Association of the ACTN3 rs1815739 Polymorphism with Physical Performance and Injury Incidence in Professional Women Football Players
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Design
2.3. Sample Collection and Genotyping
2.4. Physical Performance Testing
2.4.1. Ankle Dorsiflexion
2.4.2. Sit-and-Reach Test
2.4.3. Countermovement Jump
2.4.4. Sprint Time
2.5. Exposure Times
2.6. Injury Data Collection
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Del Coso, J.; Hiam, D.; Houweling, P.; Pérez, L.M.; Eynon, N.; Lucía, A. More than a ‘speed gene’: ACTN3 R577X genotype, trainability, muscle damage, and the risk for injuries. Eur. J. Appl. Physiol. 2019, 119, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Houweling, P.J.; Papadimitriou, I.D.; Seto, J.T.; Pérez, L.M.; Coso, J.D.; North, K.N.; Lucia, A.; Eynon, N. Is evolutionary loss our gain? The role of ACTN3 p.Arg577Ter (R577X) genotype in athletic performance, ageing, and disease. Hum. Mutat. 2018, 39, 1774–1787. [Google Scholar] [CrossRef] [PubMed]
- North, K.N.; Beggs, A.H. Deficiency of a skeletal muscle isoform of alpha-actinin (alpha-actinin-3) in merosin-positive congenital muscular dystrophy. Neuromuscul. Disord. 1996, 6, 229–235. [Google Scholar] [CrossRef]
- Houweling, P.J.; Berman, Y.D.; Turner, N.; Quinlan, K.G.R.; Seto, J.T.; Yang, N.; Lek, M.; MacArthur, D.G.; Cooney, G.; North, K.N. Exploring the relationship between α-actinin-3 deficiency and obesity in mice and humans. Int. J. Obes. 2017, 41, 1154–1157. [Google Scholar] [CrossRef]
- Hogarth, M.W.; Garton, F.C.; Houweling, P.J.; Tukiainen, T.; Lek, M.; Macarthur, D.G.; Seto, J.T.; Quinlan, K.G.R.; Yang, N.; Head, S.I.; et al. Analysis of the ACTN3 heterozygous genotype suggests that α-actinin-3 controls sarcomeric composition and muscle function in a dose-dependent fashion. Hum. Mol. Genet. 2016, 25, 866–877. [Google Scholar] [CrossRef]
- Erskine, R.M.; Williams, A.G.; Jones, D.A.; Stewart, C.E.; Degens, H. The individual and combined influence of ACE and ACTN3 genotypes on muscle phenotypes before and after strength training. Scand. J. Med. Sci. Sports 2014, 24, 642–648. [Google Scholar] [CrossRef]
- Zempo, H.; Tanabe, K.; Murakami, H.; Iemitsu, M.; Maeda, S.; Kuno, S. ACTN3 polymorphism affects thigh muscle area. Int. J. Sports Med. 2010, 31, 138–142. [Google Scholar] [CrossRef]
- Del Coso, J.; Valero, M.; Salinero, J.J.; Lara, B.; Díaz, G.; Gallo-Salazar, C.; Ruiz-Vicente, D.; Areces, F.; Puente, C.; Carril, J.C.; et al. ACTN3 genotype influences exercise-induced muscle damage during a marathon competition. Eur. J. Appl. Physiol. 2017, 117, 409–416. [Google Scholar] [CrossRef]
- Yang, N.; Schindeler, A.; McDonald, M.M.; Seto, J.T.; Houweling, P.J.; Lek, M.; Hogarth, M.; Morse, A.R.; Raftery, J.M.; Balasuriya, D.; et al. α-Actinin-3 deficiency is associated with reduced bone mass in human and mouse. Bone 2011, 49, 790–798. [Google Scholar] [CrossRef]
- Baltazar-Martins, G.; Gutiérrez-Hellín, J.; Aguilar-Navarro, M.; Ruiz-Moreno, C.; Moreno-Pérez, V.; López-Samanes, Á.; Domínguez, R.; Coso, J. Del Effect of ACTN3 Genotype on Sports Performance, Exercise-Induced Muscle Damage, and Injury Epidemiology. Sports 2020, 8, 99. [Google Scholar] [CrossRef]
- Alfred, T.; Ben-Shlomo, Y.; Cooper, R.; Hardy, R.; Cooper, C.; Deary, I.J.; Gunnell, D.; Harris, S.E.; Kumari, M.; Martin, R.M.; et al. ACTN3 genotype, athletic status, and life course physical capability: Meta-analysis of the published literature and findings from nine studies. Hum. Mutat. 2011, 32, 1008–1018. [Google Scholar] [CrossRef]
- Papadimitriou, I.D.; Lucia, A.; Pitsiladis, Y.P.; Pushkarev, V.P.; Dyatlov, D.A.; Orekhov, E.F.; Artioli, G.G.; Guilherme, J.P.L.F.; Lancha, A.H.; Ginevičiene, V.; et al. ACTN3 R577X and ACE I/D gene variants influence performance in elite sprinters: A multi-cohort study. BMC Genom. 2016, 17, 285. [Google Scholar] [CrossRef] [PubMed]
- Vincent, B.; Windelinckx, A.; Nielens, H.; Ramaekers, M.; Van Leemputte, M.; Hespel, P.; Thomis, M.A. Protective role of alpha-actinin-3 in the response to an acute eccentric exercise bout. J. Appl. Physiol. 2010, 109, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Zouhal, H.; Coso, J.D.; Jayavel, A.; Tourny, C.; Ravé, G.; Jebabli, N.; Clark, C.C.T.; Barthélémy, B.; Hackney, A.C.; Abderrahman, A. Ben Association between ACTN3 R577X genotype and risk of non-contact injury in trained athletes: A systematic review. J. Sport Health Sci. 2021. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, J.; Polman, R.; O’Donoghue, P. Physical Demands of Different Positions in FA Premier League Soccer. J. Sports Sci. Med. 2007, 6, 63–70. [Google Scholar]
- McAuley, A.B.T.; Hughes, D.C.; Tsaprouni, L.G.; Varley, I.; Suraci, B.; Roos, T.R.; Herbert, A.J.; Kelly, A.L. The association of the ACTN3 R577X and ACE I/D polymorphisms with athlete status in football: A systematic review and meta-analysis. J. Sports Sci. 2021, 39, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q. The ACE and ACTN3 polymorphisms in female soccer athletes. Genes Environ. 2021, 43, 5. [Google Scholar] [CrossRef]
- Massidda, M.; Voisin, S.; Culigioni, C.; Piras, F.; Cugia, P.; Yan, X.; Eynon, N.; Calò, C.M. ACTN3 R577X Polymorphism Is Associated with the Incidence and Severity of Injuries in Professional Football Players. Clin. J. Sport Med. 2019, 29, 57–61. [Google Scholar] [CrossRef]
- Clos, E.; Pruna, R.; Lundblad, M.; Artells, R.; Esquirol Caussa, J. ACTN3 single nucleotide polymorphism is associated with non-contact musculoskeletal soft-tissue injury incidence in elite professional football players. Knee Surg. Sport. Traumatol. Arthrosc. 2019, 27, 4055–4061. [Google Scholar] [CrossRef]
- Rodas, G.; Moreno-Pérez, V.; Del Coso, J.; Florit, D.; Osaba, L.; Lucia, A. Alpha-Actinin-3 Deficiency Might Affect Recovery from Non-Contact Muscle Injuries: Preliminary Findings in a Top-Level Soccer Team. Genes 2021, 12, 769. [Google Scholar] [CrossRef]
- Petr, M.; Thiel, D.; Kateřina, K.; Brož, P.; Malý, T.; Zahálka, F.; Vostatková, P.; Wilk, M.; Chycki, J.; Stastny, P. Speed and power-related gene polymorphisms associated with playing position in elite soccer players. Biol. Sport 2022, 39, 355–366. [Google Scholar] [CrossRef]
- Lu, D.; McCall, A.; Jones, M.; Kovalchik, S.; Steinweg, J.; Gelis, L.; Duffield, R. Injury epidemiology in Australian male professional soccer. J. Sci. Med. Sport 2020, 23, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Bahr, R.; Clarsen, B.; Derman, W.; Dvorak, J.; Emery, C.A.; Finch, C.F.; Hägglund, M.; Junge, A.; Kemp, S.; Khan, K.M.; et al. International Olympic Committee consensus statement: Methods for recording and reporting of epidemiological data on injury and illness in sport 2020 (including STROBE Extension for Sport Injury and Illness Surveillance (STROBE-SIIS)). Br. J. Sports Med. 2020, 54, 372–389. [Google Scholar] [CrossRef] [PubMed]
- Mills, M.A.; Yang, N.; Weinberger, R.; Vander Woude, D.L.; Beggs, A.H.; Easteal, S.; North, K.N. Differential expression of the actin-binding proteins, alpha-actinin-2 and -3, in different species: Implications for the evolution of functional redundancy. Hum. Mol. Genet. 2001, 10, 1335–1346. [Google Scholar] [CrossRef]
- Calatayud, J.; Martin, F.; Gargallo, P.; García-Redondo, J.; Colado, J.C.; Marín, P.J. The validity and reliability of a new instrumented device for measuring ankle dorsiflexion range of motion. Int. J. Sports Phys. Ther. 2015, 10, 197–202. [Google Scholar]
- American College of Sports Medicine. ACSM’s Resource Manual for Guidelines for Exercise Testing and Prescription—Google Books; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2014. [Google Scholar]
- Lara, B.; Gonzalez-Millán, C.; Salinero, J.J.; Abian-Vicen, J.; Areces, F.; Barbero-Alvarez, J.C.; Muñoz, V.; Portillo, L.J.; Gonzalez-Rave, J.M.; Del Coso, J. Caffeine-containing energy drink improves physical performance in female soccer players. Amino Acids 2014, 46, 1385–1392. [Google Scholar] [CrossRef]
- Vescovi, J.D. Sprint speed characteristics of high-level American female soccer players: Female Athletes in Motion (FAiM) study. J. Sci. Med. Sport 2012, 15, 474–478. [Google Scholar] [CrossRef]
- rs1815739 RefSNP Report—dbSNP—NCBI. Available online: https://www.ncbi.nlm.nih.gov/snp/rs1815739 (accessed on 27 July 2022).
- Coso, J.D.; Moreno, V.; Gutiérrez-Hellín, J.; Baltazar-Martins, G.; Ruíz-Moreno, C.; Aguilar-Navarro, M.; Lara, B.; Lucía, A. ACTN3 R577X genotype and exercise phenotypes in recreational marathon runners. Genes 2019, 10, 413. [Google Scholar] [CrossRef]
- Del Coso, J.; Salinero, J.J.; Lara, B.; Gallo-Salazar, C.; Areces, F.; Puente, C.; Herrero, D. ACTN3 X-allele carriers had greater levels of muscle damage during a half-ironman. Eur. J. Appl. Physiol. 2017, 117, 151–158. [Google Scholar] [CrossRef]
- Pimenta, E.M.; Coelho, D.B.; Cruz, I.R.; Morandi, R.F.; Veneroso, C.E.; De Azambuja Pussieldi, G.; Carvalho, M.R.S.; Silami-Garcia, E.; De Paz Fernández, J.A. The ACTN3 genotype in soccer players in response to acute eccentric training. Eur. J. Appl. Physiol. 2012, 112, 1495–1503. [Google Scholar] [CrossRef]
- Moreno, V.; Areces, F.; Ruiz-Vicente, D.; Ordovás, J.M.; Del Coso, J. Influence of the ACTN3 R577X genotype on the injury epidemiology of marathon runners. PLoS ONE 2020, 15, e0227548. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jung, E.S.; Kim, C.-H.; Youn, H.; Kim, H.R. Genetic associations of body composition, flexibility and injury risk with ACE, ACTN3 and COL5A1 polymorphisms in Korean ballerinas. J. Exerc. Nutr. Biochem. 2014, 18, 205–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, X.; Li, Z.; Cao, X.; Xie, C.; Gu, M.; Chen, P.; Yang, X.; Cai, J. The association between the ACTN3 R577X polymorphism and noncontact acute ankle sprains. J. Sports Sci. 2015, 33, 1775–1779. [Google Scholar] [CrossRef]
- Coelho, D.B.; Pimenta, E.; Rosse, I.C.; Veneroso, C.; Becker, L.K.; Carvalho, M.R.S.; Pussieldi, G.; Silami-Garcia, E. The alpha-actinin-3 r577x polymorphism and physical performance in soccer players. J. Sports Med. Phys. Fit. 2016, 56, 241–248. [Google Scholar]
- Gutiérrez-Hellín, J.; Baltazar-Martins, G.; Aguilar-Navarro, M.; Ruiz-Moreno, C.; Oliván, J.; Del Coso, J. Effect of actn3 r577x genotype on injury epidemiology in elite endurance runners. Genes 2021, 12, 76. [Google Scholar] [CrossRef]
- Costello, J.T.; Bieuzen, F.; Bleakley, C.M. Where are all the female participants in Sports and Exercise Medicine research? Eur. J. Sport Sci. 2014, 14, 847–851. [Google Scholar] [CrossRef] [PubMed]
- López-Valenciano, A.; Raya-González, J.; Garcia-Gómez, J.A.; Aparicio-Sarmiento, A.; Sainz de Baranda, P.; De Ste Croix, M.; Ayala, F. Injury Profile in Women’s Football: A Systematic Review and Meta-Analysis. Sports Med. 2021, 51, 423–442. [Google Scholar] [CrossRef]
- Pimenta, E.M.; Coelho, D.B.; Veneroso, C.E.; Coelho, E.J.B.; Cruz, I.R.; Morandi, R.F.; Pussieldi, G.D.A.; Carvalho, M.R.S.; Garcia, E.S.; De Paz Fernández, J.A. Effect of ACTN3 gene on strength and endurance in soccer players. J. Strength Cond. Res. 2013, 27, 3286–3292. [Google Scholar] [CrossRef] [PubMed]
- Clos, E.; Pruna, R.; Lundblad, M.; Artells, R.; Maffulli, N. ACTN3’s R577X Single Nucleotide Polymorphism Allele Distribution Differs Significantly in Professional Football Players according to Their Field Position. Med. Princ. Pract. 2021, 30, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.V.; Aksdal, I.M.; Stalsberg, R. Scaling Demands of Soccer According to Anthropometric and Physiological Sex Differences: A Fairer Comparison of Men’s and Women’s Soccer. Front. Psychol. 2019, 10, 762. [Google Scholar] [CrossRef]
- Bradley, P.S.; Dellal, A.; Mohr, M.; Castellano, J.; Wilkie, A. Gender differences in match performance characteristics of soccer players competing in the UEFA Champions League. Hum. Mov. Sci. 2014, 33, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Cardoso de Araújo, M.; Baumgart, C.; Jansen, C.T.; Freiwald, J.; Hoppe, M.W. Sex Differences in Physical Capacities of German Bundesliga Soccer Players. J. Strength Cond. Res. 2020, 34, 2329–2337. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, C.; Freiwald, J.; Hoppe, M.W. Sprint Mechanical Properties of Female and Different Aged Male Top-Level German Soccer Players. Sports 2018, 6, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitrotasios, M.; González-Rodenas, J.; Armatas, V.; Malavés, R.A. Creating goal scoring opportunities in men and women UEFA Champions League Soccer Matches. Tactical similarities and differences. Retos 2022, 43, 154–161. [Google Scholar] [CrossRef]
- Casal, C.A.; Losada, J.L.; Maneiro, R.; Ardá, A. Gender differences in technical-tactical behaviour of La Liga Spanish football teams. J. Hum. Sport Exerc. 2021, 16, 37–52. [Google Scholar] [CrossRef]
- Massidda, M.; Corrias, L.; Bachis, V.; Cugia, P.; Piras, F.; Scorcu, M.; Calò, C.M. Vitamin D receptor gene polymorphisms and musculoskeletal injuries in professional football players. Exp. Ther. Med. 2015, 9, 1974–1978. [Google Scholar] [CrossRef]
- Massidda, M.; Myamoto-Mikami, E.; Kumagai, H.; Ikeda, H.; Shimasaki, Y.; Yoshimura, M.; Cugia, P.; Piras, F.; Scorcu, M.; Kikuchi, N.; et al. Association between the ACE I/D polymorphism and muscle injuries in Italian and Japanese elite football players. J. Sports Sci. 2020, 38, 2423–2429. [Google Scholar] [CrossRef]
- Massidda, M.; Bachis, V.; Corrias, L.; Piras, F.; Scorcu, M.; Calò, C.M. Influence of the COL5A1 rs12722 on musculoskeletal injuries in professional soccer players. J. Sport. Med. Phys. Fit. 2015, 55, 1348–1353. [Google Scholar]
- Del Coso, J.; Salinero, J.J.; Lara, B.; Gallo-Salazar, C.; Areces, F.; Herrero, D.; Puente, C. Polygenic Profile and Exercise-Induced Muscle Damage by a Competitive Half-Ironman. J. Strength Cond. Res. 2020, 34, 1400–1408. [Google Scholar] [CrossRef]
- Coso, J.D.; Valero, M.; Salinero, J.J.; Lara, B.; Gallo-Salazar, C.; Areces, F. Optimum polygenic profile to resist exertional rhabdomyolysis during a marathon. PLoS ONE 2017, 12, e0172965. [Google Scholar]
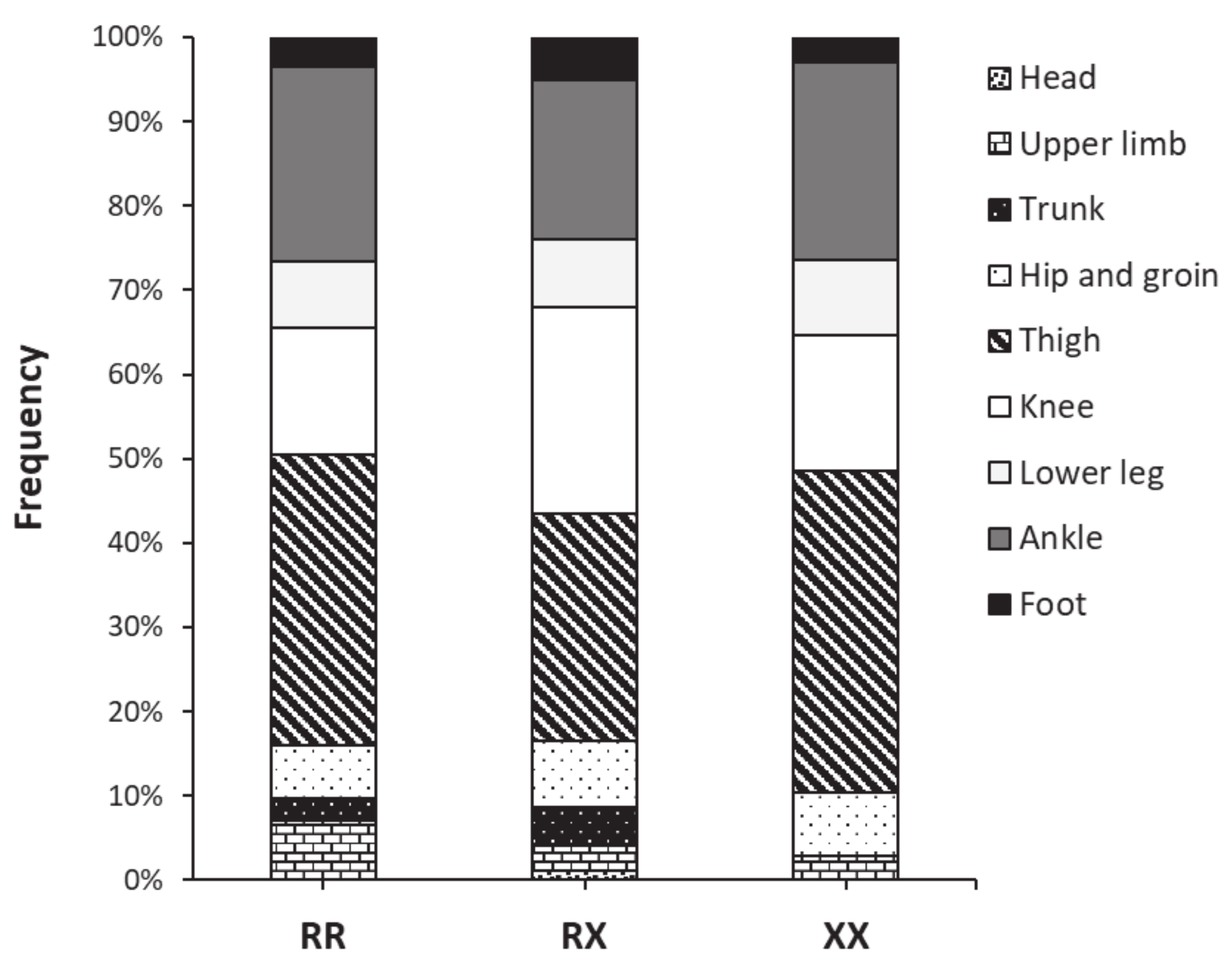
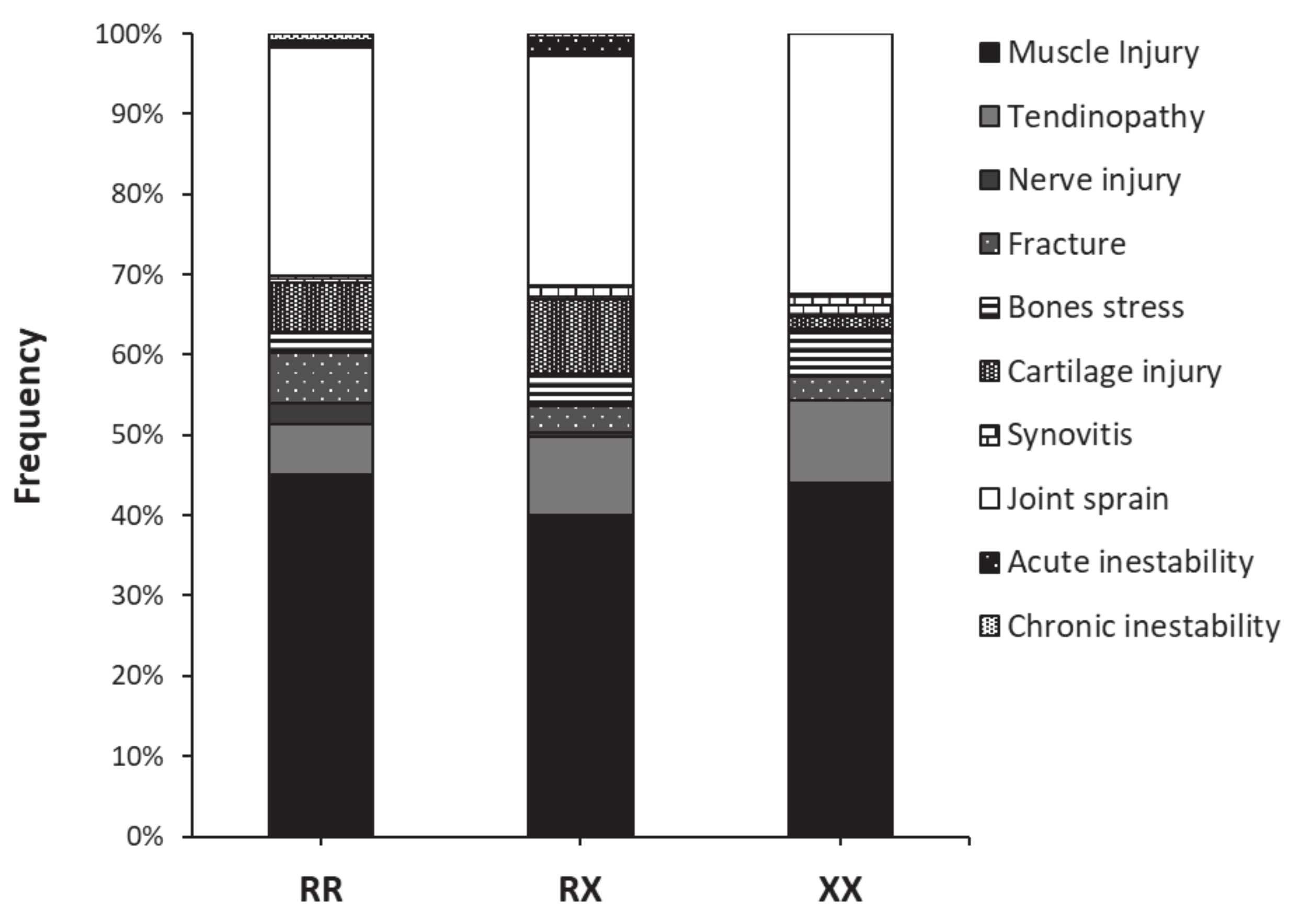
| Variable (Units) | RR | RX | XX | p Value |
|---|---|---|---|---|
| Number (frequency, %) | 54 (28.3) | 101 (52.9) | 36 (18.8) | - |
| Age (years) | 23.8 ± 4.6 | 23.1 ± 3.9 | 24.3 ± 4.1 | 0.304 |
| Height (cm) | 167.7 ± 6.1 | 166.5 ± 6.5 | 167.0 ± 5.2 | 0.438 |
| Body mass (kg) | 61.7 ± 6.7 | 59.9 ± 6.3 | 61.0 ± 5.5 | 0.220 |
| Body mass index (kg/m2) | 21.9 ± 2.1 | 21.6 ± 1.7 | 21.9 ± 1.6 | 0.422 |
| Forward (frequency, %) | 20 (37.0) | 28 (27.7) | 9 (25.0) | 0.638 |
| Midfielder (frequency, %) | 13 (24.1) | 29 (28.7) | 10 (27.8) | |
| Defender (frequency, %) | 16 (29.6) | 31 (30.7) | 15 (41.7) | |
| Goalkeeper (frequency, %) | 5 (9.3) | 13 (12.9) | 2 (5.6) | |
| International level (frequency, %) | 14 (25.9) | 23 (22.8) | 6 (16.7) | 0.586 |
| National level (frequency, %) | 40 (74.1) | 78 (77.2) | 30 (83.3) | |
| Total exposure time (h) | 202 ± 65 | 212 ± 71 | 213 ± 78 | 0.722 |
| Training exposure time (h) | 179 ± 64 | 192 ± 67 | 189 ± 74 | 0.614 |
| Match exposure time (h) | 23 ± 15 | 20 ± 14 | 24 ± 12 | 0.337 |
| Variable (Units) | RR | RX | XX | RR vs. RX vs. XX | Dominant RR vs. RX + XX | Recessive RR + RX vs. XX |
|---|---|---|---|---|---|---|
| Right ankle dorsiflexion (cm) | 10.0 ± 2.1 | 10.6 ± 2.8 | 9.9 ± 2.2 | 0.550 | 0.508 | 0.522 |
| Left ankle dorsiflexion (cm) | 10.3 ± 2.8 | 10.2 ± 2.0 | 10.3 ± 2.0 | 0.992 | 0.914 | 0.974 |
| Sit-and-reach distance (cm) | 10.9 ± 6.1 | 7.1 ± 8.1 | 8.2 ± 7.1 | 0.361 | 0.172 | 0.941 |
| Countermovement jump height (cm) | 34.2 ± 5.5 | 33.8 ± 4.0 | 33.4 ± 3.5 | 0.087 | 0.594 | 0.599 |
| 30 m sprint time (s) | 4.80 ± 0.32 | 4.72 ± 0.77 | 4.47 ± 0.27 | 0.210 | 0.316 | 0.089 |
| Variable (Units) | RR | RX | XX | RR vs. RX vs. XX | Dominant RR vs. RX + XX | Recessive RR + RX vs. XX |
|---|---|---|---|---|---|---|
| Players with injury (frequency, %) | 41 (75.9) | 73 (73.3) | 30 (83.3) | 0.415 | 0.914 | 0.219 |
| Players without injury (frequency, %) | 13 (24.1) | 28 (27.7) | 6 (16.7) | |||
| Players with muscle injury (frequency, %) | 20 (37.0) | 29 (28.7) | 15 (41.7) | 0.298 | 0.517 | 0.250 |
| Players without muscle injury (frequency, %) | 34 (63.0) | 72 (71.3) | 21 (58.3) | |||
| Players with ligament injury (frequency, %) | 10 (18.5) | 23 (22.8) | 10 (27.8) | 0.586 | 0.407 | 0.401 |
| Players without ligament injury (frequency, %) | 44 (81.5) | 78 (77.2) | 26 (72.2) | |||
| Players with bone injury (frequency, %) | 3 (5.6) | 8 (7.9) | 2 (5.6) | 0.811 | 0.667 | 0.741 |
| Players without bone injury (frequency, %) | 51 (94.4) | 93 (92.1) | 34 (94.4) |
| Variable (Units) | RR | RX | XX | RR vs. RX vs. XX | Dominant RR vs. RX + XX | Recessive RR + RX vs. XX |
|---|---|---|---|---|---|---|
| Incidence | ||||||
| /1000 h of exposure | 10.4 ± 8.6 | 8.2 ± 5.7 | 8.9 ± 5.3 | 0.222 | 0.112 | 0.714 |
| /1000 h of training | 4.8 ± 2.1 | 3.6 ± 3.7 | 3.8 ± 3.5 | 0.100 | 0.090 | 0.401 |
| /1000 h of match | 54.1 ± 6.3 | 51.8 ± 9.4 | 47.8 ± 9.5 | 0.209 | 0.163 | 0.329 |
| Number of injuries | ||||||
| No injury (%) | 24.1 | 27.7 | 16.7 | 0.074 | 0.056 | 0.884 |
| 1 injury (%) | 37.0 | 29.7 | 27.8 | |||
| 2 injuries (%) | 11.1 | 23.8 | 25.0 | |||
| ≥3 injuries (%) | 27.8 | 10.9 | 16.7 | |||
| Return to play | ||||||
| Severity (days) | 39 ± 60 | 36 ± 65 | 36 ± 51 | 0.679 | 0.543 | 0.422 |
| Minor (%) | 19.5 | 32.0 | 29.4 | 0.127 | 0.056 | 0.422 |
| Moderate (%) | 45.1 | 38.9 | 47.1 | |||
| Serious (%) | 35.4 | 29.1 | 23.5 | |||
| Exposure | ||||||
| Training (%) | 40.7 | 39.4 | 38.2 | 0.945 | 0.772 | 0.797 |
| Competition (%) | 59.3 | 60.6 | 61.8 | |||
| Recurrence | ||||||
| New onset (%) | 85.8 | 90.9 | 92.6 | 0.261 | 0.112 | 0.361 |
| Recurrent (%) | 14.2 | 9.1 | 7.4 | |||
| Mode of onset | ||||||
| Acute sudden onset (%) | 76.1 | 77.1 | 79.4 | 0.795 | 0.615 | 0.536 |
| Repetitive gradual (%) | 4.4 | 5.7 | 7.4 | |||
| Repetitive sudden onset (%) | 19.5 | 17.1 | 13.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Coso, J.; Rodas, G.; Buil, M.Á.; Sánchez-Sánchez, J.; López, P.; González-Ródenas, J.; Gasulla-Anglés, P.; López-Samanes, Á.; Hernández-Sánchez, S.; Iztueta, A.; et al. Association of the ACTN3 rs1815739 Polymorphism with Physical Performance and Injury Incidence in Professional Women Football Players. Genes 2022, 13, 1635. https://doi.org/10.3390/genes13091635
Del Coso J, Rodas G, Buil MÁ, Sánchez-Sánchez J, López P, González-Ródenas J, Gasulla-Anglés P, López-Samanes Á, Hernández-Sánchez S, Iztueta A, et al. Association of the ACTN3 rs1815739 Polymorphism with Physical Performance and Injury Incidence in Professional Women Football Players. Genes. 2022; 13(9):1635. https://doi.org/10.3390/genes13091635
Chicago/Turabian StyleDel Coso, Juan, Gil Rodas, Miguel Ángel Buil, Javier Sánchez-Sánchez, Pedro López, Joaquín González-Ródenas, Pablo Gasulla-Anglés, Álvaro López-Samanes, Sergio Hernández-Sánchez, Ane Iztueta, and et al. 2022. "Association of the ACTN3 rs1815739 Polymorphism with Physical Performance and Injury Incidence in Professional Women Football Players" Genes 13, no. 9: 1635. https://doi.org/10.3390/genes13091635
APA StyleDel Coso, J., Rodas, G., Buil, M. Á., Sánchez-Sánchez, J., López, P., González-Ródenas, J., Gasulla-Anglés, P., López-Samanes, Á., Hernández-Sánchez, S., Iztueta, A., & Moreno-Pérez, V. (2022). Association of the ACTN3 rs1815739 Polymorphism with Physical Performance and Injury Incidence in Professional Women Football Players. Genes, 13(9), 1635. https://doi.org/10.3390/genes13091635












