DNA Barcoding of Wild Plants with Potential Medicinal Properties from Faifa Mountains in Saudi Arabia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection
2.3. DNA Extraction, PCR Amplification, and Sequencing
2.4. Sequence Alignment and Data Analysis
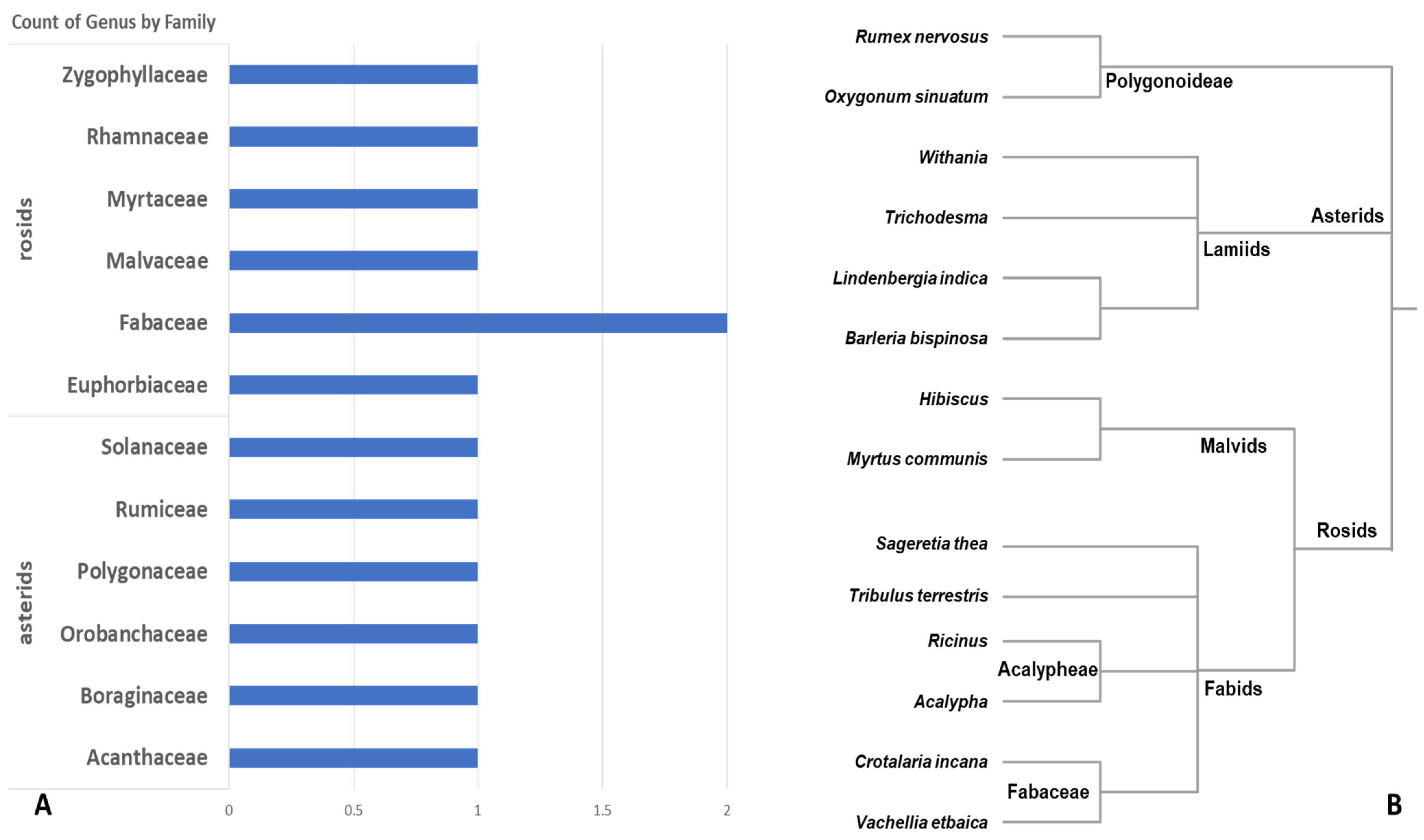
3. Results
3.1. Morphological Observation and Provisional Identification
3.2. Amplification, Sequencing, and Identification
3.2.1. Chloroplast rbcL Gene
- A. BLAST-based identification
- B. Phylogeny-based identification
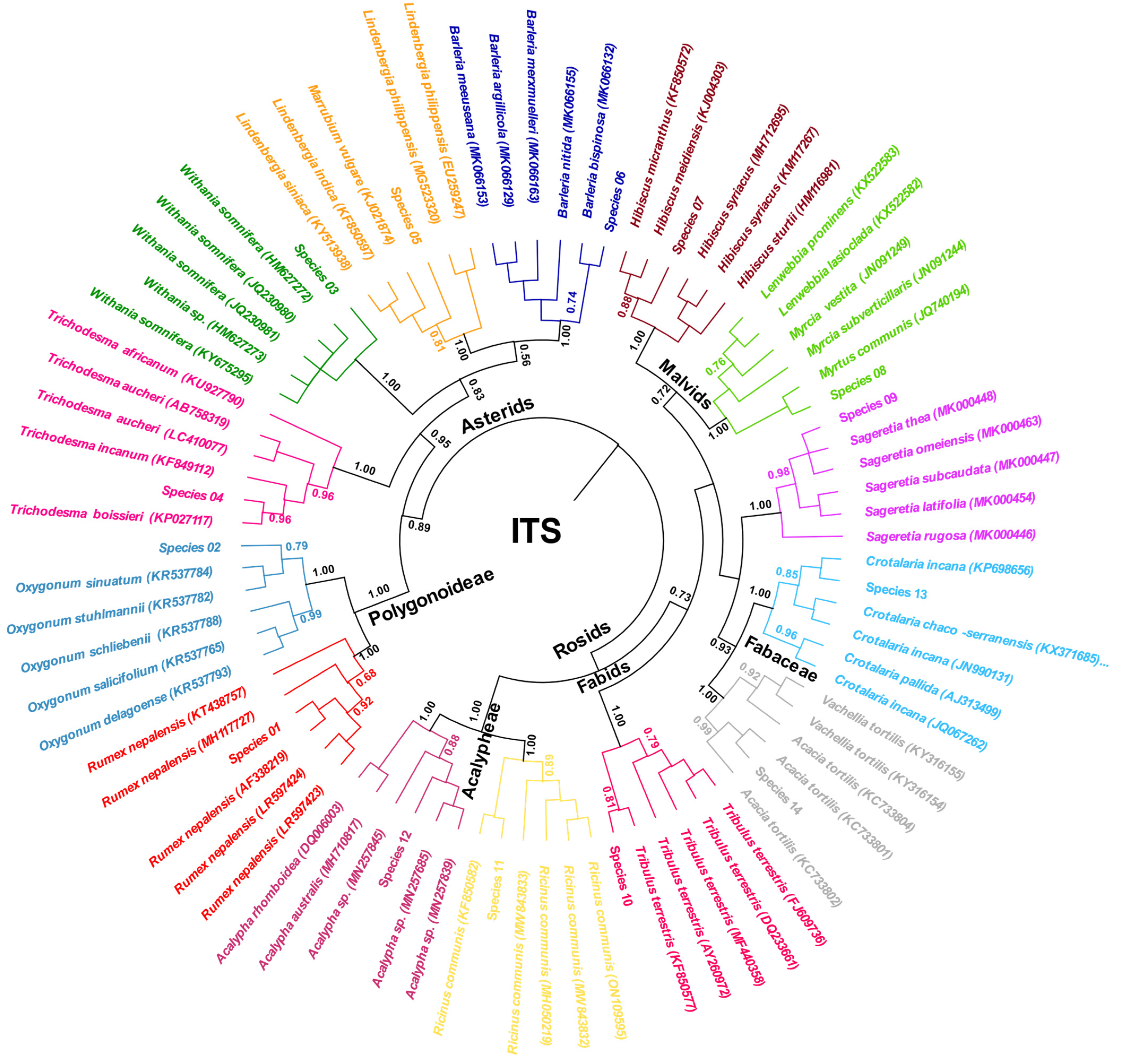
3.2.2. Nuclear ITS Region
- A. BLAST-based identification
- B. Phylogeny-Based Identification
3.3. Integrative Comparative Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Veldman, S.; Otieno, J.; Gravendeel, B.; van Andel, T.; de Boer, H. Conservation of Endangered Wild Harvested Medicinal Plants: Use of DNA Barcoding. In Novel Plant Bioresources; Gurib-Fakim, A., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2014; pp. 81–88. ISBN 978-1-118-46056-6. [Google Scholar]
- Lambert, J.; Srivastava, J.P.; Vietmeyer, N. Medicinal Plants: Rescuing a Global Heritage; World Bank Technical Papers; The World Bank: Washington, DC, USA, 1997; ISBN 978-0-8213-3856-8. [Google Scholar]
- Hamilton, A.C. Medicinal Plants, Conservation and Livelihoods. Biodivers. Conserv. 2004, 13, 1477–1517. [Google Scholar] [CrossRef]
- Techen, N.; Parveen, I.; Pan, Z.; Khan, I.A. DNA Barcoding of Medicinal Plant Material for Identification. Curr. Opin. Biotechnol. 2014, 25, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, M.; Heinrich, M.; Booker, A. Medicinal Plant Analysis: A Historical and Regional Discussion of Emergent Complex Techniques. Front. Pharmacol. 2020, 10, 1480. [Google Scholar] [CrossRef]
- Chen, S.; Yao, H.; Han, J.; Liu, C.; Song, J.; Shi, L.; Zhu, Y.; Ma, X.; Gao, T.; Pang, X.; et al. Validation of the ITS2 Region as a Novel DNA Barcode for Identifying Medicinal Plant Species. PLoS ONE 2010, 5, e8613. [Google Scholar] [CrossRef]
- Heubl, G. New Aspects of DNA-Based Authentication of Chinese Medicinal Plants by Molecular Biological Techniques. Planta Med. 2010, 76, 1963–1974. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; deWaard, J.R. Biological Identifications through DNA Barcodes. Proc. R. Soc. Lond. B 2003, 270, 313–321. [Google Scholar] [CrossRef]
- Blaxter, M. Counting Angels with DNA. Nature 2003, 421, 122–123. [Google Scholar] [CrossRef] [PubMed]
- Gregory, T.R. DNA Barcoding Does Not Compete with Taxonomy. Nature 2005, 434, 1067. [Google Scholar] [CrossRef] [PubMed]
- Schindel, D.E.; Miller, S.E. DNA Barcoding a Useful Tool for Taxonomists. Nature 2005, 435, 17. [Google Scholar] [CrossRef]
- Chase, M.W.; Fay, M.F. Barcoding of Plants and Fungi. Science 2009, 325, 682–683. [Google Scholar] [CrossRef] [PubMed]
- de Vere, N.; Rich, T.C.G.; Ford, C.R.; Trinder, S.A.; Long, C.; Moore, C.W.; Satterthwaite, D.; Davies, H.; Allainguillaume, J.; Ronca, S.; et al. DNA Barcoding the Native Flowering Plants and Conifers of Wales. PLoS ONE 2012, 7, e37945. [Google Scholar] [CrossRef]
- Pang, X.; Chen, S. Identification of Medicinal Plants Using DNA Barcoding Technique. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; Wiley: Hoboken, NJ, USA, 2021; pp. 1–5. ISBN 978-0-471-97670-7. [Google Scholar]
- Gao, T.; Yao, H.; Song, J.; Liu, C.; Zhu, Y.; Ma, X.; Pang, X.; Xu, H.; Chen, S. Identification of Medicinal Plants in the Family Fabaceae Using a Potential DNA Barcode ITS2. J. Ethnopharmacol. 2010, 130, 116–121. [Google Scholar] [CrossRef]
- Safhi, F.A.; Alshamrani, S.M.; Fiteha, Y.G.; Abd El-Moneim, D. DNA Barcoding of Endangered and Rarely Occurring Plants in Faifa Mountains (Jazan, Saudi Arabia). Agriculture 2022, 12, 1931. [Google Scholar] [CrossRef]
- Safhi, F.A.; ALshamrani, S.M.; Jalal, A.S.; El-Moneim, D.A.; Alyamani, A.A.; Ibrahim, A.A. Genetic Characterization of Some Saudi Arabia’s Accessions from Commiphora gileadensis Using Physio-Biochemical Parameters, Molecular Markers, DNA Barcoding Analysis and Relative Gene Expression. Genes 2022, 13, 2099. [Google Scholar] [CrossRef]
- Cahyaningsih, R.; Compton, L.J.; Rahayu, S.; Magos Brehm, J.; Maxted, N. DNA Barcoding Medicinal Plant Species from Indonesia. Plants 2022, 11, 1375. [Google Scholar] [CrossRef]
- Moritz, C.; Cicero, C. DNA Barcoding: Promise and Pitfalls. PLoS Biol. 2004, 2, e354. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A. DNA Barcoding Demystified. Aust. J. Entomol. 2008, 47, 169–173. [Google Scholar] [CrossRef]
- Mallet, J.; Willmott, K. Taxonomy: Renaissance or Tower of Babel? Trends Ecol. Evol. 2003, 18, 57–59. [Google Scholar] [CrossRef]
- Mesfer ALshamrani, S.; Safhi, F.A.; Alshaya, D.S.; Ibrahim, A.A.; Mansour, H.; Abd El Moneim, D. Genetic diversity using biochemical, physiological, karyological and molecular markers of Sesamum indicum L. Front. Genet 2022, 13, 1035977. [Google Scholar] [CrossRef] [PubMed]
- Pham, N.T.T.; Le, D.P.; Pham, K.T.N.; Thipphavong, X.; Chu, M.H. DNA Barcode of MatK Combined with ITS Effectively Distinguishes the Medicinal Plant Stephania Brachyandra Diels Collected in Laocai, Vietnam. J. Appl. Biol. Biotechnol. 2021, 9, 63–70. [Google Scholar] [CrossRef]
- Rahman, M.A.; Mossa, J.S.; Al-Said, M.S.; Al-Yahya, M.A. Medicinal Plant Diversity in the Flora of Saudi Arabia 1: A Report on Seven Plant Families. Fitoterapia 2004, 75, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Masrahi, Y.S.; Al-Turki, T.A.; Sayed, O.H. Wolffiella Hyalina (Delile) Monod (Lemnaceae)—A New Record for the Flora of Saudi Arabia. Feddes Repert. 2010, 121, 189–193. [Google Scholar] [CrossRef]
- Levin, R.A.; Wagner, W.L.; Hoch, P.C.; Nepokroeff, M.; Pires, J.C.; Zimmer, E.A.; Sytsma, K.J. Family-level Relationships of Onagraceae Based on Chloroplast Rbc L and Ndh F Data. Am. J. Bot. 2003, 90, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Xu, C.; Lei, L.; Li, C.; Zhang, Y.; Zhou, S. Barcoding the Kingdom Plantae: New PCR Primers for ITS Regions of Plants with Improved Universality and Specificity. Mol. Ecol. Resour. 2016, 16, 138–149. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An Integrated and Extendable Desktop Software Platform for the Organization and Analysis of Sequence Data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- CBOL Plant Working Group; Hollingsworth, P.M.; Forrest, L.L.; Spouge, J.L.; Hajibabaei, M.; Ratnasingham, S.; van der Bank, M.; Chase, M.W.; Cowan, R.S.; Erickson, D.L.; et al. A DNA Barcode for Land Plants. Proc. Natl. Acad. Sci. USA 2009, 106, 12794–12797. [Google Scholar] [CrossRef]
- Fazekas, A.J.; Kuzmina, M.L.; Newmaster, S.G.; Hollingsworth, P.M. DNA Barcoding Methods for Land Plants. In DNA Barcodes; Kress, W.J., Erickson, D.L., Eds.; Methods in Molecular BiologyTM; Humana Press: Totowa, NJ, USA, 2012; Volume 858, pp. 223–252. ISBN 978-1-61779-590-9. [Google Scholar]
- Kress, W.J.; Wurdack, K.J.; Zimmer, E.A.; Weigt, L.A.; Janzen, D.H. Use of DNA Barcodes to Identify Flowering Plants. Proc. Natl. Acad. Sci. USA 2005, 102, 8369–8374. [Google Scholar] [CrossRef] [PubMed]
- El-Atroush, H.; Magdy, M.; Werner, O. DNA Barcoding of Two Endangered Medicinal Plants from Abou Galoom Protectorate. Life Sci. J. 2015, 12, 101–109. [Google Scholar]
- El-Sakaty, S.I.; Magdy, M.; Rizk, S.; Hashim, A.; Abu-Elhamd, M.; Elateek, S. Ex-Situ Conservation of the Micro and Macro Flora of Omayed Biosphere Reserve (OBR): A Survey Report. Egypt. J. Bot. 2022, 62, 149–158. [Google Scholar] [CrossRef]
- Abd El-Moneim, D.; ELsarag, E.I.S.; Aloufi, S.; El-Azraq, A.M.; ALshamrani, S.M.; Safhi, F.A.A.; Ibrahim, A.A. Quinoa (Chenopodium quinoa Willd.): Genetic Diversity According to ISSR and SCoT Markers, Relative Gene Expression, and Morpho-Physiological Variation under Salinity Stress. Plants 2021, 10, 2802. [Google Scholar] [CrossRef] [PubMed]
- Al-Asmari, A.R.K.; Siddiqui, Y.M.; Athar, M.T.; Al-Buraidi, A.; Al-Eid, A.S.; Horaib, G.B. Antimicrobial Activity of Aqueous and Organic Extracts of a Saudi Medicinal Plant: Rumex Nervosus. J. Pharm. Bioallied Sci. 2015, 7, 300–303. [Google Scholar] [CrossRef] [PubMed]
- Collenette, S. Wildflowers of Saudi Arabia, 1st ed.; National Commission for Wildlife Conservation and Development (NCWCD): Riyadh, Saudi Arabia, 1999; ISBN 978-9960-614-09-0. [Google Scholar]
- Gangaram, S.; Naidoo, Y.; Dewir, Y.H.; El-Hendawy, S. Phytochemicals and Biological Activities of Barleria (Acanthaceae). Plants 2021, 11, 82. [Google Scholar] [CrossRef]
- Kayed, A.M.; Genady, E.A.M.; Kadry, H.A.; Elghaly, E.-S.M. New Phytoconstituents, Anti-Microbial and Cytotoxic Activities of Acacia Etbaica Schweinf. Nat. Prod. Res. 2021, 35, 5571–5580. [Google Scholar] [CrossRef]
- Esmail, S.M.; Aboulila, A.A.; Abd El-Moneim, D. Variation in several pathogenesis-related (PR) protein genes in wheat (Triticum aestivum) involved in defense against Puccinia striiformis f. sp. tritici. Physiol. Mol. Plant Pathol. 2020, 112, 101545. [Google Scholar] [CrossRef]
- McLachlan, J.S.; Clark, J.S.; Manos, P.S. Molecular Indicators of Tree Migration Capacity under Rapid Climate Change. Ecology 2005, 86, 2088–2098. [Google Scholar] [CrossRef]


| Sample | Species | PI% | Accession No. |
|---|---|---|---|
| Species_01 | Rumex nepalensis | 99.70% | KX015758 |
| Species_02 | Oxygonum sinuatum | 100% | KR736460 |
| Species_03 | Withania somnifera | 98.00% | MK142783 |
| Withania coagulans | NC_047176 | ||
| Species_04 | Trichodesma africanum | 100% | AM234930 |
| Species_05 | Lindenbergia sp. | 97.90% | AJ001768 |
| Species_06 | Barleria prionitis | 99.50% | MZ461574 |
| Species_07 | Hibiscus sabiensis | 99.80% | MZ461583 |
| Species_08 | Myrtus communis | 100% | MN662653 |
| Species_09 | Sageretia thea | 100% | OL537744 |
| Sageretia paucicostata | MN722394 | ||
| Sageretia lucida | MN205213 | ||
| Species_10 | Tribulus terrestris | 99.50% | MN205307 |
| Species_11 | Ricinus communis | 99.70% | MT555092 |
| Species_12 | Acalypha indica | 99% | KF381097 |
| Species_13 | Crotalaria sp. | 100% | KR737341 |
| Crotalaria incana | JQ591662 | ||
| Species_14 | Vachellia tortilis | 99.60% | KX015750 |
| Vachellia reficiens | MK285283 |
| Sample | Species | PI% | Accession No. |
|---|---|---|---|
| Species_01 | Rumex nepalensis | 100% | AF338219 |
| Species_02 | Oxygonum sinuatum | 99.50% | KR537784 |
| Species_03 | Withania somnifera | 99.40% | KY675295 |
| Species_04 | Trichodesma boissieri | 97.20% | KP027117 |
| Species_05 | Lindenbergia siniaca | 94.50% | KY513938 |
| Lindenbergia indica | KF850597 | ||
| Species_06 | Barleria prionitis | 100% | MK066159 |
| Species_07 | Hibiscus micranthus | 98.90% | KF850572 |
| Species_08 | Myrtus communis | 100% | JQ740194 |
| Species_09 | Sageretia thea | 100% | MK000448 |
| Sageretia omeiensis | MK000463 | ||
| Species_10 | Tribulus terrestris | 99.60% | KF850577 |
| Species_11 | Ricinus communis | 99.80% | KF850582 |
| Species_12 | Acalypha sp. | 99.60% | MN257839 |
| Species_13 | Crotalaria incana | 100% | KP698656 |
| Species_14 | Vachellia tortilis | 100% | MH547553 |
| Sample | Morphology | rbcL | ITS |
|---|---|---|---|
| Species_01 | Rumex nervous | Rumex nepalensis | Rumex nepalensis |
| Species_02 | Oxygonum sinuatum | Oxygonum sinuatum | Oxygonum sinuatum |
| Species_03 | Withania sp. | Withania somnifera | Withania somnifera |
| Species_04 | Trichodesma sp. | Trichodesma calycosum | Trichodesma boissieri |
| Species_05 | Lindenbergia siniaca | Lindenbergia sp. | Lindenbergia sp. |
| Species_06 | Barleria bispinosa | Barleria prionitis | Barleria prionitis |
| Species_07 | Hibiscus sp. | Hibiscus sabiensis | Hibiscus sp. |
| Species_08 | Myrtus communis | Myrtus communis | Myrtus communis |
| Species_09 | Sageretia thea | Sageretia thea | Sageretia thea |
| Sageretia paucicostata | Sageretia omeiensis | ||
| Sageretia lucida | |||
| Species_10 | Tribulus terrestris | Tribulus terrestris | Tribulus terrestris |
| Species_11 | Ricinus sp. | Ricinus communis | Ricinus communis |
| Species_12 | Acalypha fruticosa | Acalypha sp. | Acalypha sp. |
| Species_13 | Crotalaria incana | Crotalaria incana | Crotalaria incana |
| Species_14 | Vachellia etbaica | Vachellia tortilis | Vachellia tortilis |
| Vachellia reficiens |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safhi, F.A.; Alshamrani, S.M.; Bogmaza, A.F.M.; El-Moneim, D.A. DNA Barcoding of Wild Plants with Potential Medicinal Properties from Faifa Mountains in Saudi Arabia. Genes 2023, 14, 469. https://doi.org/10.3390/genes14020469
Safhi FA, Alshamrani SM, Bogmaza AFM, El-Moneim DA. DNA Barcoding of Wild Plants with Potential Medicinal Properties from Faifa Mountains in Saudi Arabia. Genes. 2023; 14(2):469. https://doi.org/10.3390/genes14020469
Chicago/Turabian StyleSafhi, Fatmah Ahmed, Salha Mesfer Alshamrani, Abdullah Farag Mohammed Bogmaza, and Diaa Abd El-Moneim. 2023. "DNA Barcoding of Wild Plants with Potential Medicinal Properties from Faifa Mountains in Saudi Arabia" Genes 14, no. 2: 469. https://doi.org/10.3390/genes14020469
APA StyleSafhi, F. A., Alshamrani, S. M., Bogmaza, A. F. M., & El-Moneim, D. A. (2023). DNA Barcoding of Wild Plants with Potential Medicinal Properties from Faifa Mountains in Saudi Arabia. Genes, 14(2), 469. https://doi.org/10.3390/genes14020469






