Scholar] [CrossRef] [PubMed]
- Surace, A.E.A.; Hedrich, C.M. The Role of Epigenetics in Autoimmune/Inflammatory Disease. Front. Immunol. 2019, 10, 1525. [Google Scholar] [CrossRef] [PubMed]
- Keverne, E. Significance of epigenetics for understanding brain development, brain evolution and behaviour. Neuroscience 2014, 264, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Kilvitis, H.J.; Alvarez, M.; Foust, C.M.; Schrey, A.W.; Robertson, M.; Richards, C.L. Ecological Epigenetics. Ecol. Genom. Ecol. Evol. Genes Genomes 2014, 781, 191–210. [Google Scholar] [CrossRef]
- Ardekani, A.M. Role of Epigenetics in Biology and Human Diseases. Iran. Biomed. J. 2016, 20, 246–258. [Google Scholar] [CrossRef]
- Gauer, R.L.; Braun, M.M. Thrombocytopenia. Am. Fam. Physician 2012, 85, 612–622. [Google Scholar]
- George, J.N.; Aster, R.H. Drug-induced thrombocytopenia: Pathogenesis, evaluation, and management. Hematology 2009, 2009, 153–158. [Google Scholar] [CrossRef]
- Bakchoul, T.; Marini, I. Drug-associated thrombocytopenia. Hematol. 2014 Am. Soc. Hematol. Educ. Program Book 2018, 2018, 576–583. [Google Scholar] [CrossRef]
- Vayne, C.; Guéry, E.-A.; Rollin, J.; Baglo, T.; Petermann, R.; Gruel, Y. Pathophysiology and Diagnosis of Drug-Induced Immune Thrombocytopenia. J. Clin. Med. 2020, 9, 2212. [Google Scholar] [CrossRef]
- Raadsen, M.; Du Toit, J.; Langerak, T.; van Bussel, B.; van Gorp, E.; Goeijenbier, M. Thrombocytopenia in Virus Infections. J. Clin. Med. 2021, 10, 877. [Google Scholar] [CrossRef]
- Wang, Y.; Han, S.; Ran, R.; Li, A.; Liu, H.; Liu, M.; Duan, Y.; Zhang, X.; Zhao, Z.; Song, S.; et al. A longitudinal sampling study of transcriptomic and epigenetic profiles in patients with thrombocytopenia syndrome. Nat. Commun. 2021, 12, 5629. [Google Scholar] [CrossRef] [PubMed]
- Leung, H.H.L.; Perdomo, J.; Ahmadi, Z.; Zheng, S.S.; Rashid, F.N.; Enjeti, A.; Ting, S.B.; Chong, J.J.H.; Chong, B.H. NETosis and thrombosis in vaccine-induced immune thrombotic thrombocytopenia. Nat. Commun. 2022, 13, 5206. [Google Scholar] [CrossRef] [PubMed]
- Osborne, A. The role of epigenetics in human evolution. Biosci. Horiz. Int. J. Stud. Res. 2017, 10, 1–8. [Google Scholar] [CrossRef]
- Barsam, S.J.; Psaila, B.; Forestier, M.; Page, L.K.; Sloane, P.A.; Geyer, J.T.; Villarica, G.O.; Ruisi, M.M.; Gernsheimer, T.B.; Beer, J.H.; et al. Platelet production and platelet destruction: Assessing mechanisms of treatment effect in immune thrombocytopenia. Blood 2011, 117, 5723–5732. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.-J.; Shan, N.-N. Megakaryocytic dysfunction in immune thrombocytopenia is linked to autophagy. Cancer Cell Int. 2019, 19, 59. [Google Scholar] [CrossRef]
- Eto, K.; Kunishima, S. Linkage between the mechanisms of thrombocytopenia and thrombopoiesis. Blood 2016, 127, 1234–1241. [Google Scholar] [CrossRef]
- Italiano, J.; Hartwig, J. Chapter 1: Production and Destruction of Platelets. In The Non-Thrombotic Role of Platelets in Health and Disease; IntechOpen: London, UK, 2015. [Google Scholar] [CrossRef]
- Josefsson, E.C.; Vainchenker, W.; James, C. Regulation of Platelet Production and Life Span: Role of Bcl-xL and Potential Implications for Human Platelet Diseases. Int. J. Mol. Sci. 2020, 21, 7591. [Google Scholar] [CrossRef]
- Luckheeram, R.V.; Zhou, R.; Verma, A.D.; Xia, B. CD4+T Cells: Differentiation and Functions. J. Immunol. Res. 2012, 2012, 925135. [Google Scholar] [CrossRef]
- Kidd, P. Th1/Th2 Balance: The Hypothesis, Its Limitations, and Implications for Health and Disease. Altern. Med. Rev. 2003, 8, 223–246. [Google Scholar]
- Nishimoto, T.; Kuwana, M. CD4+CD25+Foxp3+ Regulatory T Cells in the Pathophysiology of Immune Thrombocytopenia. Semin. Hematol. 2013, 50 (Suppl. S1), S43–S49. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xu, A.; Zhou, L.; Zhao, N.; Zhang, X.; Xu, J.; Feng, S.; Zheng, C. Imbalance of T Lymphocyte Subsets in Adult Immune Thrombocytopenia. Int. J. Gen. Med. 2021, 14, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Cheng, Y. Treg cell abnormality and its potential treatments in patients with primary immune thrombocytopenia. Clin. Transl. Discov. 2022, 2, e277. [Google Scholar] [CrossRef]
- Chen, W.; Qiao, J.; Zong, S.F.; Liang, D.D.; Sun, H.M.; Li, M. Changes of Inflammasome in Children with Immune Thrombocytopenia before and after Treatment. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2021, 29, 1566–1569. [Google Scholar] [CrossRef]
- Van Eden, W.; van der Zee, R.; van Kooten, P.; E Berlo, S.; Cobelens, P.M.; Kavelaars, A.; Heijnen, C.J.; Prakken, B.; Roord, S.; Albani, S. Balancing the immune system: Th1 and Th2. Ann. Rheum. Dis. 2002, 61 (Suppl. S2), ii25–ii28. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, Y.; Wang, X.; Sun, M.; Wang, L.; Wang, X.; Liu, Y.; Fan, W.; Zhang, K.; Sui, X.; et al. Regulation of Th1/Th2 and Th17/Treg by pDC/mDC imbalance in primary immune thrombocytopenia. Exp. Biol. Med. 2021, 246, 1688–1697. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Yu, T.-S.; Liu, Y.; Li, K.; Liu, S.; Liu, Y.; Feng, Q.; Zhang, L.; Li, G.-S.; et al. Disrupted balance of CD4+ T-cell subsets in bone marrow of patients with primary immune thrombocytopenia. Int. J. Biol. Sci. 2019, 15, 2798–2814. [Google Scholar] [CrossRef]
- Ma, L.M.L.; Liang, Y.L.Y.; Fang, M.F.M.; Guan, Y.G.Y.; Si, Y.S.Y.; Jiang, F.J.F.; Wang, F.W.F. The cytokines(IFN-y, IL-2, IL-4, IL-10, IL-17) and Treg cytokine (TGF-ß1) levels inadults with immune thrombocytopenia. Pharmazie 2014, 69, 694–697. [Google Scholar] [CrossRef]
- Li, F.; Ji, L.; Wang, W.; Hua, F.; Zhan, Y.; Zou, S.; Yuan, L.; Ke, Y.; Min, Z.; Song, D.; et al. Insufficient secretion of IL-10 by Tregs compromised its control on over-activated CD4+ T effector cells in newly diagnosed adult immune thrombocytopenia patients. Immunol. Res. 2014, 61, 269–280. [Google Scholar] [CrossRef]
- Pehlivan, M.; Okan, V.; Sever, T.; Balci, S.O.; Yilmaz, M.; Babacan, T.; Pehlıvan, S. Investigation of TNF-alpha, TGF-beta 1, IL-10, IL-6, IFN-gamma, MBL, GPIA, and IL1A gene polymorphisms in patients with idiopathic thrombocytopenic purpura. Platelets 2011, 22, 588–595. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, P.; Liu, H.; Liu, X.; Xie, S.; Wang, X.; Wu, Y.; Chang, J.; Ma, L. Assessment of Th17/Treg cells and Th cytokines in an improved immune thrombocytopenia mouse model. Hematology 2017, 22, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Talaat, R.M.; Elmaghraby, A.M.; Barakat, S.S.; El-Shahat, M. Alterations in immune cell subsets and their cytokine secretion profile in childhood idiopathic thrombocytopenic purpura (ITP). Clin. Exp. Immunol. 2014, 176, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Saitoh, T.; Gotoh, N.; Nitta, Y.; Alkebsi, L.; Kasamatsu, T.; Minato, Y.; Yokohama, A.; Tsukamoto, N.; Handa, H.; et al. The Cytokine Polymorphisms Affecting Th1/Th2 Increase the Susceptibility to, and Severity of, Chronic ITP. BMC Immunol. 2017, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Maben, Z.; Wang, C.; Lindquist, K.C.; Li, M.; Rayannavar, V.; Armenta, I.L.; Nager, A.; Pascua, E.; Dominik, P.K.; et al. Structural delineation and phase-dependent activation of the costimulatory CD27:CD70 complex. J. Biol. Chem. 2021, 297, 101102. [Google Scholar] [CrossRef] [PubMed]
- Borst, J.; Hendriks, J.; Xiao, Y. CD27 and CD70 in T cell and B cell activation. Curr. Opin. Immunol. 2005, 17, 275–281. [Google Scholar] [CrossRef]
- Jiang, H.; Xiao, R.; Lian, X.; Kanekura, T.; Luo, Y.; Yin, Y.; Zhang, G.; Yang, Y.; Wang, Y.; Zhao, M.; et al. Demethylation of TNFSF7 contributes to CD70 overexpression in CD4+ T cells from patients with systemic sclerosis. Clin. Immunol. 2012, 143, 39–44. [Google Scholar] [CrossRef]
- Hornero, R.A.; Georgiadis, C.; Hua, P.; Trzupek, D.; He, L.-Z.; Qasim, W.; Todd, J.A.; Ferreira, R.C.; Wood, K.J.; Issa, F.; et al. CD70 expression determines the therapeutic efficacy of expanded human regulatory T cells. Commun. Biol. 2020, 3, 375. [Google Scholar] [CrossRef]
- Brandstadter, J.D.; Maillard, I. Notch signalling in T cell homeostasis and differentiation. Open Biol. 2019, 9, 190187. [Google Scholar] [CrossRef]
- Ma, D.; Dai, J.; Zhu, X.; Yan, S.; Zhao, P.; Zhang, J.; Zhu, Y.; Sun, J.; Peng, J.; Ji, C.; et al. Aberrant expression of Notch signaling molecules in patients with immune thrombocytopenic purpura. Ann. Hematol. 2010, 89, 155–161. [Google Scholar] [CrossRef]
- Yu, S.; Liu, C.; Li, L.; Tian, T.; Wang, M.; Hu, Y.; Yuan, C.; Zhang, L.; Ji, C.; Ma, D. Inactivation of Notch signaling reverses the Th17/Treg imbalance in cells from patients with immune thrombocytopenia. Lab. Investig. 2015, 95, 157–167. [Google Scholar] [CrossRef]
- Ma, L.; Xue, H.; Gao, T.; Gao, M.; Zhang, Y. Notch1 Signaling Regulates the Th17/Treg Immune Imbalance in Patients with Psoriasis Vulgaris. Mediat. Inflamm. 2018, 2018, 3069521. [Google Scholar] [CrossRef]
- Ali, M.; Shaker, O.; Khalefa, A.; Abdelwahed, M.; Ali, E.; Ezzat, E.; Elghobary, H.; Awaji, A.; Fouad, N.; Ayoub, S. Serum long noncoding RNAs FAS-AS1 & PVT1 are novel biomarkers for systemic lupus erythematous. Br. J. Biomed. Sci. 2020, 77, 208–212. [Google Scholar] [CrossRef]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-γ: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef] [PubMed]
- Collier, S.P.; Collins, P.L.; Williams, C.L.; Boothby, M.R.; Aune, T.M. Cutting Edge: Influence of Tmevpg1, a Long Intergenic Noncoding RNA, on the Expression of Ifng by Th1 Cells. J. Immunol. 2012, 189, 2084–2088. [Google Scholar] [CrossRef] [PubMed]
- Collier, S.P.; Henderson, M.A.; Tossberg, J.T.; Aune, T.M. Regulation of the Th1 Genomic Locus from Ifng through Tmevpg1 by T-bet. J. Immunol. 2014, 193, 3959–3965. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Peng, H.; Tian, J.; Ma, J.; Tang, X.; Rui, K.; Tian, X.; Wang, Y.; Chen, J.-G.; Lu, L.; et al. Upregulation of long noncoding RNA TMEVPG1 enhances T helper type 1 cell response in patients with Sjögren syndrome. Immunol. Res. 2015, 64, 489–496. [Google Scholar] [CrossRef]
- Crane, I.J.; Forrester, J.V. Th1 and Th2 Lymphocytes in Autoimmune Disease. Crit. Rev. Immunol. 2005, 25, 75–102. [Google Scholar] [CrossRef]
- Skapenko, A.; Leipe, J.; E Lipsky, P.; Schulze-Koops, H. The role of the T cell in autoimmune inflammation. Arthritis Res. Ther. 2005, 7, S4–S14. [Google Scholar] [CrossRef]
- Marks, K.E.; Cho, K.; Stickling, C.; Reynolds, J.M. Toll-like Receptor 2 in Autoimmune Inflammation. Immune Netw. 2021, 21, e18. [Google Scholar] [CrossRef]
- Han, J.M.; Patterson, S.J.; Levings, M.K. The Role of the PI3K Signaling Pathway in CD4+ T Cell Differentiation and Function. Front. Immunol. 2012, 3, 245. [Google Scholar] [CrossRef]
- Pompura, S.L.; Dominguez-Villar, M. The PI3K/AKT signaling pathway in regulatory T-cell development, stability, and function. J. Leukoc. Biol. 2018, 103, 1065–1076. [Google Scholar] [CrossRef]
- Kim, E.H.; Suresh, M. Role of PI3K/Akt signaling in memory CD8 T cell differentiation. Front. Immunol. 2013, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Chiang, L.; Kang, C.; Winoto, A. The role of the PI3K-AKT kinase pathway in T-cell development beyond the β checkpoint. Eur. J. Immunol. 2008, 38, 3200–3207. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Liu, X.; Chen, Q.; Liu, T.; Lu, C.; Yu, J.; Miao, Y.; Wei, J. Downregulated miR-98-5p promotes PDAC proliferation and metastasis by reversely regulating MAP4K4. J. Exp. Clin. Cancer Res. 2018, 37, 130. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Yu, Q.; Chu, Y.; Zhu, X.; Lu, W.; Liu, Q.; Wang, Q. MicroRNA-98-5p inhibits proliferation and metastasis in non-small cell lung cancer by targeting TGFBR1. Int. J. Oncol. 2019, 54, 128–138. [Google Scholar] [CrossRef]
- Wang, Z.; Han, Z.; Zhang, L.; Zhang, S.; Wang, B. MicroRNA-98-5p regulates the proliferation and apoptosis of A549 cells by targeting MAP4K3. Oncol. Lett. 2019, 18, 4288–4293. [Google Scholar] [CrossRef]
- Denoeud, J.; Moser, M. Role of CD27/CD70 pathway of activation in immunity and tolerance. J. Leuk. Biol. 2011, 89, 195–203. [Google Scholar] [CrossRef]
- Goronzy, J.J.; Weyand, C.M. T-cell co-stimulatory pathways in autoimmunity. Thromb. Haemost. 2008, 10, S3. [Google Scholar] [CrossRef]
- Han, B.K.; Olsen, N.J.; Bottaro, A. The CD27–CD70 pathway and pathogenesis of autoimmune disease. Semin. Arthritis Rheum. 2016, 45, 496–501. [Google Scholar] [CrossRef]
- Xiao, Y.; Su, X.; Huang, W.; Zhang, J.; Peng, C.; Huang, H.; Wu, X.; Huang, H.; Xia, M.; Ling, W. Role of S-adenosylhomocysteine in cardiovascular disease and its potential epigenetic mechanism. Int. J. Biochem. Cell Biol. 2015, 67, 158–166. [Google Scholar] [CrossRef]
- Goggs, R.; Jeffery, U.; Levine, D.N.; Li, R.H.L. Neutrophil-Extracellular Traps, Cell-Free DNA, and Immunothrombosis in Companion Animals: A Review. Veter. Pathol. 2020, 57, 6–23. [Google Scholar] [CrossRef] [PubMed]
- Thålin, C.; Lundström, S.; Seignez, C.; Daleskog, M.; Lundström, A.; Henriksson, P.; Helleday, T.; Phillipson, M.; Wallén, H.; Demers, M. Citrullinated histone H3 as a novel prognostic blood marker in patients with advanced cancer. PLoS ONE 2018, 13, e0191231. [Google Scholar] [CrossRef] [PubMed]
- Parackova, Z.; Zentsova, I.; Malcova, H.; Cebecauerova, D.; Sediva, A.; Horvath, R. Increased histone citrullination in juvenile idiopathic arthritis. Front. Med. 2022, 9, 2509. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kaplan, M.J. The role of neutrophils and NETosis in autoimmune and renal diseases. Nat. Rev. Nephrol. 2016, 12, 402–413. [Google Scholar] [CrossRef]
- Mutua, V.; Gershwin, L.J. A Review of Neutrophil Extracellular Traps (NETs) in Disease: Potential Anti-NETs Therapeutics. Clin. Rev. Allergy Immunol. 2021, 61, 194–211. [Google Scholar] [CrossRef]
- Glennon-Alty, L.; Hackett, A.P.; Chapman, E.A.; Wright, H.L. Neutrophils and redox stress in the pathogenesis of autoimmune disease. Free Radic. Biol. Med. 2018, 125, 25–35. [Google Scholar] [CrossRef]
- Kahlenberg, J.M.; Carmona-Rivera, C.; Smith, C.K.; Kaplan, M.J. Neutrophil extracellular trap-associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. J. Immunol. 2013, 190, 1217–1226. [Google Scholar] [CrossRef]
- Granger, V.; Peyneau, M.; Chollet-Martin, S.; de Chaisemartin, L. Neutrophil Extracellular Traps in Autoimmunity and Allergy: Immune Complexes at Work. Front. Immunol. 2019, 10, 2824. [Google Scholar] [CrossRef]
- Perdomo, J.; Leung, H.H.L.; Ahmadi, Z.; Yan, F.; Chong, J.J.H.; Passam, F.H.; Chong, B.H. Neutrophil activation and NETosis are the major drivers of thrombosis in heparin-induced thrombocytopenia. Nat. Commun. 2019, 10, 1322. [Google Scholar] [CrossRef]
- Jin, J.; Qiao, S.; Liu, J.; Li, W.; Wang, F.; Gao, X.; Tian, J.; Wang, N.; Zhang, J.; Dong, J.; et al. Neutrophil extracellular traps promote thrombogenicity in cerebral venous sinus thrombosis. Cell Biosci. 2022, 12, 114. [Google Scholar] [CrossRef]
- Hendrich, B.; Hardeland, U.; Ng, H.-H.; Jiricny, J.; Bird, A. The thymine glycosylase MBD4 can bind to the product of deamination at methylated CpG sites. Nature 1999, 401, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.K.; Ramchandani, S.; Cervoni, N.; Szyf, M. A mammalian protein with specific demethylase activity for mCpG DNA. Nature 1999, 397, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Menafra, R.; Stunnenberg, H.G. MBD2 and MBD3: Elusive functions and mechanisms. Front. Genet. 2014, 5, 428. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Li, M.; Wu, H.; Jia, S.; Zhang, N.; Dai, Y.; Zhao, M.; Lu, Q. Down-regulation of MBD4 contributes to hypomethylation and overexpression of CD70 in CD4+ T cells in systemic lupus erythematosus. Clin. Epigenet. 2017, 9, 104. [Google Scholar] [CrossRef]
- Gkoutsias, A.; Makis, A. The role of epigenetics in childhood autoimmune diseases with hematological manifestations. Pediatr. Investig. 2022, 6, 36–46. [Google Scholar] [CrossRef]
- Peters, A.H.; Kubicek, S.; Mechtler, K.; O’Sullivan, R.J.; Derijck, A.A.; Perez-Burgos, L.; Kohlmaier, A.; Opravil, S.; Tachibana, M.; Shinkai, Y.; et al. Partitioning and Plasticity of Repressive Histone Methylation States in Mammalian Chromatin. Mol. Cell 2003, 12, 1577–1589. [Google Scholar] [CrossRef] [PubMed]
- A Rosenfeld, J.; Wang, Z.; E Schones, D.; Zhao, K.; DeSalle, R.; Zhang, M.Q. Determination of enriched histone modifications in non-genic portions of the human genome. BMC Genom. 2009, 10, 143. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Shan, N.-N. DNA methylation plays an important role in immune thrombocytopenia. Int. Immunopharmacol. 2020, 83, 106390. [Google Scholar] [CrossRef]
- Araki, Y.; Mimura, T. The Histone Modification Code in the Pathogenesis of Autoimmune Diseases. Mediat. Inflamm. 2017, 2017, 2608605. [Google Scholar] [CrossRef]
- Stewart, M.D.; Li, J.; Wong, J. Relationship between Histone H3 Lysine 9 Methylation, Transcription Repression, and Heterochromatin Protein 1 Recruitment. Mol. Cell. Biol. 2005, 25, 2525–2538. [Google Scholar] [CrossRef]
- Wang, Z.; Yin, H.; Lau, C.S.; Lu, Q. Histone Posttranslational Modifications of CD4+ T Cell in Autoimmune Diseases. Int. J. Mol. Sci. 2016, 17, 1547. [Google Scholar] [CrossRef] [PubMed]
- Renaude, E.; Kroemer, M.; Borg, C.; Peixoto, P.; Hervouet, E.; Loyon, R.; Adotévi, O. Epigenetic Reprogramming of CD4+ Helper T Cells as a Strategy to Improve Anticancer Immunotherapy. Front. Immunol. 2021, 12, 669992. [Google Scholar] [CrossRef]
- Weirich, S.; Khella, M.; Jeltsch, A. Structure, Activity and Function of the Suv39h1 and Suv39h2 Protein Lysine Methyltransferases. Life 2021, 11, 703. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S. Advances in Diagnosis and Treatments for Immune Thrombocytopenia. Clin. Med. Insights Blood Disord. 2016, 9, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Terrell, D.; Neunert, C.; Cooper, N.; Heitink-Pollé, K.; Kruse, C.; Imbach, P.; Kühne, T.; Ghanima, W. Immune Thrombocytopenia (ITP): Current Limitations in Patient Management. Medicina 2020, 56, 667. [Google Scholar] [CrossRef]
- Garabet, L.; Ghanima, W.; Rangberg, A.; Teruel-Montoya, R.; Martinez, C.; Lozano, M.L.; Nystrand, C.F.; Bussel, J.B.; Sandset, P.M.; Jonassen, C.M. Circulating microRNAs in patients with immune thrombocytopenia before and after treatment with thrombopoietin-receptor agonists. Platelets 2020, 31, 198–205. [Google Scholar] [CrossRef]
- Li, C.; Li, L.; Sun, M.; Sun, J.; Shao, L.; Xu, M.; Hou, Y.; Peng, J.; Wang, L.; Hou, M. Predictive Value of High ICAM-1 Level for Poor Treatment Response to Low-Dose Decitabine in Adult Corticosteroid Resistant ITP Patients. Front. Immunol. 2021, 12, 689663. [Google Scholar] [CrossRef]
- Zhou, H.; Qin, P.; Liu, Q.; Yuan, C.; Hao, Y.; Zhang, H.; Wang, Z.; Ran, X.; Chu, X.; Yu, W.; et al. A prospective, multicenter study of low dose decitabine in adult patients with refractory immune thrombocytopenia. Am. J. Hematol. 2019, 94, 1374–1381. [Google Scholar] [CrossRef]
- Zhou, H.; Hou, Y.; Liu, X.; Qiu, J.; Feng, Q.; Wang, Y.; Zhang, X.; Min, Y.; Shao, L.; Liu, X.; et al. Low-dose decitabine promotes megakaryocyte maturation and platelet production in healthy controls and immune thrombocytopenia. Thromb. Haemost. 2015, 113, 1021–1034. [Google Scholar] [CrossRef]
- Han, P.; Yu, T.; Hou, Y.; Zhao, Y.; Liu, Y.; Sun, Y.; Wang, H.; Xu, P.; Li, G.; Sun, T.; et al. Low-Dose Decitabine Inhibits Cytotoxic T Lymphocytes-Mediated Platelet Destruction via Modulating PD-1 Methylation in Immune Thrombocytopenia. Front. Immunol. 2021, 12, 630693. [Google Scholar] [CrossRef]
- He, Y.-Z.; Lu, R.-F.; Zhu, C.; Hua, J.-Y. Qian Five Rhinoceros Gindeng (QFRG) protects against development of immune thrombocytopenia via miR-181a inhibition of TLR-4 expression. Int. J. Clin. Exp. Med. 2015, 8, 6986–6993. [Google Scholar] [PubMed]
- Jiang, Y.; Liu, N.; Zhu, S.; Hu, X.; Chang, D.; Liu, J. Elucidation of the Mechanisms and Molecular Targets of Yiqi Shexue Formula for Treatment of Primary Immune Thrombocytopenia Based on Network Pharmacology. Front. Pharmacol. 2019, 10, 1136. [Google Scholar] [CrossRef]
- Borjigin, M.; Huo, W.; Gong, C.; Zhang, G.; Li, M.; Zhang, X.; Sun, X.; Yang, J.; Wang, S.; Narisu, N.; et al. Profiling of miRNA expression in immune thrombocytopenia patients before and after Qishunbaolier (QSBLE) treatment. Biomed. Pharmacother. 2015, 75, 196–204. [Google Scholar] [CrossRef]
- Burenbatu, B.; Wang, Y.; Wang, S.; Na, R.; Wuritunashun, W.; Gong, C.; Hashengaowa, H.; Eerdunduleng, E.; Sarula, S.; Guihua, G.; et al. iTRAQ-based quantitative proteomics analysis of immune thrombocytopenia patients before and after Qishunbaolier treatment. Rapid Commun. Mass Spectrom. 2021, 35, e8993. [Google Scholar] [CrossRef] [PubMed]

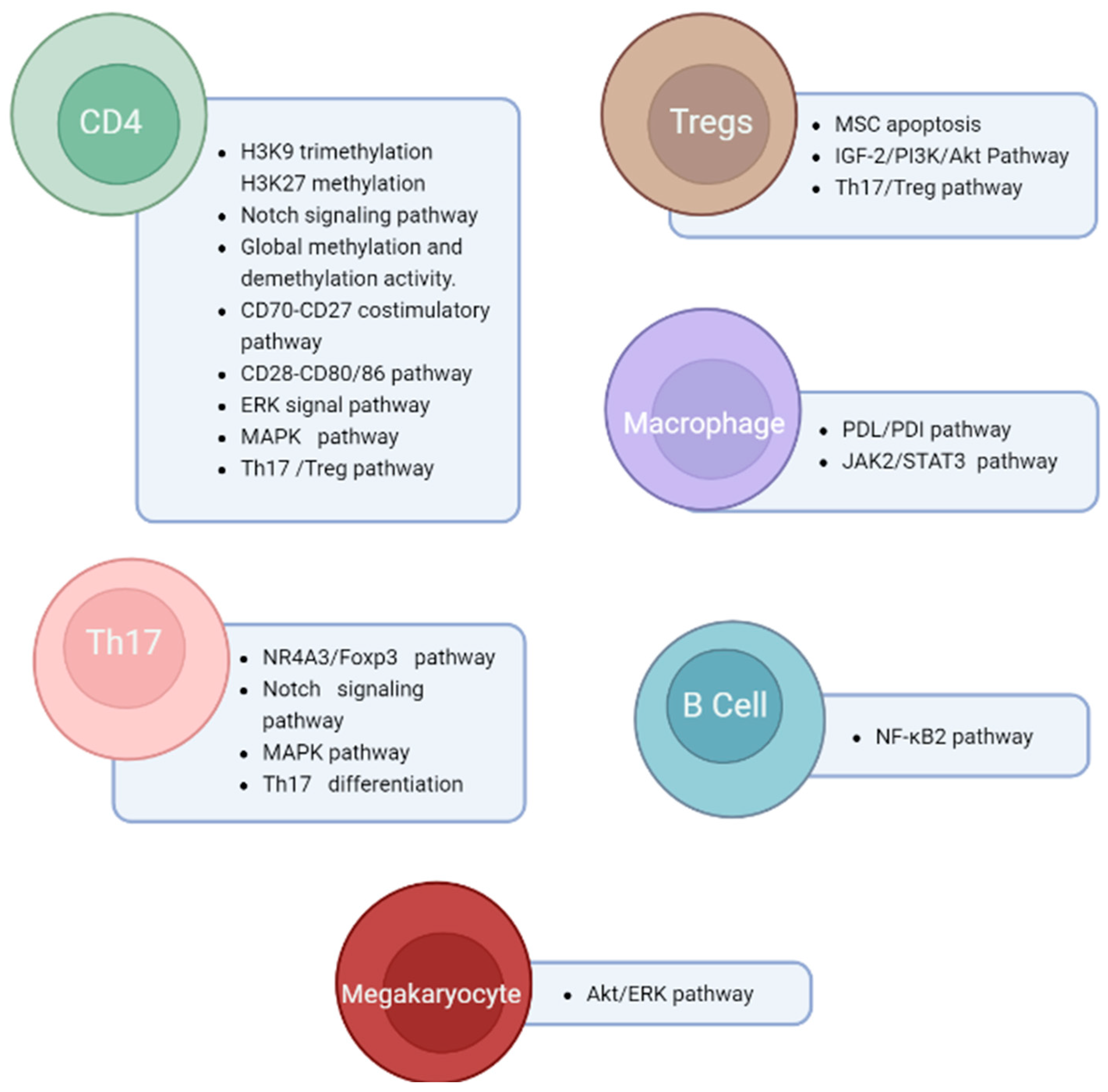
| Potential Epi-Biomarker | Known Related Functions | Reference(s) |
|---|---|---|
| CitH3 | NETosis | [37] |
| MALAT1, THRIL | TLR2 signaling Pathway | [42] |
| CXCL13, IL-21 | Plasma cells and B-memory lymphocytes | [48] |
| miR409-3p, miR-146, miR-106b-5p, miR-302c-3p, miR-483-5p, miR-223-3p, miR-597, miR-544a, miR-302a-3p, miR-410, miR-320c, miR-642b-3p, miR-1275, miR-3141, miR-4270, miR-4499, miR-4739, miR-6126, miR-144-3p, miR-1281 and miR-3162-3p, miR-106b-5-p, miR200c–3p, NR_038920 and ENST00000528366. | Immune-related | [43,49,53,61,63,64,66,70] |
| hsa-miR-548a-5p, hsa-miR-1185-2-3p, hsa-miR-30a-3p, hsa-miR-6867-5p, hsa-miR-765 and hsa-miR-3125 | Platelet apoptosis and adhesion | [59] |
| miR-584-5p, miR-142-5p and miR-29b-3p | Plasma-derived exosomes | [65] |
| miR-155–5p, miR-146b–5p, and miR-142–3p CD70 H3K9 DNMT3A, DNMT3B, MBD2 and MBD4 | Tregs Oxidative stress Histone modification Gene regulation | [58] [34] [38] [34,35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, J.H.; Ahmad Azahari, A.H.S.; Ali, A.; Ismail, N.A.S. Scoping Review on Epigenetic Mechanisms in Primary Immune Thrombocytopenia. Genes 2023, 14, 555. https://doi.org/10.3390/genes14030555
Tan JH, Ahmad Azahari AHS, Ali A, Ismail NAS. Scoping Review on Epigenetic Mechanisms in Primary Immune Thrombocytopenia. Genes. 2023; 14(3):555. https://doi.org/10.3390/genes14030555
Chicago/Turabian StyleTan, Jian Hong, Ahmad Hazim Syakir Ahmad Azahari, Adli Ali, and Noor Akmal Shareela Ismail. 2023. "Scoping Review on Epigenetic Mechanisms in Primary Immune Thrombocytopenia" Genes 14, no. 3: 555. https://doi.org/10.3390/genes14030555
APA StyleTan, J. H., Ahmad Azahari, A. H. S., Ali, A., & Ismail, N. A. S. (2023). Scoping Review on Epigenetic Mechanisms in Primary Immune Thrombocytopenia. Genes, 14(3), 555. https://doi.org/10.3390/genes14030555








