SPACR Encoded by IMPG1 Is Essential for Photoreceptor Survival by Interplaying between the Interphotoreceptor Matrix and the Retinal Pigment Epithelium
Abstract
1. Introduction
2. Materials and Methods
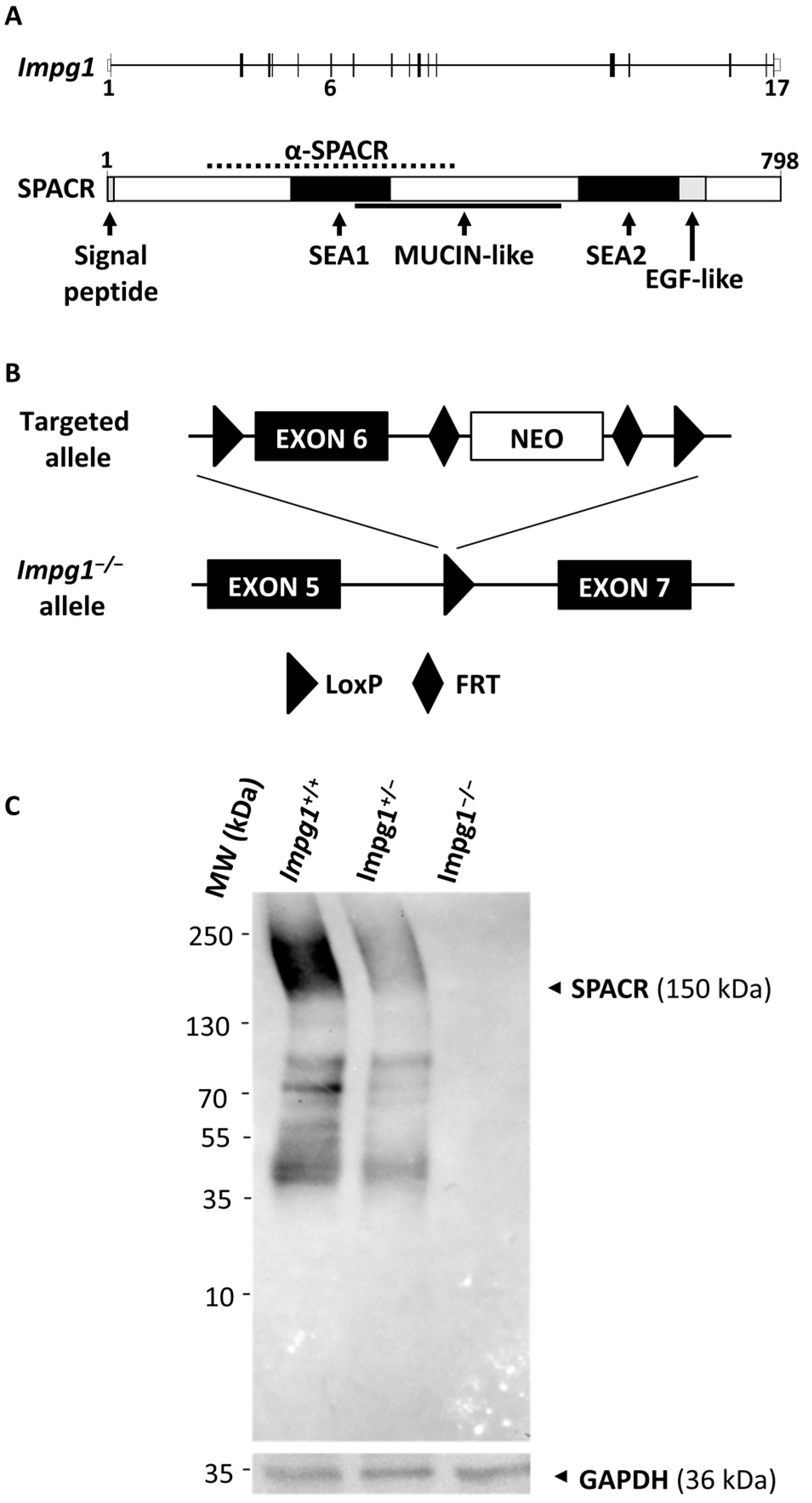
2.1. Generation of the Impg1−/− Mouse Model
2.2. Western Blotting Analysis
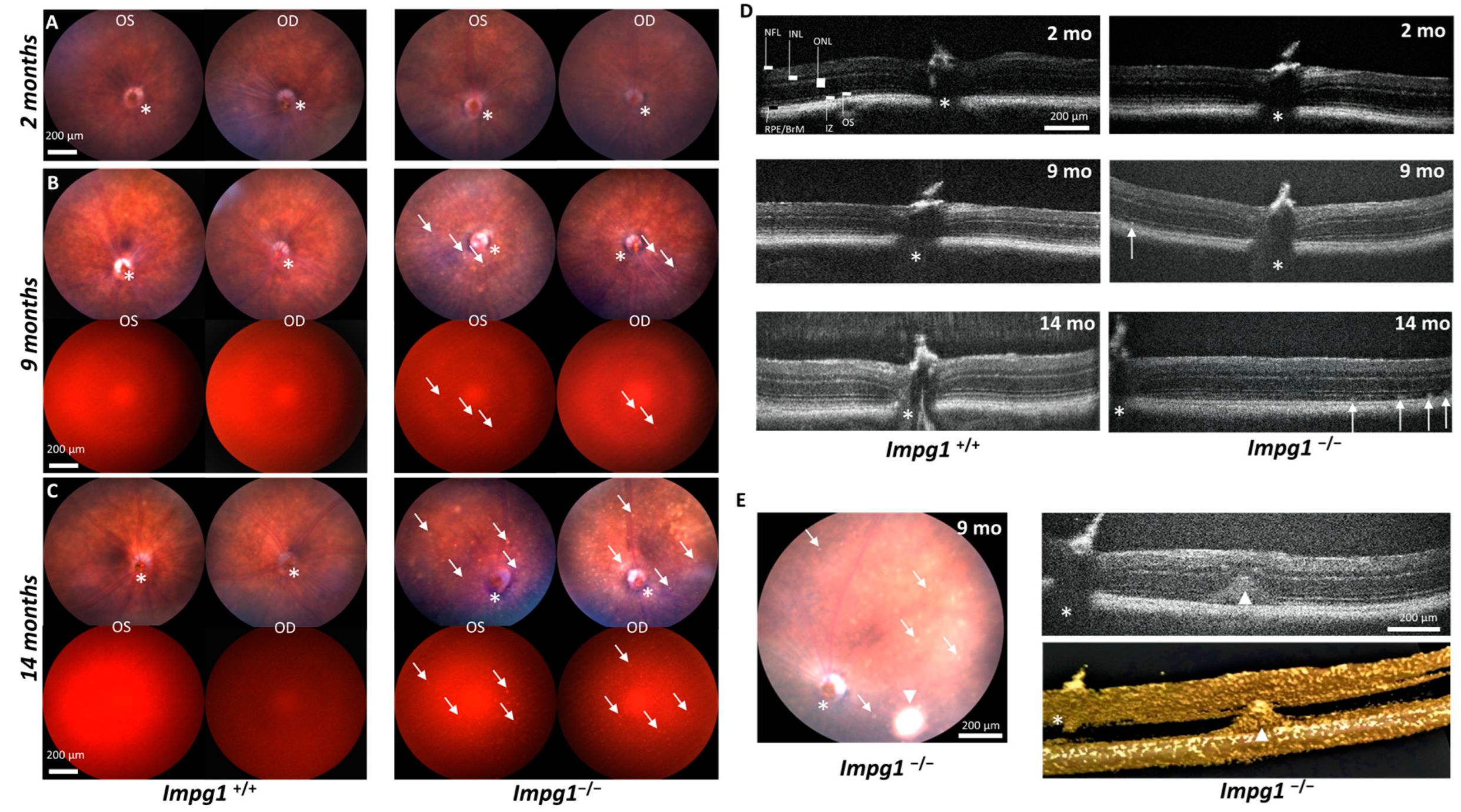
2.3. In Vivo Imaging
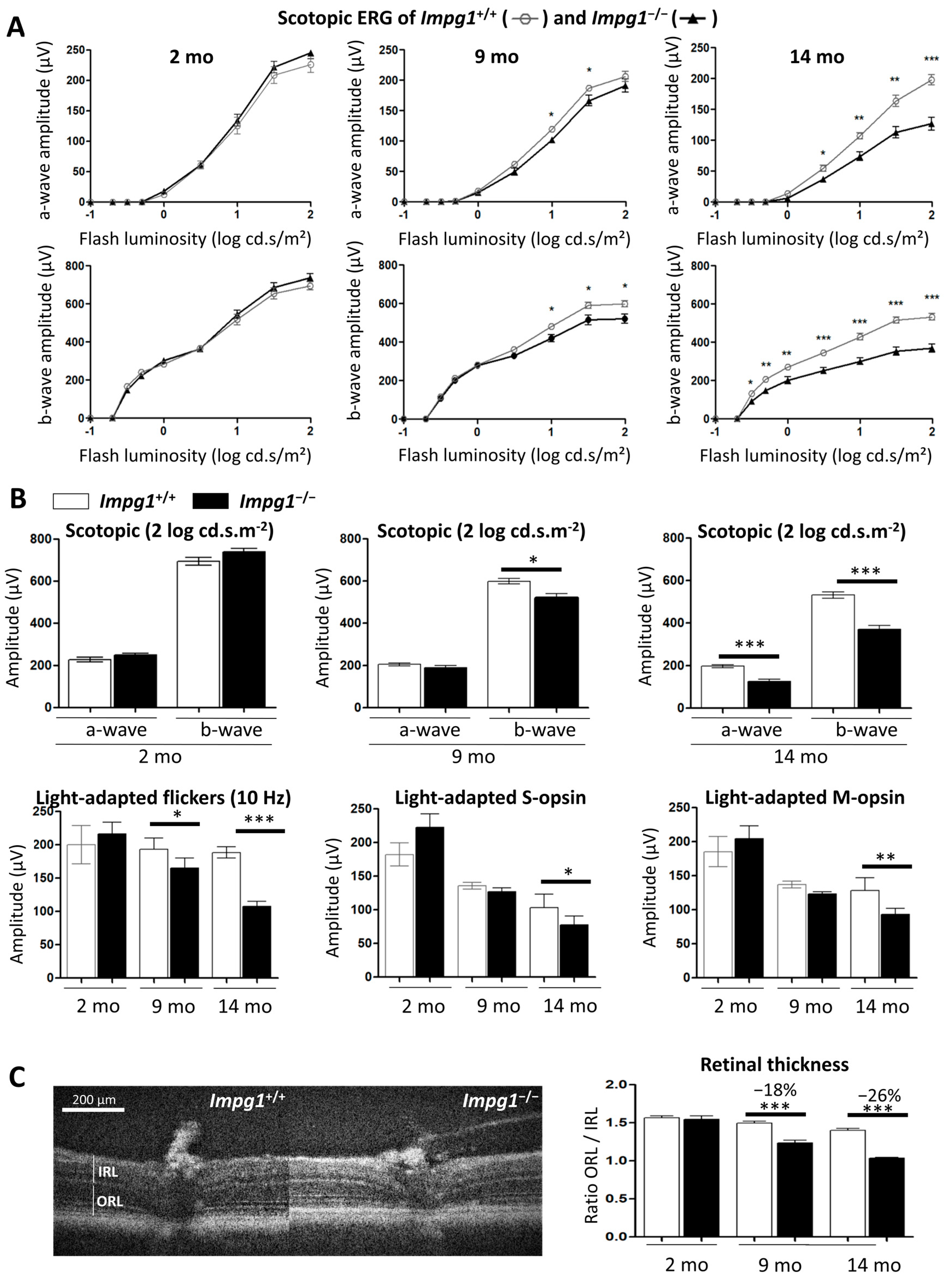
2.4. Electroretinogram (ERG) Recordings
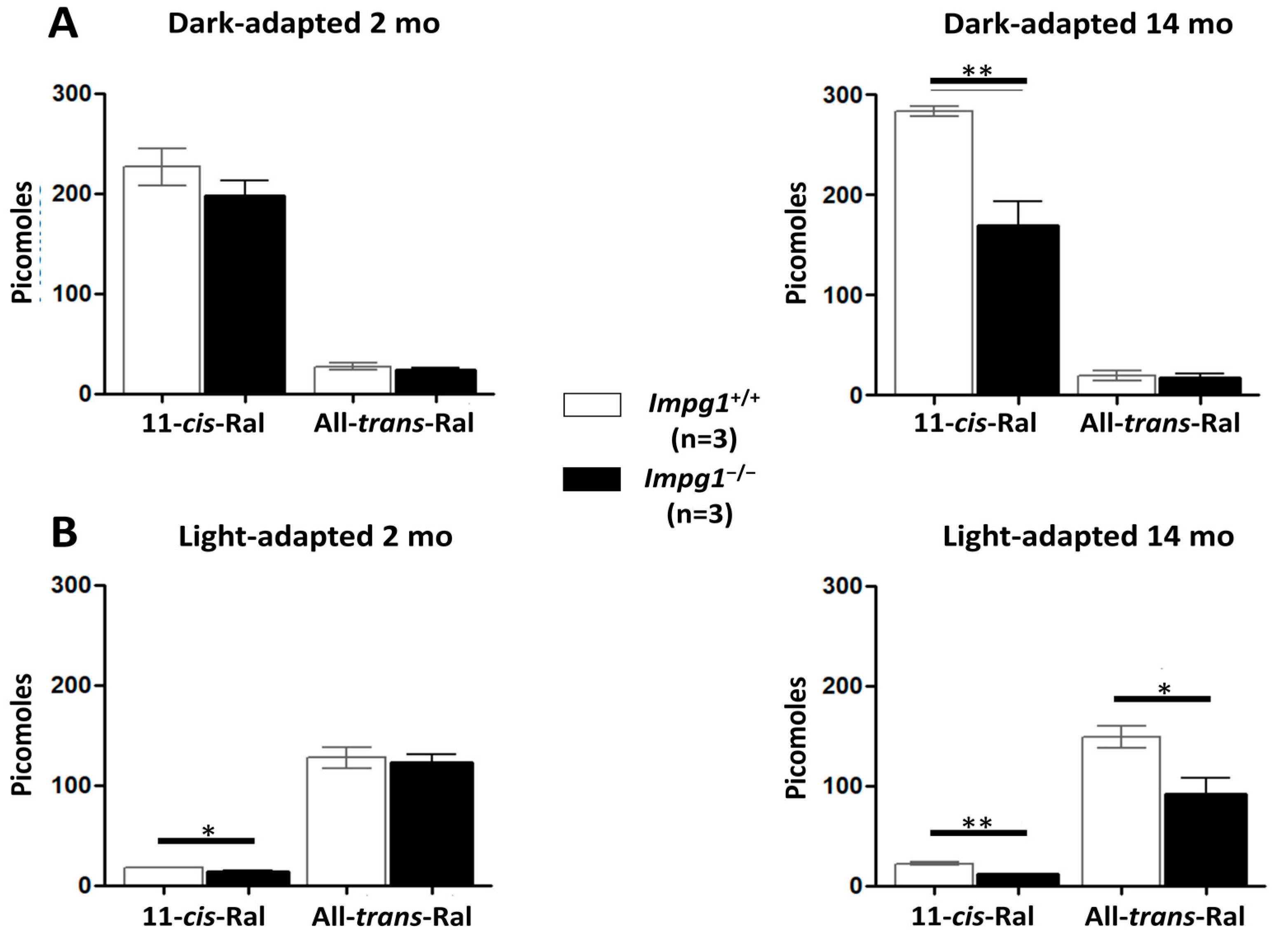
2.5. Retinoid Quantification
2.6. Histochemistry
2.7. Transmission Electron Microscopy (TEM)
2.8. Statistics
3. Results
3.1. Generation of an Impg1−/− Murine Model
3.2. Autofluorescent Material Accumulates Subretinally in Impg1−/− Mice
3.3. Subretinal Lesions Lead to a Decrease in Photoreceptor Function
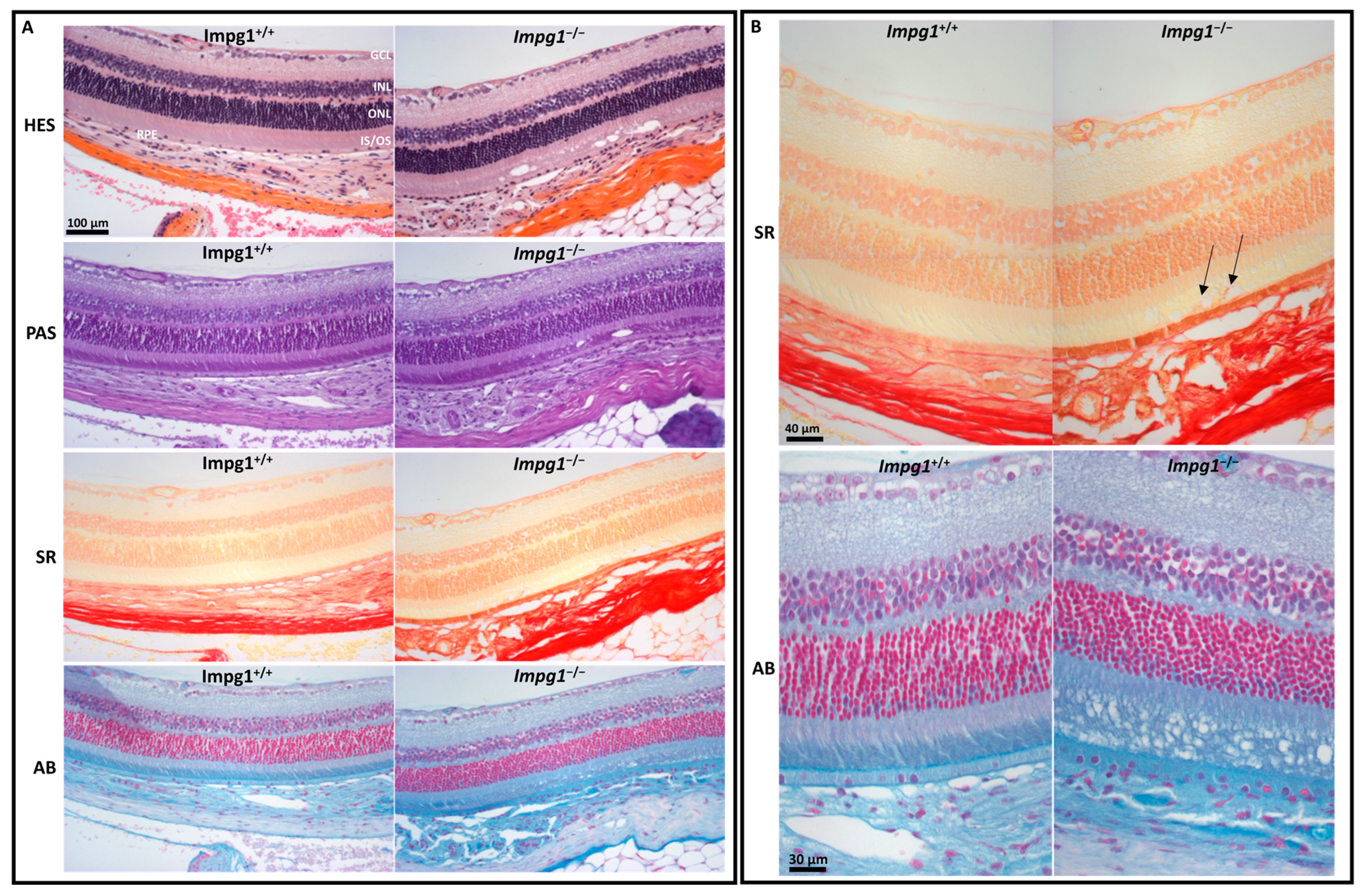
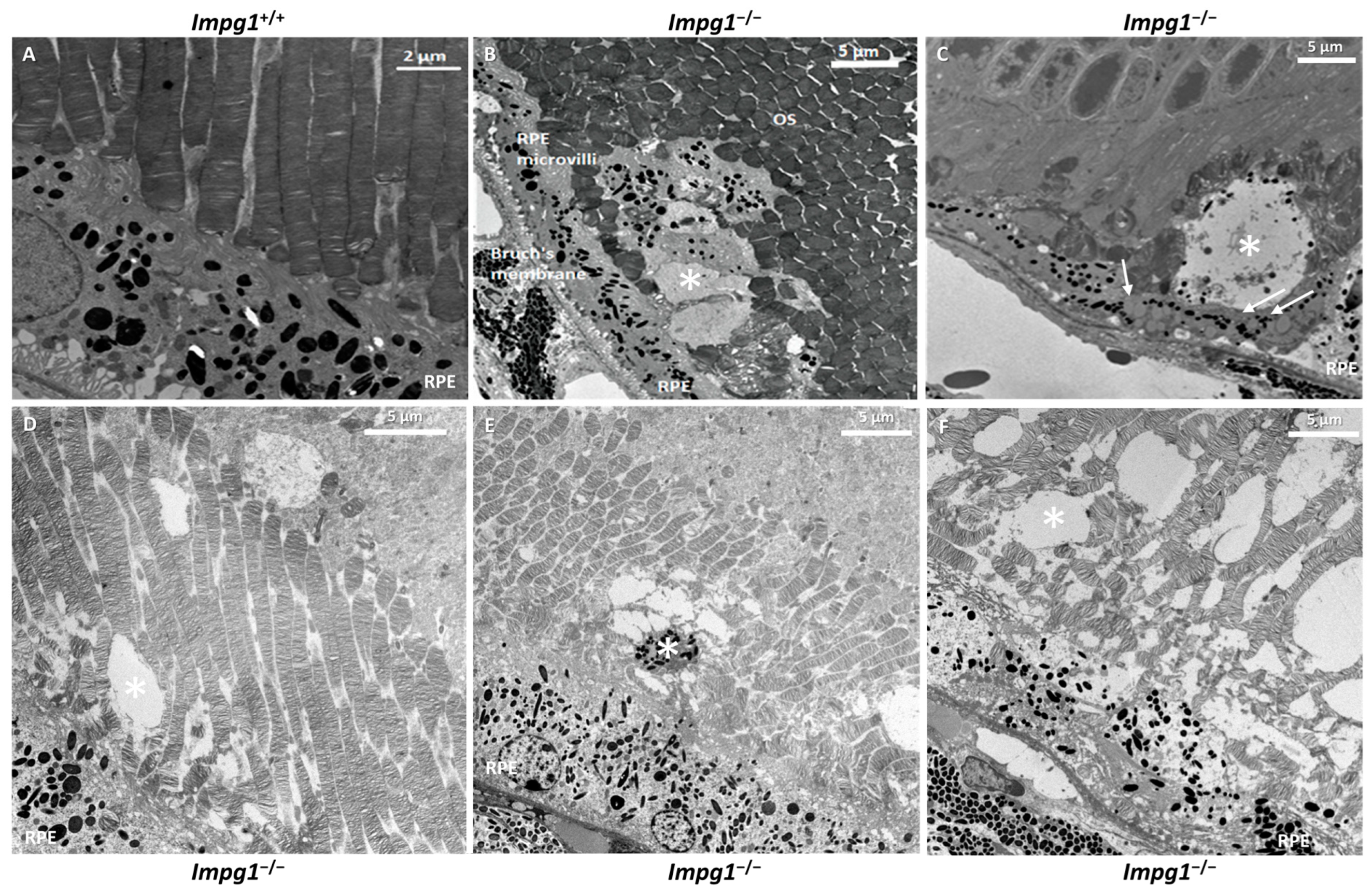
3.4. Accumulation of Cellular Material in the IPM Leads to Photoreceptor Death
4. Discussion
4.1. Impg1−/− Retinas Accumulate Autofluorescent Material Subretinally
4.2. Subretinal Material Leads to Moderate Physiological Impairment
4.3. IPM Disorganization Leads to Alteration of the RPE
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, I.; Dityatev, A. Crosstalk between Glia, Extracellular Matrix and Neurons. Brain Res. Bull. 2018, 136, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Sawada, Y.; Yoshitomi, T. Structure and Function of the Interphotoreceptor Matrix Surrounding Retinal Photoreceptor Cells. Exp. Eye Res. 2015, 133, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Rodriguez, I.R.; Moreira, E.F.; Midura, R.J.; Misono, K.; Todres, E.; Hollyfield, J.G. SPACR, a Novel Interphotoreceptor Matrix Glycoprotein in Human Retina That Interacts with Hyaluronan. J. Biol. Chem. 1998, 273, 31599–31606. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, M.H.; Hageman, G.S. Expression and Characterization of the IPM 150 Gene (IMPG1) Product, a Novel Human Photoreceptor Cell-Associated Chondroitin-Sulfate Proteoglycan. Matrix Biol. 1999, 18, 509–518. [Google Scholar] [CrossRef]
- Zhao, J.; Yoneda, M.; Takeyama, M.; Miyaishi, O.; Inoue, Y.; Kataoka, T.; Ohno-Jinno, A.; Isogai, Z.; Kimata, K.; Iwaki, M.; et al. Characterization of a Motif for Specific Binding to Hyaluronan in Chicken SPACR. J. Neurochem. 2008, 106, 1117–1124. [Google Scholar] [CrossRef]
- Palmai-Pallag, T.; Khodabukus, N.; Kinarsky, L.; Leir, S.-H.; Sherman, S.; Hollingsworth, M.A.; Harris, A. The Role of the SEA (Sea Urchin Sperm Protein, Enterokinase and Agrin) Module in Cleavage of Membrane-Tethered Mucins. FEBS J. 2005, 272, 2901–2911. [Google Scholar] [CrossRef]
- Acharya, S.; Rayborn, M.E.; Hollyfield, J.G. Characterization of SPACR, a Sialoprotein Associated with Cones and Rods Present in the Interphotoreceptor Matrix of the Human Retina: Immunological and Lectin Binding Analysis. Glycobiology 1998, 8, 997–1006. [Google Scholar] [CrossRef][Green Version]
- Acharya, S.; Foletta, V.C.; Lee, J.W.; Rayborn, M.E.; Rodriguez, I.R.; Young, W.S., 3rd; Hollyfield, J.G. SPACRCAN, a Novel Human Interphotoreceptor Matrix Hyaluronan-Binding Proteoglycan Synthesized by Photoreceptors and Pinealocytes. J. Biol. Chem. 2000, 275, 6945–6955. [Google Scholar] [CrossRef]
- Felemban, M.; Dorgau, B.; Hunt, N.C.; Hallam, D.; Zerti, D.; Bauer, R.; Ding, Y.; Collin, J.; Steel, D.; Krasnogor, N.; et al. Extracellular Matrix Component Expression in Human Pluripotent Stem Cell-Derived Retinal Organoids Recapitulates Retinogenesis in Vivo and Reveals an Important Role for IMPG1 and CD44 in the Development of Photoreceptors and Interphotoreceptor Matrix. Acta Biomater. 2018, 74, 207–221. [Google Scholar] [CrossRef]
- Chen, Q.; Lee, J.W.; Nishiyama, K.; Shadrach, K.G.; Rayborn, M.E.; Hollyfield, J.G. SPACRCAN in the Interphotoreceptor Matrix of the Mouse Retina: Molecular, Developmental and Promoter Analysis. Exp. Eye Res. 2003, 76, 1–14. [Google Scholar] [CrossRef]
- Olivier, G.; Corton, M.; Intartaglia, D.; Verbakel, S.K.; Sergouniotis, P.I.; Le Meur, G.; Dhaenens, C.-M.; Naacke, H.; Avila-Fernández, A.; Hoyng, C.B.; et al. Pathogenic Variants in IMPG1 Cause Autosomal Dominant and Autosomal Recessive Retinitis Pigmentosa. J. Med. Genet. 2020, 58, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Manes, G.; Meunier, I.; Avila-Fernández, A.; Banfi, S.; Le Meur, G.; Zanlonghi, X.; Corton, M.; Simonelli, F.; Brabet, P.; Labesse, G.; et al. Mutations in IMPG1 Cause Vitelliform Macular Dystrophies. Am. J. Hum. Genet. 2013, 93, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Meunier, I.; Manes, G.; Bocquet, B.; Marquette, V.; Baudoin, C.; Puech, B.; Defoort-Dhellemmes, S.; Audo, I.; Verdet, R.; Arndt, C.; et al. Frequency and Clinical Pattern of Vitelliform Macular Dystrophy Caused by Mutations of Interphotoreceptor Matrix IMPG1 and IMPG2 Genes. Ophthalmology 2014, 121, 2406–2414. [Google Scholar] [CrossRef]
- Bandah-Rozenfeld, D.; Collin, R.W.J.; Banin, E.; van den Born, L.I.; Coene, K.L.M.; Siemiatkowska, A.M.; Zelinger, L.; Khan, M.I.; Lefeber, D.J.; Erdinest, I.; et al. Mutations in IMPG2, Encoding Interphotoreceptor Matrix Proteoglycan 2, Cause Autosomal-Recessive Retinitis Pigmentosa. Am. J. Hum. Genet. 2010, 87, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Querques, G.; Zerbib, J.; Georges, A.; Massamba, N.; Forte, R.; Querques, L.; Rozet, J.-M.; Kaplan, J.; Souied, E.H. Multimodal Analysis of the Progression of Best Vitelliform Macular Dystrophy. Mol. Vis. 2014, 20, 575–592. [Google Scholar] [PubMed]
- Verbakel, S.K.; van Huet, R.A.C.; Boon, C.J.F.; den Hollander, A.I.; Collin, R.W.J.; Klaver, C.C.W.; Hoyng, C.B.; Roepman, R.; Klevering, B.J. Non-Syndromic Retinitis Pigmentosa. Prog. Retin. Eye Res. 2018, 66, 157–186. [Google Scholar] [CrossRef]
- Xu, H.; Qu, C.; Gan, L.; Sun, K.; Tan, J.; Liu, X.; Jiang, Z.; Tian, W.; Liu, W.; Zhang, S.; et al. Deletion of the Impg2 Gene Causes the Degeneration of Rod and Cone Cells in Mice. Hum. Mol. Genet. 2020, 29, 1624–1634. [Google Scholar] [CrossRef]
- Salido, E.M.; Ramamurthy, V. Proteoglycan IMPG2 Shapes the Interphotoreceptor Matrix and Modulates Vision. J. Neurosci. 2020, 40, 4059–4072. [Google Scholar] [CrossRef]
- Jagodzinska, J.; Sarzi, E.; Cavalier, M.; Seveno, M.; Baecker, V.; Hamel, C.; Péquignot, M.; Delettre, C. Optical Coherence Tomography: Imaging Mouse Retinal Ganglion Cells In Vivo. J. Vis. Exp. 2017, 127, 55865. [Google Scholar] [CrossRef]
- Chekroud, K.; Arndt, C.; Basset, D.; Hamel, C.P.; Brabet, P.; Pequignot, M.O. Simple and Efficient: Validation of a Cotton Wick Electrode for Animal Electroretinography. Ophthalmic Res. 2011, 45, 174–179. [Google Scholar] [CrossRef]
- Suzuki, T.; Fujita, Y.; Noda, Y.; Miyata, S. A Simple Procedure for the Extraction of the Native Chromophore of Visual Pigments: The Formaldehyde Method. Vis. Res. 1986, 26, 425–429. [Google Scholar] [CrossRef]
- Sweat, F.; Puchtler, H.; Rosenthal, S.I. Sirius red F3BA as a stain for connective tissue. Arch. Pathol. 1964, 78, 69–72. [Google Scholar] [PubMed]
- Frangieh, G.T.; Green, W.R.; Fine, S.L. A Histopathologic Study of Best’s Macular Dystrophy. Arch. Ophthalmol. 1982, 100, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Delori, F.C.; Dorey, C.K.; Staurenghi, G.; Arend, O.; Goger, D.G.; Weiter, J.J. In Vivo Fluorescence of the Ocular Fundus Exhibits Retinal Pigment Epithelium Lipofuscin Characteristics. Invest. Ophthalmol. Vis. Sci. 1995, 36, 718–729. [Google Scholar]
- Porrello, K.; LaVail, M.M. Immunocytochemical Localization of Chondroitin Sulfates in the Interphotoreceptor Matrix of the Normal and Dystrophic Rat Retina. Curr. Eye Res. 1986, 5, 981–993. [Google Scholar] [CrossRef]
- Imanishi, Y.; Gerke, V.; Palczewski, K. Retinosomes: New Insights into Intracellular Managing of Hydrophobic Substances in Lipid Bodies. J. Cell Biol. 2004, 166, 447–453. [Google Scholar] [CrossRef]
- Nandrot, E.F.; Chang, Y.; Finnemann, S.C. Avβ5 Integrin Receptors at the Apical Surface of the RPE: One Receptor, Two Functions. Recent Adv. Retin. Degener. 2008, 613, 369–375. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olivier, G.; Brabet, P.; Pirot, N.; Broyon, M.; Guillou, L.; Cazevieille, C.; Sar, C.; Quiles, M.; Sarzi, E.; Pequignot, M.; et al. SPACR Encoded by IMPG1 Is Essential for Photoreceptor Survival by Interplaying between the Interphotoreceptor Matrix and the Retinal Pigment Epithelium. Genes 2022, 13, 1508. https://doi.org/10.3390/genes13091508
Olivier G, Brabet P, Pirot N, Broyon M, Guillou L, Cazevieille C, Sar C, Quiles M, Sarzi E, Pequignot M, et al. SPACR Encoded by IMPG1 Is Essential for Photoreceptor Survival by Interplaying between the Interphotoreceptor Matrix and the Retinal Pigment Epithelium. Genes. 2022; 13(9):1508. https://doi.org/10.3390/genes13091508
Chicago/Turabian StyleOlivier, Guillaume, Philippe Brabet, Nelly Pirot, Morgane Broyon, Laurent Guillou, Chantal Cazevieille, Chamroeun Sar, Melanie Quiles, Emmanuelle Sarzi, Marie Pequignot, and et al. 2022. "SPACR Encoded by IMPG1 Is Essential for Photoreceptor Survival by Interplaying between the Interphotoreceptor Matrix and the Retinal Pigment Epithelium" Genes 13, no. 9: 1508. https://doi.org/10.3390/genes13091508
APA StyleOlivier, G., Brabet, P., Pirot, N., Broyon, M., Guillou, L., Cazevieille, C., Sar, C., Quiles, M., Sarzi, E., Pequignot, M., Andreo, E., Roubertie, A., Meunier, I., Muller, A., Kalatzis, V., & Manes, G. (2022). SPACR Encoded by IMPG1 Is Essential for Photoreceptor Survival by Interplaying between the Interphotoreceptor Matrix and the Retinal Pigment Epithelium. Genes, 13(9), 1508. https://doi.org/10.3390/genes13091508






