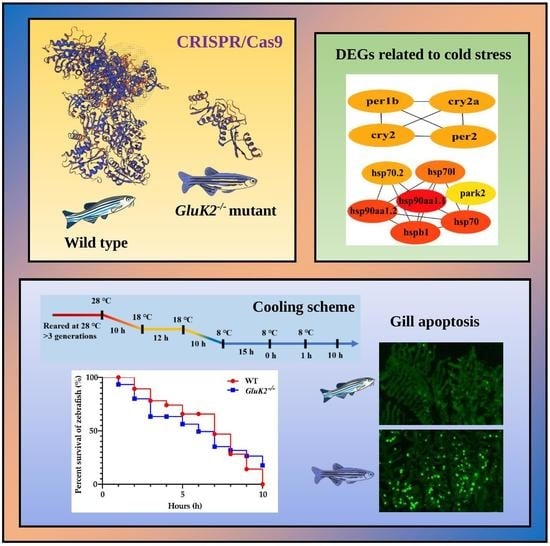
Transcriptomic and Phenotypic Analysis of CRISPR/Cas9-Mediated gluk2 Knockout in Zebrafish
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Embryos
2.2. Quantitative Real-Time PCR
2.3. Gene Knockout
2.4. Transcriptome Sequencing and RNA-Seq Data Processing
2.5. Behavior Analysis
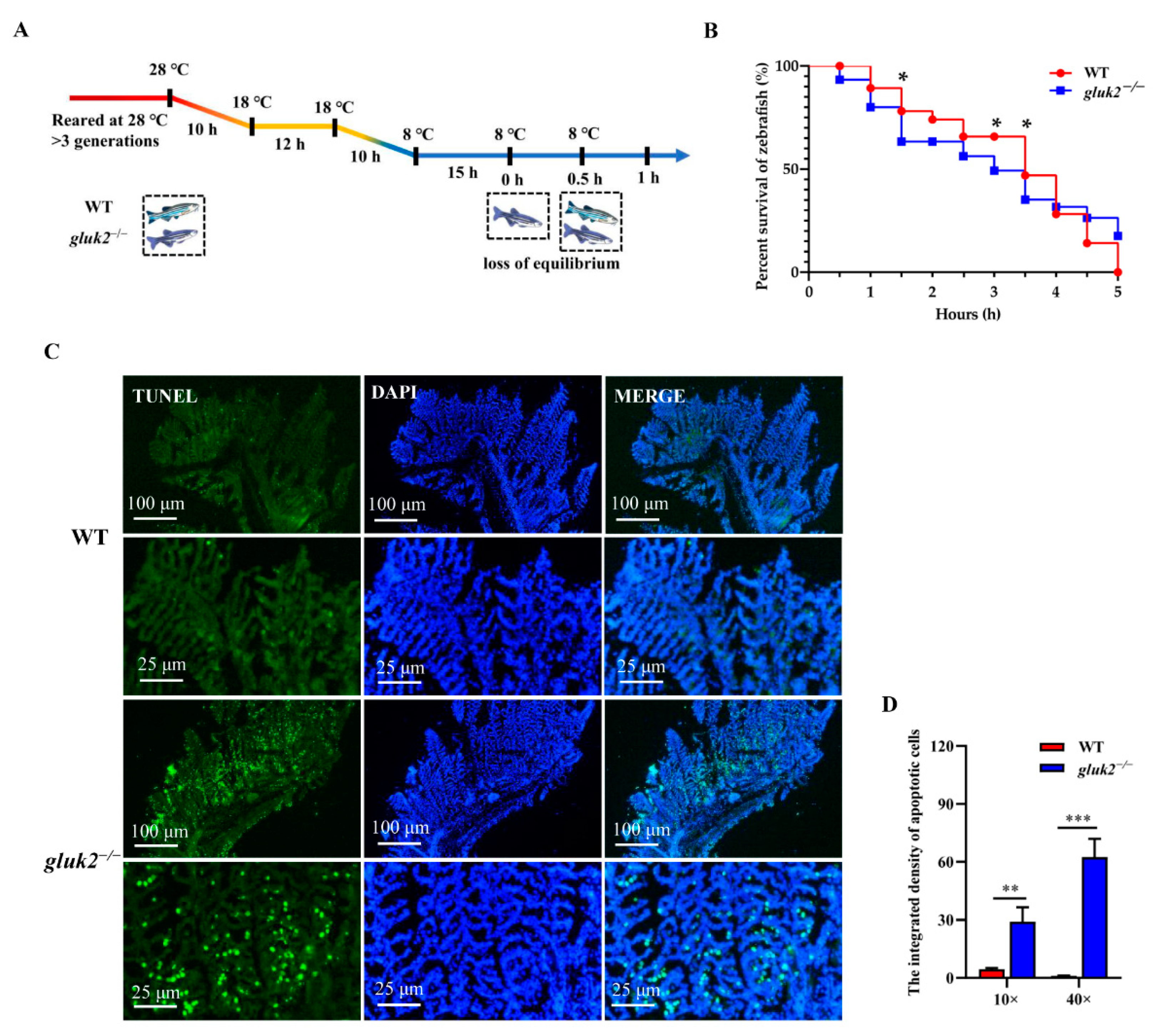
2.6. TUNEL Staining
2.7. Statistical Analysis
3. Results
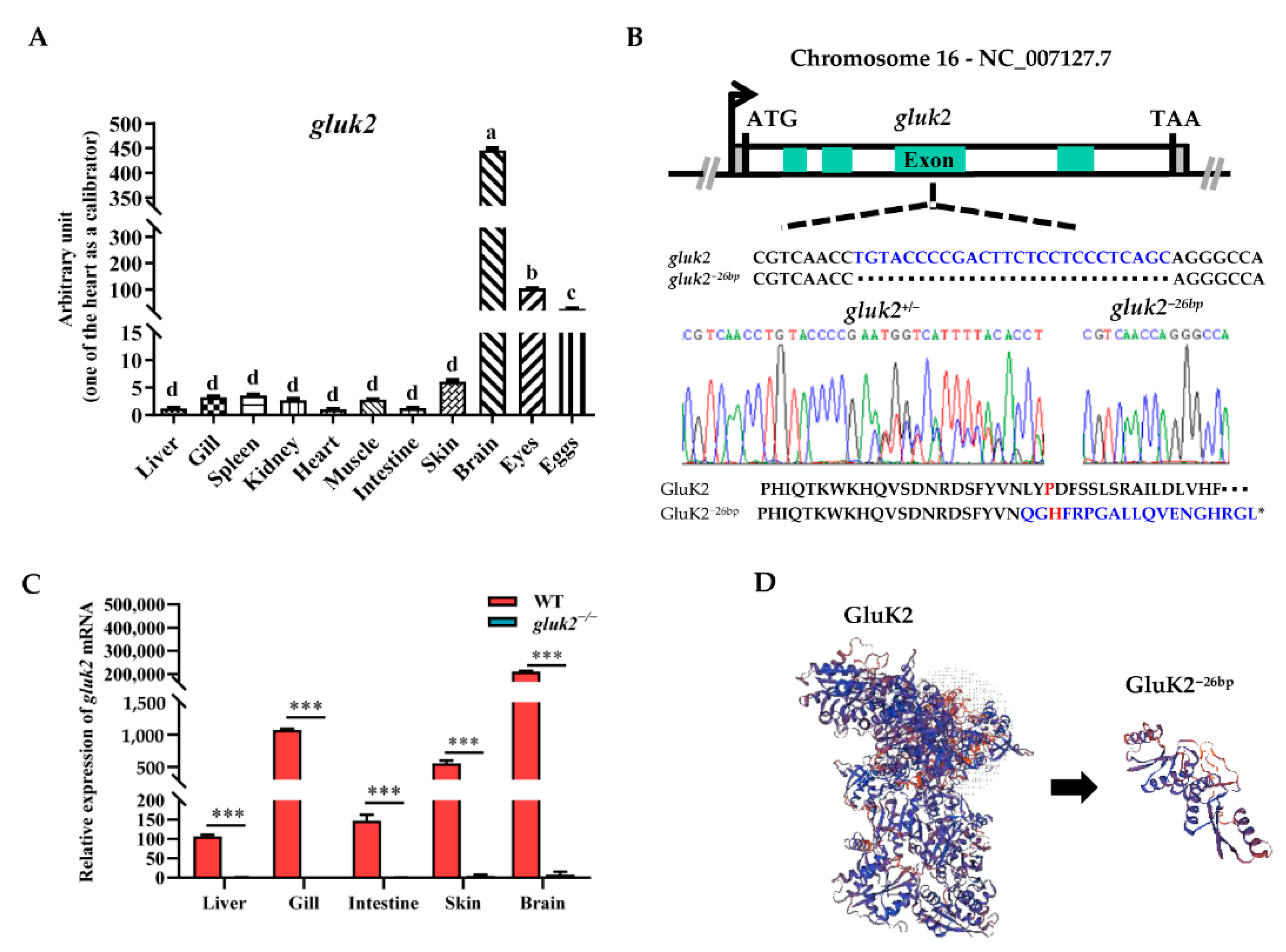
3.1. Distribution and Knockout of the gluk2 Gene in Zebrafish
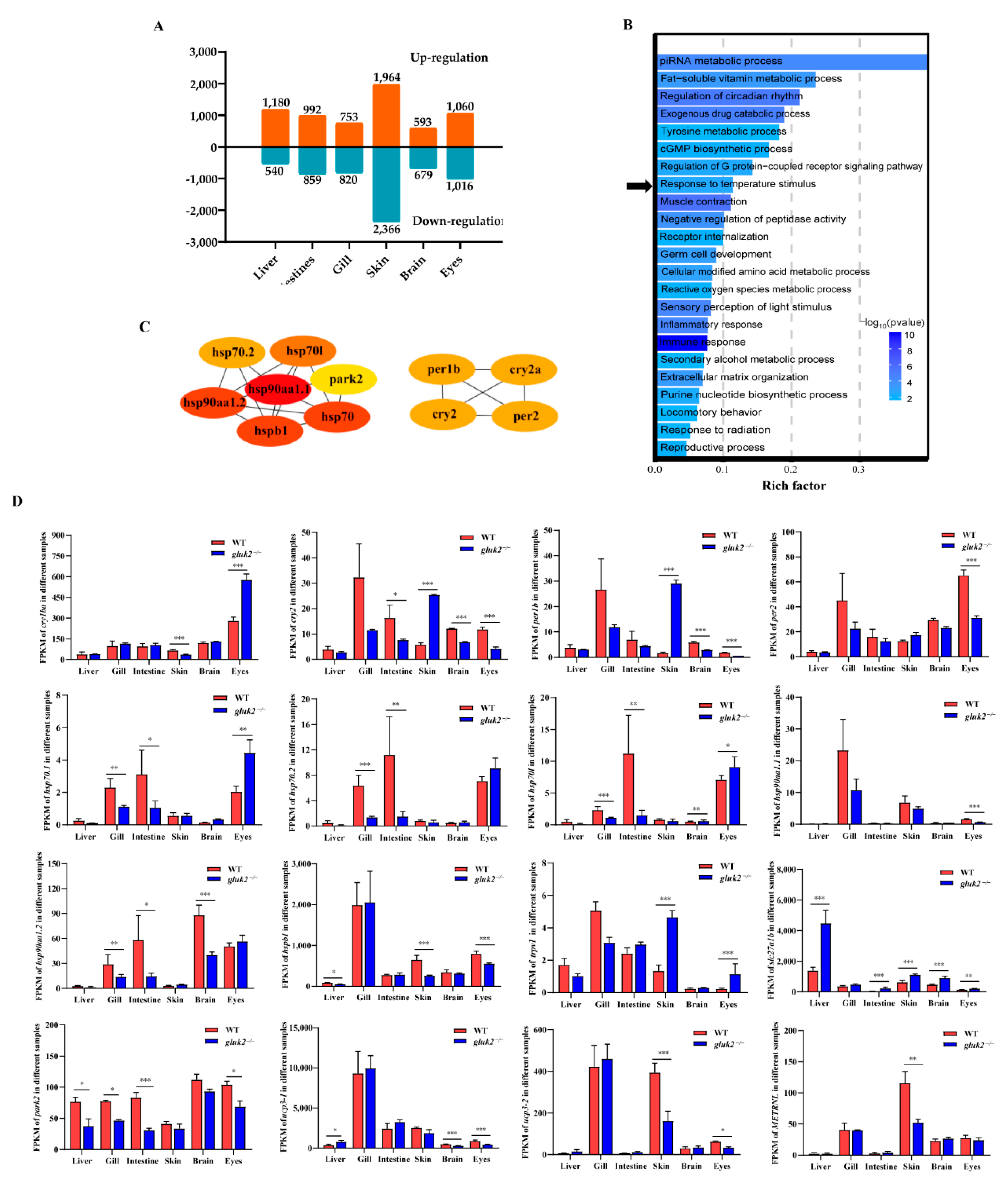
3.2. Functional Enrichment of DEGs
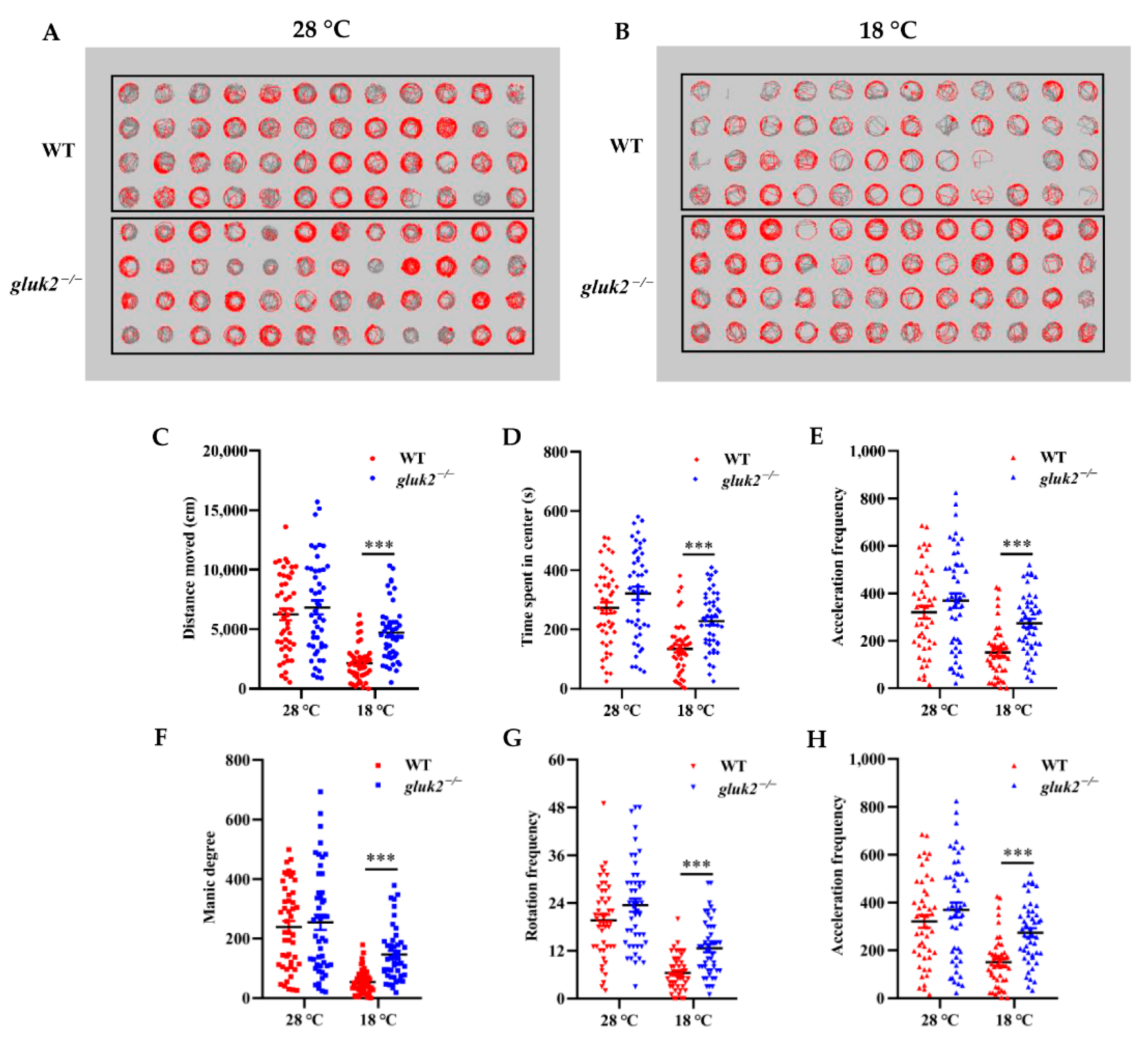
3.3. Behavioral Profiling of Cold Stress in Zebrafish Larvae
3.4. Decreased Cold Tolerance of gluk2−/− Mutant
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haesemeyer, M. Thermoregulation in fish. Mol. Cell Endocrinol. 2020, 518, 110986. [Google Scholar] [CrossRef] [PubMed]
- van den Burg, E.H.; Verhoye, M.; Peeters, R.R.; Meek, J.; Flik, G.; Van der Linden, A. Activation of a sensorimotor pathway in response to a water temperature drop in a teleost fish. J. Exp. Biol. 2006, 209 Pt 11, 2015–2024. [Google Scholar] [CrossRef] [PubMed]
- Lan, Z.; Xu, J.; Wang, Y.; Lu, W. Modulatory effect of glutamate GluR2 receptor on the caudal neurosecretory Dahlgren cells of the olive flounder, Paralichthys olivaceus. Gen. Comp. Endocrinol. 2018, 261, 9–22. [Google Scholar] [CrossRef] [PubMed]
- McKemy, D.D. Temperature sensing across species. Pflugers. Arch. 2007, 454, 777–791. [Google Scholar] [CrossRef]
- Castillo, K.; Diaz-Franulic, I.; Canan, J.; Gonzalez-Nilo, F.; Latorre, R. Thermally activated TRP channels: Molecular sensors for temperature detection. Phys. Biol. 2018, 15, 021001. [Google Scholar] [CrossRef] [PubMed]
- Paricio-Montesinos, R.; Schwaller, F.; Udhayachandran, A.; Rau, F.; Walcher, J.; Evangelista, R.; Vriens, J.; Voets, T.; Poulet, J.; Lewin, G.R. The Sensory Coding of Warm Perception. Neuron 2020, 106, 830–841.e3. [Google Scholar] [CrossRef]
- Saito, S.; Shingai, R. Evolution of thermoTRP ion channel homologs in vertebrates. Physiol. Genom. 2006, 27, 219–230. [Google Scholar] [CrossRef]
- Reiner, A.; Levitz, J. Glutamatergic Signaling in the Central Nervous System: Ionotropic and Metabotropic Receptors in Concert. Neuron 2018, 98, 1080–1098. [Google Scholar] [CrossRef]
- Traynelis, S.F.; Wollmuth, L.P.; McBain, C.J.; Menniti, F.S.; Vance, K.M.; Ogden, K.K.; Hansen, K.B.; Yuan, H.; Myers, S.J.; Dingledine, R. Glutamate receptor ion channels: Structure, regulation, and function. Pharmacol. Rev. 2010, 62, 405–496. [Google Scholar] [CrossRef]
- Wyllie, D.J.A.; Bowie, D. Ionotropic glutamate receptors: Structure, function and dysfunction. J. Physiol. 2022, 600, 175–179. [Google Scholar] [CrossRef]
- Egbenya, D.L.; Aidoo, E.; Kyei, G. Glutamate receptors in brain development. Childs Nerv. Syst. 2021, 37, 2753–2758. [Google Scholar] [CrossRef]
- Pinheiro, P.S.; Perrais, D.; Coussen, F.; Barhanin, J.; Bettler, B.; Mann, J.R.; Malva, J.O.; Heinemann, S.F.; Mulle, C. GluR7 is an essential subunit of presynaptic kainate autoreceptors at hippocampal mossy fiber synapses. Proc. Natl. Acad. Sci. USA 2007, 104, 12181–12186. [Google Scholar] [CrossRef] [PubMed]
- Niswender, C.M.; Conn, P.J. Metabotropic glutamate receptors: Physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 295–322. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.; Zhao, M.G.; Toyoda, H.; Qiu, C.S.; Zhuo, M. Altered behavioral responses to noxious stimuli and fear in glutamate receptor 5 (GluR5)- or GluR6-deficient mice. J. Neurosci. 2005, 25, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Bautista, D.M.; Siemens, J.; Glazer, J.M.; Tsuruda, P.R.; Basbaum, A.I.; Stucky, C.L.; Jordt, S.E.; Julius, D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 2007, 448, 204–208. [Google Scholar] [CrossRef]
- Gong, J.; Liu, J.; Ronan, E.A.; He, F.; Cai, W.; Fatima, M.; Zhang, W.; Lee, H.; Li, Z.; Kim, G.; et al. A Cold-Sensing Receptor Encoded by a Glutamate Receptor Gene. Cell 2019, 178, 1375–1386.e11. [Google Scholar] [CrossRef]
- Palacios-Filardo, J.; Aller, M.I.; Lerma, J. Synaptic targeting of kainate receptors. Cereb. Cortex. 2016, 26, 1464–1472. [Google Scholar] [CrossRef]
- Li, Q.; Ma, T.L.; Qiu, Y.Q.; Cui, W.Q.; Chen, T.; Zhang, W.W.; Wang, J.; Mao-Ying, Q.L.; Mi, W.L.; Wang, Y.Q.; et al. Connexin 36 Mediates Orofacial Pain Hypersensitivity Through GluK2 and TRPA1. Neurosci. Bull. 2020, 36, 1484–1499. [Google Scholar] [CrossRef]
- Li, Y.; Jia, Z.; Zhang, S.; He, X. Progress in Gene-Editing Technology of Zebrafish. Biomolecules 2021, 11, 1300. [Google Scholar] [CrossRef]
- Matsui, M.S.; Pelle, E.; Dong, K.; Pernodet, N. Biological Rhythms in the Skin. Int. J. Mol. Sci. 2016, 17, 801. [Google Scholar] [CrossRef]
- Burrows, D.; Samarut, É.; Liu, J.; Baraban, S.C.; Richardson, M.P.; Meyer, M.P.; Rosch, R.E. Imaging epilepsy in larval zebrafish. Eur. J. Paediatr. Neurol. 2020, 24, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Córdoba, M.; Rodriguez, S.; González Morón, D.; Medina, N.; Kauffman, M.A. Expanding the spectrum of Grik2 mutations: Intellectual disability, behavioural disorder, epilepsy and dystonia. Clin. Genet. 2015, 87, 293–295. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Liu, M.; Liu, Y.; Wang, J.; Zhang, D.; Niu, H.; Jiang, S.; Wang, J.; Zhang, D.; Han, B.; et al. Transcriptome comparison reveals a genetic network regulating the lower temperature limit in fish. Sci. Rep. 2016, 6, 28952. [Google Scholar] [CrossRef] [PubMed]
- Gau, P.; Poon, J.; Ufret-Vincenty, C.; Snelson, C.D.; Gordon, S.E.; Raible, D.W.; Dhaka, A. The zebrafish ortholog of TRPV1 is required for heat-induced locomotion. J. Neurosci. 2013, 33, 5249–5260. [Google Scholar] [CrossRef] [PubMed]
- Guderley, H. Metabolic responses to low temperature in fish muscle. Biol. Rev. 2004, 79, 409–427. [Google Scholar] [CrossRef]
- Bernáth, G.; Milla, S.; Várkonyi, L.; Ledoré, Y.; Griffitts, J.D.; Fontaine, P.; Urbányi, B.; Bokor, Z. The effect of two different experimental rearing temperatures on the quality and the large-scale cryopreservation of Eurasian perch (Perca fluviatilis) sperm. Theriogenology 2022, 185, 127–133. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, F.; Chen, K.; Guo, Y.; Liang, Y.; Zhao, H.; Chen, S. Exposure of zebrafish to a cold environment triggered cellular autophagy in zebrafish liver. J. Fish Dis. 2022, 45, 991–1000. [Google Scholar] [CrossRef]
- Khieokhajonkhet, A.; Sangphrom, S.; Aeksiri, N.; Tatsapong, P.; Wuthijaree, K.; Kaneko, G. Effects of long-term exposure to high temperature on growth performance, chemical composition, hematological and histological changes, and physiological responses in hybrid catfish [♂Clarias gariepinus (Burchell, 1822) ×♀C. macrocephalus (Günther, 1864)]. J. Therm. Biol. 2022, 105, 103226. [Google Scholar] [CrossRef]
- Crawshaw, L.I. Effect of rapid temperature change on mean body temperature and gill ventilation in carp. Am. J. Physiol. 1976, 231, 837–841. [Google Scholar] [CrossRef]
- Selvakumar, P.; Lee, J.; Khanra, N.; He, C.; Munguba, H.; Kiese, L.; Broichhagen, J.; Reiner, A.; Levitz, J.; Meyerson, J.R. Structural and compositional diversity in the kainate receptor family. Cell Rep. 2021, 37, 109891. [Google Scholar] [CrossRef]
- Chen, H.; Liang, Y.; Han, Y.; Liu, T.; Chen, S. Genome-wide analysis of Toll-like receptors in zebrafish and the effect of rearing temperature on the receptors in response to stimulated pathogen infection. J. Fish Dis. 2021, 44, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Le Morvan, C.; Troutaud, D.; Deschaux, P. Differential effects of temperature on specific and nonspecific immune defences in fish. J. Exp. Biol. 1998, 201 Pt 2, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Boltana, S.; Sanhueza, N.; Donoso, A.; Aguilar, A.; Crespo, D.; Vergara, D.; Arriagada, G.; Morales-Lange, B.; Mercado, L.; Rey, S.; et al. The expression of TRPV channels, prostaglandin E2 and pro-inflammatory cytokines during behavioural fever in fish. Brain Behav. Immun. 2018, 71, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.L.; Messner, H.N.; Cleverdon, R.; Baranowski, R.W.; Hamstra, S.I.; Geromella, M.S.; Stuart, J.A.; Fajardo, V.A. Heterozygous SOD2 deletion selectively impairs SERCA function in the soleus of female mice. Physiol. Rep. 2022, 10, e15285. [Google Scholar] [CrossRef]
- Lauri, S.E.; Segerstråle, M.; Vesikansa, A.; Maingret, F.; Mulle, C.; Collingridge, G.L.; Isaac, J.T.; Taira, T. Endogenous activation of kainate receptors regulates glutamate release and network activity in the developing hippocampus. J. Neurosci. 2005, 25, 4473–4484. [Google Scholar] [CrossRef]
- Zwiebel, L.J.; Hardin, P.E.; Liu, X.; Hall, J.C.; Rosbash, M. A post-transcriptional mechanism contributes to circadian cycling of a per-β-galactosidase fusion protein. Proc. Natl. Acad. Sci. USA 1991, 88, 3882–3886. [Google Scholar] [CrossRef]
- Hardin, P.E. Molecular genetic analysis of circadian timekeeping in Drosophila. Adv. Genet. 2011, 74, 141–173. [Google Scholar] [CrossRef]
- Pelham, J.F.; Dunlap, J.C.; Hurley, J.M. Intrinsic disorder is an essential characteristic of components in the conserved circadian circuit. Cell Commun. Signal. 2020, 18, 181. [Google Scholar] [CrossRef]
- Zhang, Y.; Iiams, S.E.; Menet, J.S.; Hardin, P.E.; Merlin, C. TRITHORAX-dependent arginine methylation of HSP68 mediates circadian repression by PERIOD in the monarch butterfly. Proc. Natl. Acad. Sci. USA. 2022, 119, e2115711119. [Google Scholar] [CrossRef]
- Hori, T.S.; Gamperl, A.K.; Afonso, L.O.; Johnson, S.C.; Hubert, S.; Kimball, J.; Bowman, S.; Rise, M.L. Heat-shock responsive genes identified and validated in Atlantic cod (Gadus morhua) liver, head kidney and skeletal muscle using genomic techniques. BMC Genom. 2010, 11, 72. [Google Scholar] [CrossRef]
- de Alba, G.; López-Olmeda, J.F.; Sánchez-Vázquez, F.J. Rearing temperature conditions (constant vs. thermocycle) affect daily rhythms of thermal tolerance and sensing in zebrafish. J. Therm. Biol. 2021, 97, 102880. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Jeong, T.H.; Choi, M.J.; Kim, J.M.; Lim, H.K. Heat shock protein 70 gene expression and stress response of red-spotted (Epinephelus akaara) and hybrid (E. akaara female x E. lanceolatus male) groupers to heat and cold shock exposure. Fish Physiol. Biochem. 2021, 47, 2067–2080. [Google Scholar] [CrossRef] [PubMed]
- Jeronimo, R.; Moraes, M.N.; de Assis, L.V.M.; Ramos, B.C.; Rocha, T.; Castrucci, A.M.L. Thermal stress in Danio rerio: A link between temperature, light, thermo-TRP channels, and clock genes. J. Therm. Biol. 2017, 68 Pt A, 128–138. [Google Scholar] [CrossRef]
- Manzon, L.A.; Zak, M.A.; Agee, M.; Boreham, D.R.; Wilson, J.Y.; Somers, C.M.; Manzon, R.G. Thermal acclimation alters both basal heat shock protein gene expression and the heat shock response in juvenile lake whitefish (Coregonus clupeaformis). J. Therm. Biol. 2022, 104, 103185. [Google Scholar] [CrossRef] [PubMed]
- Kelly, N.I.; Wilson, C.C.; Currie, S.; Burness, G. Acclimation capacity of the cardiac HSP70 and HSP90 response to thermal stress in lake trout (Salvelinus namaycush), a stenothermal ice-age relict. Comp. Biochem. Physiol. B Biochem. Mol Biol. 2018, 224, 53–60. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Q.; Li, W.; Gong, X.; Hu, R.; Chen, L. Transcriptomic and Phenotypic Analysis of CRISPR/Cas9-Mediated gluk2 Knockout in Zebrafish. Genes 2022, 13, 1441. https://doi.org/10.3390/genes13081441
Yan Q, Li W, Gong X, Hu R, Chen L. Transcriptomic and Phenotypic Analysis of CRISPR/Cas9-Mediated gluk2 Knockout in Zebrafish. Genes. 2022; 13(8):1441. https://doi.org/10.3390/genes13081441
Chicago/Turabian StyleYan, Qianqian, Wenhao Li, Xiaoting Gong, Ruiqin Hu, and Liangbiao Chen. 2022. "Transcriptomic and Phenotypic Analysis of CRISPR/Cas9-Mediated gluk2 Knockout in Zebrafish" Genes 13, no. 8: 1441. https://doi.org/10.3390/genes13081441
APA StyleYan, Q., Li, W., Gong, X., Hu, R., & Chen, L. (2022). Transcriptomic and Phenotypic Analysis of CRISPR/Cas9-Mediated gluk2 Knockout in Zebrafish. Genes, 13(8), 1441. https://doi.org/10.3390/genes13081441