Impact of Advanced Paternal Age on Fertility and Risks of Genetic Disorders in Offspring
Abstract
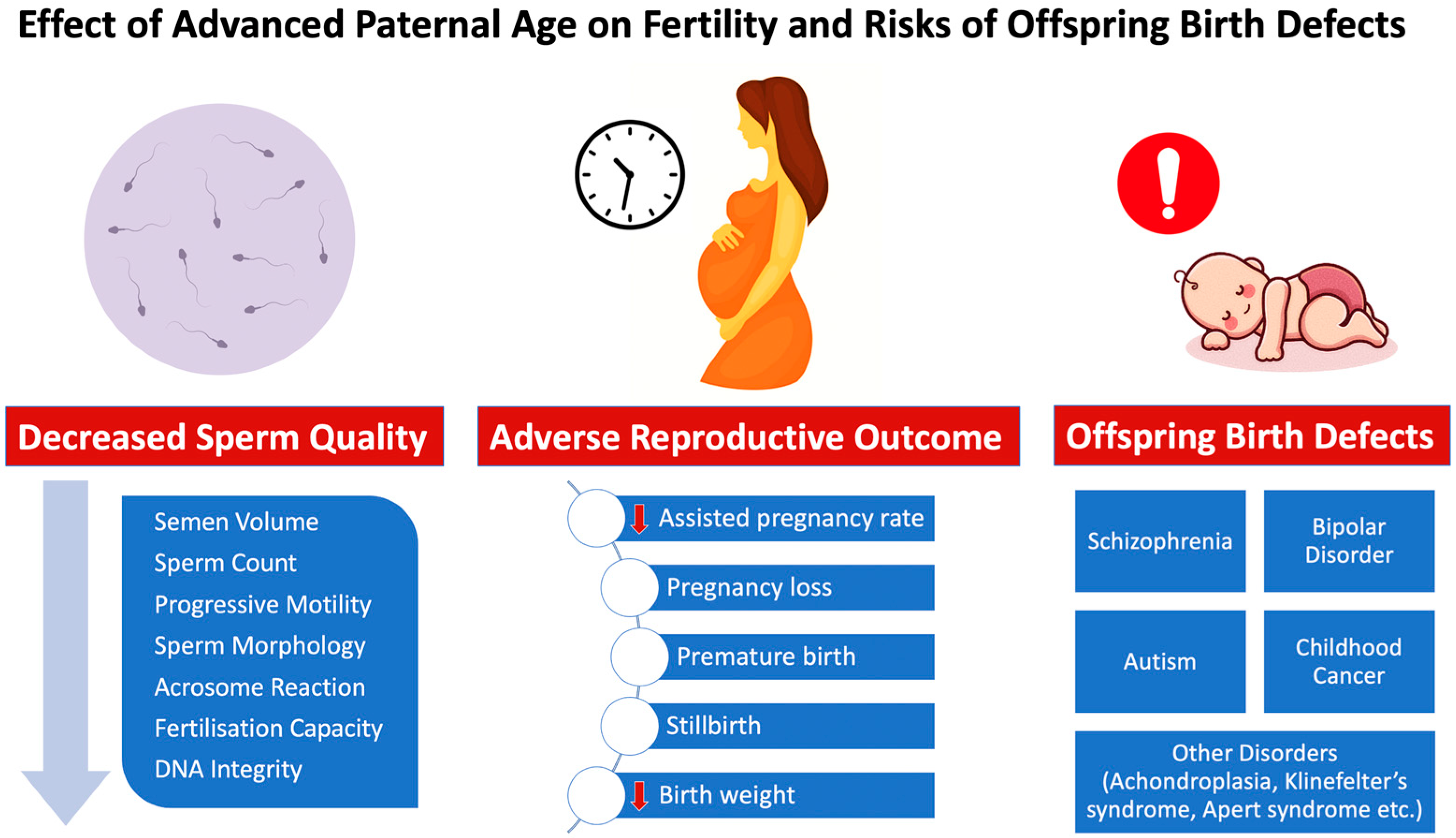
1. Introduction
2. Advanced Paternal Age Effects on Testicular Function and Sperm Quality
2.1. Reproduction and Male Hormones: The Testes’ Crucial Role
2.2. Parameters of Semen Analysis
2.3. At What Age Do the Fertility Levels Start to Drop?
3. Effects of Advanced Paternal Age on Sperm Genetic and Epigenetic Changes
3.1. Sperm DNA Damage
3.2. Telomere Analysis
3.3. Centrosome Aberrations
3.4. Male Gamete Nucleus DNA Mutations
3.5. Chromosomal Abnormalities
3.6. Instability in Molecular Structure and Aeging-Related Reduction in Gene Function
3.7. Epigenetics Alterations during Male Ageing
3.8. Wide-Scale Genome Investigations
4. Advanced Paternal Age Effect on Reproductive and Fertility Outcome
4.1. Decreased Pregnancy Rate in Assisted Reproductive Technology
4.2. Pregnancy Loss
4.3. Premature Birth
4.4. Low Birth Weight
4.5. Stillbirth
5. Advanced Paternal Age Effect on the Offspring
5.1. Schizophrenia
5.2. Bipolar Disorder
5.3. Autism
5.4. Childhood Cancer
5.5. Other Disorders
6. Conclusions
7. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cedars, M.I. Introduction: Childhood implications of parental aging. Fertil. Steril. 2015, 103, 1379–1380. [Google Scholar] [CrossRef] [PubMed]
- Szamatowicz, M. Assisted reproductive technology in reproductive medicine—Possibilities and limitations. Ginekol. Polska 2016, 87, 820–823. [Google Scholar] [CrossRef] [PubMed]
- Handelsman, D.J.; Staraj, S. Testicular Size: The Effects of Aging, Malnutrition, and Illness. J. Androl. 1985, 6, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Feldman, H.A.; Longcope, C.; Derby, C.A.; Johannes, C.B.; Araujo, A.B.; Coviello, A.D.; Bremner, W.J.; McKinlay, J.B. Age Trends in the Level of Serum Testosterone and Other Hormones in Middle-Aged Men: Longitudinal Results from the Massachusetts Male Aging Study. J. Clin. Endocrinol. Metab. 2002, 87, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Brahem, S.; Mehdi, M.; Elghezal, H.; Saad, A. The effects of male aging on semen quality, sperm DNA fragmentation and chromosomal abnormalities in an infertile population. J. Assist. Reprod. Genet. 2011, 28, 425–432. [Google Scholar] [CrossRef]
- Moskovtsev, S.I.; Jarvi, K.; Mullen, J.B.M.; Cadesky, K.I.; Hannam, T.; Lo, K.C. Testicular spermatozoa have statistically significantly lower DNA damage compared with ejaculated spermatozoa in patients with unsuccessful oral antioxidant treatment. Fertil. Steril. 2010, 93, 1142–1146. [Google Scholar] [CrossRef]
- Agarwal, A.; Majzoub, A.; Esteves, S.C.; Ko, E.; Ramasamy, R.; Zini, A. Clinical utility of sperm DNA fragmentation testing: Practice recommendations based on clinical scenarios. Transl. Androl. Urol. 2016, 5, 935–950. [Google Scholar] [CrossRef]
- Broer, L.; Codd, V.; Nyholt, D.R.; Deelen, J.; Mangino, M.; Willemsen, G.; Albrecht, E.; Amin, N.; Beekman, M.; de Geus, E.J.; et al. Meta-analysis of telomere length in 19,713 subjects reveals high heritability, stronger maternal inheritance and a paternal age effect. Eur. J. Hum. Genet. 2013, 21, 1163–1168. [Google Scholar] [CrossRef]
- Crow, J.F. The origins, patterns and implications of human spontaneous mutation. Nat. Rev. Genet. 2000, 1, 40–47. [Google Scholar] [CrossRef]
- Curley, J.P.; Mashoodh, R.; Champagne, F.A. Epigenetics and the origins of paternal effects. Horm. Behav. 2011, 59, 306–314. [Google Scholar] [CrossRef]
- Lian, Z.H.; Zack, M.M.; Erickson, J.D. Paternal age and the occurrence of birth defects. Am. J. Hum. Genet. 1986, 39, 648–660. [Google Scholar]
- Orioli, I.M.; Castilla, E.E.; Scarano, G.; Mastroiacovo, P. Effect of paternal age in achondroplasia, thanatophoric dysplasia, and osteogenesis imperfecta. Am. J. Med. Genet. 1995, 59, 209–217. [Google Scholar] [CrossRef]
- D’Onofrio, B.M.; Rickert, M.E.; Frans, E.; Kuja-Halkola, R.; Almqvist, C.; Sjölander, A.; Larsson, H.; Lichtenstein, P. Paternal Age at Childbearing and Offspring Psychiatric and Academic Morbidity. JAMA Psychiatry 2014, 71, 432–438. [Google Scholar] [CrossRef]
- Neaves, W.B.; Johnson, L.; Porter, J.C.; Parker, C.R.; Petty, C.S. Leydig Cell Numbers, Daily Sperm Production, and Serum Gonadotropin Levels in Aging Men*. J. Clin. Endocrinol. Metab. 1984, 59, 756–763. [Google Scholar] [CrossRef]
- Johnson, L.; Abdo, J.G.; Petty, C.S.; Neaves, W.B. Effect of age on the composition of seminiferous tubular boundary tissue and on the volume of each component in humans. Fertil. Steril. 1988, 49, 1045–1051. [Google Scholar] [CrossRef]
- Plas, E. Effects of aging on male fertility? Exp. Gerontol. 2000, 35, 543–551. [Google Scholar] [CrossRef]
- Gunes, S.; Hekim, G.N.T.; Arslan, M.A.; Asci, R. Effects of aging on the male reproductive system. J. Assist. Reprod. Genet. 2016, 33, 441–454. [Google Scholar] [CrossRef]
- Nie, X.; Munyoki, S.K.; Sukhwani, M.; Schmid, N.; Missel, A.; Emery, B.R.; Connect, D.; Stukenborg, J.-B.; Mayerhofer, A.; Orwig, K.E.; et al. Single-cell analysis of human testis aging and correlation with elevated body mass index. Dev. Cell 2022, 57, 1160–1176.e5. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Goemaere, S.; El-Garem, Y.; Van Pottelbergh, I.; Comhaire, F.H.; Kaufman, J.M. Testicular Volume in Relation to Hormonal Indices of Gonadal Function in Community-Dwelling Elderly Men. J. Clin. Endocrinol. Metab. 2003, 88, 179–184. [Google Scholar] [CrossRef]
- Dimitriadis, F.; Tsiampali, C.; Chaliasos, N.; Tsounapi, P.; Takenaka, A.; Sofikitis, N. The Sertoli cell as the orchestra conductor of spermatogenesis: Spermatogenic cells dance to the tune of testosterone. Hormones 2015, 14, 479–503. [Google Scholar] [CrossRef]
- Sasano, N.; Ichijo, S. Vascular Patterns of the Human Testis with Special Reference to Its Senile Changes. Tohoku J. Exp. Med. 1969, 99, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; Grumbles, J.S.; Bagheri, A.; Petty, C.S. Increased Germ Cell Degeneration during Postprophase of Meiosis is Related to Increased Serum Follicle-Stimulating Hormone Concentrations and Reduced Daily Sperm Production in Aged Men1. Biol. Reprod. 1990, 42, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.M.; T’Sjoen, G. The effects of testosterone deficiency on male sexual function. Aging Male 2002, 5, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Handelsman, D.J. Male Reproductive Ageing: Human Fertility, Androgens and Hormone Dependent Disease. Novartis Found Symp. 2002, 242, 66–77; discussion 77–81. [Google Scholar] [CrossRef]
- Mirone, V.; Ricci, E.; Gentile, V.; Fasolo, C.B.; Parazzini, F. Determinants of Erectile Dysfunction Risk in a Large Series of Italian Men Attending Andrology Clinics. Eur. Urol. 2004, 45, 87–91. [Google Scholar] [CrossRef]
- Mastrogiacomo, I.; Feghali, G.; Foresta, C.; Ruzza, G. Andropause: Incidence and Pathogenesis. Arch. Androl. 1982, 9, 293–296. [Google Scholar] [CrossRef]
- Gray, A.; Feldman, H.A.; McKinlay, J.B.; Longcope, C. Age, Disease, and Changing Sex Hormone Levels in Middle-Aged Men: Results of the Massachusetts Male Aging Study*. J. Clin. Endocrinol. Metab. 1991, 73, 1016–1025. [Google Scholar] [CrossRef]
- Wu, F.C.W.; Tajar, A.; Pye, S.R.; Silman, A.J.; Finn, J.D.; O’Neill, T.W.; Bartfai, G.; Casanueva, F.; Forti, G.; Giwercman, A.; et al. Hypothalamic-Pituitary-Testicular Axis Disruptions in Older Men Are Differentially Linked to Age and Modifiable Risk Factors: The European Male Aging Study. J. Clin. Endocrinol. Metab. 2008, 93, 2737–2745. [Google Scholar] [CrossRef]
- Dong, S.; Chen, C.; Zhang, J.; Gao, Y.; Zeng, X.; Zhang, X. Testicular aging, male fertility and beyond. Front. Endocrinol. 2022, 13, 1012119. [Google Scholar] [CrossRef]
- World Health Organziation. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; World Health Organization: Geneva, Switzerland, 2021.
- Agarwal, A.; Sekhon, L.H. Oxidative stress and antioxidants for idiopathic oligoasthenoteratospermia: Is it justified? Indian J. Urol. 2011, 27, 74–85. [Google Scholar] [CrossRef]
- Schlegel, P.N.; Sigman, M.; Collura, B.; De Jonge, C.J.; Eisenberg, M.L.; Lamb, D.J.; Mulhall, J.P.; Niederberger, C.; Sandlow, J.I.; Sokol, R.Z.; et al. Diagnosis and Treatment of Infertility in Men: AUA/ASRM Guideline Part I. J. Urol. 2021, 205, 36–43. [Google Scholar] [CrossRef]
- Salonia, A.; Bettocchi, C.; Carvalho, J.; Corona, G.; Jones, T.H.; Kadioğlu, A.; Martinez-Salamanca, J.I.; Minhas, S.; Serefoğlu, E.C.; Verze, P.; et al. EAU Guidelines on Sexual and Reproductive Health. Available online: https://uroweb.org/guideline/sexual-and-reproductive-health/ (accessed on 9 January 2023).
- Kidd, S.A.; Eskenazi, B.; Wyrobek, A.J. Effects of male age on semen quality and fertility: A review of the literature. Fertil. Steril. 2001, 75, 237–248. [Google Scholar] [CrossRef]
- Hossain, M.M.; Fatima, P.; Rahman, D.; Hossain, H.B. Semen parameters at different age groups of male partners of infertile couples. Mymensingh Med. J. 2012, 21, 306–315. [Google Scholar]
- Mukhopadhyay, D.; Varghese, A.C.; Pal, M.; Banerjee, S.K.; Bhattacharyya, A.K.; Sharma, R.; Agarwal, A. Semen quality and age-specific changes: A study between two decades on 3729 male partners of couples with normal sperm count and attending an andrology laboratory for infertility-related problems in an Indian city. Fertil. Steril. 2010, 93, 2247–2254. [Google Scholar] [CrossRef]
- Stone, B.A.; Alex, A.; Werlin, L.B.; Marrs, R.P. Age thresholds for changes in semen parameters in men. Fertil. Steril. 2013, 100, 952–958. [Google Scholar] [CrossRef]
- Johnson, S.L.; Dunleavy, J.; Gemmell, N.J.; Nakagawa, S. Consistent age-dependent declines in human semen quality: A systematic review and meta-analysis. Ageing Res. Rev. 2015, 19, 22–33. [Google Scholar] [CrossRef]
- Li, Y.; Lin, H.; Li, Y.; Cao, J. Association between socio-psycho-behavioral factors and male semen quality: Systematic review and meta-analyses. Fertil. Steril. 2011, 95, 116–123. [Google Scholar] [CrossRef]
- Molina, R.I.; Martini, A.C.; Tissera, A.; Olmedo, J.; Senestrari, D.; De Cuneo, M.F.; Ruiz, R.D. Semen quality and aging: Analysis of 9.168 samples in Cordoba. Argentina. Arch. Esp. Urol. 2010, 63, 214–222. [Google Scholar]
- Eskenazi, B.; Wyrobek, A.; Sloter, E.; Kidd, S.; Moore, L.; Young, S.; Moore, D. The association of age and semen quality in healthy men. Hum. Reprod. 2003, 18, 447–454. [Google Scholar] [CrossRef]
- Levitas, E.; Lunenfeld, E.; Weisz, N.; Friger, M.; Potashnik, G. Relationship between age and semen parameters in men with normal sperm concentration: Analysis of 6022 semen samples. Andrologia 2007, 39, 45–50. [Google Scholar] [CrossRef]
- Zhu, Q.-X.; Meads, C.; Lu, M.-L.; Wu, J.-Q.; Zhou, W.-J.; Gao, E.-S. Turning point of age for semen quality: A population-based study in Chinese men. Fertil. Steril. 2011, 96, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Marcon, L.; Boissonneault, G. Transient DNA Strand Breaks during Mouse and Human Spermiogenesis:New Insights in Stage Specificity and Link to Chromatin Remodeling1. Biol. Reprod. 2004, 70, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Sloter, E.; Schmid, T.; Marchetti, F.; Eskenazi, B.; Nath, J.; Wyrobek, A. Quantitative effects of male age on sperm motion. Hum. Reprod. 2006, 21, 2868–2875. [Google Scholar] [CrossRef] [PubMed]
- Guzick, D.S.; Overstreet, J.W.; Factor-Litvak, P.; Brazil, C.K.; Nakajima, S.T.; Coutifaris, C.; Carson, S.A.; Cisneros, P.; Steinkampf, M.P.; Hill, J.A.; et al. Sperm Morphology, Motility, and Concentration in Fertile and Infertile Men. N. Engl. J. Med. 2001, 345, 1388–1393. [Google Scholar] [CrossRef]
- Keel, B.A. Within- and between-subject variation in semen parameters in infertile men and normal semen donors. Fertil. Steril. 2006, 85, 128–134. [Google Scholar] [CrossRef]
- Demirkol, M.K.; Barut, O.; Dogan, N.T.; Hamarat, M.B.; Resim, S. At What Age Threshold does the Decline in Semen Parameters Begin? J. Coll. Physicians Surg. Pak. 2021, 31, 4–7. [Google Scholar] [CrossRef]
- Carrell, D.T.; Liu, L. Altered protamine 2 expression is uncommon in donors of known fertility, but common among men with poor fertilizing capacity, and may reflect other abnormalities of spermiogenesis. J. Androl. 2001, 22, 604–610. [Google Scholar]
- Aoki, V.W.; Moskovtsev, S.I.; Willis, J.; Liu, L.; Mullen, J.B.M.; Carrell, D.T. DNA Integrity Is Compromised in Protamine-Deficient Human Sperm. J. Androl. 2005, 26, 741–748. [Google Scholar] [CrossRef]
- Aoki, V.W.; Emery, B.R.; Liu, L.; Carrell, D.T. Protamine Levels Vary Between Individual Sperm Cells of Infertile Human Males and Correlate with Viability and DNA Integrity. J. Androl. 2006, 27, 890–898. [Google Scholar] [CrossRef]
- Oliva, R. Protamines and male infertility. Hum. Reprod. Updat. 2006, 12, 417–435. [Google Scholar] [CrossRef]
- Carrell, D.T.; Emery, B.R.; Hammoud, S. The aetiology of sperm protamine abnormalities and their potential impact on the sperm epigenome. Int. J. Androl. 2008, 31, 537–545. [Google Scholar] [CrossRef]
- Erenpreiss, J.; Spano, M.; Bungum, M.; Giwercman, A. Sperm chromatin structure and male fertility: Biological and clinical aspects. Asian J. Androl. 2006, 8, 11–29. [Google Scholar] [CrossRef]
- Zhang, X.; Gabriel, M.S.; Zini, A. Sperm Nuclear Histone to Protamine Ratio in Fertile and Infertile Men: Evidence of Heterogeneous Subpopulations of Spermatozoa in the Ejaculate. J. Androl. 2006, 27, 414–420. [Google Scholar] [CrossRef]
- Agarwal, A. Role of sperm chromatin abnormalities and DNA damage in male infertility. Hum. Reprod. Updat. 2003, 9, 331–345. [Google Scholar] [CrossRef]
- Sharma, R.; Said, T.; Agarwal, A. Sperm DNA damage and its clinical relevance in assessing reproductive outcome. Asian J. Androl. 2004, 6, 139–148. [Google Scholar]
- Moustafa, M.H.; Sharma, R.; Thornton, J.; Mascha, E.; Abdel-Hafez, M.A.; Thomas, A.J.; Agarwal, A. Relationship between ROS production, apoptosis and DNA denaturation in spermatozoa from patients examined for infertility. Hum. Reprod. 2004, 19, 129–138. [Google Scholar] [CrossRef]
- Evenson, D.P.; Larson, K.L.; Jost, L.K. Sperm Chromatin Structure Assay: Its Clinical Use for Detecting Sperm DNA Fragmentation in Male Infertility and Comparisons with Other Techniques. J. Androl. 2002, 23, 25–43. [Google Scholar] [CrossRef]
- Evenson, D.; Wixon, R. Meta-analysis of sperm DNA fragmentation using the sperm chromatin structure assay. Reprod. Biomed. Online 2006, 12, 466–472. [Google Scholar] [CrossRef]
- Sharma, R.; Sabanegh, E.; Mahfouz, R.Z.; Gupta, S.; Thiyagarajan, A.; Agarwal, A. TUNEL as a Test for Sperm DNA Damage in the Evaluation of Male Infertility. Urology 2010, 76, 1380–1386. [Google Scholar] [CrossRef]
- Wolf, B.B.; Schuler, M.; Echeverri, F.; Green, D.R. Caspase-3 Is the Primary Activator of Apoptotic DNA Fragmentation via DNA Fragmentation Factor-45/Inhibitor of Caspase-activated DNase Inactivation. J. Biol. Chem. 1999, 274, 30651–30656. [Google Scholar] [CrossRef]
- Baccetti, B.; Collodel, G.; Piomboni, P. Apoptosis in human ejaculated sperm cells (notulae seminologicae 9). J. Submicrosc. Cytol. Pathol. 1996, 28, 587–596. [Google Scholar] [PubMed]
- Sakkas, D.; Mariethoz, E.; Manicardi, G.C.; Bizzaro, D.; Bianchi, P.G.; Bianchi, U. Origin of DNA damage in ejaculated human spermatozoa. Rev. Reprod. 1999, 4, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, D.; Moffatt, O.; Manicardi, G.C.; Mariethoz, E.; Tarozzi, N.; Bizzaro, D. Nature of DNA Damage in Ejaculated Human Spermatozoa and the Possible Involvement of Apoptosis1. Biol. Reprod. 2002, 66, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, D.; Seli, E.; Bizzaro, D.; Tarozzi, N.; Manicardi, G.C. Abnormal spermatozoa in the ejaculate: Abortive apoptosis and faulty nuclear remodelling during spermatogenesis. Reprod. Biomed. Online 2003, 7, 428–432. [Google Scholar] [CrossRef] [PubMed]
- El-Domyati, M.M.; Al-Din, A.-B.M.; Barakat, M.T.; El-Fakahany, H.M.; Xu, J.; Sakkas, D. Deoxyribonucleic acid repair and apoptosis in testicular germ cells of aging fertile men: The role of the poly(adenosine diphosphate-ribosyl)ation pathway. Fertil. Steril. 2009, 91, 2221–2229. [Google Scholar] [CrossRef]
- Moskovtsev, S.I.; Alladin, N.; Lo, K.C.; Jarvi, K.; Mullen, J.B.M.; Librach, C.L. A comparison of ejaculated and testicular spermatozoa aneuploidy rates in patients with high sperm DNA damage. Syst. Biol. Reprod. Med. 2012, 58, 142–148. [Google Scholar] [CrossRef]
- Brahem, S.; Mehdi, M.; Elghezal, H.; Saad, A. Analysis of Sperm Aneuploidies and DNA Fragmentation in Patients with Globozoospermia or with Abnormal Acrosomes. Urology 2011, 77, 1343–1348. [Google Scholar] [CrossRef]
- Brahem, S.; Mehdi, M.; Elghezal, H.; Saad, A. Study of aneuploidy rate and sperm DNA fragmentation in large-headed, multiple-tailed spermatozoa. Andrologia 2011, 44, 130–135. [Google Scholar] [CrossRef]
- Moskovtsev, S.I.; Willis, J.; White, J.; Mullen, J.B.M. Sperm DNA Damage: Correlation to Severity of Semen Abnormalities. Urology 2009, 74, 789–793. [Google Scholar] [CrossRef]
- Singh, N.P.; Muller, C.H.; Berger, R.E. Effects of age on DNA double-strand breaks and apoptosis in human sperm. Fertil. Steril. 2003, 80, 1420–1430. [Google Scholar] [CrossRef]
- Oliveira, J.; Petersen, C.; Mauri, A.; Vagnini, L.; Baruffi, R.; Jose, J.F., Jr. The effects of age on sperm quality: An evaluation of 1500 semen samples. JBRA Assist. Reprod. 2014, 18, 34–41. [Google Scholar] [CrossRef]
- Moskovtsev, S.I.; Willis, J.; Mullen, J.B.M. Age-related decline in sperm deoxyribonucleic acid integrity in patients evaluated for male infertility. Fertil. Steril. 2006, 85, 496–499. [Google Scholar] [CrossRef]
- Spanò, M.; Bonde, J.P.; Hjøllund, H.I.; Kolstad, H.A.; Cordelli, E.; Leter, G. Sperm chromatin damage impairs human fertility. Fertil. Steril. 2000, 73, 43–50. [Google Scholar] [CrossRef]
- Das, M.; Al-Hathal, N.; San-Gabriel, M.; Phillips, S.; Kadoch, I.-J.; Bissonnette, F.; Holzer, H.; Zini, A. High prevalence of isolated sperm DNA damage in infertile men with advanced paternal age. J. Assist. Reprod. Genet. 2013, 30, 843–848. [Google Scholar] [CrossRef]
- Barroso, G.; Morshedi, M.; Oehninger, S. Analysis of DNA fragmentation, plasma membrane translocation of phosphatidylserine and oxidative stress in human spermatozoa. Hum. Reprod. 2000, 15, 1338–1344. [Google Scholar] [CrossRef]
- Colin-Valenzuela, A.; Gómez-López, N.; Avila-Lombardo, R.; Barroso-Villa, G. Impact of male aging in the functional capacity of sperm through the expression of phosphatidyl serine and oligonucleomas. Ginecol. Obstet. México 2010, 78, 669–676. [Google Scholar]
- Loft, S.; Kold-Jensen, T.; Hjollund, N.H.I.; Giwercman, A.; Gyllemborg, J.; Ernst, E.; Olsen, J.; Scheike, T.; Poulsen, H.E.; Bonde, J.P. Oxidative DNA damage in human sperm influences time to pregnancy. Hum. Reprod. 2003, 18, 1265–1272. [Google Scholar] [CrossRef]
- Giwercman, A.; Lindstedt, L.; Larsson, M.; Bungum, M.; Spano, M.; Levine, R.J.; Rylander, L. Sperm chromatin structure assay as an independent predictor of fertility in vivo: A case-control study. Int. J. Androl. 2010, 33, e221–e227. [Google Scholar] [CrossRef]
- Blackburn, E.H.; Gall, J.G. A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena. J. Mol. Biol. 1978, 120, 33–53. [Google Scholar] [CrossRef]
- Blackburn, E.H. Switching and Signaling at the Telomere. Cell 2001, 106, 661–673. [Google Scholar] [CrossRef]
- Lindsey, J.; McGill, N.I.; Lindsey, L.A.; Green, D.K.; Cooke, H.J. In vivo loss of telomeric repeats with age in humans. Mutat. Res. 1991, 256, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Daniali, L.; Benetos, A.; Susser, E.; Kark, J.D.; Labat, C.; Kimura, M.; Desai, K.K.; Granick, M.; Aviv, A. Telomeres shorten at equivalent rates in somatic tissues of adults. Nat. Commun. 2013, 4, 1597. [Google Scholar] [CrossRef] [PubMed]
- Aubert, G.; Lansdorp, P.M.; Forrester, S.J.; Booz, G.W.; Sigmund, C.D.; Coffman, T.M.; Kawai, T.; Rizzo, V.; Scalia, R.; Eguchi, S.; et al. Telomeres and Aging. Physiol. Rev. 2008, 88, 557–579. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, E.H.; Greider, C.W.; Szostak, J.W. Telomeres and telomerase: The path from maize, Tetrahymena and yeast to human cancer and aging. Nat. Med. 2006, 12, 1133–1138. [Google Scholar] [CrossRef]
- Dolcetti, R.; De Rossi, A. Telomere/telomerase interplay in virus-driven and virus-independent lymphomagenesis: Pathogenic and clinical implications. Med. Res. Rev. 2012, 32, 233–253. [Google Scholar] [CrossRef]
- Unryn, B.M.; Cook, L.S.; Riabowol, K.T. Paternal age is positively linked to telomere length of children. Aging Cell 2005, 4, 97–101. [Google Scholar] [CrossRef]
- De Meyer, T.; Rietzschel, E.R.; De Buyzere, M.L.; De Bacquer, D.; Van Criekinge, W.; De Backer, G.G.; Gillebert, T.C.; Van Oostveldt, P.; Bekaert, S.; on behalf of the Asklepios investigators. Paternal age at birth is an important determinant of offspring telomere length. Hum. Mol. Genet. 2007, 16, 3097–3102. [Google Scholar] [CrossRef]
- Njajou, O.T.; Cawthon, R.M.; Damcott, C.M.; Wu, S.-H.; Ott, S.; Garant, M.J.; Blackburn, E.H.; Mitchell, B.D.; Shuldiner, A.R.; Hsueh, W.-C. Telomere length is paternally inherited and is associated with parental lifespan. Proc. Natl. Acad. Sci. USA 2007, 104, 12135–12139. [Google Scholar] [CrossRef]
- Prescott, J.; Du, M.; Wong, J.; Han, J.; De Vivo, I. Paternal age at birth is associated with offspring leukocyte telomere length in the nurses’ health study. Hum. Reprod. 2012, 27, 3622–3631. [Google Scholar] [CrossRef]
- Aviv, A.; Susser, E. Leukocyte Telomere Length and the Father’s Age Enigma: Implications for Population Health and for Life Course. Int. J. Epidemiol. 2013, 42, 457–462. [Google Scholar] [CrossRef]
- Arbeev, K.G.; Hunt, S.C.; Kimura, M.; Aviv, A.; Yashin, A.I. Leukocyte telomere length, breast cancer risk in the offspring: The relations with father’s age at birth. Mech. Ageing Dev. 2011, 132, 149–153. [Google Scholar] [CrossRef]
- Kimura, M.; Cherkas, L.F.; Kato, B.S.; Demissie, S.; Hjelmborg, J.B.; Brimacombe, M.; Cupples, A.; Hunkin, J.L.; Gardner, J.P.; Lu, X.; et al. Offspring’s Leukocyte Telomere Length, Paternal Age, and Telomere Elongation in Sperm. PLoS Genet. 2008, 4, e37. [Google Scholar] [CrossRef]
- Aston, K.I.; Hunt, S.C.; Susser, E.; Kimura, M.; Factor-Litvak, P.; Carrell, D.; Aviv, A. Divergence of sperm and leukocyte age-dependent telomere dynamics: Implications for male-driven evolution of telomere length in humans. Mol. Hum. Reprod. 2012, 18, 517–522. [Google Scholar] [CrossRef]
- Ferlin, A.; Rampazzo, E.; Rocca, M.S.; Keppel, S.; Frigo, A.C.; De Rossi, A.; Foresta, C. In young men sperm telomere length is related to sperm number and parental age. Hum. Reprod. 2013, 28, 3370–3376. [Google Scholar] [CrossRef]
- Eisenberg, D.T.A.; Hayes, M.G.; Kuzawa, C.W. Delayed paternal age of reproduction in humans is associated with longer telomeres across two generations of descendants. Proc. Natl. Acad. Sci. USA 2012, 109, 10251–10256. [Google Scholar] [CrossRef]
- Nordfjäll, K.; Svenson, U.; Norrback, K.-F.; Adolfsson, R.; Roos, G. Large-scale parent–child comparison confirms a strong paternal influence on telomere length. Eur. J. Hum. Genet. 2010, 18, 385–389. [Google Scholar] [CrossRef]
- Zalenskaya, I.A.; Zalensky, A.O. Telomeres in Mammalian Male Germline Cells. Int. Rev. Cytol. 2002, 218, 37–67. [Google Scholar] [CrossRef]
- Riou, L.; Bastos, H.; Lassalle, B.; Coureuil, M.; Testart, J.; Boussin, F.; Allemand, I.; Fouchet, P. The Telomerase Activity of Adult Mouse Testis Resides in the Spermatogonial α6-Integrin-Positive Side Population Enriched in Germinal Stem Cells. Endocrinology 2005, 146, 3926–3932. [Google Scholar] [CrossRef]
- Thilagavathi, J.; Kumar, M.; Mishra, S.S.; Venkatesh, S.; Kumar, R.; Dada, R. Analysis of sperm telomere length in men with idiopathic infertility. Arch. Gynecol. Obstet. 2013, 287, 803–807. [Google Scholar] [CrossRef]
- Siderakis, M.; Tarsounas, M. Telomere regulation and function during meiosis. Chromosom. Res. 2007, 15, 667–679. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Sofikitis, N.; Ono, K.; Kaki, T.; Isoyama, T.; Suzuki, N.; Miyagawa, I. Postmeiotic modifications of spermatogenic cells are accompanied by inhibition of telomerase activity. Urol. Res. 1999, 27, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, S.; Seyama, A. Cellular aging and centrosome aberrations. Ann. N. Y. Acad. Sci. 2010, 1197, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Ly, D.H.; Lockhart, D.J.; Lerner, R.A.; Schultz, P.G. Mitotic Misregulation and Human Aging. Science 2000, 287, 2486–2492. [Google Scholar] [CrossRef] [PubMed]
- Cande, W. Centrosomes: Composition and reproduction. Curr. Opin. Cell Biol. 1990, 2, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Huang, B. Genetics and biochemistry of centrosomes and spindle poles. Curr. Opin. Cell Biol. 1990, 2, 28–32. [Google Scholar] [CrossRef]
- Cheng, J.; Türkel, N.; Hemati, N.; Fuller, M.T.; Hunt, A.J.; Yamashita, Y.M. Centrosome misorientation reduces stem cell division during ageing. Nature 2008, 456, 599–604. [Google Scholar] [CrossRef]
- Grégoire, M.-C.; Massonneau, J.; Simard, O.; Gouraud, A.; Brazeau, M.-A.; Arguin, M.; LeDuc, F.; Boissonneault, G. Male-driven de novo mutations in haploid germ cells. Mol. Hum. Reprod. 2013, 19, 495–499. [Google Scholar] [CrossRef]
- Lowe, X.; Eskenazi, B.; Nelson, D.O.; Kidd, S.; Alme, A.; Wyrobek, A.J. Frequency of XY Sperm Increases with Age in Fathers of Boys with Klinefelter Syndrome. Am. J. Hum. Genet. 2001, 69, 1046–1054. [Google Scholar] [CrossRef]
- Kong, A.; Frigge, M.L.; Masson, G.; Besenbacher, S.; Sulem, P.; Magnusson, G.; Gudjonsson, S.A.; Sigurdsson, A.; Jonasdottir, A.; Jonasdottir, A.; et al. Rate of de novo mutations and the importance of father’s age to disease risk. Nature 2012, 488, 471–475. [Google Scholar] [CrossRef]
- Drost, J.B.; Lee, W.R. Biological basis of germline mutation: Comparisons of spontaneous germline mutation rates among drosophila, mouse, and human. Environ. Mol. Mutagen. 1995, 25, 48–64. [Google Scholar] [CrossRef]
- Chianese, C.; Brilli, S.; Krausz, C. Genomic Changes in Spermatozoa of the Aging Male. Adv. Exp. Med. Biol. 2014, 791, 13–26. [Google Scholar] [CrossRef]
- Glaser, R.L.; Jiang, W.; Boyadjiev, S.A.; Tran, A.K.; Zachary, A.A.; Van Maldergem, L.; Johnson, D.; Walsh, S.; Oldridge, M.; Wall, S.A.; et al. Paternal Origin of FGFR2 Mutations in Sporadic Cases of Crouzon Syndrome and Pfeiffer Syndrome. Am. J. Hum. Genet. 2000, 66, 768–777. [Google Scholar] [CrossRef]
- Goriely, A.; Wilkie, A.O. Paternal Age Effect Mutations and Selfish Spermatogonial Selection: Causes and Consequences for Human Disease. Am. J. Hum. Genet. 2012, 90, 175–200. [Google Scholar] [CrossRef]
- Rannan-Eliya, S.V.; Taylor, I.B.; De Heer, I.M.; Van den Ouweland, A.M.W.; Wall, S.A.; Wilkie, A.O.M. Paternal origin of FGFR3 mutations in Muenke-type craniosynostosis. Hum. Genet. 2004, 115, 200–207. [Google Scholar] [CrossRef]
- Wyrobek, A.J.; Eskenazi, B.; Young, S.; Arnheim, N.; Tiemann-Boege, I.; Jabs, E.W.; Glaser, R.L.; Pearson, F.S.; Evenson, D. Advancing age has differential effects on DNA damage, chromatin integrity, gene mutations, and aneuploidies in sperm. Proc. Natl. Acad. Sci. USA 2006, 103, 9601–9606. [Google Scholar] [CrossRef]
- McLachlan, R.I.; O’Bryan, M.K. State of the Art for Genetic Testing of Infertile Men. J. Clin. Endocrinol. Metab. 2010, 95, 1013–1024. [Google Scholar] [CrossRef]
- Johnson, R.E.; Washington, M.T.; Prakash, S.; Prakash, L. Fidelity of Human DNA Polymerase η. J. Biol. Chem. 2000, 275, 7447–7450. [Google Scholar] [CrossRef]
- Makova, K.D.; Yang, S.; Chiaromonte, F. Insertions and Deletions Are Male Biased Too: A Whole-Genome Analysis in Rodents. Genome Res. 2004, 14, 567–573. [Google Scholar] [CrossRef]
- Kato, T.; Yamada, K.; Inagaki, H.; Kogo, H.; Ohye, T.; Emanuel, B.S.; Kurahashi, H. Age has no effect on de novo constitutional t(11;22) translocation frequency in sperm. Fertil. Steril. 2007, 88, 1446–1448. [Google Scholar] [CrossRef]
- Bashamboo, A.; Ferraz-De-Souza, B.; Lourenço, D.; Lin, L.; Sebire, N.J.; Montjean, D.; Bignon-Topalovic, J.; Mandelbaum, J.; Siffroi, J.-P.; Christin-Maitre, S.; et al. Human Male Infertility Associated with Mutations in NR5A1 Encoding Steroidogenic Factor 1. Am. J. Hum. Genet. 2010, 87, 505–512. [Google Scholar] [CrossRef]
- Sun, C.; Skaletsky, H.; Birren, B.; Devon, K.; Tang, Z.; Silber, S.; Oates, R.; Page, D.C. An azoospermic man with a de novo point mutation in the Y-chromosomal gene USP9Y. Nat. Genet. 1999, 23, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Templado, C.; Donate, A.; Giraldo, J.; Bosch, M.; Estop, A. Advanced age increases chromosome structural abnormalities in human spermatozoa. Eur. J. Hum. Genet. 2011, 19, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Hassold, T.; Hunt, P. To err (meiotically) is human: The genesis of human aneuploidy. Nat. Rev. Genet. 2001, 2, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Regan, L.; Rai, R. Epidemiology and the medical causes of miscarriage. Baillieres Best Pract. Res. Clin. Obstet. Gynaecol. 2000, 14, 839–854. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.K.; Abruzzo, M.A.; Millie, E.A.; Feingold, E.; Hassold, T.J. Sex ratio in normal and disomic sperm: Evidence that the extra chromosome 21 preferentially segregates with the Y chromosome. Am. J. Hum. Genet. 1996, 59, 1108–1113. [Google Scholar]
- McIntosh, G.C.; Olshan, A.F.; Baird, P.A. Paternal age and the risk of birth defects in offspring. Epidemiology 1995, 6, 282–288. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Moskalev, A.A.; Shaposhnikov, M.V.; Plyusnina, E.N.; Zhavoronkov, A.; Budovsky, A.; Yanai, H.; Fraifeld, V.E. The role of DNA damage and repair in aging through the prism of Koch-like criteria. Ageing Res. Rev. 2013, 12, 661–684. [Google Scholar] [CrossRef]
- Fumagalli, M.; Rossiello, F.; Clerici, M.; Barozzi, S.; Cittaro, D.; Kaplunov, J.M.; Bucci, G.; Dobreva, M.; Matti, V.; Beausejour, C.M.; et al. Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nature 2012, 14, 355–365. [Google Scholar] [CrossRef]
- Rossiello, F.; Herbig, U.; Longhese, M.P.; Fumagalli, M.; di Fagagna, F.D. Irreparable telomeric DNA damage and persistent DDR signalling as a shared causative mechanism of cellular senescence and ageing. Curr. Opin. Genet. Dev. 2014, 26, 89–95. [Google Scholar] [CrossRef]
- Sikora, E.; Arendt, T.; Bennett, M.; Narita, M. Impact of cellular senescence signature on ageing research. Ageing Res. Rev. 2011, 10, 146–152. [Google Scholar] [CrossRef]
- Kotaja, N. MicroRNAs and spermatogenesis. Fertil. Steril. 2014, 101, 1552–1562. [Google Scholar] [CrossRef]
- Harries, L.W. MicroRNAs as Mediators of the Ageing Process. Genes 2014, 5, 656–670. [Google Scholar] [CrossRef]
- Belleannee, C.; Légaré, C.; Calvo, E.; Thimon, V.; Sullivan, R. microRNA signature is altered in both human epididymis and seminal microvesicles following vasectomy. Hum. Reprod. 2013, 28, 1455–1467. [Google Scholar] [CrossRef]
- Turner, T.T. On the epididymis and its role in the development of the fertile ejaculate. J. Androl. 1995, 16, 292–298. [Google Scholar] [PubMed]
- Jones, R.C. To store or mature spermatozoa? The primary role of the epididymis. Int. J. Androl. 1999, 22, 57–67. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Q.; Zhang, W.; Li, J.; Li, Z.; Tang, Z.; Li, Y.; Han, C.; Hall, S.H.; Zhang, Y. Comparative profiling of genes and miRNAs expressed in the newborn, young adult, and aged human epididymides. Acta Biochim. et Biophys. Sin. 2010, 42, 145–153. [Google Scholar] [CrossRef]
- Zitzmann, M. Effects of age on male fertility. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 617–628. [Google Scholar] [CrossRef]
- Dada, R.; Kumar, M.; Jesudasan, R.; Fernández, J.L.; Gosálvez, J.; Agarwal, A. Epigenetics and its role in male infertility. J. Assist. Reprod. Genet. 2012, 29, 213–223. [Google Scholar] [CrossRef]
- Marques, C.J.; Carvalho, F.; Sousa, M.; Barros, A. Genomic imprinting in disruptive spermatogenesis. Lancet 2004, 363, 1700–1702. [Google Scholar] [CrossRef]
- El Hajj, N.; Zechner, U.; Schneider, E.; Tresch, A.; Gromoll, J.; Hahn, T.; Schorsch, M.; Haaf, T. Methylation Status of Imprinted Genes and Repetitive Elements in Sperm DNA from Infertile Males. Sex. Dev. 2011, 5, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Sato, A.; Otsu, E.; Hiura, H.; Tomatsu, C.; Utsunomiya, T.; Sasaki, H.; Yaegashi, N.; Arima, T. Aberrant DNA methylation of imprinted loci in sperm from oligospermic patients. Hum. Mol. Genet. 2007, 16, 2542–2551. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Hiura, H.; Okae, H.; Miyauchi, N.; Abe, Y.; Utsunomiya, T.; Yaegashi, N.; Arima, T. Assessing loss of imprint methylation in sperm from subfertile men using novel methylation polymerase chain reaction Luminex analysis. Fertil. Steril. 2011, 95, 129–134.e4. [Google Scholar] [CrossRef] [PubMed]
- Houshdaran, S.; Cortessis, V.K.; Siegmund, K.; Yang, A.; Laird, P.W.; Sokol, R.Z. Widespread Epigenetic Abnormalities Suggest a Broad DNA Methylation Erasure Defect in Abnormal Human Sperm. PLoS ONE 2007, 2, e1289. [Google Scholar] [CrossRef] [PubMed]
- Montjean, D.; Ravel, C.; Benkhalifa, M.; Cohen-Bacrie, P.; Berthaut, I.; Bashamboo, A.; McElreavey, K. Methylation changes in mature sperm deoxyribonucleic acid from oligozoospermic men: Assessment of genetic variants and assisted reproductive technology outcome. Fertil. Steril. 2013, 100, 1241–1247.e2. [Google Scholar] [CrossRef]
- Benchaib, M.; Braun, V.; Lornage, J.; Hadj, S.; Salle, B.; Lejeune, H.; Guérin, J.F. Sperm DNA fragmentation decreases the pregnancy rate in an assisted reproductive technique. Hum. Reprod. 2003, 18, 1023–1028. [Google Scholar] [CrossRef]
- Benchaib, M.; Braun, V.; Ressnikof, D.; Lornage, J.; Durand, P.; Niveleau, A.; Guérin, J. Influence of global sperm DNA methylation on IVF results. Hum. Reprod. 2005, 20, 768–773. [Google Scholar] [CrossRef]
- Beard, C.; Li, E.; Jaenisch, R. Loss of methylation activates Xist in somatic but not in embryonic cells. Genes Dev. 1995, 9, 2325–2334. [Google Scholar] [CrossRef]
- Katz-Jaffe, M.G.; Parks, J.; McCallie, B.; Schoolcraft, W.B. Aging sperm negatively impacts in vivo and in vitro reproduction: A longitudinal murine study. Fertil. Steril. 2013, 100, 262–268.e2. [Google Scholar] [CrossRef]
- Nanassy, L.; Liu, L.; Griffin, J.; Carrell, D.T. The Clinical Utility of the Protamine 1/Protamine 2 Ratio in Sperm. Protein Pept. Lett. 2011, 18, 772–777. [Google Scholar] [CrossRef]
- Jenkins, T.G.; Aston, K.I.; Cairns, B.R.; Carrell, D.T. Paternal aging and associated intraindividual alterations of global sperm 5-methylcytosine and 5-hydroxymethylcytosine levels. Fertil. Steril. 2013, 100, 945–951.e2. [Google Scholar] [CrossRef]
- Dan, B.; Pelc, K.; Christophe, C. What would the brain look like in Angelman syndrome? Eur. J. Paediatr. Neurol. 2009, 13, 269–270. [Google Scholar] [CrossRef]
- DeBaun, M.R.; Siegel, M.J.; Choyke, P.L. Nephromegaly in infancy and early childhood: A risk factor for Wilms tumor in Beckwith-Wiedemann syndrome. J. Pediatr. 1998, 132, 401–404. [Google Scholar] [CrossRef]
- Gosden, R.; Trasler, J.; Lucifero, D.; Faddy, M. Rare congenital disorders, imprinted genes, and assisted reproductive technology. Lancet 2003, 361, 1975–1977. [Google Scholar] [CrossRef]
- Lin, Y.-N.; Matzuk, M.M. Genetics of Male Fertility. Hum. Fertil. Methods Protoc. 2014, 1154, 25–37. [Google Scholar] [CrossRef]
- Summerer, D. Enabling technologies of genomic-scale sequence enrichment for targeted high-throughput sequencing. Genomics 2009, 94, 363–368. [Google Scholar] [CrossRef]
- Katib, A.A.; Al–Hawsawi, K.; Motair, W.; Bawa, A.M. Secondary infertility and the aging male, overview. Central Eur. J. Urol. 2014, 67, 184–188. [Google Scholar] [CrossRef]
- Yuen, R.K.; Merkoulovitch, A.; MacDonald, J.R.; Vlasschaert, M.; Lo, K.; Grober, E.; Marshall, C.R.; Jarvi, K.A.; Kolomietz, E.; Scherer, S.W. Development of a high-resolution Y-chromosome microarray for improved male infertility diagnosis. Fertil. Steril. 2014, 101, 1079–1085.e3. [Google Scholar] [CrossRef]
- Mathieu, C.; Ecochard, R.; Bied, V.; Lornage, J.; Czyba, J. Andrology: Cumulative conception rate following intrauterine artificial insemination with husband’s spermatozoa: Influence of husband’s age. Hum. Reprod. 1995, 10, 1090–1097. [Google Scholar] [CrossRef]
- Belloc, S.; Cohen-Bacrie, P.; Benkhalifa, M.; Cohen-Bacrie, M.; De Mouzon, J.; Hazout, A.; Menezo, Y. Effect of maternal and paternal age on pregnancy and miscarriage rates after intrauterine insemination. Reprod. Biomed. Online 2008, 17, 392–397. [Google Scholar] [CrossRef]
- Demir, B.; Dilbaz, B.; Cinar, O.; Karadag, B.; Tasci, Y.; Kocak, M.; Dilbaz, S.; Goktolga, U. Factors affecting pregnancy outcome of intrauterine insemination cycles in couples with favourable female characteristics. J. Obstet. Gynaecol. 2011, 31, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Klonoff-Cohen, H.S.; Natarajan, L. The effect of advancing paternal age on pregnancy and live birth rates in couples undergoing in vitro fertilization or gamete intrafallopian transfer. Am. J. Obstet. Gynecol. 2004, 191, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Nijs, M.; De Jonge, C.; Cox, A.; Janssen, M.; Bosmans, E.; Ombelet, W. Correlation between male age, WHO sperm parameters, DNA fragmentation, chromatin packaging and outcome in assisted reproduction technology. Andrologia 2011, 43, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, Q.; Wang, Y.; Li, Y. Whether sperm deoxyribonucleic acid fragmentation has an effect on pregnancy and miscarriage after in vitro fertilization/intracytoplasmic sperm injection: A systematic review and meta-analysis. Fertil. Steril. 2014, 102, 998–1005.e8. [Google Scholar] [CrossRef]
- Kühnert, B.; Nieschlag, E. Reproductive functions of the ageing male. Hum. Reprod. Updat. 2004, 10, 327–339. [Google Scholar] [CrossRef]
- Morris, I.D.; Ilott, S.; Dixon, L.; Brison, D.R. The spectrum of DNA damage in human sperm assessed by single cell gel electrophoresis (Comet assay) and its relationship to fertilization and embryo development. Hum. Reprod. 2002, 17, 990–998. [Google Scholar] [CrossRef]
- Borini, A.; Tarozzi, N.; Bizzaro, D.; Bonu, M.; Fava, L.; Flamigni, C.; Coticchio, G. Sperm DNA fragmentation: Paternal effect on early post-implantation embryo development in ART. Hum. Reprod. 2006, 21, 2876–2881. [Google Scholar] [CrossRef]
- Simon, L.; Murphy, K.; Shamsi, M.B.; Liu, L.; Emery, B.; Aston, K.I.; Hotaling, J.; Carrell, D.T. Paternal influence of sperm DNA integrity on early embryonic development. Hum. Reprod. 2014, 29, 2402–2412. [Google Scholar] [CrossRef]
- Frattarelli, J.L.; Miller, K.A.; Miller, B.T.; Elkind-Hirsch, K.; Scott, R.T., Jr. Male age negatively impacts embryo development and reproductive outcome in donor oocyte assisted reproductive technology cycles. Fertil. Steril. 2008, 90, 97–103. [Google Scholar] [CrossRef]
- Bellver, J.; Garrido, N.; Remohí, J.; Pellicer, A.; Meseguer, M. Influence of paternal age on assisted reproduction outcome. Reprod. Biomed. Online 2008, 17, 595–604. [Google Scholar] [CrossRef]
- Belloc, S.; Hazout, A.; Zini, A.; Merviel, P.; Cabry, R.; Chahine, H.; Copin, H.; Benkhalifa, M. How to overcome male infertility after 40: Influence of paternal age on fertility. Maturitas 2014, 78, 22–29. [Google Scholar] [CrossRef]
- Spandorfer, S.D.; Avrech, O.M.; Colombero, L.T.; Palermo, G.D.; Rosenwaks, Z. Effect of parental age on fertilization and pregnancy characteristics in couples treated by intracytoplasmic sperm injection. Hum. Reprod. 1998, 13, 334–338. [Google Scholar] [CrossRef]
- Dain, L.; Auslander, R.; Dirnfeld, M. The effect of paternal age on assisted reproduction outcome. Fertil. Steril. 2011, 95, 1–8. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. The clinical utility of sperm DNA integrity testing: A guideline. Fertil. Steril. 2013, 99, 673–677. [Google Scholar] [CrossRef]
- de La Rochebrochard, E.; Thonneau, P. Paternal age and maternal age are risk factors for miscarriage; results of a multicentre European study. Hum. Reprod. 2002, 17, 1649–1656. [Google Scholar] [CrossRef]
- Kleinhaus, K.; Perrin, M.; Friedlander, Y.; Paltiel, O.; Malaspina, D.; Harlap, S. Paternal Age and Spontaneous Abortion. Obstet. Gynecol. 2006, 108, 369–377. [Google Scholar] [CrossRef]
- Slama, R.; Bouyer, J.; Windham, G.; Fenster, L.; Werwatz, A.; Swan, S.H. Influence of Paternal Age on the Risk of Spontaneous Abortion. Am. J. Epidemiol. 2005, 161, 816–823. [Google Scholar] [CrossRef]
- Luna, M.; Finkler, E.; Barritt, J.; Bar-Chama, N.; Sandler, B.; Copperman, A.B.; Grunfeld, L. Paternal age and assisted reproductive technology outcome in ovum recipients. Fertil. Steril. 2009, 92, 1772–1775. [Google Scholar] [CrossRef]
- Ferreira, R.C.; Braga, D.P.D.A.F.; Bonetti, T.C.D.S.; Pasqualotto, F.F.; Iaconelli, A.; Borges, E. Negative influence of paternal age on clinical intracytoplasmic sperm injection cycle outcomes in oligozoospermic patients. Fertil. Steril. 2010, 93, 1870–1874. [Google Scholar] [CrossRef]
- Spong, C.Y. Defining “Term” Pregnancy: Recommendations from the Defining “Term” Pregnancy Workgroup. JAMA 2013, 309, 2445–2446. [Google Scholar] [CrossRef]
- Lawn, J.E.; Gravett, M.G.; Nunes, T.M.; Rubens, C.E.; Stanton, C.; the GAPPS Review Group. Global report on preterm birth and stillbirth (1 of 7): Definitions, description of the burden and opportunities to improve data. BMC Pregnancy Childbirth 2010, 10, S1. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.L.; Madsen, K.M.; Vestergaard, M.; Basso, O.; Olsen, J. Paternal Age and Preterm Birth. Epidemiology 2005, 16, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Astolfi, P.; De Pasquale, A.; Zonta, L.A. Paternal Age and Preterm Birth in Italy, 1990 to 1998. Epidemiology 2006, 17, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Abel, E.L.; Kruger, M.; Burd, L. Effects of Maternal and Paternal Age on Caucasian and Native American Preterm Births and Birth Weights. Am. J. Perinatol. 2002, 19, 49–54. [Google Scholar] [CrossRef]
- Olshan, A.F.; Ananth, C.V.; Savitz, D.A. Intrauterine growth retardation as an endpoint in mutation epidemiology: An evaluation based on paternal age. Mutat. Res. Toxicol. 1995, 344, 89–94. [Google Scholar] [CrossRef]
- Kinzler, W.; Ananth, C.V.; Smulian, J.C.; Vintzileos, A.M. Parental age difference and adverse perinatal outcomes in the United States. Paediatr. Périnat. Epidemiol. 2002, 16, 320–327. [Google Scholar] [CrossRef]
- Tough, S.C.; Faber, A.J.; Svenson, L.W.; Johnston, D.W. Is Paternal Age Associated with an Increased Risk of Low Birthweight, Preterm Delivery, and Multiple Birth? Can. J. Public Health 2003, 94, 88–92. [Google Scholar] [CrossRef]
- Hack, M.; Klein, N.K.; Taylor, H.G. Long-Term Developmental Outcomes of Low Birth Weight Infants. Futur. Child. 1995, 5, 176–196. [Google Scholar] [CrossRef]
- Alio, A.P.; Salihu, H.M.; McIntosh, C.; August, E.; Weldeselasse, H.; Sanchez, E.; Mbah, A.K. The Effect of Paternal Age on Fetal Birth Outcomes. Am. J. Men’s Health 2012, 6, 427–435. [Google Scholar] [CrossRef]
- Cartlidge, P.; Stewart, J. Effect of changing the stillbirth definition on evaluation of perinatal mortality rates. Lancet 1995, 346, 486–488. [Google Scholar] [CrossRef]
- Andersen, A.-M.N.; Hansen, K.D.; Andersen, P.K.; Smith, G.D. Advanced Paternal Age and Risk of Fetal Death: A Cohort Study. Am. J. Epidemiol. 2004, 160, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Astolfi, P.; De Pasquale, A.; Zonta, L. Late paternity and stillbirth risk. Hum. Reprod. 2004, 19, 2497–2501. [Google Scholar] [CrossRef] [PubMed]
- Crow, J.F. Age and Sex Effects on Human Mutation Rates: An Old Problem with New Complexities. J. Radiat. Res. 2006, 47, B75–B82. [Google Scholar] [CrossRef] [PubMed]
- Clarke, H.; Harrison, S.; Perez, M.J.; Kirkman-Brown, J. UK guidelines for the medical and laboratory procurement and use of sperm, oocyte and embryo donors (2019). Hum. Fertil. 2021, 24, 3–13. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive, M.; the Practice Committee for the Society for Assisted Reproductive Technology. Guidance regarding gamete and embryo donation. Fertil. Steril. 2021, 115, 1395–1410. [Google Scholar] [CrossRef]
- Torrey, E.F.; Buka, S.; Cannon, T.D.; Goldstein, J.M.; Seidman, L.J.; Liu, T.; Hadley, T.; Rosso, I.M.; Bearden, C.; Yolken, R.H. Paternal age as a risk factor for schizophrenia: How important is it? Schizophr. Res. 2009, 114, 1–5. [Google Scholar] [CrossRef]
- Buizer-Voskamp, J.E.; Laan, W.; Staal, W.G.; Hennekam, E.A.; Aukes, M.F.; Termorshuizen, F.; Kahn, R.S.; Boks, M.P.; Ophoff, R.A. Paternal age and psychiatric disorders: Findings from a Dutch population registry. Schizophr. Res. 2011, 129, 128–132. [Google Scholar] [CrossRef]
- Miller, B.; Messias, E.; Miettunen, J.; Alaräisänen, A.; Järvelin, M.-R.; Koponen, H.; Räsänen, P.; Isohanni, M.; Kirkpatrick, B. Meta-analysis of Paternal Age and Schizophrenia Risk in Male Versus Female Offspring. Schizophr. Bull. 2011, 37, 1039–1047. [Google Scholar] [CrossRef]
- Frans, E.M.; McGrath, J.J.; Sandin, S.; Lichtenstein, P.; Reichenberg, A.; Långström, N.; Hultman, C.M. Advanced paternal and grandpaternal age and schizophrenia: A three-generation perspective. Schizophr. Res. 2011, 133, 120–124. [Google Scholar] [CrossRef]
- Owen, M.J.; Sawa, A.; Mortensen, P.B. Schizophrenia. Lancet 2016, 388, 86–97. [Google Scholar] [CrossRef]
- Malaspina, D.; Corcoran, C.; Fahim, C.; Berman, A.; Harkavy-Friedman, J.; Yale, S.; Goetz, D.; Goetz, R.; Harlap, S.; Gorman, J. Paternal age and sporadic schizophrenia: Evidence for de novo mutations. Am. J. Med. Genet. 2002, 114, 299–303. [Google Scholar] [CrossRef]
- Xu, B.; Roos, J.; Levy, S.; van Rensburg, E.; Gogos, J.A.; Karayiorgou, M. Strong association of de novo copy number mutations with sporadic schizophrenia. Nat. Genet. 2008, 40, 880–885. [Google Scholar] [CrossRef]
- Malaspina, D.; Harlap, S.; Fennig, S.; Heiman, D.; Nahon, D.; Feldman, D.; Susser, E.S. Advancing Paternal Age and the Risk of Schizophrenia. Arch. Gen. Psychiatry 2001, 58, 361–367. [Google Scholar] [CrossRef]
- Sipos, A.; Rasmussen, F.; Harrison, G.; Tynelius, P.; Lewis, G.; Leon, D.; Gunnell, D. Paternal age and schizophrenia: A population based cohort study. BMJ 2004, 329, 1070. [Google Scholar] [CrossRef]
- Tsuchiya, K.J.; Takagai, S.; Kawai, M.; Matsumoto, H.; Nakamura, K.; Minabe, Y.; Mori, N.; Takei, N. Advanced paternal age associated with an elevated risk for schizophrenia in offspring in a Japanese population. Schizophr. Res. 2005, 76, 337–342. [Google Scholar] [CrossRef]
- Petersen, L.; Mortensen, P.B.; Pedersen, C.B. Paternal Age at Birth of First Child and Risk of Schizophrenia. Am. J. Psychiatry 2011, 168, 82–88. [Google Scholar] [CrossRef]
- Moore, T. Genomic imprinting in mammalian development: A parental tug-of-war. Trends Genet. 1991, 7, 45–49. [Google Scholar] [CrossRef]
- Perrin, M.C.; Brown, A.S.; Malaspina, D. Aberrant Epigenetic Regulation Could Explain the Relationship of Paternal Age to Schizophrenia. Schizophr. Bull. 2007, 33, 1270–1273. [Google Scholar] [CrossRef]
- Anderson, I.M.; Haddad, P.M.; Scott, J. Bipolar disorder. BMJ 2012, 345, e8508. [Google Scholar] [CrossRef]
- Frans, E.M.; Sandin, S.; Reichenberg, A.; Lichtenstein, P.; Långström, N.; Hultman, C.M. Advancing Paternal Age and Bipolar Disorder. Arch. Gen. Psychiatry 2008, 65, 1034–1040. [Google Scholar] [CrossRef]
- Menezes, P.R.; Lewis, G.; Rasmussen, F.; Zammit, S.; Sipos, A.; Harrison, G.L.; Tynelius, P.; Gunnell, D. Paternal and maternal ages at conception and risk of bipolar affective disorder in their offspring. Psychol. Med. 2010, 40, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Kirov, G.; Pocklington, A.J.; Holmans, P.; Ivanov, D.; Ikeda, M.; Ruderfer, D.; Moran, J.; Chambert, K.; Toncheva, D.; Georgieva, L.; et al. De novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia. Mol. Psychiatry 2012, 17, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, Z.; Tochigi, M.; Jia, P.; Pal, M.; Mill, J.; Kwan, A.; Ioshikhes, I.; Vincent, J.B.; Kennedy, J.L.; Strauss, J.; et al. A multi-tissue analysis identifies HLA complex group 9 gene methylation differences in bipolar disorder. Mol. Psychiatry 2012, 17, 728–740. [Google Scholar] [CrossRef] [PubMed]
- Anagnostou, E.; Taylor, M.J. Review of neuroimaging in autism spectrum disorders: What have we learned and where we go from here. Mol. Autism 2011, 2, 4. [Google Scholar] [CrossRef]
- Reichenberg, A.; Gross, R.; Weiser, M.; Bresnahan, M.; Silverman, J.; Harlap, S.; Rabinowitz, J.; Shulman, C.; Malaspina, D.; Lubin, G.; et al. Advancing Paternal Age and Autism. Arch. Gen. Psychiatry 2006, 63, 1026–1032. [Google Scholar] [CrossRef]
- Hultman, C.M.; Sandin, S.; Levine, S.Z.; Lichtenstein, P.; Reichenberg, A. Advancing paternal age and risk of autism: New evidence from a population-based study and a meta-analysis of epidemiological studies. Mol. Psychiatry 2011, 16, 1203–1212. [Google Scholar] [CrossRef]
- Durkin, M.S.; Maenner, M.J.; Newschaffer, C.J.; Lee, L.-C.; Cunniff, C.M.; Daniels, J.L.; Kirby, R.S.; Leavitt, L.; Miller, L.; Zahorodny, W.; et al. Advanced Parental Age and the Risk of Autism Spectrum Disorder. Am. J. Epidemiol. 2008, 168, 1268–1276. [Google Scholar] [CrossRef]
- O’Roak, B.J.; Vives, L.; Girirajan, S.; Karakoc, E.; Krumm, N.; Coe, B.P.; Levy, R.; Ko, A.; Lee, C.; Smith, J.D.; et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature 2012, 485, 246–250. [Google Scholar] [CrossRef]
- Sanders, S.J.; Murtha, M.T.; Gupta, A.R.; Murdoch, J.D.; Raubeson, M.J.; Willsey, A.J.; Ercan-Sencicek, A.G.; DiLullo, N.M.; Parikshak, N.N.; Stein, J.L.; et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature 2012, 485, 237–241. [Google Scholar] [CrossRef]
- Teras, L.R.; Gaudet, M.M.; Blase, J.L.; Gapstur, S.M. Parental Age at Birth and Risk of Hematological Malignancies in Older Adults. Am. J. Epidemiol. 2015, 182, 41–48. [Google Scholar] [CrossRef]
- Urhoj, S.K.; Raaschou-Nielsen, O.; Hansen, A.V.; Mortensen, L.H.; Andersen, P.K.; Andersen, A.-M.N. Advanced paternal age and childhood cancer in offspring: A nationwide register-based cohort study. Int. J. Cancer 2017, 140, 2461–2472. [Google Scholar] [CrossRef]
- Hemminki, K.; Kyyrönen, P.; Vaittinen, P. Parental Age As a Risk Factor of Childhood Leukemia and Brain Cancer in Offspring. Epidemiology 1999, 10, 271–275. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Lee, K.-M.; Park, S.K.; Noh, D.-Y.; Ahn, S.-H.; Yoo, K.-Y.; Kang, D. Association of paternal age at birth and the risk of breast cancer in offspring: A case control study. BMC Cancer 2005, 5, 143. [Google Scholar] [CrossRef]
- Xue, F.; Colditz, G.A.; Willett, W.C.; Rosner, B.A.; Michels, K.B. Parental age at delivery and incidence of breast cancer: A prospective cohort study. Breast Cancer Res. Treat. 2007, 104, 331–340. [Google Scholar] [CrossRef]
- Printz, C. Father’s age at birth of child may increase child’s blood cancer risk. Cancer 2015, 121, 2863. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaltsas, A.; Moustakli, E.; Zikopoulos, A.; Georgiou, I.; Dimitriadis, F.; Symeonidis, E.N.; Markou, E.; Michaelidis, T.M.; Tien, D.M.B.; Giannakis, I.; et al. Impact of Advanced Paternal Age on Fertility and Risks of Genetic Disorders in Offspring. Genes 2023, 14, 486. https://doi.org/10.3390/genes14020486
Kaltsas A, Moustakli E, Zikopoulos A, Georgiou I, Dimitriadis F, Symeonidis EN, Markou E, Michaelidis TM, Tien DMB, Giannakis I, et al. Impact of Advanced Paternal Age on Fertility and Risks of Genetic Disorders in Offspring. Genes. 2023; 14(2):486. https://doi.org/10.3390/genes14020486
Chicago/Turabian StyleKaltsas, Aris, Efthalia Moustakli, Athanasios Zikopoulos, Ioannis Georgiou, Fotios Dimitriadis, Evangelos N. Symeonidis, Eleftheria Markou, Theologos M. Michaelidis, Dung Mai Ba Tien, Ioannis Giannakis, and et al. 2023. "Impact of Advanced Paternal Age on Fertility and Risks of Genetic Disorders in Offspring" Genes 14, no. 2: 486. https://doi.org/10.3390/genes14020486
APA StyleKaltsas, A., Moustakli, E., Zikopoulos, A., Georgiou, I., Dimitriadis, F., Symeonidis, E. N., Markou, E., Michaelidis, T. M., Tien, D. M. B., Giannakis, I., Ioannidou, E. M., Papatsoris, A., Tsounapi, P., Takenaka, A., Sofikitis, N., & Zachariou, A. (2023). Impact of Advanced Paternal Age on Fertility and Risks of Genetic Disorders in Offspring. Genes, 14(2), 486. https://doi.org/10.3390/genes14020486









