Functional Association between Storage Protein Mobilization and Redox Signaling in Narrow-Leafed Lupin (Lupinus angustifolius L.) Seed Germination and Seedling Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Microscopy Study of NLL Cotyledon during Seed Germination
2.1.1. Imbibition and Germination of NLL Seeds
2.1.2. Preparation of NLL Seed Samples for Light Microscopy
2.1.3. Observation of NLL Seed Samples via Light Microscopy
2.1.4. Immunocytochemical Analysis
2.2. Gene Expression Analyses during the NLL Seed Germination Process
2.2.1. Total RNA Isolation and cDNA Amplification
2.2.2. Real-Time PCR Analysis
2.2.3. Data Collection and Controls
2.2.4. Data Analysis
2.3. Biochemical Analysis of β-Conglutin Proteins during NLL Seed Germination Process
- -
- SDS-PAGE separation: Initially, protein samples containing 10 µg of protein per sample were subjected to electrophoresis on 4–20% Mini-PROTEAN® TGX™ Precast Gels (Bio-Rad, Hercules, CA, USA), utilizing the Mini-PROTEAN® Tetra Cell apparatus (Bio-Rad). Following electrophoresis, the proteins were visualized using Coomassie Brilliant Blue staining following established protocols.
- -
- Transfer onto PVDF membrane: To facilitate further analysis, the proteins were subsequently transferred from the gel onto polyvinylidene fluoride (PVDF) membranes using the Mini-Trans-Blot Electrophoretic Transfer Cell system (Bio-Rad). This step allowed for the subsequent immunodetection of specific proteins.
- -
- Blocking and primary antibody incubation: The PVDF membranes were blocked for 2 h with a blocking solution containing 5% (w/v) non-fat dry milk in Tris-buffered saline (TBS) buffer at pH 7.4. The immunodetection of β-conglutin proteins was achieved by incubating the membranes with a rabbit polyclonal antiserum developed in-house. The antiserum was diluted to a ratio of 1:1000 in TBS buffer containing 5% (w/v) non-fat dry milk and 0.5% Tween-20.
- -
- Secondary antibody and signal detection: Following primary antibody incubation, a secondary antibody, horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (Bio-Rad), was utilized at a dilution ratio of 1:3000 in TBS buffer with 0.5% Tween-20. The secondary antibody was incubated for 2 h, followed by three 15 min washing steps with TBS containing 0.5% Tween-20 to remove any unbound antibodies.
- -
- Chemiluminescence detection: The presence of β-conglutin proteins was detected through chemiluminescence. The membranes were exposed to the SuperSignal® West Pico Chemiluminescent substrate (Thermo Scientific, Waltham, MA, USA), and the resulting chemiluminescent signal, indicative of the presence of β-conglutin proteins, was captured on X-ray films (Kodak, Rochester, NY, USA). This comprehensive procedure allowed for the detailed examination of β-conglutin proteins and their variations throughout the Narrow-Leafed Lupin seed germination process.
2.4. Statistical Analysis
2.5. Bioinformatic Analysis
2.5.1. Genetic Resources
2.5.2. Primer Design
3. Results and Discussion
3.1. Microscopy Study of PBs during Lupin Seed Germination Reveals the Presence of β-Conglutins
3.2. Biochemical Analysis of β-Conglutins during NLL Seed Germination Process
3.3. Oxidative Metabolism Promotes the Mobilization of Reserve Proteins and Seed Germination
SSPs Are Degraded during Seed Maturation
3.4. Oxidative Enzyme Modifications during Seed Germination
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bailly, C.; El-Maarouf-Bouteau, H.; Corbineau, F. From intracellular signaling networks to cell death: The dual role of reactive oxygen species in seed physiology. Comptes Rendus Biol. 2008, 331, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Niu, Y.; Zheng, Y.; Wang, Z. Advances in the Understanding of Reactive Oxygen Species-Dependent Regulation on Seed Dormancy, Germination, and Deterioration in Crops. Front. Plant Sci. 2022, 13, 826809. [Google Scholar] [CrossRef]
- Smolikova, G.; Leonova, T.; Vashurina, N.; Frolov, A.; Medvedev, S. Desiccation Tolerance as the Basis of Long-Term Seed Viability. Int. J. Mol. Sci. 2020, 22, 101. [Google Scholar] [CrossRef] [PubMed]
- Bewley, J. Seed Germination and Dormancy. Plant Cell 1997, 9, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A. Seed Aging. Implications for Seed Storage and Persistence in the Soil. David A. Priestly. Q. Rev. Biol. 1987, 62, 196–197. [Google Scholar] [CrossRef]
- Matilla, A.J. The Orthodox Dry Seeds Are Alive: A Clear Example of Desiccation Tolerance. Plants 2021, 11, 20. [Google Scholar] [CrossRef]
- Oracz, K.; El-Maarouf Bouteau, H.; Farrant, J.M.; Cooper, K.; Belghazi, M.; Job, C.; Job, D.; Corbineau, F.; Bailly, C. ROS production and protein oxidation as a novel mechanism for seed dormancy alleviation. Plant J. Cell Mol. Biol. 2007, 50, 452–465. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Vranová, E.; Inzé, D.; Van Breusegem, F. Signal transduction during oxidative stress. J. Exp. Bot. 2002, 53, 1227–1236. [Google Scholar] [CrossRef]
- El-Maarouf-Bouteau, H.; Bailly, C. Oxidative signaling in seed germination and dormancy. Plant Signal. Behav. 2008, 3, 175–182. [Google Scholar] [CrossRef]
- Bailly, C. The signalling role of ROS in the regulation of seed germination and dormancy. Biochem. J. 2019, 476, 3019–3032. [Google Scholar] [CrossRef] [PubMed]
- Walters, C. Understanding the mechanisms and kinetics of seed aging. Seed Sci. Res. 1998, 8, 223–244. [Google Scholar] [CrossRef]
- Kurek, K.; Plitta-Michalak, B.; Ratajczak, E. Reactive Oxygen Species as Potential Drivers of the Seed Aging Process. Plants 2019, 8, 174. [Google Scholar] [CrossRef]
- Buitink, J.; Hoekstra, F.A.; Leprince, O. Biochemistry and biophysics of tolerance systems. Desiccation Surviv. Plants Dry. Dying 2002, 293–318. [Google Scholar] [CrossRef]
- Bewley, J.D.; Black, M.; Pritchard, H.W. (Eds.) Desiccation and survival in plants. Drying without dying. In Annals of Botany; CAB International: Wallington, UK, 2003; Volume 91, p. 105. [Google Scholar] [CrossRef]
- Dekkers, B.J.W.; Costa, M.C.D.; Maia, J.; Bentsink, L.; Ligterink, W.; Hilhorst, H.W.M. Acquisition and loss of desiccation tolerance in seeds: From experimental model to biological relevance. Planta 2015, 241, 563–577. [Google Scholar] [CrossRef]
- Bykova, N.V.; Hoehn, B.; Rampitsch, C.; Banks, T.; Stebbing, J.-A.; Fan, T.; Knox, R. Redox-sensitive proteome and antioxidant strategies in wheat seed dormancy control. Proteomics 2011, 11, 865–882. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; Leymarie, J.; Lehner, A.; Rousseau, S.; Côme, D.; Corbineau, F. Catalase activity and expression in developing sunflower seeds as related to drying. J. Exp. Bot. 2004, 55, 475–483. [Google Scholar] [CrossRef]
- Bailly, C.; Audigier, C.; Ladonne, F.; Wagner, M.H.; Coste, F.; Corbineau, F.; Côme, D. Changes in oligosaccharide content and antioxidant enzyme activities in developing bean seeds as related to acquisition of drying tolerance and seed quality. J. Exp. Bot. 2001, 52, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Zhang, J. Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol. 2001, 42, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Buijs, G.; Kodde, J.; Groot, S.P.C.; Bentsink, L. Seed dormancy release accelerated by elevated partial pressure of oxygen is associated with DOG loci. J. Exp. Bot. 2018, 69, 3601–3608. [Google Scholar] [CrossRef]
- Jeevan Kumar, S.P.; Rajendra Prasad, S.; Banerjee, R.; Thammineni, C. Seed birth to death: Dual functions of reactive oxygen species in seed physiology. Ann. Bot. 2015, 116, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.; Lin, T. Mechanism of hydrogen peroxide in improving the germination of Cinnamomum camphora seed. Seed Sci. Technol. 1994, 22, 231–236. [Google Scholar]
- Dormancy Breakate of Hordeum Vulgare Seeds: Effects of Hydrogen Peroxide and Scarification on Glutathione Level and Glutathione Reductase Activity—ScienceOpen. Available online: https://www.scienceopen.com/document?vid=94f17eb1-227e-4e1e-b983-598765f13a0a (accessed on 1 August 2023).
- Barba-Espin, G.; Diaz-Vivancos, P.; Clemente-Moreno, M.J.; Albacete, A.; Faize, L.; Faize, M.; Pérez-Alfocea, F.; Hernández, J.A. Interaction between hydrogen peroxide and plant hormones during germination and the early growth of pea seedlings. Plant Cell Environ. 2010, 33, 981–994. [Google Scholar] [CrossRef] [PubMed]
- Polle, A. Dissecting the superoxide dismutase-ascorbate-glutathione-pathway in chloroplasts by metabolic modeling. Computer simulations as a step towards flux analysis. Plant Physiol. 2001, 126, 445–462. [Google Scholar] [CrossRef]
- Chung, H.S.; Wang, S.-B.; Venkatraman, V.; Murray, C.I.; Van Eyk, J.E. Cysteine Oxidative Post-translational Modifications: Emerging Regulation in the Cardiovascular System. Circ. Res. 2013, 112, 382–392. [Google Scholar] [CrossRef]
- Cysteine-Mediated Redox Signaling: Chemistry, Biology, and Tools for Discovery|Chemical Reviews. Available online: https://pubs.acs.org/doi/10.1021/cr300163e (accessed on 23 February 2023).
- Barba-Espin, G.; Nicolas, E.; Almansa, M.S.; Cantero-Navarro, E.; Albacete, A.; Hernández, J.A.; Díaz-Vivancos, P. Role of thioproline on seed germination: Interaction ROS-ABA and effects on antioxidative metabolism. Plant Physiol. Biochem. PPB 2012, 59, 30–36. [Google Scholar] [CrossRef]
- Job, C.; Rajjou, L.; Lovigny, Y.; Belghazi, M.; Job, D. Patterns of protein oxidation in Arabidopsis seeds and during germination. Plant Physiol. 2005, 138, 790–802. [Google Scholar] [CrossRef]
- Hui, Z.; DongLi, H.; Ming, L.; PingFang, Y. Carbonylated protein changes between active germinated embryos and quiescent embryos give insights into rice seed germination regulation. Plant Growth Regul. 2017, 83, 335–350. [Google Scholar]
- Nyström, T. Role of oxidative carbonylation in protein quality control and senescence. EMBO J. 2005, 24, 1311–1317. [Google Scholar] [CrossRef]
- He, H.; Yan, J.; Yu, X.; Liang, Y.; Fang, L.; Scheller, H.V.; Zhang, A. The NADPH-oxidase AtRbohI plays a positive role in drought-stress response in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2017, 491, 834–839. [Google Scholar] [CrossRef]
- Herman, E.; Larkins, B. Protein storage bodies and vacuoles. Plant Cell 1999, 11, 601–614. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Lopez, J.C.; Hernandez-Soriano, M.C. Protein Bodies in Cotyledon Cells Exhibit Differential Patterns of Legumin-Like Proteins Mobilization during Seedling Germinating States. Am. J. Plant Sci. 2013, 4, 2444–2454. [Google Scholar] [CrossRef][Green Version]
- Shewry, P.R.; Halford, N.G. Cereal seed storage proteins: Structures, properties and role in grain utilization. J. Exp. Bot. 2002, 53, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Foley, R.C.; Jimenez-Lopez, J.C.; Kamphuis, L.G.; Hane, J.K.; Melser, S.; Singh, K.B. Analysis of conglutin seed storage proteins across lupin species using transcriptomic, protein and comparative genomic approaches. BMC Plant Biol. 2015, 15, 106. [Google Scholar] [CrossRef]
- Jimenez-Lopez, J.C.; Melser, S.; DeBoer, K.; Thatcher, L.F.; Kamphuis, L.G.; Foley, R.C.; Singh, K.B. Narrow-Leafed Lupin (Lupinus angustifolius) β1- and β6-Conglutin Proteins Exhibit Antifungal Activity, Protecting Plants against Necrotrophic Pathogen Induced Damage from Sclerotinia sclerotiorum and Phytophthora nicotianae. Front. Plant Sci. 2016, 7, 1856. [Google Scholar] [CrossRef]
- Lima-Cabello, E.; Robles, P.; Alche, J.; Jimenez-Lopez, J. Gene expression and localization of narrow-leafed lupin seed proteins evidence the functional interplay between conglutin protein families driving seed germination. In Proceedings of the 8th ICLGG Conference, Siófok, Hungary, 6–11 August 2023; Jimenez-Lopez’s Personal Communication ICLGG2017/P/67. ISBN 978-615-5270-39-0. [Google Scholar]
- Bailly, C.; Merendino, L. Oxidative signalling in seed germination and early seedling growth: An emerging role for ROS trafficking and inter-organelle communication. Biochem. J. 2021, 478, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Czubinski, J.; Feder, S. Lupin seeds storage protein composition and their interactions with native flavonoids. J. Sci. Food Agric. 2019, 99, 4011–4018. [Google Scholar] [CrossRef] [PubMed]
- Cabello-Hurtado, F.; Keller, J.; Ley, J.; Sanchez-Lucas, R.; Jorrín-Novo, J.V.; Aïnouche, A. Proteomics for exploiting diversity of lupin seed storage proteins and their use as nutraceuticals for health and welfare. J. Proteomics 2016, 143, 57–68. [Google Scholar] [CrossRef]
- Gulisano, A.; Alves, S.; Martins, J.N.; Trindade, L.M. Genetics and Breeding of Lupinus mutabilis: An Emerging Protein Crop. Front. Plant Sci. 2019, 10, 1385. [Google Scholar] [CrossRef] [PubMed]
- Tahmasian, A.; Juhász, A.; Broadbent, J.A.; Nye-Wood, M.G.; Le, T.T.; Colgrave, M.L. Evaluation of the Major Seed Storage Proteins, the Conglutins, Across Genetically Diverse Narrow-Leafed Lupin Varieties. Front. Nutr. 2022, 9, 842168. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Lopez, J.C.; Hernandez-Soriano, M.C. Functional differences of storage proteins are reflected in their mobilization patterns from protein bodies in cotyledon cells during olive (Olea europaea L.) seed germination. Commun. Agric. Appl. Biol. Sci. 2014, 79, 187–192. [Google Scholar] [PubMed]
- Wawrzyńska, A.; Sirko, A. The Role of Selective Protein Degradation in the Regulation of Iron and Sulfur Homeostasis in Plants. Int. J. Mol. Sci. 2020, 21, 2771. [Google Scholar] [CrossRef]
- Zheng, H.; Staehelin, L.A. Protein storage vacuoles are transformed into lytic vacuoles in root meristematic cells of germinating seedlings by multiple, cell type-specific mechanisms. Plant Physiol. 2011, 155, 2023–2035. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Kim, W.-S.; Song, B.; Oehrle, N.W.; Liu, S.; Krishnan, H.B. Soybean Mutants Lacking Abundant Seed Storage Proteins Are Impaired in Mobilization of Storage Reserves and Germination. ACS Omega 2020, 5, 8065–8075. [Google Scholar] [CrossRef] [PubMed]
- Foley, R.C.; Gao, L.-L.; Spriggs, A.; Soo, L.Y.C.; Goggin, D.E.; Smith, P.M.C.; Atkins, C.A.; Singh, K.B. Identification and characterisation of seed storage protein transcripts from Lupinus angustifolius. BMC Plant Biol. 2011, 11, 59. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Ascorbate and Glutathione: Keeping Active Oxygen Under Control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of Ascorbate-Glutathione Pathway in Mitigating Oxidative Damage in Plants under Abiotic Stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef] [PubMed]
- Carmo de Carvalho e Martins, M.D.; Martins; da Silva Santos Oliveira, A.S.; da Silva, L.A.A.; Primo, M.G.S.; de Carvalho Lira, V.B. Biological Indicators of Oxidative Stress [Malondialdehyde, Catalase, Glutathione Peroxidase, and Superoxide Dismutase] and Their Application in Nutrition. In Biomarkers in Nutrition; Patel, V.B., Preedy, V.R., Eds.; Springer International Publishing: Cham, Germany, 2022; pp. 833–856. ISBN 978-3-031-07389-2. [Google Scholar]
- Wulff, A.; Oliveira, H.C.; Saviani, E.E.; Salgado, I. Nitrite reduction and superoxide-dependent nitric oxide degradation by Arabidopsis mitochondria: Influence of external NAD(P)H dehydrogenases and alternative oxidase in the control of nitric oxide levels. Nitric Oxide Biol. Chem. 2009, 21, 132–139. [Google Scholar] [CrossRef]
- Ma, Z.; Marsolais, F.; Bykova, N.V.; Igamberdiev, A.U. Nitric Oxide and Reactive Oxygen Species Mediate Metabolic Changes in Barley Seed Embryo during Germination. Front. Plant Sci. 2016, 7, 138. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Yang, L.; Tian, M.; Chen, J.; Shi, J.; Yang, Y.; Hu, X. Nitric oxide enhances desiccation tolerance of recalcitrant Antiaris toxicaria seeds via protein S-nitrosylation and carbonylation. PLoS ONE 2011, 6, e20714. [Google Scholar] [CrossRef]
- Aravind, P.; Prasad, M.N.V. Modulation of cadmium-induced oxidative stress in Ceratophyllum demersum by zinc involves ascorbate-glutathione cycle and glutathione metabolism. Plant Physiol. Biochem. PPB 2005, 43, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Luo, Z.; Dong, H.; Li, W.; Chen, Y. Non-uniform salinity in the root zone alleviates salt damage by increasing sodium, water and nutrient transport genes expression in cotton. Sci. Rep. 2017, 7, 2879. [Google Scholar] [CrossRef] [PubMed]
- Nagalakshmi, N.; Prasad, M.N.V. Responses of glutathione cycle enzymes and glutathione metabolism to copper stress in Scenedesmus bijugatus. Plant Sci. Int. J. Exp. Plant Biol. 2001, 160, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.M.; Nemat Alla, M.M. Kinetics of inhibition of isoproturon to glutathione-associated enzymes in wheat. Physiol. Mol. Biol. Plants 2020, 26, 1505–1518. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiol. Mol. Biol. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Hossain, M.A.; Teixeira da Silva, J.; Fujita, M. Plant Response and Tolerance to Abiotic Oxidative Stress: Antioxidant Defense Is a Key Factor. In Crop Stress and Its Management: Perspectives and Strategies; Springer: Berlin/Heidelberg, Germany, 2012; Volume 8, pp. 261–315. ISBN 978-94-007-2219-4. [Google Scholar]
- Chen, J.-H.; Jiang, H.-W.; Hsieh, E.-J.; Chen, H.-Y.; Chien, C.-T.; Hsieh, H.-L.; Lin, T.-P. Drought and salt stress tolerance of an Arabidopsis glutathione S-transferase U17 knockout mutant are attributed to the combined effect of glutathione and abscisic acid. Plant Physiol. 2012, 158, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.; Umar, S.; Chan, M.-T. Ascorbate-Glutathione Pathway and Stress Tolerance in Plants; Springer: New York, NY, USA, 2010; ISBN 978-90-481-9403-2. [Google Scholar]
- Pawlak, S.; Firych, A.; Rymer, K.; Deckert, J. Cu,Zn-superoxide dismutase is differently regulated by cadmium and lead in roots of soybean seedlings. Acta Physiol. Plant. 2009, 31, 741–747. [Google Scholar] [CrossRef]
- Xi, D.-M.; Liu, W.-S.; Yang, G.-D.; Wu, C.-A.; Zheng, C.-C. Seed-specific overexpression of antioxidant genes in Arabidopsis enhances oxidative stress tolerance during germination and early seedling growth. Plant Biotechnol. J. 2010, 8, 796–806. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Sun, W.Q. Desiccation sensitivity and activities of free radical-scavenging enzymes in recalcitrant Theobroma cacao seeds. Seed Sci. Res. 1999, 9, 209–217. [Google Scholar] [CrossRef]
- Smirnoff, N. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol. 1993, 125, 27–58. [Google Scholar] [CrossRef] [PubMed]
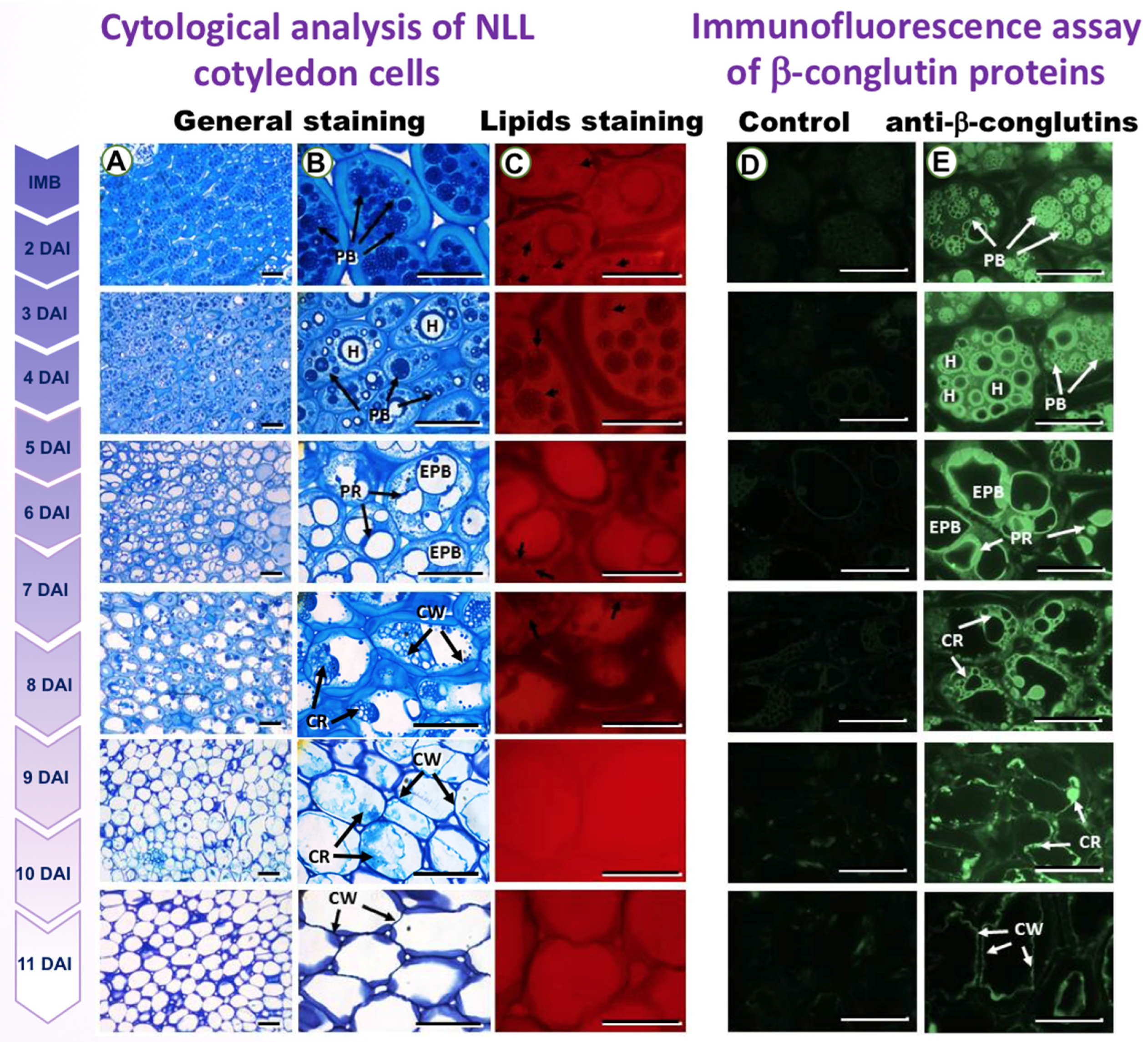
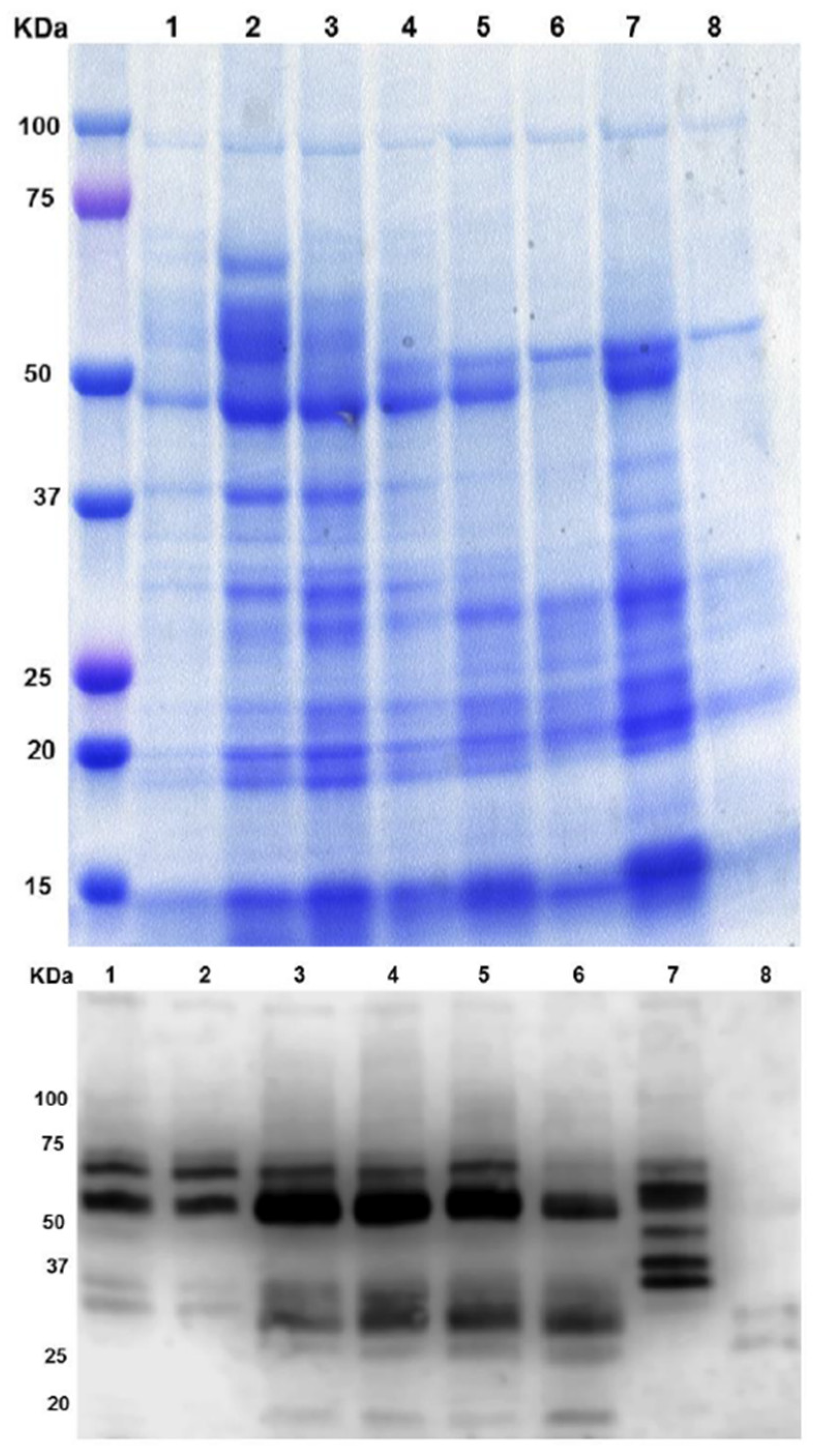
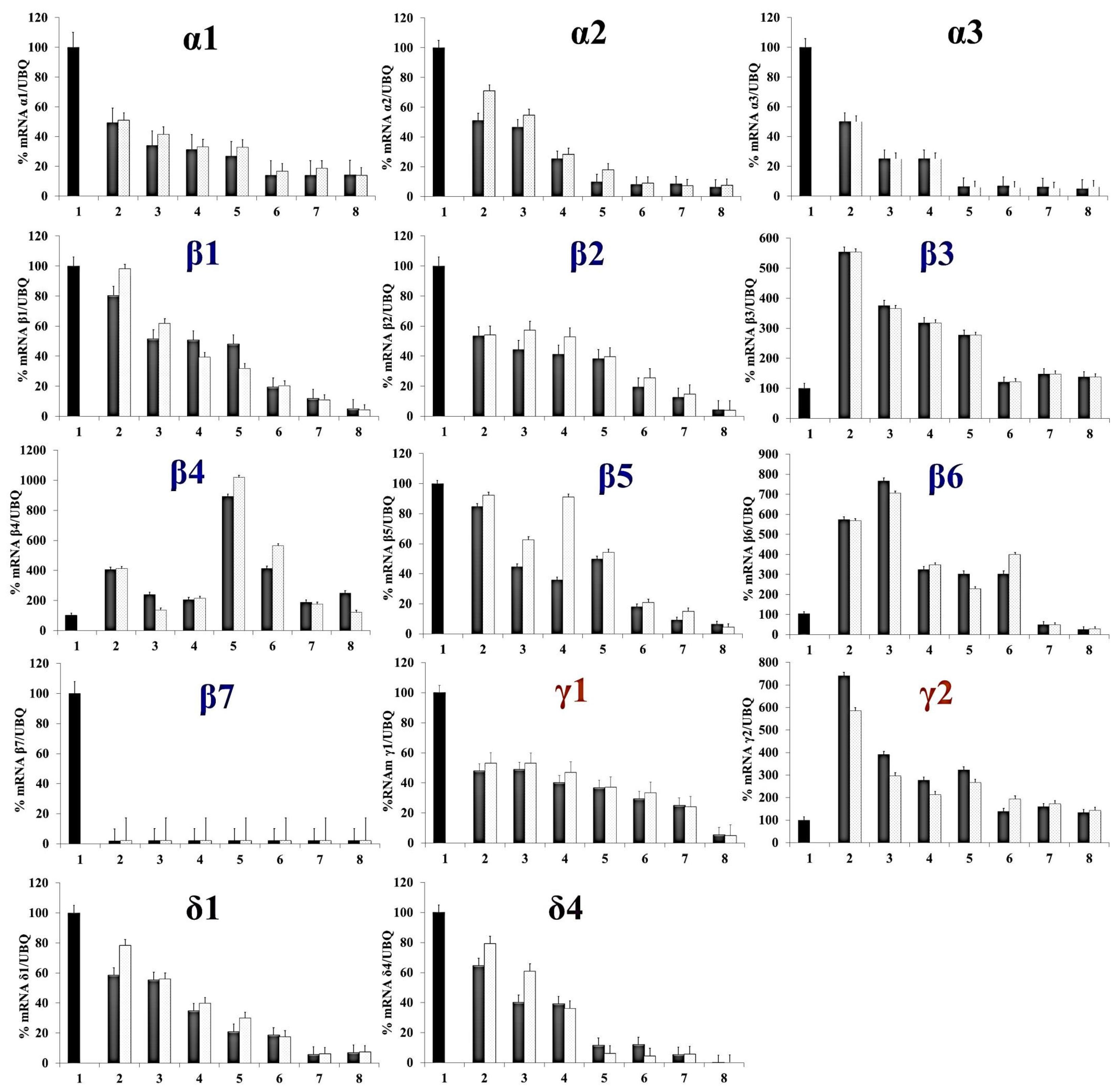
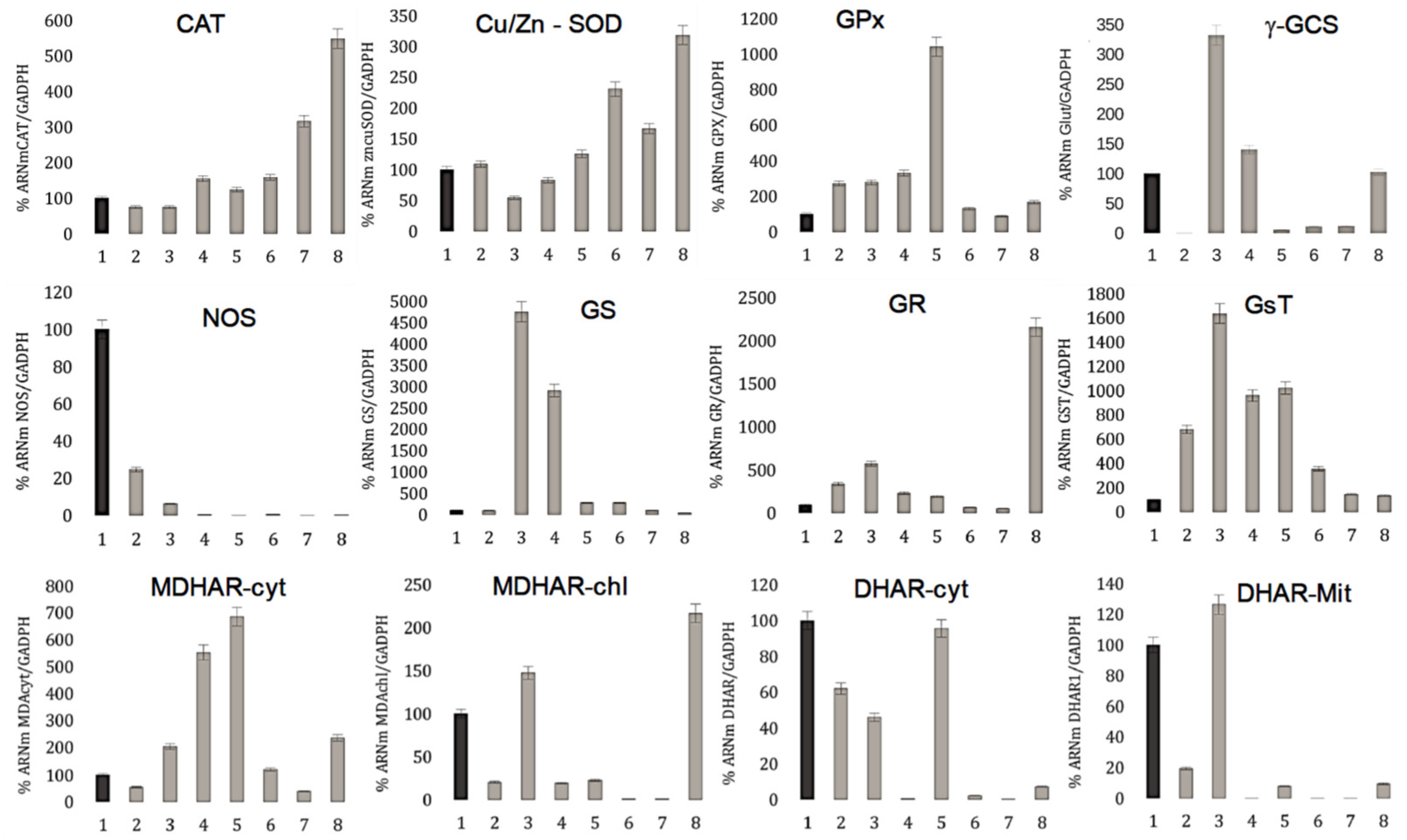
| Primer | Forward | Reverse |
|---|---|---|
| CAT | GGA ACT ATC CCG AGT GGA AAC | CCT CAG GCC AAG TCT TAGT TAC |
| GsT | GGA CCC AAA TGA TGG AAC AGA | GCC AAA CCC AAG TCA ACA AC |
| GS | CAC TAC CAC CAC CAC TCA AA | TGC GAG GTT CAC GGA TTT |
| GPx | CAA GGA TGA TGC GGA GTA TGT | TGA AAC CTC CTG TGC CAT AAA |
| GR | GGA GCC AAG GTT GGG ATT T | GGG AAC ACA GCC ACG AAT AA |
| Cu/Zn-SOD | GGG TCA CCT GGG AAA CAT AG | CCA CTA AGG CTC TTC CAA CTA C |
| NOS | CCA GAG GTT TGC CTC AGA TT | TTC ACC AGA TGA ACG GAT TG |
| MDHAR-chl | GAA ACC TAT CCG GTG TTC ACT AT | TTC ACC TCC AAC AAC TAC AAC |
| MDHAR_cyt | GGA CGA GAG CAG ATT TCC ATA A | AAA GGA GAA GGG AAA GTG TGA G |
| DHAR-chl | CTC CTC CTT CCC AAC CAT TT | TTC CTC CAG TGT CAG CAA TAC |
| DHAR_cyt | GAG ACA AGG CTG AGG GTA TTT | GGA GAT AAG TCC AGA AGG GAA AG |
| GAPDH | CGT GTC CCT ACA GTT GAT GTT | CCT CCT TGA TGG CAG CTT TA |
| Primer | Forward | Reverse |
|---|---|---|
| α1 | GAA CAA GGG CAA GGT CCT A | CAC CAG TAG GCA CTG CTA |
| α2 | GAA GAA ACA AGT ACT CGA G | GTT CTT GRC CTT GTT CTT G |
| α3 | CAC GGC GAA GAC ATA CAC G | CTC FFT FFF FAT TFT TCC T |
| β1 | AGG AAT ACG AGC AAG GAG AGG | GAA AGT GGA TGA CCT GCC G |
| β2 | AAG AAG GGG TTT ATC TTT TCC | CAT GAT AGT AGA TAG CAG CTT AG |
| β3 | GGA ACA GAG GGA AGA GAG AGA AC | CAT TGG CTC GTC ATC TTC CTG C |
| β4 | CAG AAG TTA AAG GGC TCA | TTG TTG TTG TTG GGG T |
| β5 | CAG AGT GGT GAT GAG AGA AAG AC | CAT TGT CTG CCT GAG CT |
| β6 | GAG CGT GAT CGT GAG CCT T | GTT CTC TCT CTT CCC TCT GTT CC |
| β7 | GTG AGA TTT CCA ACC TTA GTC | TTG TTT ACG CGG ACG TG |
| γ1 | ATG GCA CGA AAT ATG GCT CA | AGA AGT GGG TAG AGG ATA ATG C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escudero-Feliu, J.; Lima-Cabello, E.; Rodríguez de Haro, E.; Morales-Santana, S.; Jimenez-Lopez, J.C. Functional Association between Storage Protein Mobilization and Redox Signaling in Narrow-Leafed Lupin (Lupinus angustifolius L.) Seed Germination and Seedling Development. Genes 2023, 14, 1889. https://doi.org/10.3390/genes14101889
Escudero-Feliu J, Lima-Cabello E, Rodríguez de Haro E, Morales-Santana S, Jimenez-Lopez JC. Functional Association between Storage Protein Mobilization and Redox Signaling in Narrow-Leafed Lupin (Lupinus angustifolius L.) Seed Germination and Seedling Development. Genes. 2023; 14(10):1889. https://doi.org/10.3390/genes14101889
Chicago/Turabian StyleEscudero-Feliu, Julia, Elena Lima-Cabello, Esther Rodríguez de Haro, Sonia Morales-Santana, and Jose C. Jimenez-Lopez. 2023. "Functional Association between Storage Protein Mobilization and Redox Signaling in Narrow-Leafed Lupin (Lupinus angustifolius L.) Seed Germination and Seedling Development" Genes 14, no. 10: 1889. https://doi.org/10.3390/genes14101889
APA StyleEscudero-Feliu, J., Lima-Cabello, E., Rodríguez de Haro, E., Morales-Santana, S., & Jimenez-Lopez, J. C. (2023). Functional Association between Storage Protein Mobilization and Redox Signaling in Narrow-Leafed Lupin (Lupinus angustifolius L.) Seed Germination and Seedling Development. Genes, 14(10), 1889. https://doi.org/10.3390/genes14101889







