Effects of Heat Stress on Motion Characteristics and Metabolomic Profiles of Boar Spermatozoa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Heat Stress Treatment for Boar Sperm
2.2. Evaluation of Sperm Quality
2.3. Purification of Spermatozoa
2.4. Metabolite Extraction
2.5. Liquid Chromatography–Mass Spectrometry (LC-MS) Analysis
2.6. Data Preprocessing and Annotation
2.7. Statistical Analysis
3. Results
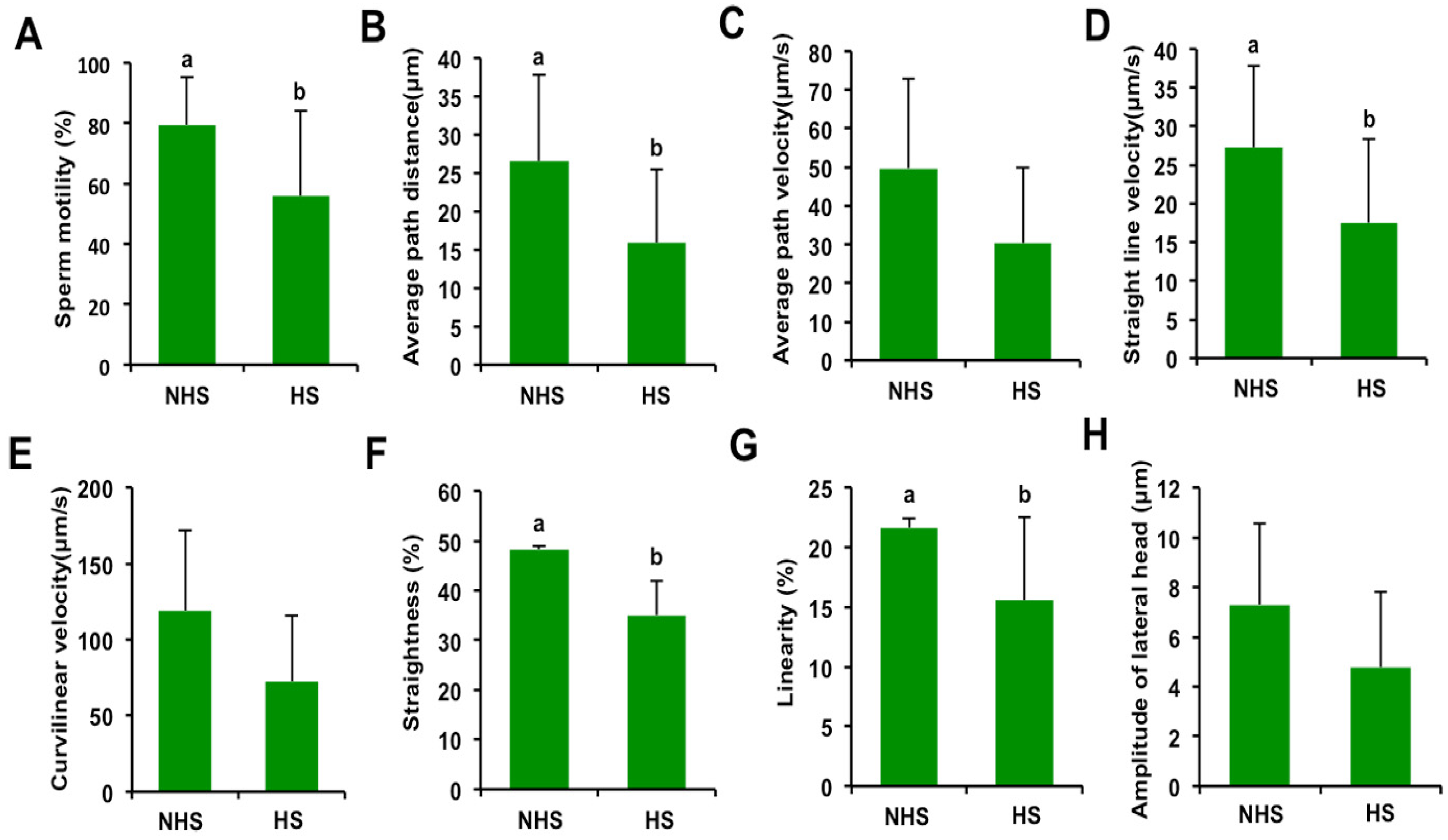
3.1. Effect of Elevated Ambient Temperature on Motion Characteristics in Boar Spermatozoa
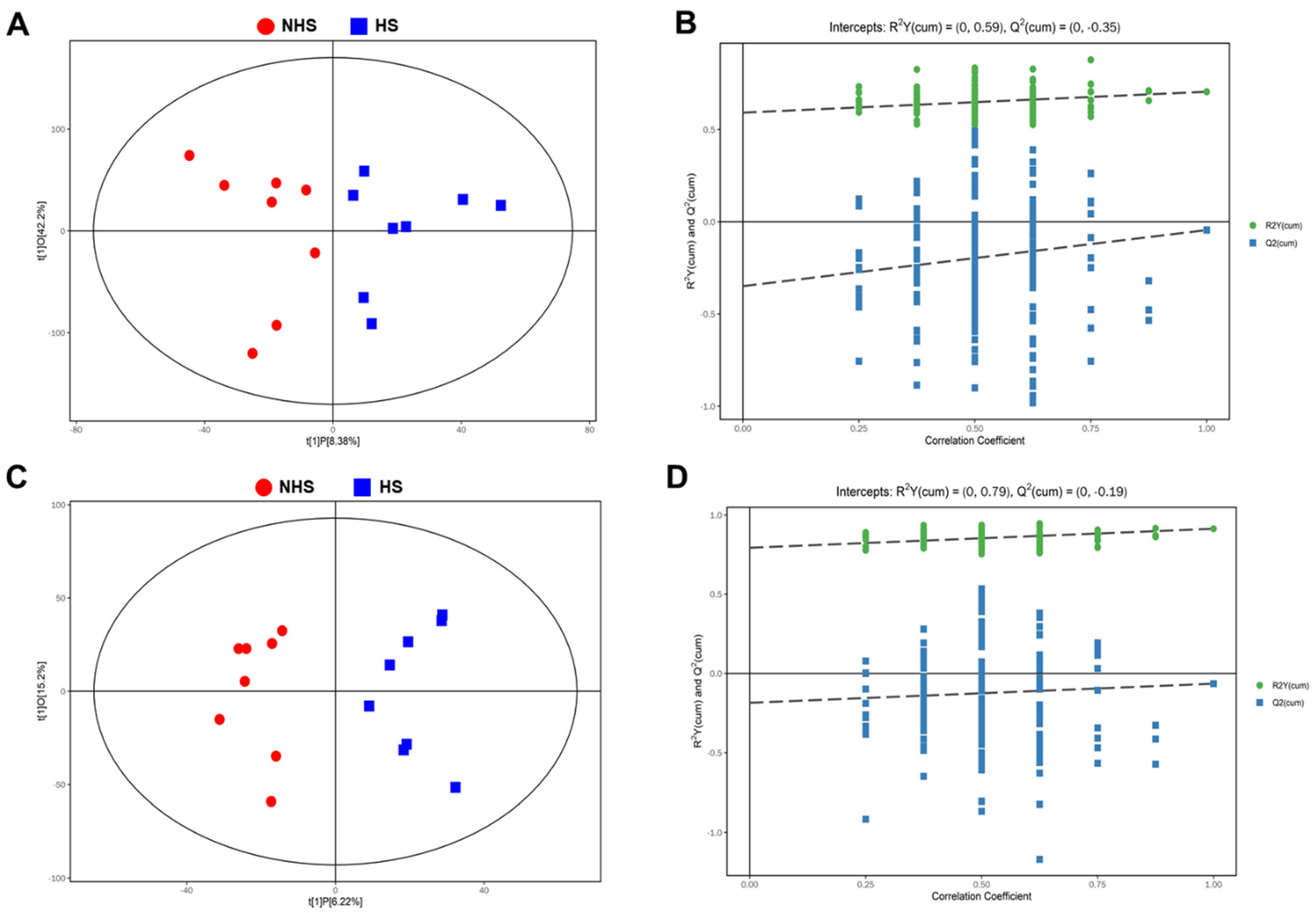
3.2. Changes of Metabolomics Features in Boar Spermatozoa
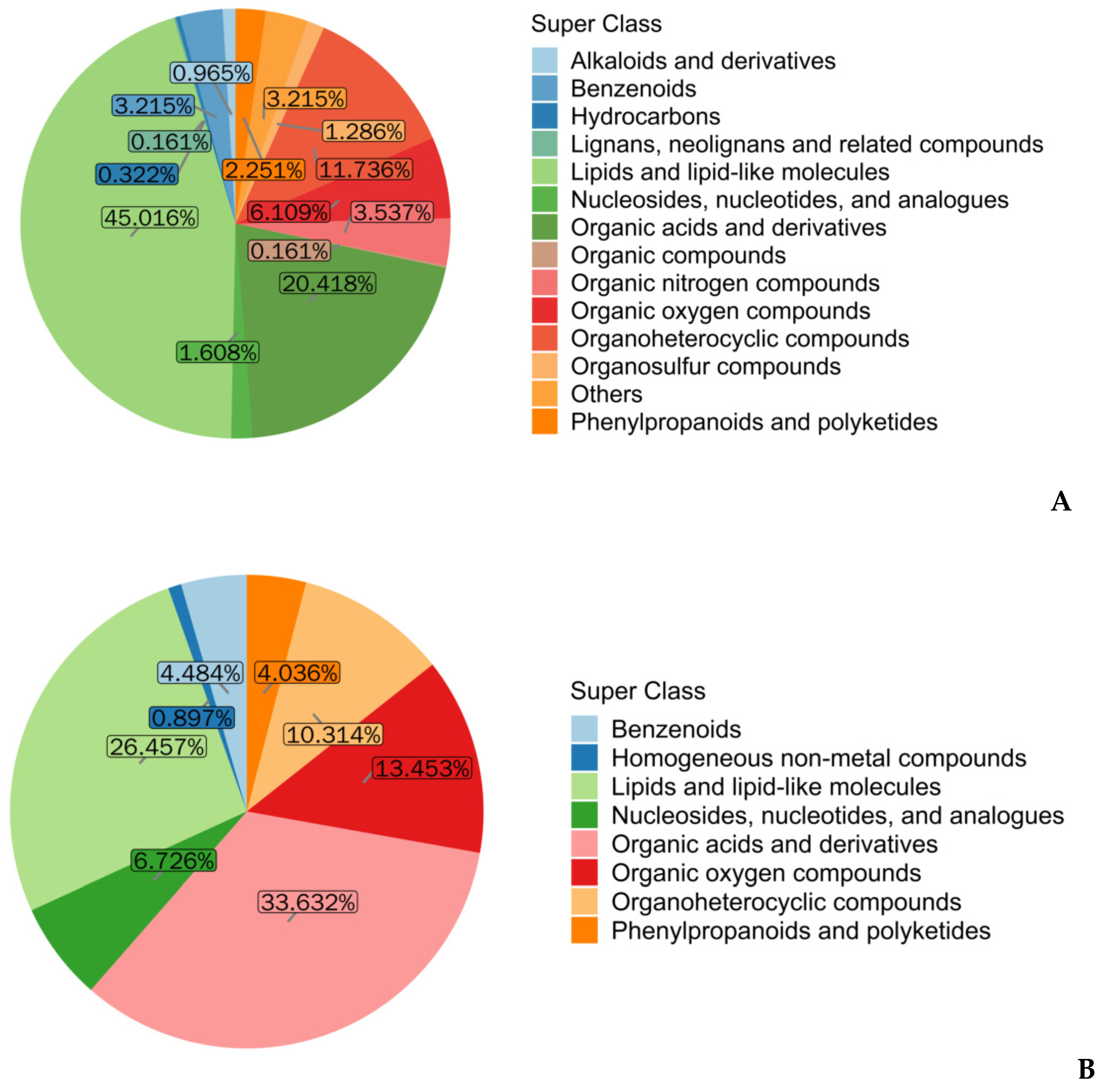
3.3. Classification of Sperm Metabolites in the Positive and Negative Ion Modes
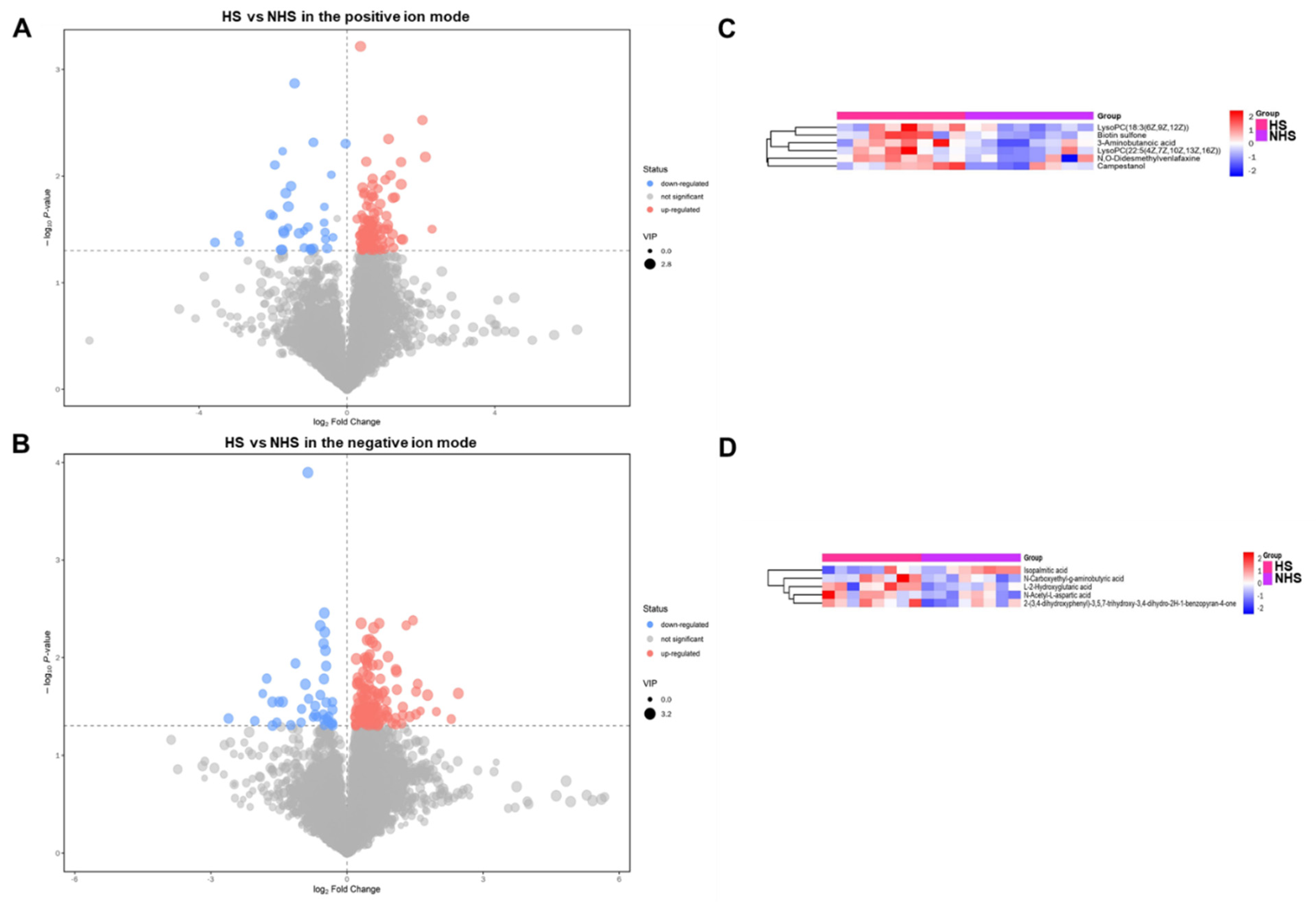
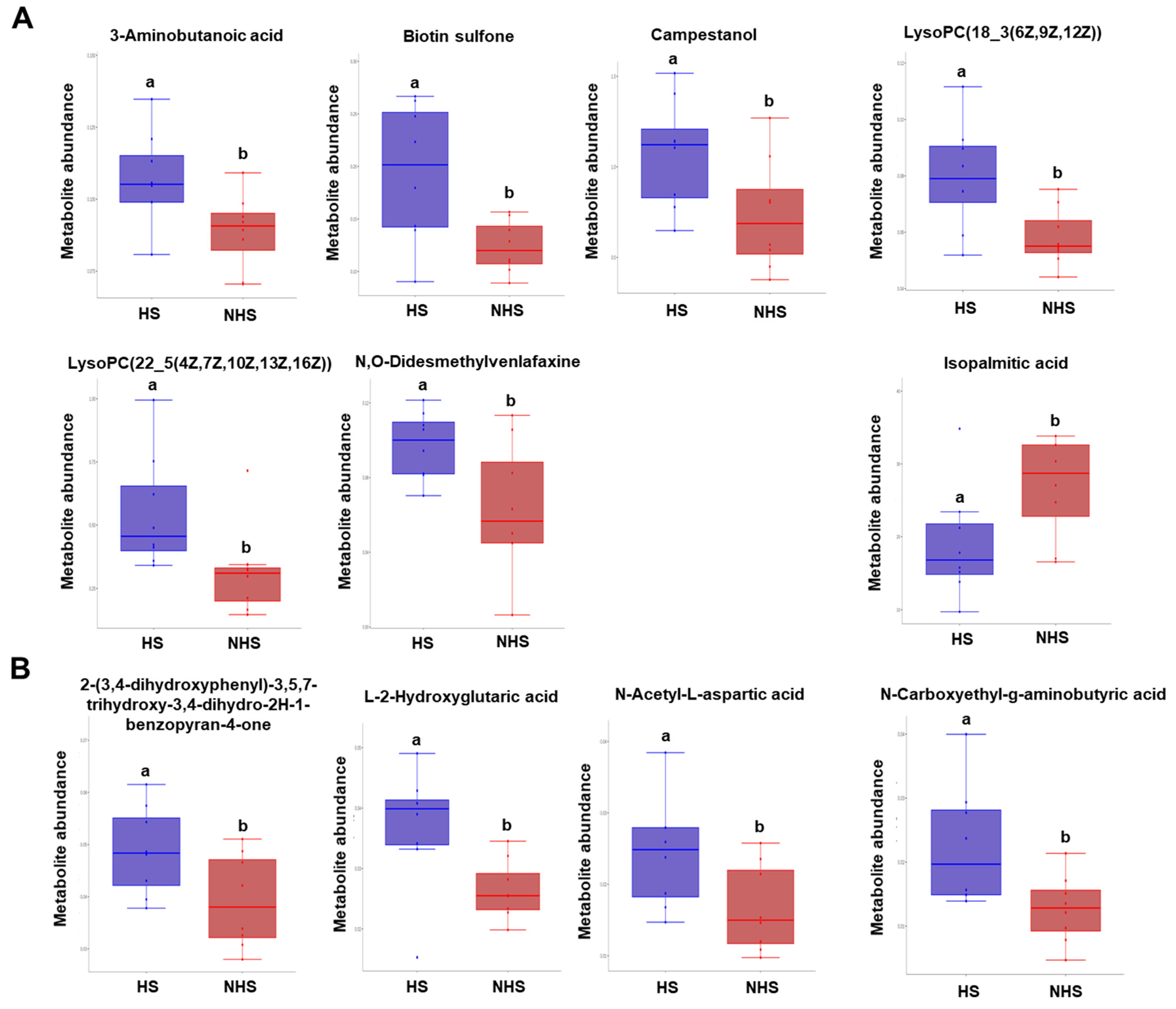
3.4. Identification and Clustering Analysis of Differential Metabolites in Sperm
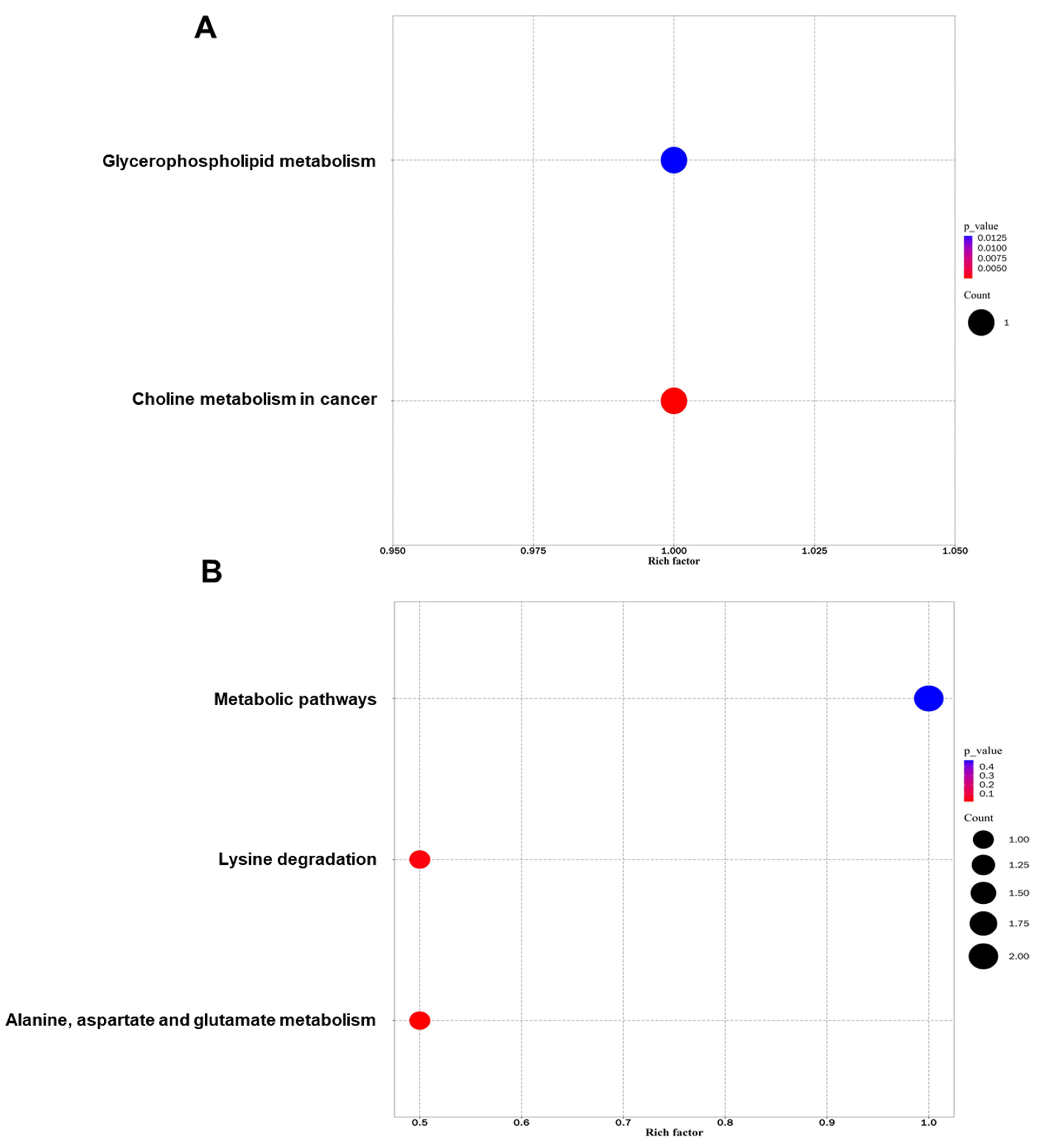
3.5. Metabolic Pathway Analysis for Differential Metabolites in Sperm
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mayorga, E.J.; Renaudeau, D.; Ramirez, B.C.; Ross, J.W.; Baumgard, L.H. Heat stress adaptations in pigs. Anim. Front. 2019, 9, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Wettemann, R.P.; Wells, M.E.; Omtvedt, I.T.; Pope, C.E.; Turman, E.J. Influence of elevated ambient temperature on reproductive performance of boars. J. Anim. Sci. 1976, 42, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Malmgren, L.; Larsson, K. Semen quality and fertility after heat stress in boars. Acta Vet Scand. 1984, 25, 425–435. Available online: https://www.ncbi.nlm.nih.gov/pubmed/6524574 (accessed on 1 September 1984). [CrossRef] [PubMed]
- Ciereszko, A.; Ottobre, J.S.; Glogowski, J. Effects of season and breed on sperm acrosin activity and semen quality of boars. Anim. Reprod. Sci. 2000, 64, 89–96. [Google Scholar] [CrossRef]
- Flowers, W.L. Genetic and phenotypic variation in reproductive traits of AI boars. Theriogenology 2008, 70, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Pena, S.T., Jr.; Stone, F.; Gummow, B.; Parker, A.J.; Paris, D. Susceptibility of boar spermatozoa to heat stress using in vivo and in vitro experimental models. Trop. Anim. Health Prod. 2021, 53, 97. [Google Scholar] [CrossRef]
- Pena, S.T., Jr.; Stone, F.; Gummow, B.; Parker, A.J.; Paris, D. Tropical summer induces DNA fragmentation in boar spermatozoa: Implications for evaluating seasonal infertility. Reprod. Fertil. Dev. 2019, 31, 590–601. [Google Scholar] [CrossRef]
- Li, Y.; Cao, Y.; Zhou, X.; Wang, F.; Shan, T.; Li, Z.; Xu, W.; Li, C. Effects of zinc sulfate pretreatment on heat tolerance of Bama miniature pig under high ambient temperature. J. Anim. Sci. 2015, 93, 3421–3430. [Google Scholar] [CrossRef]
- Wu, Y.Q.; Rao, M.; Hu, S.F.; Ke, D.D.; Zhu, C.H.; Xia, W. Effect of transient scrotal hyperthermia on human sperm: An iTRAQ-based proteomic analysis. Reprod. Biol. Endocrinol. 2020, 18, 83. [Google Scholar] [CrossRef]
- Martin-Hidalgo, D.; Macias-Garcia, B.; Garcia-Marin, L.J.; Bragado, M.J.; Gonzalez-Fernandez, L. Boar spermatozoa proteomic profile varies in sperm collected during the summer and winter. Anim. Reprod. Sci. 2020, 219, 106513. [Google Scholar] [CrossRef]
- Rocha, D.R.; Martins, J.A.; van Tilburg, M.F.; Oliveira, R.V.; Moreno, F.B.; Monteiro-Moreira, A.C.; Moreira, R.A.; Araujo, A.A.; Moura, A.A. Effect of increased testicular temperature on seminal plasma proteome of the ram. Theriogenology 2015, 84, 1291–1305. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.R.; de Lazari, F.L.; Dalberto, P.F.; Bizarro, C.V.; Sontag, E.R.; Koetz Junior, C.; Menegassi, S.R.O.; Barcellos, J.O.J.; Bustamante-Filho, I.C. Effect of scrotal insulation on sperm quality and seminal plasma proteome of Brangus bulls. Theriogenology 2020, 144, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Argov-Argaman, N.; Mahgrefthe, K.; Zeron, Y.; Roth, Z. Season-induced variation in lipid composition is associated with semen quality in Holstein bulls. Reproduction 2013, 145, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wang, X.; Lei, Z.; Ping, J.; Liu, J.; Ma, Z.; Zhang, Z.; Jia, C.; Jin, M.; Li, X.; et al. Heat-stress-induced metabolic changes and altered male reproductive function. J. Proteome Res. 2015, 14, 1495–1503. [Google Scholar] [CrossRef]
- Sun, L.; Cui, Z.; Huang, S.; Xue, Q.; Rehman, S.U.; Luo, X.; Shi, D.; Li, X. Effect of environmental temperature on semen quality and seminal plasma metabolites of Mediterranean buffalo bulls. Anim. Biotechnol. 2022, 1–11. [Google Scholar] [CrossRef]
- Kathiravan, P.; Kalatharan, J.; Karthikeya, G.; Rengarajan, K.; Kadirvel, G. Objective sperm motion analysis to assess dairy bull fertility using computer-aided system--a review. Reprod. Domest. Anim. 2011, 46, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Maya-Soriano, M.J.; Taberner, E.; Sabes-Alsina, M.; Ramon, J.; Rafel, O.; Tusell, L.; Piles, M.; Lopez-Bejar, M. Daily exposure to summer temperatures affects the motile subpopulation structure of epididymal sperm cells but not male fertility in an in vivo rabbit model. Theriogenology 2015, 84, 384–389. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, H.; Liu, Y.; Zhao, M.; Xu, Q.; Liu, Z.; Weng, X. Metabolomic Analysis and Identification of Sperm Freezability-Related Metabolites in Boar Seminal Plasma. Animals 2021, 11, 1939. [Google Scholar] [CrossRef]
- Ugur, M.R.; Dinh, T.; Hitit, M.; Kaya, A.; Topper, E.; Didion, B.; Memili, E. Amino Acids of Seminal Plasma Associated With Freezability of Bull Sperm. Front. Cell Dev. Biol. 2019, 7, 347. [Google Scholar] [CrossRef]
- Nimptsch, A.; Pyttel, S.; Paasch, U.; Mohr, C.; Heinrich, J.M.; Schiller, J. A MALDI MS investigation of the lysophosphatidylcholine/phosphatidylcholine ratio in human spermatozoa and erythrocytes as a useful fertility marker. Lipids 2014, 49, 287–293. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, R.; Feng, C.; Liu, R.; Zheng, Y.; Hoque, S.A.M.; Wu, D.; Lu, H.; Zhang, T.; Zeng, W. Exogenous Oleic Acid and Palmitic Acid Improve Boar Sperm Motility via Enhancing Mitochondrial Beta-Oxidation for ATP Generation. Animals 2020, 10, 591. [Google Scholar] [CrossRef] [PubMed]
- Di Giorgio, N.P.; Bizzozzero-Hiriart, M.; Libertun, C.; Lux-Lantos, V. Unraveling the connection between GABA and kisspeptin in the control of reproduction. Reproduction 2019, 157, R225–R233. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.K.; Im, K.S.; Zheng, X.; Foote, R.H. In vitro capacitation and fertilizing ability of ejaculated rabbit sperm treated with lysophosphatidylcholine. Gamete Res. 1989, 22, 131–141. [Google Scholar] [CrossRef]
- Perez Aguirreburualde, M.S.; Fernandez, S.; Cordoba, M. Acrosin activity regulation by protein kinase C and tyrosine kinase in bovine sperm acrosome exocytosis induced by lysophosphatidylcholine. Reprod. Domest. Anim. 2012, 47, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, K.E.; Hofmo, P.O.; Tverdal, A.; Miller, R.R., Jr. Within and between breed differences in freezing tolerance and plasma membrane fatty acid composition of boar sperm. Reproduction 2006, 131, 887–894. [Google Scholar] [CrossRef]
- Kurata, S.; Hiradate, Y.; Umezu, K.; Hara, K.; Tanemura, K. Capacitation of mouse sperm is modulated by gamma-aminobutyric acid (GABA) concentration. J. Reprod. Dev. 2019, 65, 327–334. [Google Scholar] [CrossRef]
- Kurata, S.; Umezu, K.; Takamori, H.; Hiradate, Y.; Hara, K.; Tanemura, K. Exogenous gamma-aminobutyric acid addition enhances porcine sperm acrosome reaction. Anim. Sci. J. 2022, 93, e13744. [Google Scholar] [CrossRef]
- Ritta, M.N.; Bas, D.E.; Tartaglione, C.M. In vitro effect of gamma-aminobutyric acid on bovine spermatozoa capacitation. Mol. Reprod. Dev. 2004, 67, 478–486. [Google Scholar] [CrossRef]
- Shi, Q.X.; Yuan, Y.Y.; Roldan, E.R. gamma-Aminobutyric acid (GABA) induces the acrosome reaction in human spermatozoa. Mol. Hum. Reprod. 1997, 3, 677–683. [Google Scholar] [CrossRef]
- Medica, A.J.; Aitken, R.J.; Nicolson, G.L.; Sheridan, A.R.; Swegen, A.; De Iuliis, G.N.; Gibb, Z. Glycerophospholipids protect stallion spermatozoa from oxidative damage in vitro. Reprod. Fertil. 2021, 2, 199–209. [Google Scholar] [CrossRef]
- Hezavehei, M.; Sharafi, M.; Fathi, R.; Shahverdi, A.; Gilani, M.A.S. Membrane lipid replacement with nano-micelles in human sperm cryopreservation improves post-thaw function and acrosome protein integrity. Reprod. Biomed. Online 2021, 43, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.; Costa, C.; Bassaizteguy, V.; Santos, M.; Cardozo, R.; Montes, J.; Settineri, R.; Nicolson, G.L. Incubation of human sperm with micelles made from glycerophospholipid mixtures increases sperm motility and resistance to oxidative stress. PLoS ONE 2018, 13, e0197897. [Google Scholar] [CrossRef] [PubMed]
- Geer, B.W. Dietary choline requirements for sperm motility and normal mating activity in Drosophila melanogaster. Biol. Bull. 1967, 133, 548–566. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ren, L.; Chang, Z.; Zhao, Y.; Zhang, Y.; Xia, D.; Zhao, R.; He, B. Lysine acetylation participates in boar spermatozoa motility and acrosome status regulation under different glucose conditions. Theriogenology 2021, 159, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Ritagliati, C.; Luque, G.M.; Stival, C.; Baro Graf, C.; Buffone, M.G.; Krapf, D. Lysine acetylation modulates mouse sperm capacitation. Sci. Rep. 2018, 8, 13334. [Google Scholar] [CrossRef]
- He, S.; Woods, L.C., 3rd. Effects of glycine and alanine on short-term storage and cryopreservation of striped bass (Morone saxatilis) spermatozoa. Cryobiology 2003, 46, 17–25. [Google Scholar] [CrossRef]
- Ansari, M.; Zhandi, M.; Kohram, H.; Zaghari, M.; Sadeghi, M.; Sharafi, M. Improvement of post-thawed sperm quality and fertility of Arian rooster by oral administration of d-aspartic acid. Theriogenology 2017, 92, 69–74. [Google Scholar] [CrossRef]
- Macchia, G.; Topo, E.; Mangano, N.; D’Aniello, E.; Boni, R. DL-Aspartic acid administration improves semen quality in rabbit bucks. Anim. Reprod. Sci. 2010, 118, 337–343. [Google Scholar] [CrossRef]
- Raspa, M.; Paoletti, R.; Mahabir, E.; Scavizzi, F. d-aspartate treatment in vitro improves mouse sperm fertility in young B6N mice. Theriogenology 2020, 148, 60–67. [Google Scholar] [CrossRef]
- Boni, R. Heat stress, a serious threat to reproductive function in animals and humans. Mol. Reprod. Dev. 2019, 86, 1307–1323. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Meng, T.; Wu, L.; Duan, Y.; Li, G.; Shi, C.; Zhang, H.; Peng, Z.; Fan, C.; Ma, J.; et al. Association between ambient temperature and semen quality: A longitudinal study of 10 802 men in China. Environ. Int. 2020, 135, 105364. [Google Scholar] [CrossRef] [PubMed]
- Hoang-Thi, A.P.; Dang-Thi, A.T.; Phan-Van, S.; Nguyen-Ba, T.; Truong-Thi, P.L.; Le-Minh, T.; Nguyen-Vu, Q.H.; Nguyen-Thanh, T. The Impact of High Ambient Temperature on Human Sperm Parameters: A Meta-Analysis. Iran. J. Public Health 2022, 51, 710–723. [Google Scholar] [CrossRef] [PubMed]
- Kaewman, P.; Nudmamud-Thanoi, S.; Amatyakul, P.; Thanoi, S. High mRNA expression of GABA receptors in human sperm with oligoasthenoteratozoospermia and teratozoospermia and its association with sperm parameters and intracytoplasmic sperm injection outcomes. Clin. Exp. Reprod. Med. 2021, 48, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Talevi, R.; Barbato, V.; Fiorentino, I.; Braun, S.; Longobardi, S.; Gualtieri, R. Protective effects of in vitro treatment with zinc, d-aspartate and coenzyme q10 on human sperm motility, lipid peroxidation and DNA fragmentation. Reprod. Biol. Endocrinol. 2013, 11, 81. [Google Scholar] [CrossRef]
- Cheng, Y.M.; Hu, X.N.; Peng, Z.; Pan, T.T.; Wang, F.; Chen, H.Y.; Chen, W.Q.; Zhang, Y.; Zeng, X.H.; Luo, T. Lysine glutarylation in human sperm is associated with progressive motility. Hum. Reprod. 2019, 34, 1186–1194. [Google Scholar] [CrossRef]
- Yu, H.; Diao, H.; Wang, C.; Lin, Y.; Yu, F.; Lu, H.; Xu, W.; Li, Z.; Shi, H.; Zhao, S.; et al. Acetylproteomic analysis reveals functional implications of lysine acetylation in human spermatozoa (sperm). Mol. Cell. Proteom. 2015, 14, 1009–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sui, H.; Wang, S.; Liu, G.; Meng, F.; Cao, Z.; Zhang, Y. Effects of Heat Stress on Motion Characteristics and Metabolomic Profiles of Boar Spermatozoa. Genes 2022, 13, 1647. https://doi.org/10.3390/genes13091647
Sui H, Wang S, Liu G, Meng F, Cao Z, Zhang Y. Effects of Heat Stress on Motion Characteristics and Metabolomic Profiles of Boar Spermatozoa. Genes. 2022; 13(9):1647. https://doi.org/10.3390/genes13091647
Chicago/Turabian StyleSui, Heming, Shiqi Wang, Gang Liu, Fei Meng, Zubing Cao, and Yunhai Zhang. 2022. "Effects of Heat Stress on Motion Characteristics and Metabolomic Profiles of Boar Spermatozoa" Genes 13, no. 9: 1647. https://doi.org/10.3390/genes13091647
APA StyleSui, H., Wang, S., Liu, G., Meng, F., Cao, Z., & Zhang, Y. (2022). Effects of Heat Stress on Motion Characteristics and Metabolomic Profiles of Boar Spermatozoa. Genes, 13(9), 1647. https://doi.org/10.3390/genes13091647





