Mutational Spectrum, Ocular and Olfactory Phenotypes of CNGB1-Related RP-Olfactory Dysfunction Syndrome in a Multiethnic Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Classification of CNGB1 Variants
2.3. Clinical/Demographic Features
2.4. Ophthalmic Examination and Multimodal Imaging
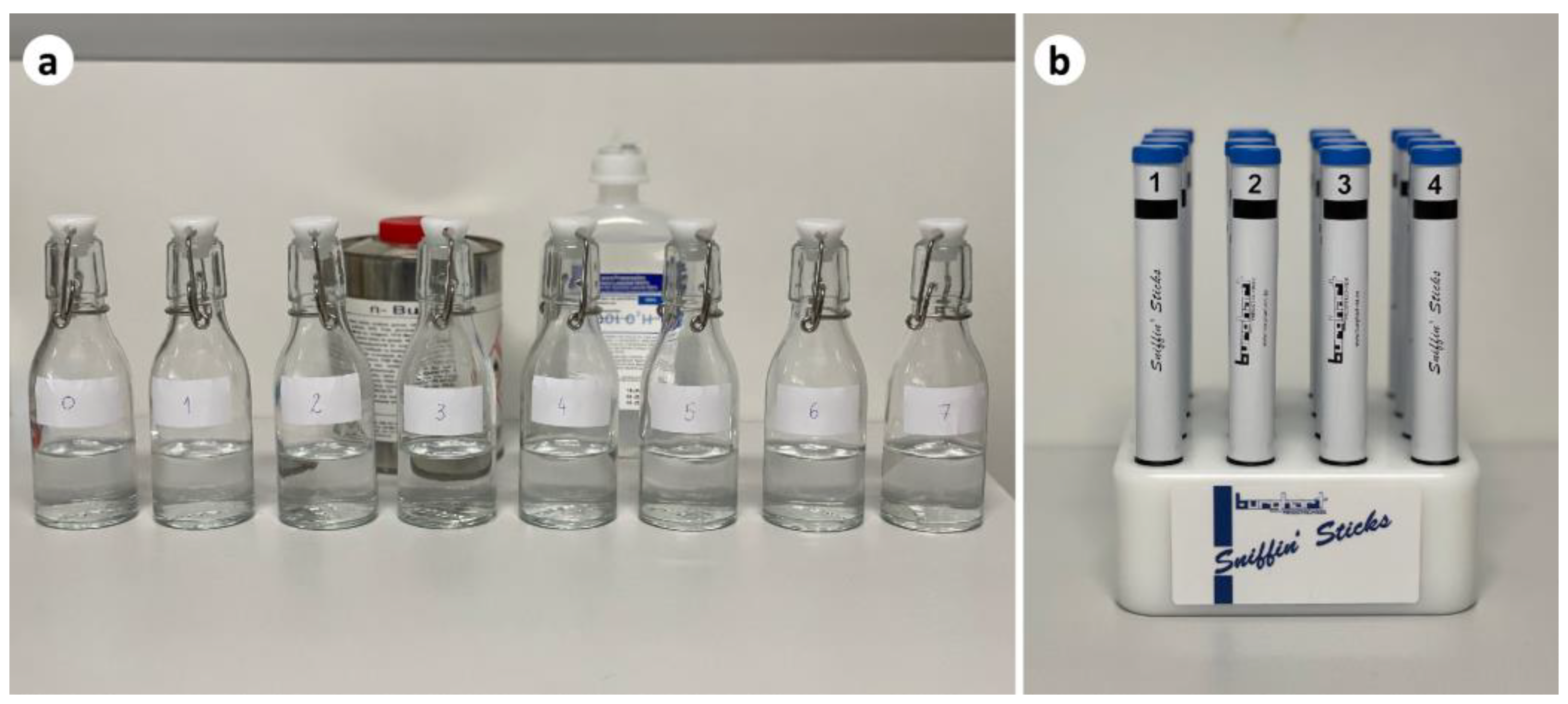
2.5. Olfactory Function Evaluation
2.6. Statistical Analysis
3. Results
3.1. CNGB1 Variants
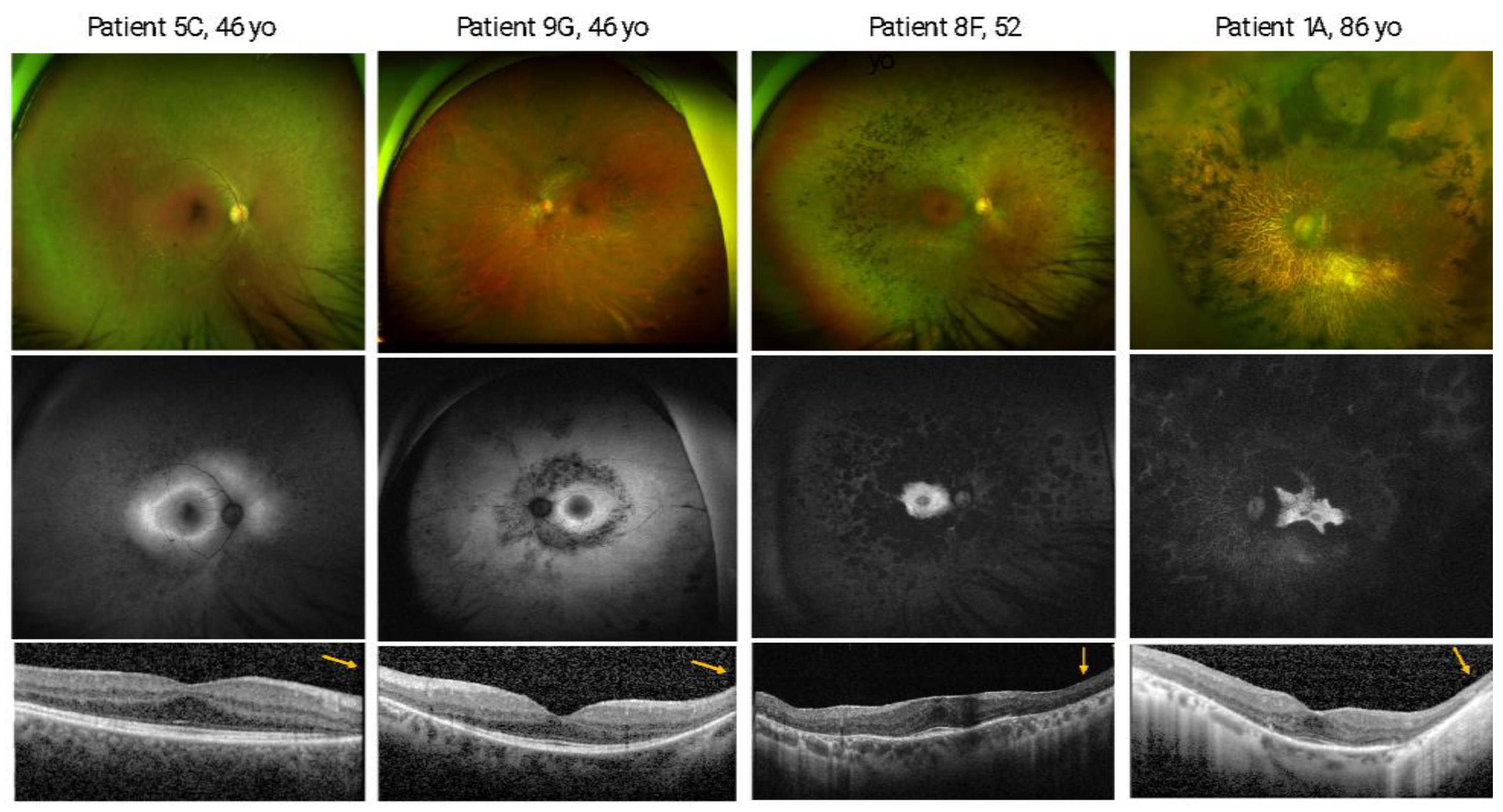
3.2. Ocular Phenotype
3.3. Olfactory Phenotype
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verbakel, S.K.; van Huet, R.A.; Boon, C.J.; den Hollander, A.I.; Collin, R.W.; Klaver, C.C.; Hoyng, C.B.; Roepman, R.; Klevering, B.J. Non-syndromic retinitis pigmentosa. Prog. Retin. Eye Res. 2018, 66, 157–186. [Google Scholar] [CrossRef]
- Hamel, C. Retinitis pigmentosa. Orphanet. J. Rare Dis. 2006, 1, 40. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.F.; Joo, K.; Kemp, J.A.; Fialho, S.L.; Cunha, A.S., Jr.; Woo, S.J.; Kwon, Y.J. Molecular genetics and emerging therapies for retinitis pigmentosa: Basic research and clinical perspectives. Prog. Retin. Eye Res. 2018, 63, 107–131. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Ouyang, J.; Sun, W.; Li, S.; Xiao, X.; Zhang, Q. Comparative exome sequencing reveals novel candidate genes for retinitis pigmentosa. EBioMedicine 2020, 56, 102792. [Google Scholar] [CrossRef] [PubMed]
- Schneider, N.; Sundaresan, Y.; Gopalakrishnan, P.; Beryozkin, A.; Hanany, M.; Levanon, E.Y.; Banin, E.; Ben-Aroya, S.; Sharon, D. Inherited retinal diseases: Linking genes, disease-causing variants, and relevant therapeutic modalities. Prog. Retin. Eye Res. 2021, 89, 101029. [Google Scholar] [CrossRef]
- Issa, P.C.; Reuter, P.; Kühlewein, L.; Birtel, J.; Gliem, M.; Tropitzsch, A.; Whitcroft, K.L.; Bolz, H.J.; Ishihara, K.; MacLaren, R.E.; et al. Olfactory dysfunction in patients with CNGB1-associated retinitis pigmentosa. JAMA Ophthalmol. 2018, 136, 761–769. [Google Scholar] [CrossRef]
- Zheng, J.; Trudeau, M.C.; Zagotta, W.N. Rod cyclic nucleotide-gated channels have a stoichiometry of three CNGA1 subunits and one CNGB1 subunit. Neuron 2002, 36, 891–896. [Google Scholar] [CrossRef]
- Zheng, J.; Zagotta, W.N. Stoichiometry and assembly of olfactory cyclic nucleotide-gated channels. Neuron 2004, 42, 411–421. [Google Scholar] [CrossRef]
- Kaupp, U.B.; Seifert, R. Cyclic nucleotide-gated ion channels. Physiol. Rev. 2002, 82, 769–824. [Google Scholar] [CrossRef]
- Biel, M.; Zong, X.; Ludwig, A.; Sautter, A.; Hofmann, F. Molecular cloning and expression of the modulatory subunit of the cyclic nucleotide-gated cation channel. J. Biol. Chem. 1996, 271, 6349–6355. [Google Scholar] [CrossRef]
- Bönigk, W.; Bradley, J.; Müller, F.; Sesti, F.; Boekhoff, I.; Ronnett, G.V.; Kaupp, U.B.; Frings, S. The native rat olfactory cyclic nucleotide-gated channel is composed of three distinct subunits. J. Neurosci. 1999, 19, 5332–5347. [Google Scholar] [CrossRef] [PubMed]
- Ardell, M.D.; Bedsole, D.L.; Schoborg, R.V.; Pittler, S.J. Genomic organization of the human rod photoreceptor cGMP-gated cation channel β-subunit gene. Gene 2000, 245, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Nassisi, M.; Smirnov, V.; Hernandez, C.S.; Mohand-Saïd, S.; Condroyer, C.; Antonio, A.; Kühlewein, L.; Kempf, M.; Kohl, S.; Wissinger, B.; et al. CNGB1-related rod-cone dystrophy: A mutation review and update. Hum. Mutat. 2021, 42, 641–666. [Google Scholar] [CrossRef] [PubMed]
- Radojevic, B.; Jones, K.; Klein, M.; Mauro-Herrera, M.; Kingsley, R.; Birch, D.G.; Bennett, L.D. Variable expressivity in patients with autosomal recessive retinitis pigmentosa associated with the gene CNGB1. Ophthalmic Genet. 2020, 42, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Afshar, F.; Arno, G.; Ba-Abbad, R.; Degli Esposti, S.; Michaelides, M.; Webster, A.R.; Mahroo, O.A. Awareness of olfactory impairment in a cohort of patients with CNGB1-associated retinitis pigmentosa. Eye 2019, 34, 783–784. [Google Scholar] [CrossRef]
- Hüttl, S.; Michalakis, S.; Seeliger, M.; Luo, D.-G.; Acar, N.; Geiger, H.; Hudl, K.; Mader, R.; Haverkamp, S.; Moser, M.; et al. Impaired channel targeting and retinal degeneration in mice lacking the cyclic nucleotide-gated channel subunit CNGB1. J. Neurosci. 2005, 25, 130–138. [Google Scholar] [CrossRef]
- Michalakis, S.; Reisert, J.; Geiger, H.; Wetzel, C.; Zong, X.; Bradley, J.; Spehr, M.; Hüttl, S.; Gerstner, A.; Pfeifer, A.; et al. Loss of CNGB1 protein leads to olfactory dysfunction and subciliary cyclic nucleotide-gated channel trapping. J. Biol. Chem. 2006, 281, 35156–35166. [Google Scholar] [CrossRef]
- Hull, S.; Attanasio, M.; Arno, G.; Carss, K.; Robson, A.G.; Thompson, D.A.; Plagnol, V.; Michaelides, M.; Holder, G.E.; Henderson, R.H.; et al. Clinical characterization of CNGB1-related autosomal recessive retinitis pigmentosa. JAMA Ophthalmol. 2017, 135, 137–144. [Google Scholar] [CrossRef]
- Marques, J.P.; Carvalho, A.L.; Henriques, J.; Murta, J.N.; Saraiva, J.; Silva, R. Design, development and deployment of a web-based interoperable registry for inherited retinal dystrophies in Portugal: The IRD-PT. Orphanet. J. Rare Dis. 2020, 15, 304. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Jones, K.D.; Wheaton, D.K.; Bowne, S.J.; Sullivan, L.S.; Birch, D.G.; Chen, R.; Daiger, S.P. Next-generation sequencing to solve complex inherited retinal dystrophy: A case series of multiple genes contributing to disease in extended families. Mol. Vis. 2017, 23, 470–481. [Google Scholar] [PubMed]
- Lynn, J.; Raney, A.; Britton, N.; Ramoin, J.; Yang, R.W.; Radojevic, B.; McClard, C.K.; Kingsley, R.; Coussa, R.G.; Bennett, L.D. Genetic Diagnosis for 64 Patients with Inherited Retinal Disease. Genes 2023, 14, 74. [Google Scholar] [CrossRef] [PubMed]
- Hummel, T.; Whitcroft, K.L.; Andrews, P.; Altundag, A.; Cinghi, C.; Costanzo, R.M.; Damm, M.; Frasnelli, J.; Gudziol, H.; Gupta, N.; et al. Position paper on olfactory dysfunction. Rhinology 2017, 25, 1–30. [Google Scholar]
- Cain, W.S.; Gent, J.F.; Goodspeed, R.B.; Leonard, G. Evaluation of olfactory dysfunction in the Connecticut Chemosensory Clinical Research Center. Laryngoscope 1988, 98, 83–88. [Google Scholar] [CrossRef]
- Hummel, T.; Sekinger, B.; Wolf, S.R.; Pauli, E.; Kobal, G. “Sniffin’ Sticks”: Olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem. Senses 1997, 22, 39–52. [Google Scholar] [CrossRef]
- Ribeiro, J.C.; Simões, J.; Silva, F.; Silva, E.D.; Hummel, C.; Hummel, T.; Paiva, A. Cultural adaptation of the Portuguese version of the “Sniffin’ Sticks” smell test: Reliability, validity, and normative data. PLoS ONE 2016, 11, e0148937. [Google Scholar] [CrossRef]
- Rumeau, C.; Nguyen, D.T.; Jankowski, R. How to assess olfactory performance with the Sniffin’ Sticks test. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2016, 133, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Oleszkiewicz, A.; Schriever, V.A.; Croy, I.; Hähner, A.; Hummel, T. Updated Sniffin’ Sticks normative data based on an extended sample of 9139 subjects. Eur. Arch. Oto-Rhino-Laryngol. 2019, 276, 719–728. [Google Scholar] [CrossRef]
- Tatour, Y.; Ben-Yosef, T. Syndromic Inherited Retinal Diseases: Genetic, Clinical and Diagnostic Aspects. Diagnostics 2020, 10, 779. [Google Scholar] [CrossRef]
- Karstensen, H.G.; Tommerup, N. Isolated and syndromic forms of congenital anosmia. Clin. Genet. 2012, 81, 210–215. [Google Scholar] [CrossRef]
- Landis, B.N.; Hummel, T.; Hugentobler, M.; Giger, R.; Lacroix, J.S. Ratings of overall olfactory function. Chem. Senses 2003, 28, 691–694. [Google Scholar] [CrossRef] [PubMed]
- Philpott, C.M.; Rimal, D.; Tassone, P.; Prinsley, P.R.; Premachandra, D.J. A study of olfactory testing in patients with rhinological pathology in the ENT clinic. Rhinology 2008, 46, 34–39. [Google Scholar] [PubMed]
- Delank, K.W.; Stoll, W. Olfactory function after functional endoscopic sinus surgery for chronic sinusitis. Rhinology 1998, 36, 15–19. [Google Scholar] [PubMed]
- Doty, R.L. Psychophysical testing of smell and taste function. Handb. Clin. Neurol. 2019, 164, 229–246. [Google Scholar]
- Trudeau, M.C.; Zagotta, W.N. An intersubunit interaction regulates trafficking of rod cyclic nucleotide-gated channels and is disrupted in an inherited form of blindness. Neuron 2002, 34, 197–207. [Google Scholar] [CrossRef]
- Marques, J.P.; Neves, E.; Geada, S.; Carvalho, A.L.; Murta, J.; Saraiva, J.; Silva, R. Frequency of cystoid macular edema and vitreomacular interface disorders in genetically solved syndromic and non-syndromic retinitis pigmentosa. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 2859–2866. [Google Scholar] [CrossRef]
| Patient (Gender; Age) | Family | Nationality | CNGB1 Variants (NM_001297.4) (ACMG Classification) | Consanguinity | Family History | Age of Onset | Age at Diagnosis (Years) | VA—First Visit (ETDRS Letters) | VA—Last Visit (ETDRS Letters) | RPE/EZ Foveal Sparing on SD-OCT (Last Visit) | Olfactory Phenotype | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V1 | V2 | OD | OS | OD | OS | OD | OS | Odor Threshold—CCCRC Test Score (Classification) | Odor Identification—SnSt 16 Pens Score (Classification) a | |||||||
| 1 (F; 86) | A | PT | c.1958-1G > A p.? (V) | c.1958-1G > A p.? (V) | - | + | Childhood | 84 | 66 | 69 | 66 | 69 | + | + | 0 (Anosmia) | 1 (Functional anosmia) |
| 2 (M;77) | A | + | Childhood | 74 | 65 | 65 | 67 | 62 | +; VMT | +;VMT | 0 (Anosmia) | 2 (Functional anosmia) | ||||
| 3 (M;55) | B | PT | c.3150del p.(Phe1051Leufs*12) (V) | c.3150del p.(Phe1051Leufs*12) (V) | - | - | Childhood | 52 | 81 | 81 | 85 | 75 | + | + | 0 (Anosmia) | 5 (Functional anosmia) |
| 4 (M;42) | C | PT | c.1958-1G > A p.? (V) | c.2565_2566del p.(Phe856*) (IV) | - | + | Childhood | 11 | 85 | 85 | 85 | 85 | +; CMO | +; CMO | 2 (Severe hyposmia) | 8 (Hyposmia) |
| 5 (F;46) | C | + | Childhood | 15 | 85 | 85 | 85 | 80 | + | + | 5 (Mild hyposmia) | 8 (Hyposmia) | ||||
| 6 (M;71) | D | PT | c.1958-1G > A p.? (V) | c.2285G > T p.(Arg762Leu) (IV) | - | + | Mid adulthood | 49 | 75 | 55 | 70 | 0 | +;VMT | -;VMT | 5 (Mild hyposmia) | 12 (Normosmia) |
| 7 (F;54) | E | PT | c.1958-1G > A p.? (V) | c.1958-1G > A p.? (V) | + | - | Childhood | 48 | 80 | 80 | 77 | 76 | + | + | 1 (Anosmia) | 8 (Functional anosmia) |
| 8 (F;52) | F | PT | c.1958-1G > A p.? (V) | c.1958-1G > A p.? (V) | - | + | Adolescence | 44 | 77 | 79 | 65 | 65 | +;ERM | +;ERM | 2 (Severe hyposmia) | 11 (Hyposmia) |
| 9 (M;46) | G | PT | c.1958-1G > A p.? (V) | c.1958-1G > A p.? (V) | + | + | Childhood | 16 | 85 | 85 | 0 | 80 | Impossible to evaluate (dense cataract) | + | 5 (Mild hyposmia) | 10 (Hyposmia) |
| 10 (F;77) | H | PT | c.1958-1G > A p.? (V) | c.1958-1G > A p.? (V) | - | + | Childhood | 67 | 70 | 60 | 55 | 44 | +;CMO | +; CMO | 0 (Anosmia) | 2 (Functional anosmia) |
| 11 (M;63) | I | FR | c.2978G > T, p.Gly993Val (IV) | c.2978G > T, p.Gly993Val (IV) | - | + | Adolescence | 20 | 50 | 75 | 50 | 75 | + | +; LH | 6 (Normosmia) | 14 (Normosmia) |
| 12 (M;60) | I | FR | + | Childhood | 38 | 65 | 80 | 35 | 75 | -; LH | +; LH | 6 (Normosmia) | 14 (Normosmia) | |||
| 13 (F;55) | I | FR | + | Childhood | 30 | 75 | 85 | 65 | 75 | -; CMO | +; CMO, ERM | 4 (Moderate hyposmia) | 13 (Normosmia) | |||
| 14 (M;49) | J | TR | c.2492 + 2T > G, p.? (V) | c.2492 + 2T > G, p.? (V) | + | + | Adolescence | 44 | 85 | 85 | 75 | 80 | + | + | 1 (Anosmia) | 4 (Functional anosmia) |
| 15 (F;24) | J | TR | c.1917G > A, p.(Trp639*) (V) | c.2492 + 2T > G, p.? (V) | + | Childhood | 18 | 75 | 80 | 75 | 80 | +; CMO | +; CMO | 2 (Severe hyposmia) | 8 (Functional anosmia) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geada, S.; Teixeira-Marques, F.; Teixeira, B.; Carvalho, A.L.; Lousan, N.; Saraiva, J.; Murta, J.; Silva, R.; Zanlonghi, X.; Defoort-Dhellemmes, S.; et al. Mutational Spectrum, Ocular and Olfactory Phenotypes of CNGB1-Related RP-Olfactory Dysfunction Syndrome in a Multiethnic Cohort. Genes 2023, 14, 830. https://doi.org/10.3390/genes14040830
Geada S, Teixeira-Marques F, Teixeira B, Carvalho AL, Lousan N, Saraiva J, Murta J, Silva R, Zanlonghi X, Defoort-Dhellemmes S, et al. Mutational Spectrum, Ocular and Olfactory Phenotypes of CNGB1-Related RP-Olfactory Dysfunction Syndrome in a Multiethnic Cohort. Genes. 2023; 14(4):830. https://doi.org/10.3390/genes14040830
Chicago/Turabian StyleGeada, Sara, Francisco Teixeira-Marques, Bruno Teixeira, Ana Luísa Carvalho, Nuno Lousan, Jorge Saraiva, Joaquim Murta, Rufino Silva, Xavier Zanlonghi, Sabine Defoort-Dhellemmes, and et al. 2023. "Mutational Spectrum, Ocular and Olfactory Phenotypes of CNGB1-Related RP-Olfactory Dysfunction Syndrome in a Multiethnic Cohort" Genes 14, no. 4: 830. https://doi.org/10.3390/genes14040830
APA StyleGeada, S., Teixeira-Marques, F., Teixeira, B., Carvalho, A. L., Lousan, N., Saraiva, J., Murta, J., Silva, R., Zanlonghi, X., Defoort-Dhellemmes, S., Smirnov, V., Dhaenens, C.-M., Blanchet, C., Meunier, I., & Marques, J. P. (2023). Mutational Spectrum, Ocular and Olfactory Phenotypes of CNGB1-Related RP-Olfactory Dysfunction Syndrome in a Multiethnic Cohort. Genes, 14(4), 830. https://doi.org/10.3390/genes14040830






