Abstract
Sulfate transporters (SULTRs) are an essential plant transporter class responsible for the absorption and distribution of sulfur, an essential plant growth element. SULTRs are also involved in processes related to growth and development and in response to environmental stimuli. In the present study, 22 TdSULTR family members were identified and characterized in the genome of Triticum turgidum L. ssp. durum (Desf.) using available bioinformatics tools. The expression levels of candidate TdSULTR genes were investigated under salt treatments of 150 and 250 mM NaCl after several different exposure times. TdSULTRs showed diversity in terms of physiochemical properties, gene structure, and pocket sites. TdSULTRs and their orthologues were classified into the known five main plant groups of highly diverse subfamilies. In addition, it was noted that segmental duplication events could lengthen TdSULTR family members under evolutionary processes. Based on pocket site analysis, the amino acids leucine (L), valine (V), and serine (S) were most often detected in TdSULTR protein binding sites. Moreover, it was predicted that TdSULTRs have a high potential to be targeted by phosphorylation modifications. According to promoter site analysis, the plant bioregulators ABA and MeJA were predicted to affect TdSULTR expression patterns. Real-time PCR analysis revealed TdSULTR genes are differentially expressed at 150 mM NaCl but show similar expression in response to 250 mM NaCl. TdSULTR reached a maximum level of expression 72 h after the 250 mM salt treatment. Overall, we conclude that TdSULTR genes are involved in the response to salinity in durum wheat. However, additional studies of functionality are needed to determine their precise function and linked-interaction pathways.
1. Introduction
Sulfur, an important and necessary element for optimal plant growth and development, is involved in many cellular processes [1]. In addition, sulfur is present in the structure of hormones, vitamins, amino acids, and coenzymes, and its deficiency will reduce the quantity and quality of plant production [2]. Sulfur is also involved in the formation of glutathione, which is sensitive to oxidative stress and functions to regulate oxidant-dependent signaling pathways along with stress-related responses [1,3,4,5]. Also, sulfur-dependent metabolites are effective at increasing the tolerance of plants to abiotic stresses such as salt stress by controlling and regulating related molecular and physiological processes. Plants absorb sulfate through root cells and use it for sulfur-dependent metabolism. Absorption and distribution of sulfate occur through sulfate transporters (SULTRs) located in cell membranes and those of organelles such as the vacuole and plastid [6,7]. SULTRs contain a STAS (Sulfate Transporter/AntiSigma-factor) domain in its carboxyl-terminal region and 12 membrane-spanning domains [8,9,10].
Due to the importance of sulfur to subsequent plant growth processes, the SULTR gene family has been subjected to detailed molecular investigation in model plants such as Arabidopsis [11,12,13,14,15]. Moreover, the SULTR gene family is classified into five groups and four putative subfamilies, including subfamily SULTR 1, 2, 3, and 4 [11,12,13,14,15]. The function of some members of these subfamilies has been determined. For example, subfamily SULTR 1 members, 1.1, 1.2, and 1.3, are primarily active in roots and are responsible for sulfate absorption and transport [11,12]. SULTR 2.1 and SULTR 2.2 belong to group 2, which play principal roles in transferring sulfate from roots to other plant organs [14]. Functional activities for the five members of the SULTR 3 subfamily (SULTR 3.1, 3.2, 3.3, 3.4, and 3.5) vary and a specific function has not been determined [16]. SULTR 4.1 and SULTR 4.2, members of group 4, are involved in the transport and transfer of sulfate between the vacuole and the cytosol [6,17]. SULTRs are predominantly involved in growth and development processes, but studies have revealed that members of this gene family are also involved in responding to environmental stress [18,19,20,21]. For example, subfamily SULTR 3 members have been found to interact with transcription factors (TFs) associated with stress responses in potatoes [19]. It was reported that SULTR genes in maize are induced as a response to heat and drought stress [22]. Heavy metal stress will also alter SULTR expression [20,21].
Triticum turgidum L. ssp. durum (Desf.) is a tetraploid wheat, 2 n = 4 × = 28, that is well adapted to arid regions [23,24]. As mentioned above, SULTRs play critical roles in the absorption and transfer of sulfate and in responses to environmental stimuli. Some SULTR gene family members have been identified and characterized in several plant models and the functionality of some gene family members has been determined. However, this gene family has not been studied in T. turgidum L. ssp. durum (Desf.). The SULTR family genes of T. turgidum L. ssp. durum (Desf.) (TdSULTRs) studied here were identified and characterized with the expression patterns of candidate TdSULTRs evaluated under salinity.
2. Materials and Methods
2.1. Identification of SULTRs
To recognize the SULTR family members in the genome of T. turgidum L. ssp. durum (Desf.) (TdSULTRs), two domains Sulfate_transp (PF00916), and STAS (PF01740) were applied as queries with the BLASTP tool of Ensembl Plants database (Accessed: 20 December 2022) [25]. SULTR proteins were also obtained for the Oryza sativa Indica group, Triticum urartu, Triticum aestivum, Hordeum vulgare, Sorghum bicolor, and Zea mays. The presence of SULTR domains in identified proteins was investigated using the Pfam database (Accessed: 20 December 2022) [26] and the Conserved Domain Database (CDD) (Accessed: 20 December 2022) [27]. Several physiochemical properties of TdSULTRs, such as isoelectric points (pI), molecular weight (MW), GRAVY, and instability index were evaluated by the ExpasyProtParam tool (Accessed: 20 December 2022) [28]. The subcellular localization of each TdSULTR was predicted using the BUSCA server [29].
2.2. Phylogenetics and Conserved Motif Analyses
To construct a phylogeny tree, the SULTR proteins from T. turgidum L. ssp. durum (Desf.), O. sativa, T. urartu, T. aestivum, H. vulgare, S. bicolor, and Z. mays were analyzed by Clustal-Omega, an online multiple alignment tool [30]. Next, the aligned sequences were used to construct a phylogeny tree using the Maximum likelihood (ML) with 1000 bootstrap replication and the default setting with IQ-TREE web server [31]. The resultant file was imported into the iTOL online tool [32] to illustrate the phylogeny tree.
2.3. Duplication Analysis of TdSULTR Genes
To identify duplicated TdSULTR genes, the coding sequence of pairs of TdSULTR genes was compared and screened for genes with an identity ≥0.85 [33]. Furthermore, the non-synonymous (Ka) and synonymous (Ks) indexes were calculated for each duplicated gene by MEGAX software [34]. In addition, the time of divergence for duplicated genes was calculated using the following equation, T = (Ks/2λ) × 10−6. (λ = 6.5 × 10−9).
2.4. Promoter Analysis
To identify putative cis-regulatory elements in the promoter regions of TdSULTR genes, the Plant CARE tool (Accessed: 14 December 2022) [35] screened the 1500 bp region upstream of each gene. All discovered cis-regulatory elements were separated by function.
2.5. Prediction of Three-Dimensional Structure of TdSULTR Proteins
In the current study, the Phyre2 [36] database (Accessed: 17 December 2022) was used to predict the 3D structure of TdSULTR proteins. Predictions of pocket site locations as ligand binding regions in the 3D structure of TdSULTR proteins were generated with the Phyre investigator tool from the Phyre2 server (Accessed: 17 December 2022).
2.6. Prediction of Phosphorylation Region in TdSULTR Proteins
The region of phosphorylation events based on three amino acids, serine, tyrosine, and threonine, was predicted in TdSULTR proteins using the NetPhos 3-1 server (Accessed: 18 December 2022) [37], and the potential value of each region was set with a high probability, more than 0.90.
2.7. Interaction Network of SULTR Family
Protein-protein interaction of TdSULTRs was investigated with the String database (Accessed: 13 December 2022) based on orthologues from the model plant Arabidopsis [38]. Direct and indirect interactions were discerned by setting the first layer of the network to 5 and the second layer to 20 nodes. In addition, significant gene ontology (GO) terms (FDR ≤0.01) were identified based on the molecular function and biological processes of the interaction network nodes. The final interaction network was designed using Cytoscape software (v3.9.1) [39].
2.8. Plant Materials and Treatments
The present study utilized the T. turgidum L. ssp. durum (Desf.) cultivar Yavaros. Sterilized seeds were planted three per pot in peat moss. Seedlings were grown under photoperiodic lighting (16 h of light: 8 h of dark) at a temperature of 24 ± 3 °C. Six-week-old seedlings were separately treated with two salt concentrations, 150 and 250 mM NaCl, by irrigation. The process was repeated after 24 h with three additional pots irrigated without salt for use as controls. In the next step, seedling shoots were collected after 6, 24 and 72 h of salt treatment. The harvested shoot samples were immediately frozen in liquid nitrogen before being transferred to a −65 °C freezer.
2.9. Real-Time PCR Analysis
The total RNA of collected samples was extracted using an RNX plus kit (Cinaclone, Tehran, Iran), following the included manufacturer’s protocol. Also, a reverse transcriptase (Roche, Mannheim, Germany) enzyme was used to synthesize our cDNA based on the provided manufacturer’s protocol. In this study, six TdSULTR genes were selected for expression pattern analysis in response to salinity. The Actin7 gene was selected as a housekeeping gene for data normalization. Primers (forward and reverse) for each selected gene (Table S1) were designed using an online tool, Primer3 (Accessed: 10 December 2022) [40]. The observed expression patterns for the TdSULTR genes were evaluated by ABI Step One using Maxima SYBR Green/ROX qPCR Master Mix (Thermo Fisher, Illkirch-Graffenstaden, France), according to the manufacturer’s protocol. Lastly, gene expression levels were estimated using the delta-delta ct protocol [41]. Three biological replicates were used in this experiment.
3. Results
3.1. Identification and Physicochemical Properties of the SULTR Family
Twenty-two TdSULTR genes were identified within the T. turgidum L. ssp. durum (Desf.) genome and their physicochemical properties are provided in Table 1. TdSULTR proteins ranged in length from 147 (TRITD6Av1G100790) to 698 (TRITD4Bv1G165010) amino acids. Molecular weight (MW) ranged from 16.77 (TRITD6Av1G100790) to 75.69 kDa (TRITD4Bv1G165010). In addition, exon numbers varied from 3 to 13. The endomembrane system and organelle membranes were predicted to be the subcellular localization site for TdSULTR (Table 1). Based on isoelectric point (pI) data, most members of the TdSULTR gene family, with the exception of two proteins (TRITD6Av1G100790 and TRITD7Av1G260730), were alkaline (pI ≥ 7.0) in nature. The positive value for GRAVY indicates the hydrophobic nature of the proteins, while negative values indicate the hydrophilic nature of the proteins [42]. The value of GRAVY of all TdSULTR family members was positive, which indicates the hydrophilic nature of TdSULTRs. Results showed that TdSULTR family members have diverse physiochemical and structural properties, suggesting the SULTR gene family has likely been influenced by evolutionary processes.

Table 1.
Physicochemical properties for TdSULTR family members within the T. turgidum L. ssp. durum (Desf.) genome.
3.2. Phylogenetic Analysis of SULTR Family Members
A phylogenetic tree of 22 TdSULTR proteins and associated orthologues in T. aestivum, T. urartu, S. bicolor, H. vulgare, O. sativa, A. thaliana, and Z. mays was constructed based on the maximum likelihood (ML) method (Figure 1). The results indicated SULTR family proteins could be classified into five main groups. The greatest diversity of SULTR proteins was observed in group IV. In addition, the highest number of TdSULTR proteins was observed in group III. Group IV was subdivided into two large subgroups, IV-a and IV-b. Group V was the largest clade with 32 SULTR proteins. Groups I, II, III and IV included 12, 22, 27, and 29 SULTR proteins, respectively. All SULTR 3.5 proteins were located in group I, while SULTR 3.1 and 3.2 were placed in group II. Other SULTR 3 proteins, SULTR 3.3 and 3.4, were located in group III, while all SULTR 1 proteins were in group V. Interestingly, SULTR 4 subfamily members along with SULTR 2 members were placed in group IV. A perusal of the phylogeny tree indicated the diversity in SULTR gene family members likely occurred after the derivation of monocots and dicots.
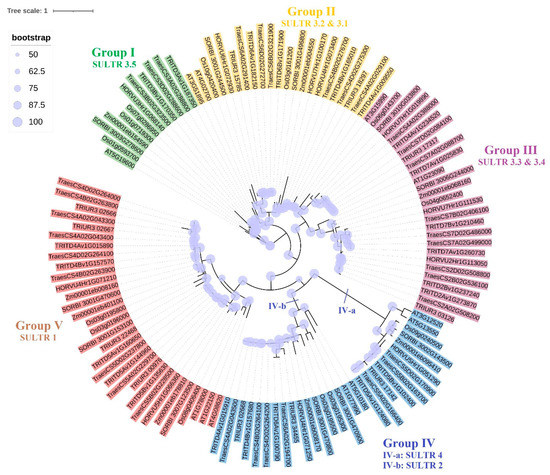
Figure 1.
Phylogenetic analysis of SULTR gene family members from T. turgidum L. ssp. durum (Desf.) (TRITD prefix), T. aestivum (Traes prefix), T. uratu (TRIUR prefix), S. bicolor (SORBI prefix), H. vulgare (HORVU prefix), O. sativa (Os prefix), A. thaliana (AT prefix), and Z. mays (Zm prefix).
3.3. Chromosome Location and Duplication Events in TdSULTR Genes
Chromosome positioning for the 22 TdSULTR genes of T. turgidum L. ssp. durum (Desf.) illustrated that all were located on 12 chromosomes, an indication that TdSULTR genes were randomly distributed among the chromosomes (Figure 2a). For instance, four genes in chromosome 4A, three chromosomes 4B and 5A, two genes in chromosomes 5B and 7A, and a single gene in the 2A, 2B, 3A, 3B, 6A, 6B, and 7B chromosomes. Based on duplication analysis, 20 genes were segmentally duplicated among TdSULTR gene family members (Table S2). The first duplication event was estimated to have been around 32 million years ago (MYB) for two SULTR3.3 genes including TRITD2Bv1G237240 and TRITD7Av1G260730 (Figure 2b). The Ka/Ks ratio was calculated for duplicated genes (Figure 2c). All duplicated TdSULTRs showed Ka/Ks < 0.40, indicating that the duplicated TdSULTR genes were under purifying (negative) selection.
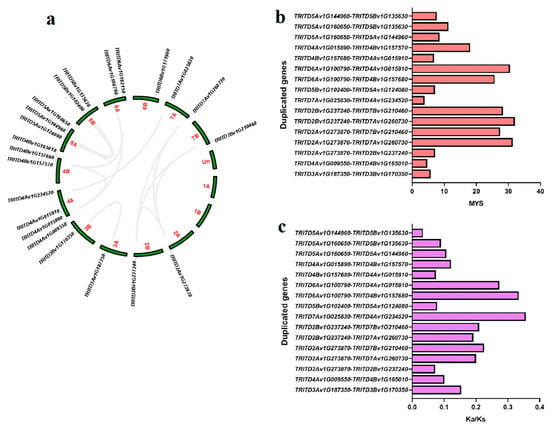
Figure 2.
Distribution of TdSULTR genes in the genome of T. turgidum L. ssp. durum (Desf.), gray lines showed the duplicated genes (a), time of divergence of duplicated TdSULTR genes based on million years ago (b), and the ratio Ka/Ks of duplicated genes of the TdSULTR gene family (c).
3.4. Analysis of TdSULTR Protein Structure
SULTR protein structures were modeled with more than 90% confidence and their ligand binding sites (pocket sites) were also predicted (Figure 3). According to the 3D structure results, different binding sites were predicted in TdSULTR proteins (Figure 3a). Variation in protein structure may reflect their transport activity in response to environmental stimuli. Based on pocket site analysis, leucine (L), valine (V), and serine (S) were frequently observed in the binding residues at the ligand binding site for nearly all of the TdSULTR proteins (Figure 3b). L was present in most TdSULTR proteins, while V and S were absent in three TdSULTR proteins each. Generally, L is known as a key residue in predicted pocket sites of TdSULTR proteins.
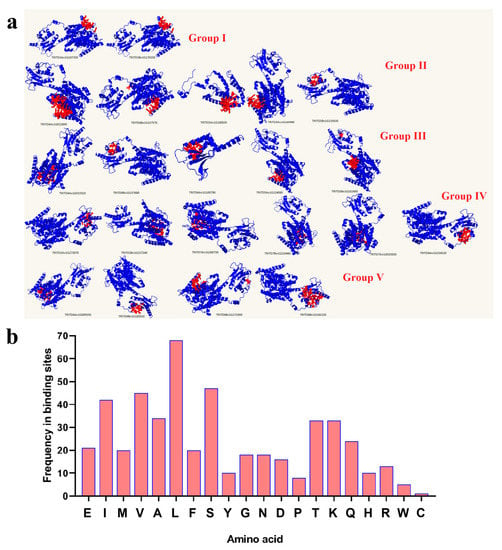
Figure 3.
Three-dimensional and pocket site analysis of TdSULTRs in T. turgidum (a), and frequency of residues located in pocket sites of TdSULTRs (b). Red fill indicates the pocket site in the 3D structure of TdSULTRs. Abbreviations: Alanine, (Ala, A); Arginine, (Arg, R); Asparagine, (Asn, N); Aspartic acid, (Asp, D); Cysteine, (Cys, C); Glutamic acid, (Glu, E); Glutamine, (Gln, Q); Glycine, (Gly, G); Histidine, (His, H); Isoleucine, (Ile, I); Leucine, (Leu, L); Lysine, (Lys, K); Methionine, (Met, M); Phenylalanine, (Phe, F); Proline, (Pro, P); Serine, (Ser, S); Threonine, (Thr, T); Tryptophan, (Trp, W); Tyrosine, (Tyr, Y); Valine, (Val, V).
3.5. TdSULTR Post-Translational Modifications and Predicted Phosphorylation Regions
Potential phosphorylation regions, important for post-translational modifications, were investigated in TdSULTR proteins. Results revealed TdSULTRs are extremely likely (>90%) to target kinases (Figure 4). TdSULTR 3 subfamily protein TRITD4Bv1G165010, with 67, possessed the highest number of potential sites. It was also uncovered that serine is more influenced by phosphorylation modifications than the amino acids tyrosine and threonine.
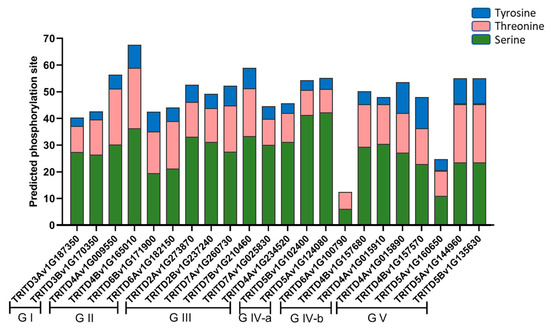
Figure 4.
Prediction of phosphorylation sites in TdSULTR proteins based on three amino acids including serine, tyrosine, and threonine. TdSULTR is separated according to the phylogeny tree from G I (group 1) to G V (group 5).
3.6. Promoter Analysis of TdSULRs
Analysis of TdSULTR gene promoter regions led to the discovery of the putative cis-regulatory elements affecting expression patterns (Table S3 and Figure 5). In the present study, cis-regulatory elements were classified into four main groups, including hormone-responsive elements (REs), growth REs, stress REs, and light REs (Figure 5a). Cis-regulatory elements involved in plant stress responses were most abundant, at 35%, with cis-regulatory elements involved in growth processes (8%) those least represented (Figure 5a). Cis-regulatory elements related to hormone responses were divided into five groups, including auxin REs, methyl jasmonate (MeJA) REs, abscisic acid (ABA) REs, GA Res, and salicylic acid (SA) REs (Figure 5b). ABA REs and MeJA REs were distributed most in the promoter region of TdSULTRs, indicating these gene family members are more induced by ABA and MeJA. In addition, cis-regulatory elements involved in response to drought, anaerobic, and low-temperature stresses are most often found upstream sites of TdSULTR genes (Figure 5c).
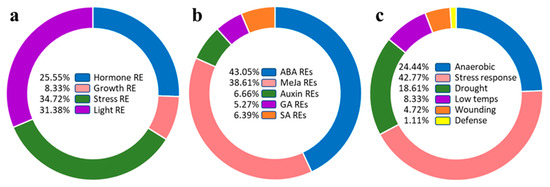
Figure 5.
Classification of putative cis-regulatory elements present in the upstream region of TdSULTR genes (a), involved in response to phytohormones (b), and stresses (c). Full details are provided in Table S3.
3.7. Expression Profiles of TdSULTR Genes
The expression patterns of members of a gene family can reveal some aspects of their function in response to different biological conditions. To clarify the potential biological roles of durum wheat TdSULTR genes, the expression patterns of six candidates were investigated under two levels of salt stress (150 and 250 mM NaCl concentration). Candidate genes showed differential expression patterns in response to salinity (Figure 6). The TRITD3Bv1G170350 gene, from the TdSULTR 3.5 subfamily, showed a significant down-regulation after 6 h of salinity in both applied salt concentrations, indicating that this gene can be an early response of durum wheat in response to salinity. Moreover, TRITD3Bv1G170350 illustrated down-regulation in all series after 250 mM of NaCl. Besides, the TRID4Av1G015890 gene (TdSULTR 1.2 subfamily) was also down-regulated in all time periods of 150 mM salt treatment, but up-regulated at 250 mM NaCl. TRID5Bv1G12400, a TdSULTR 4 subfamily member, was induced 24h after salt treatment and showed high up-regulation after 72 h of 250 mM NaCl. Thus, the TRID5Bv1G12400 gene remains inactive during the early salinity stress response. TRID7Av1G260730 (TdSULTR 3.3 subfamily), showed differential expression at 150 µM NaCl, up-regulation after 6h, and down-regulation after 24 h and 72 h of salt treatment. Two TdSULTRs, TRID4Bv1G165010 (TdSULTR 3.1 subfamily) and TRID4Bv1G157680 (TdSULTR subfamily 2), showed similar expression patterns with both genes showing upregulation at each time point, except 6 h after 150 mM NaCl. Interestingly, all candidate genes, with the exception of TRITD3Bv1G170350, displayed the highest expression levels 72 h after 250 mM salt treatment.
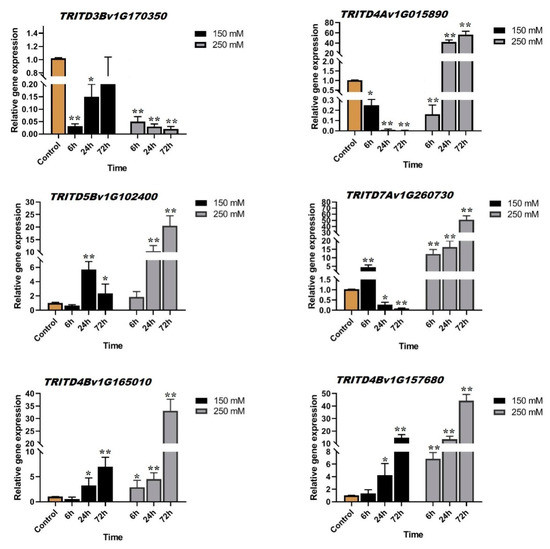
Figure 6.
Expression levels of candidate TdSULTR genes in durum wheat in response to salinity, 150 and 250 mM of NaCl. * and ** indicate a significant difference between the experimental treatments and control treatment (according to Student’s t-test) at p < 0.05 and p < 0.01, respectively.
3.8. TdSULTR Interaction Network
In this study, the SULTR protein interaction network revealed proteins associated with sulfur assimilation such as SERAT, serine O-acetyltransferase, APS (adenosine 5′-phosphosulfate), APR (adenosine 5′-phosphosulfate reductase), and APK (adenosine-5′-phosphosulfate kinase) (Figure 7a). Gene ontology enrichment analysis based on network elements revealed that molecular function terms including adenylyl sulfate transferase (ATP) activity, adenylyl sulfate kinase activity, and adenylyl sulfate reductase (glutathione) activity were significantly linked with the SULTR network (Figure 7b). Biological processes such as the hydrogen sulfide biosynthesis process and sulfate reduction process were also significantly associated with the SULTR network. Cellular component enrichment analysis showed chloroplast and chloroplast stroma were the target sites of members of the SULTR network. As expected, based on KEGG analysis, sulfur metabolism was recognized as a metabolic process related to the SULTR network.
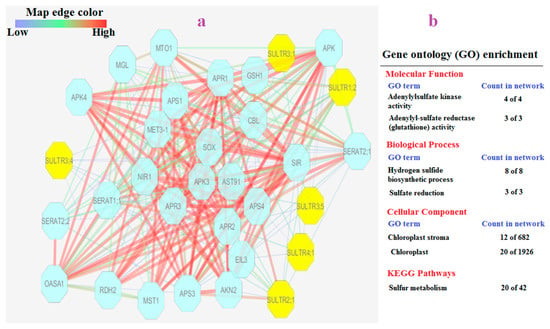
Figure 7.
Interaction network of SULTR family members (a), and list of significant GO terms (FDR < 0.01) of nodes presented in network (b). Abbreviations: SERAT (serine O-acetyltransferase); APS (adenosine 5′-phosphosulfate); APR (adenosine 5′-phosphosulfate reductase); and APK (adenosine-5′-phosphosulfate kinase).
4. Discussion
Sulfur is a nutrient factor is involved in photosynthesis and critical growth processes and in plant responses to environmental stresses. Therefore, sulfur absorption and distribution in plant organs are very important. The sulfate transporters described here, as the main factor of sulfate distribution, help to improve plant performance of [18,43]. In the present study, 22 TdSULTR family members were identified and characterized by bioinformatic analyses. Physicochemical property analysis disclosed that TdSULTR were hydrophilic proteins, indicating that all TdSULTRs probably work under the same conditions. However, TdSULTRs were diverse based on exon number, ranging from 3 to 13, and more exons were observed in the structure of subfamily SULTR 3 genes. Exon numbers can affect gene expression thus, genes with high exon numbers are slowly induced compared to genes with fewer exons [44]. The phylogeny analysis showed the evolutionary process of each TdSULTR subfamily varied. In comparisons of relationships between the subfamilies of monocot SULTRs with the dicot model, Arabidopsis, it can be concluded that diversity within this family most likely occurred after the derivation of monocots and dicots [45,46]. In addition, subfamily TdSULTR 3 showed more diversity, suggesting that this subfamily may be an ancestor of other subfamilies [47,48]. Segmental duplication was recognized as a main evolution event, which expanded the number of TdSULTR family members. However, it was predicted that duplicated TdSULTR were under purifying selection based on Ka/Ks ratio [49]. Predicting three-dimensional structures of TdSULTR proteins showed that members of this gene family are structured similarly but possess different pocket regions. Variations in protein active site location can influence interactions and levels of protein activity [50,51]. L, V, and S were most abundant in the pocket regions, which showed the importance of these amino acids to TdSULTR activity rates and possible interactions. Hence, additional research is needed to better determine the effect of divergence in these areas. The TdSULTRs were predicted to be proteins highly likely to be phosphorylated by kinases. The presence of high-potential phosphorylation regions indicates TdSULTRs may be controlled by kinase-dependent signaling pathways. Studying expression patterns of target genes can provide a relative understanding of their biological functions. Few studies have been conducted in the field that addresses the function of SULTRs [6,7,52,53] and their roles in the plant environmental stress response are unknown. In this work, expression profiles of candidate TdSULTRs were investigated under two different concentrations of salinity, 150 and 250 mM NaCl. TdSULTRs showed diverse expression patterns in response to 150 mM NaCl, while all TdSULTRs, except TRITD3Bv1G170350, were highly induced by 250 mM of NaCl after 72h. These results indicate that the expression pattern of SULTRs is dependent on salt concentration and duration of stress. Also, it seems that TdSULTRs are present in both early and delayed responses to salt stress. For instance, TRID5Bv1G12400, as a member of subfamily TdSULTR 4, could not be classified in the group of early responses to salinity. Hormones, cytosol Ca concentration, and kinases as well as ROS greatly induced plant cell signaling elements in response to stresses [54]. Cis-regulatory elements related to ABA and MeJA-responsive hormones were often found upstream of TdSULTR genes, indicating TdSULTR genes can be controlled by stress-related hormones. These results indicate sulfate transfer genes are diverse in structure and function and are involved in several different cellular pathways.
5. Conclusions
In the present study, the structure and function of sulfate transporter gene family members in T. turgidum L. ssp. durum (Desf.) (TdSULTRs) were investigated. We conclude that TdSULTRs are diverse based on their physiochemical properties and structure. It also appears that TdSULTRs were extended by segmental duplication events. Moreover, we found that TdSULTR genes are involved in the hormone response to salinity and have the potential to induce responses from phytohormones such as ABA and MeJA. However, further functional analyses are required to understand the role of TdSULTRs in durum wheat resistance to stress and to find the upstream elements induced by the TdSULTRs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes14020333/s1, Table S1: List of TdSULTR primers used in real-time PCR analysis; Table S2: List of the duplicated TdSULTR genes along with Ka and Ks values; List Table S3: List of cis elements engaged in various developmental and stress responsive pathways in TdSULTR genes.
Author Contributions
Conceptualization, P.H.; methodology, P.H. and F.P.; software, P.H. and F.P.; validation, P.H.; formal analysis, P.H. and F.P.; investigation, P.H. and S.L.; writing—original draft preparation, P.H. and S.L.; writing—review and editing, P.H. and S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nazar, R.; Iqbal, N.; Masood, A.; Syeed, S.; Khan, N.A. Understanding the significance of sulfur in improving salinity tolerance in plants. Environ. Exp. Bot. 2011, 70, 80–87. [Google Scholar] [CrossRef]
- Hawkesford, M.J. Plant responses to sulphur deficiency and the genetic manipulation of sulphate transporters to improve S-utilization efficiency. J. Exp. Bot. 2000, 51, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Rausch, T.; Wachter, A. Sulfur metabolism: A versatile platform for launching defence operations. Trends Plant Sci. 2005, 10, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Tausz, M.; Šircelj, H.; Grill, D. The glutathione system as a stress marker in plant ecophysiology: Is a stress-response concept valid? J. Exp. Bot. 2004, 55, 1955–1962. [Google Scholar] [CrossRef]
- Szalai, G.; Kellős, T.; Galiba, G.; Kocsy, G. Glutathione as an antioxidant and regulatory molecule in plants under abiotic stress conditions. J. Plant Growth Regul. 2009, 28, 66–80. [Google Scholar] [CrossRef]
- Wang, L.; Chen, K.; Zhou, M. Structure and function of an Arabidopsis thaliana sulfate transporter. Nat. Commun. 2021, 12, 4455. [Google Scholar] [CrossRef]
- Buchner, P.; Stuiver, C.E.E.; Westerman, S.; Wirtz, M.; Hell, R.; Hawkesford, M.J.; De Kok, L.J. Regulation of sulfate uptake and expression of sulfate transporter genes in Brassica oleracea as affected by atmospheric H2S and pedospheric sulfate nutrition. Plant Physiol. 2004, 136, 3396–3408. [Google Scholar] [CrossRef]
- Leustek, T.; Saito, K. Sulfate transport and assimilation in plants. Plant Physiol. 1999, 120, 637–644. [Google Scholar] [CrossRef]
- Shibagaki, N.; Grossman, A.R. Binding of cysteine synthase to the STAS domain of sulfate transporter and its regulatory consequences. J. Biol. Chem. 2010, 285, 25094–25102. [Google Scholar] [CrossRef]
- Shibagaki, N.; Rose, A.; McDermott, J.P.; Fujiwara, T.; Hayashi, H.; Yoneyama, T.; Davies, J.P. Selenate-resistant mutants of Arabidopsis thaliana identify Sultr1; 2, a sulfate transporter required for efficient transport of sulfate into roots. Plant J. 2002, 29, 475–486. [Google Scholar] [CrossRef]
- Zheng, Z.-L.; Zhang, B.; Leustek, T. Transceptors at the boundary of nutrient transporters and receptors: A new role for Arabidopsis SULTR1; 2 in sulfur sensing. Front. Plant Sci. 2014, 5, 710. [Google Scholar] [CrossRef] [PubMed]
- Aarabi, F.; Naake, T.; Fernie, A.R.; Hoefgen, R. Coordinating sulfur pools under sulfate deprivation. Trends Plant Sci. 2020, 25, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Maruyama-Nakashita, A.; Nakamura, Y.; Yamaya, T.; Takahashi, H. Regulation of high-affinity sulphate transporters in plants: Towards systematic analysis of sulphur signalling and regulation. J. Exp. Bot. 2004, 55, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Watanabe-Takahashi, A.; Smith, F.W.; Blake-Kalff, M.; Hawkesford, M.J.; Saito, K. The roles of three functional sulphate transporters involved in uptake and translocation of sulphate in Arabidopsis thaliana. Plant J. 2000, 23, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Kopriva, S.; Giordano, M.; Saito, K.; Hell, R. Sulfur assimilation in photosynthetic organisms: Molecular functions and regulations of transporters and assimilatory enzymes. Annu. Rev. Plant Biol. 2011, 62, 157–184. [Google Scholar] [CrossRef]
- Cao, M.; Wang, Z.; Zhao, Q.; Mao, J.; Speiser, A.; Wirtz, M.; Hell, R.; Zhu, J.; Xiang, C. Sulfate availability affects ABA levels and germination response to ABA and salt stress in A rabidopsis thaliana. Plant J. 2014, 77, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, T.; Watanabe-Takahashi, A.; Hayashi, N.; Ohnishi, M.; Mimura, T.; Buchner, P.; Hawkesford, M.J.; Yamaya, T.; Takahashi, H. Vacuolar sulfate transporters are essential determinants controlling internal distribution of sulfate in Arabidopsis. Plant Cell 2004, 16, 2693–2704. [Google Scholar] [CrossRef]
- Ding, Y.; Zhou, X.; Zuo, L.; Wang, H.; Yu, D. Identification and functional characterization of the sulfate transporter gene GmSULTR1; 2b in soybean. BMC Genom. 2016, 17, 636. [Google Scholar] [CrossRef]
- Vatansever, R.; Koc, I.; Ozyigit, I.I.; Sen, U.; Uras, M.E.; Anjum, N.A.; Pereira, E.; Filiz, E. Genome-wide identification and expression analysis of sulfate transporter (SULTR) genes in potato (Solanum tuberosum L.). Planta 2016, 244, 1167–1183. [Google Scholar] [CrossRef]
- Huang, S.Q.; Xiang, A.L.; Che, L.L.; Chen, S.; Li, H.; Song, J.B.; Yang, Z.M. A set of miRNAs from Brassica napus in response to sulphate deficiency and cadmium stress. Plant Biotechnol. J. 2010, 8, 887–899. [Google Scholar] [CrossRef]
- Kumar, S.; Asif, M.H.; Chakrabarty, D.; Tripathi, R.D.; Dubey, R.S.; Trivedi, P.K. Comprehensive analysis of regulatory elements of the promoters of rice sulfate transporter gene family and functional characterization of OsSul1; 1 promoter under different metal stress. Plant Signal. Behav. 2015, 10, e990843. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, M.; Xia, Z. The SULTR gene family in maize (Zea mays L.): Gene cloning and expression analyses under sulfate starvation and abiotic stress. J. Plant Physiol. 2018, 220, 24–33. [Google Scholar] [CrossRef]
- Dubcovsky, J.; Dvorak, J. Genome plasticity a key factor in the success of polyploid wheat under domestication. Science 2007, 316, 1862–1866. [Google Scholar] [CrossRef]
- Yaghobi, M.; Heidari, P. Genome-wide analysis of aquaporin gene family in Triticum turgidum and its expression profile in response to salt stress. Genes 2023, 14, 202. [Google Scholar] [CrossRef] [PubMed]
- Bolser, D.M.; Staines, D.M.; Perry, E.; Kersey, P.J. Ensembl plants: Integrating tools for visualizing, mining, and analyzing plant genomic data. In Plant Genomics Databases; Springer: New York, NY, USA, 2017; pp. 1–31. [Google Scholar]
- Finn, R.D.; Bateman, A.; Clements, J.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Heger, A.; Hetherington, K.; Holm, L.; Mistry, J. Pfam: The protein families database. Nucleic Acids Res. 2014, 42, D222–D230. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Derbyshire, M.K.; Gonzales, N.R.; Lu, S.; Chitsaz, F.; Geer, L.Y.; Geer, R.C.; He, J.; Gwadz, M.; Hurwitz, D.I. CDD: NCBI’s conserved domain database. Nucleic Acids Res. 2015, 43, D222–D226. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein identification and analysis tools on the ExPASy server. In The Proteomics Protocols Handbook; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar]
- Savojardo, C.; Martelli, P.L.; Fariselli, P.; Profiti, G.; Casadio, R. BUSCA: An integrative web server to predict subcellular localization of proteins. Nucleic Acids Res. 2018, 46, W459–W466. [Google Scholar] [CrossRef] [PubMed]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating Maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef]
- Musavizadeh, Z.; Najafi-Zarrini, H.; Kazemitabar, S.K.; Hashemi, S.H.; Faraji, S.; Barcaccia, G.; Heidari, P. Genome-Wide Analysis of Potassium Channel Genes in Rice: Expression of the OsAKT and OsKAT Genes under Salt Stress. Genes 2021, 12, 784. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J.E. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef]
- Blom, N.; Sicheritz-Pontén, T.; Gupta, R.; Gammeltoft, S.; Brunak, S. Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics 2004, 4, 1633–1649. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Franz, M.; Lopes, C.T.; Huck, G.; Dong, Y.; Sumer, O.; Bader, G.D. Cytoscape. js: A graph theory library for visualisation and analysis. Bioinformatics 2016, 32, 309–311. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Heidari, P.; Ahmadizadeh, M.; Izanlo, F.; Nussbaumer, T. In silico study of the CESA and CSL gene family in Arabidopsis thaliana and Oryza sativa: Focus on post-translation modifications. Plant Gene 2019, 19, 100189. [Google Scholar] [CrossRef]
- Smith, F.W.; Ealing, P.M.; Hawkesford, M.J.; Clarkson, D.T. Plant members of a family of sulfate transporters reveal functional subtypes. Proc. Natl. Acad. Sci. USA 1995, 92, 9373–9377. [Google Scholar] [CrossRef]
- Heidari, P.; Puresmaeli, F.; Mora-Poblete, F. Genome-Wide Identification and Molecular Evolution of the Magnesium Transporter (MGT) Gene Family in Citrullus lanatus and Cucumis sativus. Agronomy 2022, 12, 2253. [Google Scholar] [CrossRef]
- Abdullah; Faraji, S.; Mehmood, F.; Malik, H.M.T.; Ahmed, I.; Heidari, P.; Poczai, P. The GASA Gene Family in Cacao (Theobroma cacao, Malvaceae): Genome Wide Identification and Expression Analysis. Agronomy 2021, 11, 1425. [Google Scholar] [CrossRef]
- Faraji, S.; Ahmadizadeh, M.; Heidari, P. Genome-wide comparative analysis of Mg transporter gene family between Triticum turgidum and Camelina sativa. BioMetals 2021, 34, 639–660. [Google Scholar] [CrossRef]
- Ahmadizadeh, M.; Rezaee, S.; Heidari, P. Genome-wide characterization and expression analysis of fatty acid desaturase gene family in Camelina sativa. Gene Rep. 2020, 21, 100894. [Google Scholar] [CrossRef]
- Faraji, S.; Hasanzadeh, S.; Heidari, P. Comparative in silico analysis of Phosphate transporter gene family, PHT, in Camelina sativa gemome. Gene Rep. 2021, 25, 101351. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Zhao, X.-Q.; Wang, J.; Wong, G.K.-S.; Yu, J. KaKs_Calculator: Calculating Ka and Ks through model selection and model averaging. Genom. Proteom. Bioinform. 2006, 4, 259–263. [Google Scholar] [CrossRef]
- Faraji, S.; Filiz, E.; Kazemitabar, S.K.; Vannozzi, A.; Palumbo, F.; Barcaccia, G.; Heidari, P. The AP2/ERF Gene Family in Triticum durum: Genome-Wide Identification and Expression Analysis under Drought and Salinity Stresses. Genes 2020, 11, 1464. [Google Scholar] [CrossRef]
- Heidari, P.; Abdullah; Faraji, S.; Poczai, P. Magnesium transporter Gene Family: Genome-Wide Identification and Characterization in Theobroma cacao, Corchorus capsularis and Gossypium hirsutum of Family Malvaceae. Agronomy 2021, 11, 1651. [Google Scholar] [CrossRef]
- Yoshimoto, N.; Takahashi, H.; Smith, F.W.; Yamaya, T.; Saito, K. Two distinct high-affinity sulfate transporters with different inducibilities mediate uptake of sulfate in Arabidopsis roots. Plant J. 2002, 29, 465–473. [Google Scholar] [CrossRef]
- Takahashi, H.; Buchner, P.; Yoshimoto, N.; Hawkesford, M.J.; Shiu, S.-H. Evolutionary relationships and functional diversity of plant sulfate transporters. Front. Plant Sci. 2012, 2, 119. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-T.; Heidari, P. Cell Signaling in Model Plants. Int. J. Mol. Sci. 2020, 21, 6062. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).