The Circulating Level of Klotho Is Not Dependent upon Physical Fitness and Age-Associated Methylation Increases at the Promoter Region of the Klotho Gene
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Tests and Blood Collection
2.3. Physiology Tests
2.4. Klotho ELISA Kit
2.5. DNA Methylation and Promoter Region Methylation of KL
2.6. Determination of Epigenetic Clocks
2.7. Statistical Analysis
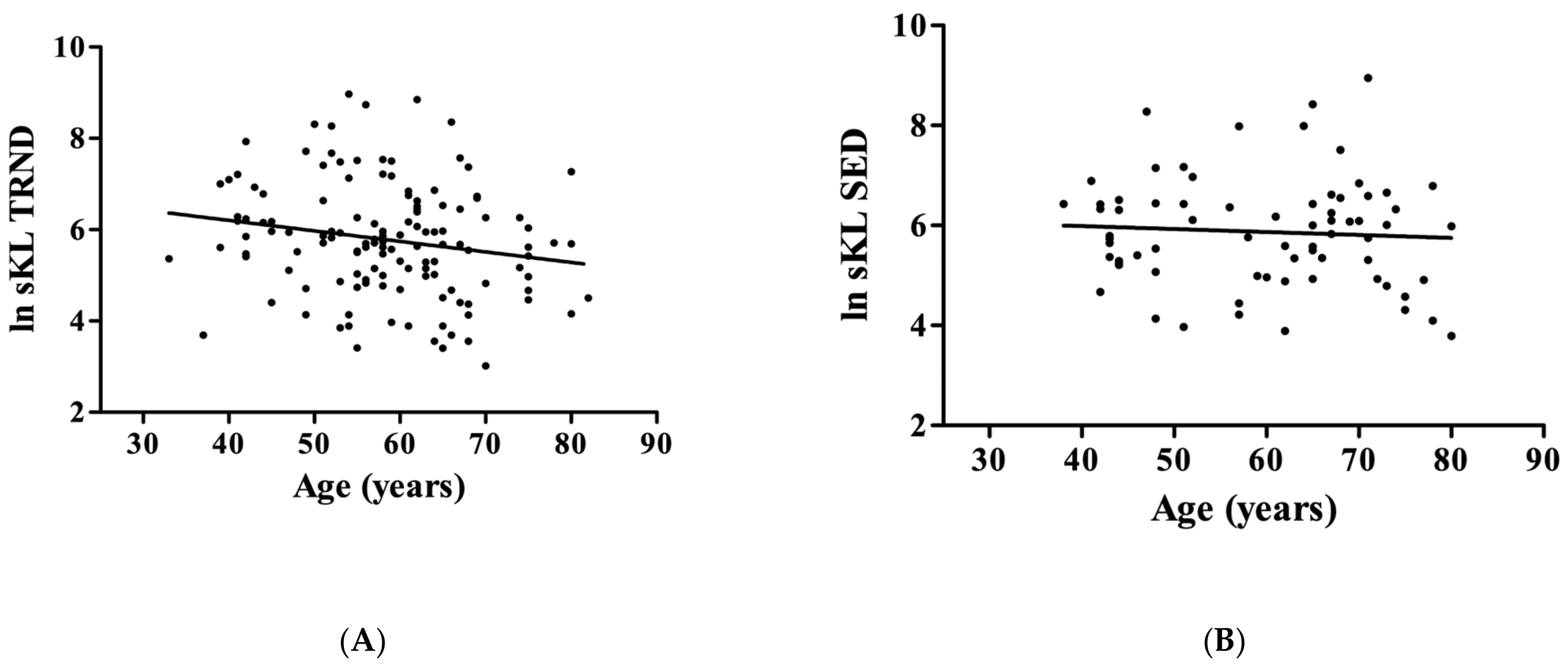
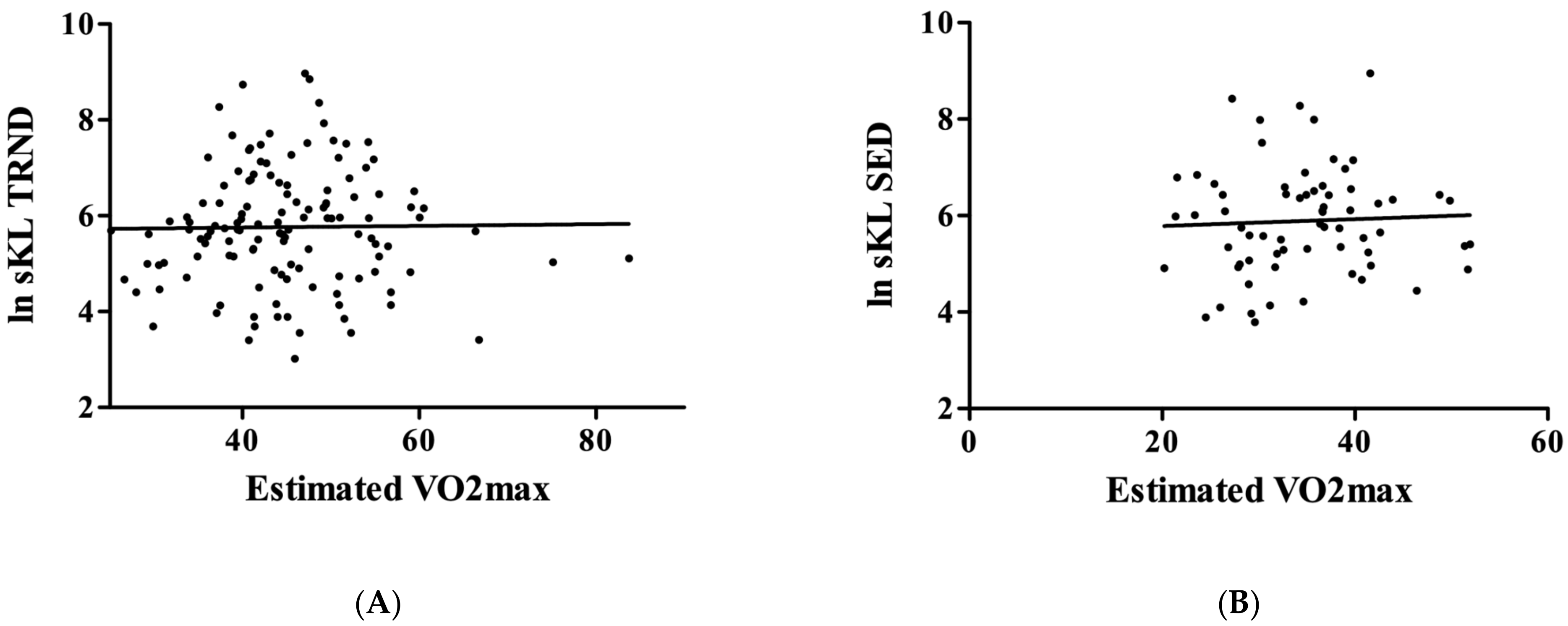
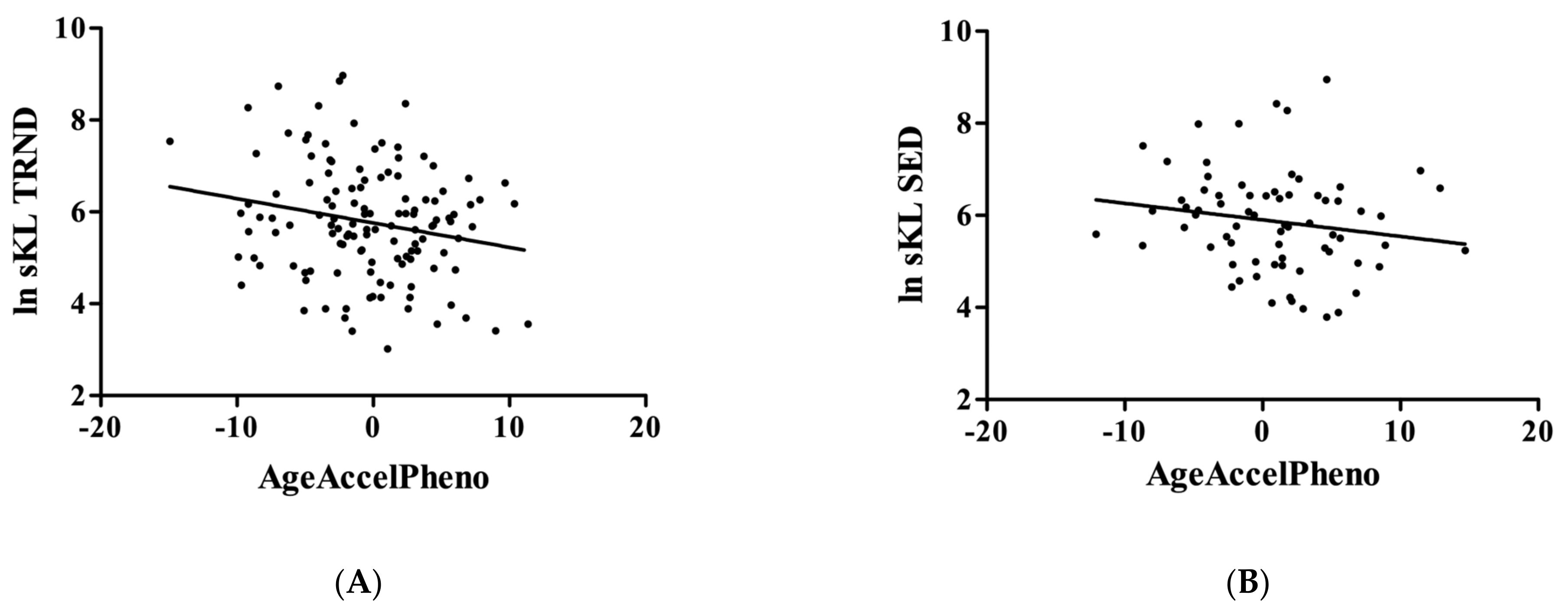
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| adrenomedullin | (ADM) |
| beta-2 microglobulin | (B2M) |
| cystatin C | (Cystatin C) |
| fibroblast growth factor | (FGF) |
| fibroblast growth factor 23 | (FGF23) |
| growth differentiation factor 15 | (GDF-15) |
| leptin | (Leptin) |
| plasminogen activation inhibitor 1 | (PAI-1) |
| insulin-like growth factor 1 | (IGF1) |
| klotho | (KL) |
| natural logarithm of klotho | (ln sKL) |
| sedentary | (SED) |
| tissue inhibitor metalloproteinases 1 | (TIMP-1) |
| soluble KL | (sKL) |
| trained | (TRND) |
References
- Gähwiler, E.K.N.; Motta, S.E.; Martin, M.; Nugraha, B.; Hoerstrup, S.P.; Emmert, M.Y. Human IPSCs and Genome Editing Technologies for Precision Cardiovascular Tissue Engineering. Front. Cell Dev. Biol. 2021, 9, 639699. [Google Scholar] [CrossRef]
- Karagiannis, P.; Takahashi, K.; Saito, M.; Yoshida, Y.; Okita, K.; Watanabe, A.; Inoue, H.; Yamashita, J.K.; Todani, M.; Nakagawa, M.; et al. Induced Pluripotent Stem Cells and Their Use in Human Models of Disease and Development. Physiol. Rev. 2019, 99, 79–114. [Google Scholar] [CrossRef]
- Kuro-o, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; et al. Mutation of the Mouse Klotho Gene Leads to a Syndrome Resembling Ageing. Nature 1997, 390, 45–51. [Google Scholar] [CrossRef]
- Château, M.T.; Araiz, C.; Descamps, S.; Galas, S. Klotho Interferes with a Novel FGF-Signalling Pathway and Insulin/Igf-like Signalling to Improve Longevity and Stress Resistance in Caenorhabditis Elegans. Aging 2010, 2, 567–581. [Google Scholar] [CrossRef]
- Kuro-o, M. Klotho as a Regulator of Oxidative Stress and Senescence. Biol. Chem. 2008, 389, 233–241. [Google Scholar] [CrossRef]
- Min, D.; Panoskaltsis-Mortari, A.; Kuro, O.M.; Holländer, G.A.; Blazar, B.R.; Weinberg, K.I. Sustained Thymopoiesis and Improvement in Functional Immunity Induced by Exogenous KGF Administration in Murine Models of Aging. Blood 2007, 109, 2529–2537. [Google Scholar] [CrossRef]
- Kawaguchi, H.; Manabe, N.; Miyaura, C.; Chikuda, H.; Nakamura, K.; Kuro-o, M. Independent Impairment of Osteoblast and Osteoclast Differentiation in Klotho Mouse Exhibiting Low-Turnover Osteopenia. J. Clin. Investig. 1999, 104, 229–237. [Google Scholar] [CrossRef]
- Sato, A.; Hirai, T.; Imura, A.; Kita, N.; Iwano, A.; Muro, S.; Nabeshima, Y.; Suki, B.; Mishima, M. Morphological Mechanism of the Development of Pulmonary Emphysema in Klotho Mice. Proc. Natl. Acad. Sci. USA 2007, 104, 2361–2365. [Google Scholar] [CrossRef]
- Nagai, T.; Yamada, K.; Kim, H.C.; Kim, Y.S.; Noda, Y.; Imura, A.; Nabeshima, Y.; Nabeshima, T. Cognition Impairment in the Genetic Model of Aging Klotho Gene Mutant Mice: A Role of Oxidative Stress. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2003, 17, 50–52. [Google Scholar] [CrossRef]
- Kamemori, M.; Ohyama, Y.; Kurabayashi, M.; Takahashi, K.; Nagai, R.; Furuya, N. Expression of Klotho Protein in the Inner Ear. Hear Res. 2002, 171, 103–110. [Google Scholar] [CrossRef]
- Kurosu, H.; Yamamoto, M.; Clark, J.D.; Pastor, J.V.; Nandi, A.; Gurnani, P.; McGuinness, O.P.; Chikuda, H.; Yamaguchi, M.; Kawaguchi, H.; et al. Suppression of Aging in Mice by the Hormone Klotho. Science 2005, 309, 1829–1833. [Google Scholar] [CrossRef]
- Semba, R.D.; Cappola, A.R.; Sun, K.; Bandinelli, S.; Dalal, M.; Crasto, C.; Guralnik, J.M.; Ferrucci, L. Relationship of Low Plasma Klotho with Poor Grip Strength in Older Community-Dwelling Adults: The InCHIANTI Study. Eur. J. Appl. Physiol. 2012, 112, 1215–1220. [Google Scholar] [CrossRef]
- Shardell, M.; Drew, D.A.; Semba, R.D.; Harris, T.B.; Cawthon, P.M.; Simonsick, E.M.; Kalyani, R.R.; Schwartz, A.V.; Kritchevsky, S.B.; Newman, A.B. Plasma Soluble AKlotho, Serum Fibroblast Growth Factor 23, and Mobility Disability in Community-Dwelling Older Adults. J. Endocr. Soc. 2020, 4, bvz032. [Google Scholar] [CrossRef]
- Shardell, M.; Semba, R.D.; Kalyani, R.R.; Bandinelli, S.; Prather, A.A.; Chia, C.W.; Ferrucci, L. Plasma Klotho and Frailty in Older Adults: Findings from the InCHIANTI Study. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1052–1057. [Google Scholar] [CrossRef]
- Veronesi, F.; Borsari, V.; Cherubini, A.; Fini, M. Association of Klotho with Physical Performance and Frailty in Middle-Aged and Older Adults: A Systematic Review. Exp. Gerontol. 2021, 154, 111518. [Google Scholar] [CrossRef]
- Prud’homme, G.J.; Kurt, M.; Wang, Q. Pathobiology of the Klotho Antiaging Protein and Therapeutic Considerations. Front. Aging 2022, 3, 931331. [Google Scholar] [CrossRef]
- Shiraki-Iida, T.; Aizawa, H.; Matsumura, Y.; Sekine, S.; Iida, A.; Anazawa, H.; Nagai, R.; Kuro-o, M.; Nabeshima, Y. Structure of the Mouse Klotho Gene and Its Two Transcripts Encoding Membrane and Secreted Protein. FEBS Lett. 1998, 424, 6–10. [Google Scholar] [CrossRef]
- Matsumura, Y.; Aizawa, H.; Shiraki-Iida, T.; Nagai, R.; Kuro-o, M.; Nabeshima, Y. Identification of the Human Klotho Gene and Its Two Transcripts Encoding Membrane and Secreted Klotho Protein. Biochem. Biophys. Res. Commun. 1998, 242, 626–630. [Google Scholar] [CrossRef]
- Kuro-o, M. Klotho. Pflug. Arch. Eur. J. Physiol. 2010, 459, 333–343. [Google Scholar] [CrossRef]
- Kuro-o, M. Klotho and Aging. Biochim. Biophys. Acta 2009, 1790, 1049–1058. [Google Scholar] [CrossRef]
- Torres, P.U.; Prié, D.; Molina-Blétry, V.; Beck, L.; Silve, C.; Friedlander, G. Klotho: An Antiaging Protein Involved in Mineral and Vitamin D Metabolism. Kidney Int. 2007, 71, 730–737. [Google Scholar] [CrossRef]
- Imura, A.; Iwano, A.; Tohyama, O.; Tsuji, Y.; Nozaki, K.; Hashimoto, N.; Fujimori, T.; Nabeshima, Y. Secreted Klotho Protein in Sera and CSF: Implication for Post-Translational Cleavage in Release of Klotho Protein from Cell Membrane. FEBS Lett. 2004, 565, 143–147. [Google Scholar] [CrossRef]
- Wolf, I.; Levanon-Cohen, S.; Bose, S.; Ligumsky, H.; Sredni, B.; Kanety, H.; Kuro-o, M.; Karlan, B.; Kaufman, B.; Koeffler, H.P.; et al. Klotho: A Tumor Suppressor and a Modulator of the IGF-1 and FGF Pathways in Human Breast Cancer. Oncogene 2008, 27, 7094–7105. [Google Scholar] [CrossRef]
- Hu, M.C.; Shi, M.; Zhang, J.; Pastor, J.; Nakatani, T.; Lanske, B.; Razzaque, M.S.; Rosenblatt, K.P.; Baum, M.G.; Kuro-o, M.; et al. Klotho: A Novel Phosphaturic Substance Acting as an Autocrine Enzyme in the Renal Proximal Tubule. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2010, 24, 3438–3450. [Google Scholar] [CrossRef]
- Cha, S.K.; Hu, M.C.; Kurosu, H.; Kuro-o, M.; Moe, O.; Huang, C.L. Regulation of Renal Outer Medullary Potassium Channel and Renal K(+) Excretion by Klotho. Mol. Pharmacol. 2009, 76, 38–46. [Google Scholar] [CrossRef]
- Kim, J.H.; Hwang, K.H.; Park, K.S.; Kong, I.D.; Cha, S.K. Biological Role of Anti-Aging Protein Klotho. J. Lifestyle Med. 2015, 5, 1–6. [Google Scholar] [CrossRef]
- Yamamoto, M.; Clark, J.D.; Pastor, J.V.; Gurnani, P.; Nandi, A.; Kurosu, H.; Miyoshi, M.; Ogawa, Y.; Castrillon, D.H.; Rosenblatt, K.P.; et al. Regulation of Oxidative Stress by the Anti-Aging Hormone Klotho. J. Biol. Chem. 2005, 280, 38029–38034. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, X. Klotho: A Novel Biomarker for Cancer. J. Cancer Res. Clin. Oncol. 2015, 141, 961–969. [Google Scholar] [CrossRef]
- King, G.D.; Rosene, D.L.; Abraham, C.R. Promoter Methylation and Age-Related Downregulation of Klotho in Rhesus Monkey. Age 2012, 34, 1405–1419. [Google Scholar] [CrossRef]
- Lu, A.T.; Quach, A.; Wilson, J.G.; Reiner, A.P.; Aviv, A.; Raj, K.; Hou, L.; Baccarelli, A.A.; Li, Y.; Stewart, J.D.; et al. DNA Methylation GrimAge Strongly Predicts Lifespan and Healthspan. Aging 2019, 11, 303–327. [Google Scholar] [CrossRef]
- Levine, M.E.; Lu, A.T.; Quach, A.; Chen, B.H.; Assimes, T.L.; Bandinelli, S.; Hou, L.; Baccarelli, A.A.; Stewart, J.D.; Li, Y.; et al. An Epigenetic Biomarker of Aging for Lifespan and Healthspan. Aging 2018, 10, 573–591. [Google Scholar] [CrossRef]
- Iturriaga, T.; Yvert, T.; Sanchez-Lorente, I.M.; Diez-Vega, I.; Fernandez-Elias, V.E.; Sanchez-Barroso, L.; Dominguez-Balmaseda, D.; Larrosa, M.; Perez-Ruiz, M.; Santiago, C. Acute Impacts of Different Types of Exercise on Circulating α-Klotho Protein Levels. Front. Physiol. 2021, 12, 716473. [Google Scholar] [CrossRef]
- Mostafidi, E.; Moeen, A.; Nasri, H.; Ghorbani Hagjo, A.; Ardalan, M. Serum Klotho Levels in Trained Athletes. Nephro-Urol. Mon. 2016, 8, e30245. [Google Scholar] [CrossRef]
- Hawkins, S.; Wiswell, R. Rate and Mechanism of Maximal Oxygen Consumption Decline with Aging: Implications for Exercise Training. Sport. Med. 2003, 33, 877–888. [Google Scholar] [CrossRef]
- Carnethon, M.R.; Gulati, M.; Greenland, P. Prevalence and Cardiovascular Disease Correlates of Low Cardiorespiratory Fitness in Adolescents and Adults. JAMA 2005, 294, 2981–2988. [Google Scholar] [CrossRef]
- Izquierdo, M.C.; Lopes, S.; Teixeira, M.; Polónia, J.; Alves, A.J.; Mesquita-Bastos, J.; Ribeiro, F. The Chester Step Test Is a Valid Tool to Assess Cardiorespiratory Fitness in Adults with Hypertension: Reducing the Gap between Clinical Practice and Fitness Assessments. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2019, 42, 2021–2024. [Google Scholar] [CrossRef]
- Min, J.L.; Hemani, G.; Davey Smith, G.; Relton, C.; Suderman, M. Meffil: Efficient Normalization and Analysis of Very Large DNA Methylation Datasets. Bioinformatics 2018, 34, 3983–3989. [Google Scholar] [CrossRef]
- Triche, T.J.J.; Weisenberger, D.J.; Van Den Berg, D.; Laird, P.W.; Siegmund, K.D. Low-Level Processing of Illumina Infinium DNA Methylation BeadArrays. Nucleic Acids Res 2013, 41, e90. [Google Scholar] [CrossRef]
- Horvath, S. DNA Methylation Age of Human Tissues and Cell Types. Genome Biol. 2013, 14, R115. [Google Scholar] [CrossRef]
- Hannum, G.; Guinney, J.; Zhao, L.; Zhang, L.; Hughes, G.; Sadda, S.; Klotzle, B.; Bibikova, M.; Fan, J.-B.; Gao, Y.; et al. Genome-Wide Methylation Profiles Reveal Quantitative Views of Human Aging Rates. Mol. Cell 2013, 49, 359–367. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Z. Current Understanding of Klotho. Ageing Res. Rev. 2009, 8, 43–51. [Google Scholar] [CrossRef]
- Raj, K.; Horvath, S. Current Perspectives on the Cellular and Molecular Features of Epigenetic Ageing. Exp. Biol. Med. 2020, 245, 1532–1542. [Google Scholar] [CrossRef]
- Oblak, L.; van der Zaag, J.; Higgins-Chen, A.T.; Levine, M.E.; Boks, M.P. A Systematic Review of Biological, Social and Environmental Factors Associated with Epigenetic Clock Acceleration. Ageing Res. Rev. 2021, 69, 101348. [Google Scholar] [CrossRef]
- Seki, Y.; Aczel, D.; Torma, F.; Jokai, M.; Boros, A.; Suzuki, K.; Higuchi, M.; Tanisawa, K.; Boldogh, I.; Horvath, S.; et al. No Strong Association among Epigenetic Modifications by DNA Methylation, Telomere Length, and Physical Fitness in Biological Aging. Biogerontology 2023. [Google Scholar] [CrossRef]
- Ramsey, K.A.; Rojer, A.G.M.; D’Andrea, L.; Otten, R.H.J.; Heymans, M.W.; Trappenburg, M.C.; Verlaan, S.; Whittaker, A.C.; Meskers, C.G.M.; Maier, A.B. The Association of Objectively Measured Physical Activity and Sedentary Behavior with Skeletal Muscle Strength and Muscle Power in Older Adults: A Systematic Review and Meta-Analysis. Ageing Res. Rev. 2021, 67, 101266. [Google Scholar] [CrossRef]
- Batsis, J.A.; Villareal, D.T. Sarcopenic Obesity in Older Adults: Aetiology, Epidemiology and Treatment Strategies. Nat. Rev. Endocrinol. 2018, 14, 513–537. [Google Scholar] [CrossRef]
- Wullems, J.A.; Verschueren, S.M.; Degens, H.; Morse, C.I.; Onambélé, G.L. A Review of the Assessment and Prevalence of Sedentarism in Older Adults, Its Physiology/Health Impact and Non-Exercise Mobility Counter-Measures. Biogerontology 2016, 17, 547–565. [Google Scholar] [CrossRef]
- Martín-Núñez, E.; Pérez-Castro, A.; Tagua, V.G.; Hernández-Carballo, C.; Ferri, C.; Pérez-Delgado, N.; Rodríguez-Ramos, S.; Cerro-López, P.; López-Castillo, Á.; Delgado-Molinos, A.; et al. Klotho Expression in Peripheral Blood Circulating Cells Is Associated with Vascular and Systemic Inflammation in Atherosclerotic Vascular Disease. Sci. Rep. 2022, 12, 8422. [Google Scholar] [CrossRef]
- Huemer, M.-T.; Kluttig, A.; Fischer, B.; Ahrens, W.; Castell, S.; Ebert, N.; Gastell, S.; Jöckel, K.-H.; Kaaks, R.; Karch, A.; et al. Grip Strength Values and Cut-off Points Based on over 200,000 Adults of the German National Cohort—A Comparison to the EWGSOP2 Cut-off Points. Age Ageing 2023, 52, afac324. [Google Scholar] [CrossRef]
- Semba, R.D.; Ferrucci, L.; Sun, K.; Simonsick, E.; Turner, R.; Miljkovic, I.; Harris, T.; Schwartz, A.V.; Asao, K.; Kritchevsky, S.; et al. Low Plasma Klotho Concentrations and Decline of Knee Strength in Older Adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016, 71, 103–108. [Google Scholar] [CrossRef]
- Patel, M.S.; Donaldson, A.V.; Lewis, A.; Natanek, S.A.; Lee, J.Y.; Andersson, Y.M.; Haji, G.; Jackson, S.G.; Bolognese, B.J.; Foley, J.P.; et al. Klotho and Smoking--An Interplay Influencing the Skeletal Muscle Function Deficits That Occur in COPD. Respir. Med. 2016, 113, 50–56. [Google Scholar] [CrossRef]
- Adema, A.Y.; de Roij van Zuijdewijn, C.L.M.; Hoenderop, J.G.; de Borst, M.H.; Ter Wee, P.M.; Heijboer, A.C.; Vervloet, M.G. Influence of Exogenous Growth Hormone Administration on Circulating Concentrations of α-Klotho in Healthy and Chronic Kidney Disease Subjects: A Prospective, Single-Center Open Case-Control Pilot Study. BMC Nephrol. 2018, 19, 327. [Google Scholar] [CrossRef]
- Guarnotta, V.; Pizzolanti, G.; Petrancosta, R.; Radellini, S.; Baiamonte, C.; Giordano, C. Gender-Specific Soluble α-Klotho Levels as Marker of GH Deficiency in Children: A Case-Control Study. J. Endocrinol. Investig. 2022, 45, 1247–1254. [Google Scholar] [CrossRef]
- Sato, T.; Komaba, H.; Nagatani, T.; Watanabe, T.; Kishida, Y.; Fukagawa, M. The Pituitary Is a Candidate Organ That Modulates Circulating Klotho Levels. J. Endocr. Soc. 2019, 3, 52–61. [Google Scholar] [CrossRef]
- Drew, D.A.; Katz, R.; Kritchevsky, S.; Ix, J.H.; Shlipak, M.G.; Newman, A.B.; Hoofnagle, A.N.; Fried, L.F.; Sarnak, M.; Gutiérrez, O.M.; et al. Soluble Klotho and Incident Hypertension. Clin. J. Am. Soc. Nephrol. CJASN 2021, 16, 1502–1511. [Google Scholar] [CrossRef]
- Donate-Correa, J.; Ferri, C.M.; Martín-Núñez, E.; Pérez-Delgado, N.; González-Luis, A.; Mora-Fernández, C.; Navarro-González, J.F. Klotho as a Biomarker of Subclinical Atherosclerosis in Patients with Moderate to Severe Chronic Kidney Disease. Sci. Rep. 2021, 11, 15877. [Google Scholar] [CrossRef]
- Buendia-Roldan, I.; Machuca, N.; Mejía, M.; Maldonado, M.; Pardo, A.; Selman, M. Lower Levels of α-Klotho in Serum Are Associated with Decreased Lung Function in Individuals with Interstitial Lung Abnormalities. Sci. Rep. 2019, 9, 10801. [Google Scholar] [CrossRef]
- Corcillo, A.; Fountoulakis, N.; Sohal, A.; Farrow, F.; Ayis, S.; Karalliedde, J. Low Levels of Circulating Anti-Ageing Hormone Klotho Predict the Onset and Progression of Diabetic Retinopathy. Diabetes Vasc. Dis. Res. 2020, 17, 1479164120970901. [Google Scholar] [CrossRef]
- Paroni, G.; Panza, F.; De Cosmo, S.; Greco, A.; Seripa, D.; Mazzoccoli, G. Klotho at the Edge of Alzheimer’s Disease and Senile Depression. Mol. Neurobiol. 2019, 56, 1908–1920. [Google Scholar] [CrossRef]
- Shmulevich, R.; Nissim, T.B.; Wolf, I.; Merenbakh-Lamin, K.; Fishman, D.; Sekler, I.; Rubinek, T. Klotho Rewires Cellular Metabolism of Breast Cancer Cells through Alteration of Calcium Shuttling and Mitochondrial Activity. Oncogene 2020, 39, 4636–4649. [Google Scholar] [CrossRef]
- Aczel, D.; Gyorgy, B.; Bakonyi, P.; BukhAri, R.; Pinho, R.; Boldogh, I.; Yaodong, G.; Radak, Z. The Systemic Effects of Exercise on the Systemic Effects of Alzheimer’s Disease. Antioxidants 2022, 11, 1028. [Google Scholar] [CrossRef]
- Radak, Z.; Suzuki, K.; Posa, A.; Petrovszky, Z.; Koltai, E.; Boldogh, I. The Systemic Role of SIRT1 in Exercise Mediated Adaptation. Redox Biol. 2020, 35, 101467. [Google Scholar] [CrossRef]
- Radak, Z.; Torma, F.; Berkes, I.; Goto, S.; Mimura, T.; Posa, A.; Balogh, L.; Boldogh, I.; Suzuki, K.; Higuchi, M.; et al. Exercise Effects on Physiological Function during Aging. Free Radic. Biol. Med. 2019, 132, 33–41. [Google Scholar] [CrossRef]
- Radak, Z.; Chung, H.Y.; Goto, S. Systemic Adaptation to Oxidative Challenge Induced by Regular Exercise. Free Radic. Biol. Med. 2008, 44, 153–159. [Google Scholar] [CrossRef]
- Arrianti, E.E.; Natalia, F.; Ayu, M.; Afida, K.L.; Shine, L.M.; Wahyuni, N.; I Adiatmika, P.G. The Aerobic Exercise Increases Cognitive Function Biomarker, Klotho Protein, in Alzheimer Disease. Sport Fit. J. 2022, 10, 111–123. [Google Scholar]
- Rosa, T.S.; Neves, R.V.P.; Deus, L.A.; Sousa, C.V.; da Silva Aguiar, S.; de Souza, M.K.; Moraes, M.R.; Rosa, É.C.C.C.; Andrade, R.V.; Korhonen, M.T.; et al. Sprint and Endurance Training in Relation to Redox Balance, Inflammatory Status and Biomarkers of Aging in Master Athletes. Nitric Oxide 2020, 102, 42–51. [Google Scholar] [CrossRef]
- Rosa, T.D.S.; Corrêa, H.L.; Barbosa, L.P.; Santos, P.A.D.; de Araújo Leite, P.L.; Aguiar, S.S.; Deus, L.A.; Maciel, L.A.; Neves, R.V.P.; Simoes, H.G. Age-Related Decline in Renal Function Is Attenuated in Master Athletes. Int. J. Sport. Med. 2021, 42, 889–895. [Google Scholar] [CrossRef]


| Klotho (n = 202) | TRND | SED | ||
|---|---|---|---|---|
| Number of patients | 131 | 71 | ||
| Men | Women | Men | Women | |
| 80 | 51 | 27 | 44 | |
| Age (years), Mean ± SD | 59.14 (±10.8) | 57.24 (±9.4) | 55.63 (±13.4) | 61.91 (±10.5) |
| VO2 max (mL/kg/min) | 42.27 (±9.2) | 41.46 (±8.4) | 38.25 (±7.9) | 32.55 (±6.6) |
| Serum Klotho (pg/mL) | 5.92 (±0.09) | 5.39 (±0.15) | 6.13 (±0,23) | 5.71 (±0.15) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aczel, D.; Torma, F.; Jokai, M.; McGreevy, K.; Boros, A.; Seki, Y.; Boldogh, I.; Horvath, S.; Radak, Z. The Circulating Level of Klotho Is Not Dependent upon Physical Fitness and Age-Associated Methylation Increases at the Promoter Region of the Klotho Gene. Genes 2023, 14, 525. https://doi.org/10.3390/genes14020525
Aczel D, Torma F, Jokai M, McGreevy K, Boros A, Seki Y, Boldogh I, Horvath S, Radak Z. The Circulating Level of Klotho Is Not Dependent upon Physical Fitness and Age-Associated Methylation Increases at the Promoter Region of the Klotho Gene. Genes. 2023; 14(2):525. https://doi.org/10.3390/genes14020525
Chicago/Turabian StyleAczel, Dora, Ferenc Torma, Matyas Jokai, Kristen McGreevy, Anita Boros, Yasuhiro Seki, Istvan Boldogh, Steve Horvath, and Zsolt Radak. 2023. "The Circulating Level of Klotho Is Not Dependent upon Physical Fitness and Age-Associated Methylation Increases at the Promoter Region of the Klotho Gene" Genes 14, no. 2: 525. https://doi.org/10.3390/genes14020525
APA StyleAczel, D., Torma, F., Jokai, M., McGreevy, K., Boros, A., Seki, Y., Boldogh, I., Horvath, S., & Radak, Z. (2023). The Circulating Level of Klotho Is Not Dependent upon Physical Fitness and Age-Associated Methylation Increases at the Promoter Region of the Klotho Gene. Genes, 14(2), 525. https://doi.org/10.3390/genes14020525







