Abstract
In freshwater ecosystems, dynamic hydraulic events (floods or dam maintenance) lead to sediment resuspension and mixing with waters of different composition. Microbial communities living in the sediments play a major role in these leaching events, contributing to organic matter degradation and the release of trace elements. However, the dynamics of community diversity are seldom studied in the context of ecological studies. Therefore, we carried out laboratory-induced leaching experiments, using sediments from the Villerest dam reservoir (Villerest, France). To assess whole microbial community diversity, we sequenced the archaeal and bacterial 16S rRNA genes using Illumina MiSeq. Our results suggest that the degree of dissolved oxygen found in the water during these resuspension episodes influenced community dynamics, with anoxic waters leading to drastic shifts in sedimentary communities compared to oxic waters. Furthermore, the release of microbial cells from sediments to the water column were more favorable to water colonization when events were caused by oxic waters. Most of the bacteria found in the sediments were chemoorganotrophs and most of the archaea were methanogens. Methylotrophic, as well as archaeal, and bacterial chemoorganotrophs were detected in the leachate samples. These results also show that organic matter degradation occurred, likely participating in carbonate dissolution and the release of trace elements during freshwater resuspension events.
1. Introduction
In subsurface environments such as soils and sediments, microorganisms play a significant role in the weathering of minerals [1,2]. Indeed, microbial activity can enhance mineral dissolution by releasing protons and organic compounds [3,4,5], or by redox reactions [6,7]. For some microbial species, this liberates limiting nutrients/substrates required for their metabolism, such as P [8,9], Fe [10,11], or S [12,13]. However, microbial activity can inhibit element release by the development of a passivation layer at the mineral surface, such as an amorphous silicate layer [14], or by the adsorption of polysaccharides [15].
Sediments are formed of various solid phases, silicates, carbonates, iron, manganese or aluminum oxyhydroxides, sulfide, and particulate organic matter (POM). The latter originate from bedrock erosion and alteration, soil weathering, and in situ processes. Freshwater sediments (e.g., lakes, dams, streams) typically harbour intense microbial diversity and activity, which are correlated to the high amounts of organic matter issued from the water column’s biological activity [16,17]. In calm hydraulic conditions, water–sediment exchanges through porewater diffusion to the water column are weak but present [18,19]. During dynamic hydrological events (e.g., floods or maintenance of dam reservoirs), sediment resuspension and input of water with a different composition can change reaction conditions, inducing mineral phase dissolution/precipitation and organic matter degradation [20].
In dam reservoirs, large amounts of water are mixed during hydrodynamic events like river floods, or maintenance operations such as dredging, flushing, and sluicing. During flushing, withdrawal of anoxic hypolimnion water via a bottom outlet allows oxygenated epilimnion waters to descend modifying redox conditions at the sediment–water interface [21,22]. Depending on organic matter and suspended particle loads, floods may input oxic or anoxic waters into reservoirs [23,24]. In general, floods, flushing, and sluicing are short-term events (from 12 to 48 h). They may discharge large amounts of suspended sediment downstream [25,26], which can have adverse effects on the water quality and aquatic organisms [27].
Many leaching experiments (batch experiments) on sediments have focused on the influence of the sedimentary matrix (mineral composition, granulometry, organic matter content), pH, and redox conditions. Other studies have shown that microbial activity influences leaching by decreasing the pH [28,29], since microbes release protons and different organic compounds during organic matter degradation. However, most of these studies have focused on synthetic sediments and minerals with a limited number of microbial species [30,31].
Therefore, in this study we conducted a series of leaching experiments using real dam reservoir sediments to assess the changes of the in situ microbial community composition during resuspension events, as this has not been done before. Furthermore, both archaeal and bacterial community diversity and composition were sequenced and assessed to cover the total prokaryotic community. Studied sediments were collected from the Villerest dam reservoir (France) and the leaching experiments were carried out in the lab under oxic and anoxic conditions to simulate different environmental situations. Indeed, dam maintenance will lead to the mixing of either oxic or anoxic water with the lake water. We postulate that: (1) sediment leaching will affect sediment prokaryotic community composition, (2) sediment leaching will result in the discharge of cells into the water column, thus influencing the diversity of the water community, and (3) the presence/absence of oxygen will influence prokaryotic community composition during leaching events.
2. Materials and Methods
2.1. Study Site, Sampling, and Incubation Setup
Sediments used for this study were sampled in the Villerest dam reservoir, situated in the Upper Loire Basin (France) (Figure 1). This dam was built across the Loire River in the late 1970s and has been in operation since 1984 in order to regulate flood events, sustain low flows, and produce electricity. Regular sediment flushing operations are performed at least once every 2 years. The reservoir and surrounding area lie on crystalline rocks, sedimentary formations, and recent alluvial deposits. Several towns and associated industrial zones, plus an old coal-mining district, are located upstream from the reservoir. In addition to an elevated natural geochemical background [32], a high metal trace element enrichment of the reservoir sediments has been detected [33]. In addition, this reservoir has presented a high nutrient load since it was launched, leading to its eutrophication. Seven 50 cm-long sediment cores were collected in the deeper part of the reservoir in November 2016 with a gravity corer (UWITEC, fitted with 90-mm diameter plastic liners). The first 15 cm of each core was mixed and used for leaching experiments. In November 2017, bottom water was sampled at 30 m depth using a manual water sampler, to assess the in situ microbial diversity composition and to compare it with the community formed in the water column during the leaching experiments. Although communities may change over the course of 1 year, sampling was done during the same fall season, when community composition is typically stable in lake waters [34].
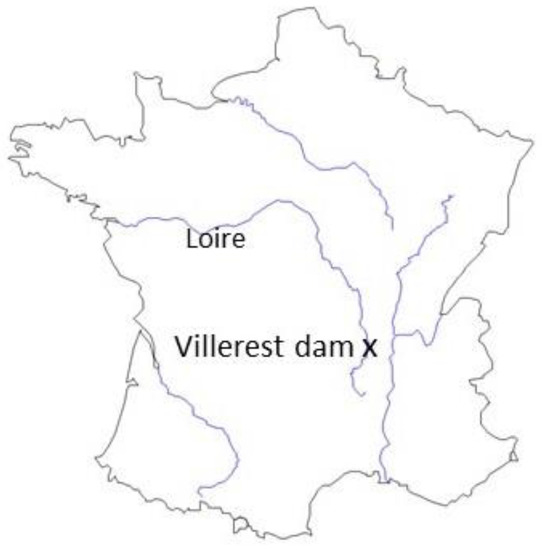
Figure 1.
Map of France, locating the Loire River and the Villerest dam.
Batch experiments were conducted with open glass beakers (oxic conditions) or PP vials (anoxic conditions) over 30 days until the electrical conductivity of the leachates reached a pseudo-plateau. To accelerate the weathering of the solid phases, deionized water (DI) was used as leaching solution. No enrichment medium was added during the experiments. Sediment samples were mixed with DI at a fixed solid/solution ratio of 1/10 (12 g of sediment per 120 mL of water). Solutions were continuously agitated to stimulate resuspension events. Water and sediment aliquots were taken after 24, 120, and 720 h incubation for both the oxic and anoxic incubations. For the aerobic experiment, a 250 mL vial was used for each step and the blank. Solutions were agitated with a magnetic stirrer (100 rpm). For the anaerobic experiment, triplicates of 50 mL PP vials closed with plastic caps and equipped with inlet and outlet connectors were used. Prior to mixing with sediment, the DI water was degassed for 4.5 h with pure N2 gas. After filling the vials, nitrogen was added at the same time to all the subsamples for 10 min to strip dissolved O2 and CO2 from the solution. Vials were agitated on a roller shaker (40 rpm). All treatments and manipulations of subsamples were performed in a portable glove box under a N2 atmosphere.
2.2. Chemical Analysis of Water Leachate
Major element and phosphorus concentrations were determined using ICP-OES at the SARM laboratory (SARM-CRPG UMR 7358, Nancy, France). The accuracy and precision of determinations were checked at regular intervals with the international standard SLRS-5 and internal references. Typical measurement uncertainties were ±2–5% for Ca. Nitrate, and sulphate concentrations were measured using ionic chromatography with chemical suppression. Typical measurement uncertainties were ±1 × 10−6 mol L−1 for NO3− and ± 2 × 10−5 mol L−1 for SO42−.
To estimate the dissolved organic matter (DOM) content, DOC (dissolved organic carbon) was determined on a Shimadzu TOC/TN analyser using a non-purgeable organic carbon (NPOC) procedure that involved acidification, sparging (inorganic carbon removal), and combustion of the sample at 680 °C. Potassium hydrogenophtalate was used to prepare the standard solution. The accuracy of DOC concentration measurements was ±0.5 mg L−1 estimated on blank samples and a range of standard concentrations. The total dissolved nitrogen (TDN) was measured in the filtered solution using the total nitrogen unit of the Shimadzu analyser with an accuracy estimated at ±0.5 mg L−1. The total dissolved organic nitrogen (DON) content (ammonium + dissolved organic matter containing nitrogen = N(-III) forms) was estimated as the difference between total dissolved nitrogen (TDN) and nitrogen-nitrate (N-NO3) concentrations
2.3. DNA Extraction, Sequencing and Sequence Analysis
Six grams of the sediment samples were used to extract DNA, using the DNeasy PowerMax Soil Kit (QIAGEN, Hilden, Germany). Samples were taken prior to the incubations (0 h, 0H), after 12 h (12H), 240 h (240H), and 720 h (720H). Three grams were used for separate extractions for each sediment sample in order to produce duplicates. Ten mL of the liquid phase/leachate (overlying DI water collected at the same different timepoints as the sediment samples) was filtered using a polyethersulfone 0.2 µM filter membrane (Sartorius, Göttingen, Germany). DNA was extracted from the filters using the DNeasy PowerWater kit (QIAGEN, Germany). Given the low amount of water, no duplicate samples were extracted. The archaeal and bacterial 16S rRNA genes were sequenced using an Illumina MiSeq at the CERMO (Centre d’excellence en recherche sur les maladies orphelines, Biological Sciences department, UQAM), with the 340F (5′-CCCTACGGGGYGCASCAG-3′, [35])-915R (5′-GTGCTCCCCCGCCAATTCC-3′, [36]) and the 341F (5′-CCTACGGGAGGCAGCA-3′, [37])-785R (5′-GACTACHVGGGTATCTAATCC-3′, [38]) primer pairs, respectively. Reads were merged, processed, and analyzed using mothur v.1.47 [39], resulting in 575 bp reads for the archaea and 444 bp reads for the bacteria. Classification of 16S rRNA genes was obtained using the SILVA reference database v.138 [40]. The archaeal 16S rRNA gene datasets were further analyzed using a personal database based on the taxonomy of the Woesearchaeota [41] and the Bathyarchaeota [42]. The obtained sequences were deposited in the National Center for Biotechnology Information (NCBI) under the BioProject ID: PRJNA805122.
2.4. Statistical Analyses
Amplicon sequence variants (ASVs) were created using mothur. ASV tables were rarefied to 3895 reads for the archaeal, and 10,636 reads for the bacterial 16S rRNA genes, to standardize sequence efforts across samples. The Shannon diversity indices (α-diversity) were calculated using mothur. Indices were compared using a Mann–Whitney–Wilcoxon test, with the wilcox.test function in R v.4.1.2 [43] when comparing 2 groups of samples, and using a Kruskal–Wallis test, with the dunnTest function of the FSA package (https://cran.r-project.org/web/packages/FSA/FSA.pdf accessed on 1 August 2022), when comparing 3 groups of samples. Samples were clustered using the pvclust package in R [44], using a correlation distance and the average agglomeration method with 1000 bootstrap replications, on a Hellinger transformed ASV table. To test whether bacterial community composition varied significantly depending on environmental parameters, we ran permutational multivariate analyses (PERMANOVA) on the rarefied ASV tables in R, using the adonis function of the vegan package (https://cran.r-project.org/web/packages/vegan/vegan.pdf accessed on 1 August 2022). Significantly different ASVs between sample groups were identified using linear discriminant effect size analyses (lefse) using the online tool from the Huttenhower lab (https://huttenhower.sph.harvard.edu/lefse/ accessed on 1 August 2022). The FEAST method (fast expectation-maximization for microbial source tracking; [45]) was used to estimate the contribution of sediment communities to water communities, and the contribution of communities in sediment and water over the different incubation time points (i.e., the source of communities in both the sediments and water samples), using raw ASV tables without transformation.
3. Results
3.1. Geochemical Analyses in the Water Leachate over Time
Under oxic conditions, pH decreased rapidly (Figure 2a) during the first 24 h (from 6.9 to 5.1), stayed stable up to 20 days, and then decreased again to 4.3 by the end of the experiment. The main weathered mineral was a calcium carbonate according to the Ca2+ release (Figure 2b), which began after 24 h and continued to increase regularly from 11.93 to 89.77 mg L−1. Under anoxic conditions, pH slightly increased during the first 10 days (from 5.4 to 5.6) then stayed stable at around 6.4 until the end of experiment. The Ca2+ release followed the same trend, increasing the first 10 days (from 11.54 to 37.10 mg L−1) then staying stable at around 30 mg L−1 until day 30. For both conditions, mineral alteration, evidenced by Ca2+ concentration increase, seemed to start 6 to 12 h after the beginning. Under oxic conditions, pH and Ca2+ release varied in opposite directions, while they both increased to a pseudo-plateau under anoxic conditions.
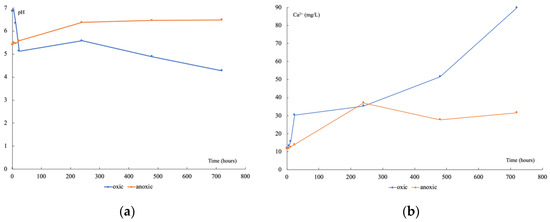
Figure 2.
Time variation of pH (a), and Ca2+ (b), during the leachate incubations, in oxic (blue) and anoxic (orange) conditions.
Under oxic conditions, the initial DOC measured corresponded to that contained in porewater and it began to be consumed after 6 h (Figure 3a), increased at 10 days, and then decreased continuously. At the same time, TDN still increased, firstly composed mainly of DON then of N-NO3. After 6 h, the P-PO4 decreased until reaching a concentration lower than the detection limit at 10 days (Figure 3b). A high input of S-SO4 was recorded continuously until reaching a plateau after 10 days when P-PO4 was no longer available. Under anoxic conditions, DOC and DON concentrations were higher than under oxic conditions (Figure 3c), but N-NO3 was very low. The input of S-SO4 was half of that under oxic conditions and decreased sharply at 30 days. From the beginning of the experiments, the P-PO4 concentration was higher than under oxic conditions (Figure 3b). It fluctuated greatly over time. Time variations of DOC, DON, N-NO3, P-PO4, and S-SO4 released during these experiments can be mainly linked to organic matter degradation.
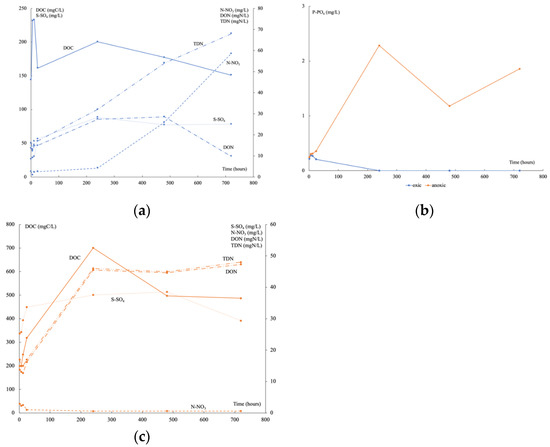
Figure 3.
Time variation of DOC, S-SO4, N-NO3, DON, and TDN in oxic (a) and anoxic conditions (c), and of P-PO4 (b) in oxic (blue) and anoxic (orange) conditions.
3.2. Archaeal 16S rRNA Gene Diversity in the Sediments and Leachate
In the sediments, for both the oxic and anoxic incubations at 3 time points (12, 240 and 720 h), there was a complete shift of the archaeal community diversity compared to the community initially present. Indeed, the S0 sample (used as a starting point for each incubation), was dominated by unclassified Archaea, Bathyarchaeota subgroup 6 (Bathy_6), and Woesarchaeota (Supplementary Material Table S1). We were unable to recover enough sequences for the second duplicate of the 12 h anoxic sample. In both the sediment oxic and anoxic incubations, there was an increase of the relative abundance of methanogens which dominated the samples: Methanosarcina, Methanosaeta, Methanoregula, Methanocella or Rice Cluster I (Figure 4). Bathy_6 archaea were also detected in both experiments, with a higher relative abundance in the oxic incubations.
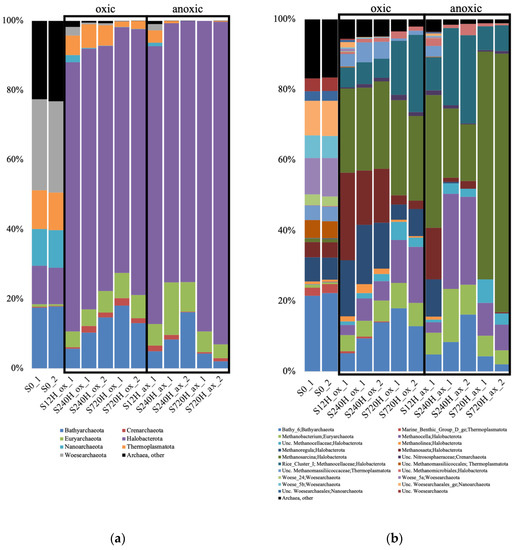
Figure 4.
Relative abundance of the archaeal taxonomic groups at the phylum (a); and genus (b) levels, in the oxic (ox) and anoxic (ax) incubations, in the sediment (S) samples, and for both duplicates (_1 and _2). Relative abundance is expressed in percentage of the total number of sequences for each sample. S0, initial sediment before incubation; Unc, unclassified.
We recovered an extremely low amount of reads in the leachate samples. Therefore, the water samples were not used for further statistical analyses. However, it is worth noting that, among these reads, Bathy_6 archaea were the highest group in the oxic samples (Supplementary Material Table S1). Also, we detected methanogens, candidatus Methanoperendens, cand. Nitrosotalea, and Nitrososphaeraceae, all three of which were not dominant in the sediment samples. The water sample collected from the dam lake (Dam) contained few reads as well, with Nitrosoarchaeum representing most of those reads.
3.3. Bacterial 16S rRNA Gene Diversity in the Sediments and Leachate
As for the archaeal 16S rRNA gene diversity, for both the oxic and anoxic incubations at all time points, there was a complete shift of the bacterial community diversity compared to the community initially present. The S0 sample was dominated by Rhodanobacter, unclassified Acidimicriia, and unc. Micropepsaceae (Supplementary Material Table S2). In the oxic incubations, the sediments were dominated by Bacillus after 12 h, then by Sphingomonas at 240 h, and 720 h (Figure 5). The leachate samples showed different dominant taxa, with Sphingomonas dominating at 12 h, Pseudoarthrobacter at 240 h, and Rhodanobacter at 720 h. In the anoxic incubations, the sediments were dominated by Bacillus after 12 h, then Clostridium at 240 h, and Bacillus at 720 h (Figure 5). As for the oxic incubations, the leachate samples showed different taxa, with Rhodanobacter dominating at 12 h, Pseudoarthrobacter at 240 h, and Flavobacterium at 720 h. The water sample collected from the dam lake (Dam) was completely different, dominated by cand. Methylopumilus, unc. Illumatobacteraceae, Xanthobacteraceae, and Gaillelales.
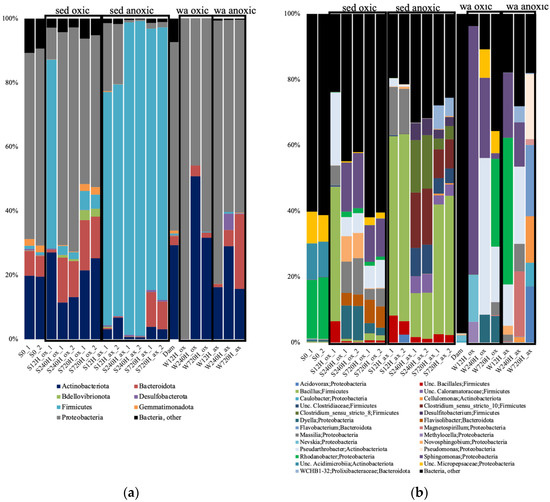
Figure 5.
Relative abundance of the bacterial taxonomic groups at the phylum (a); and genus (b) levels, in the oxic (ox) and anoxic (ax) incubations, in the sediment (S) and water (W) samples and for both duplicates (_1 and _2). Relative abundance is expressed in percentage of the total number of sequences for each sample. S0, initial sediment before incubation; Dam, dam lake water sample; Unc, unclassified; sed, sediment; wa, water.
3.4. Archaeal and Bacterial α-Diversity Indices Comparison
Archaeal (Figure 6a) and bacterial sediment sample diversity indices (Figure 6b) were not significantly different between the oxic and anoxic incubation (Mann–Whitney–Wilcoxon, p = 0.84 for Archaea and 0.1 for Bacteria). Similarly, leachate bacterial diversity indices were not significantly different between the oxic and anoxic incubations (p = 0.1). However, bacterial diversity indices were significantly different between sediments and leachate sample when comparing diversity indices in oxic conditions (p = 0.036), anoxic conditions (p = 0.024), or oxic and anoxic conditions (p = 0.00016), with sediment communities being more diverse than leachate communities.
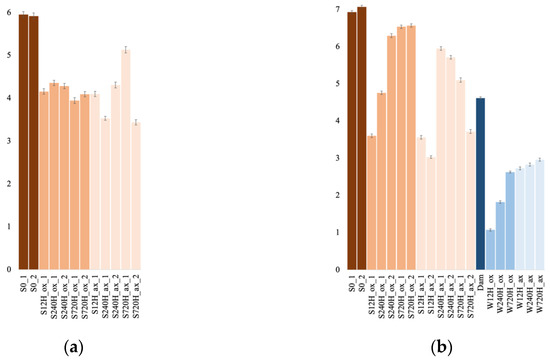
Figure 6.
Shannon diversity indices for the archaeal (a) and bacterial (b) communities, in the oxic (ox) and anoxic (ax) incubations, in the sediment (S) and water (W) samples and for both duplicates (_1 and _2). S0, initial sediment before incubation; Dam, dam lake water sample.
3.5. Bacterial Community Composition
The cluster dendrogram based on the bacterial 16S rRNA gene diversity shows a clear separation between sediment and leachate samples (Figure 7). It also indicates that leachate samples were more similar amongst themselves than the sediment samples. The first timepoints of the sediment oxic and anoxic incubations cluster together, suggesting that oxygen presence/absence did not play a major role in the community composition in the first 12 h of incubation. All other timepoints, whether in the oxic or anoxic conditions do not cluster together, indicating major community differences. The community composition of the sample collected in the dam lake water (Dam) was most similar to the leachate sample collected after 720 h in anoxic conditions. This suggests that cells leaching from the sediments into the water column explain part of the existing in situ diversity in the Villerest lake water.
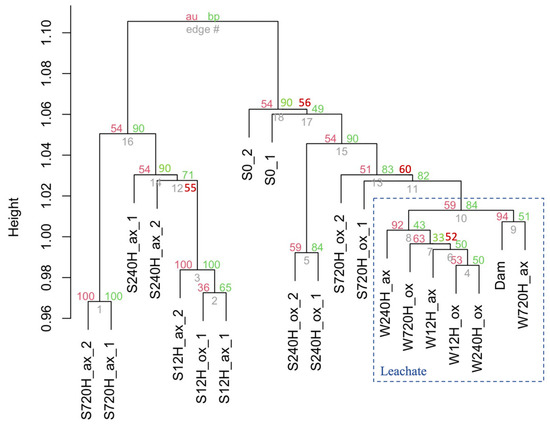
Figure 7.
Dendrogram from cluster analysis of bacterial 16S rRNA gene diversity in the oxic (ox), and anoxic (ax) incubations, in the sediment (S) and water (W) samples and for both duplicates (_1 and _2). Bar indicates dissimilarity values. Approximately unbiased (au) p values are shown in red, and bootstrap probability (bp) values are shown in green at each node. S0, initial sediment before incubation; Dam, dam lake water sample.
Environment type (sediment/leachate) explained 15.4% of the variation in all bacterial samples (PERMANOVA, p = 0.001, Table 1), whereas oxygen presence/absence explained 13.8% of the variation (p = 0.002), and incubation time explained an additional 15.6% of the variation (p = 0.04), confirming observations made with the cluster dendrograms.

Table 1.
Variation in bacterial community composition explained by oxygen (oxic/anoxic incubation conditions), environment type (sediment/leachate), or incubation time (12, 240 or 720 h), tested using PERMANOVA.
The lefse analysis comparing bacterial community composition between oxic and anoxic samples in the sediment, Sphingomonas, Pseudoarthrobacter, and Dyella were significantly higher in the oxic community, whereas Bacillus and Clostridium were significantly higher in the anoxic community (Figure 8a). In the leachate, Methylocella and Micrococcus were significantly higher in the oxic community, whereas Flavobacterium, Cellulomonas, and Caulobacter were significantly higher in the anoxic community. When comparing the sediment and leachate samples in the oxic incubations, Bacillus and Novosphingobium were significantly higher in the sediments (Figure 8b). In the anoxic incubations, Bacillus and Clostridium were significantly higher in the sediments, and Sphingomonas, Flavobacterium, and Magnetospirillum were significantly higher in the leachate.
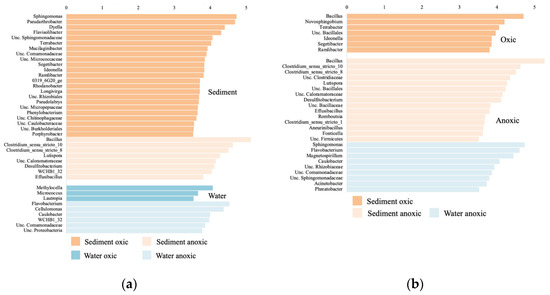
Figure 8.
Linear discriminant analysis (LDA) score comparing significantly different bacterial genera between (a) the oxic and anoxic samples in the sediment and leachate samples separately; (b) the sediment and leachate samples in the oxic and anoxic samples separately; calculated using the lefse analysis. Genera with a LDA score > 3.5 are displayed.
3.6. Source of Leachate and Sediment Archaeal and Bacterial Communities
For the Archaea, we only used the sediment dataset for the FEAST analysis, as the number of sequences in the leachate samples were too low. Also, the analysis could only be done for the 240 h and 720 h samples, as the FEAST analysis requires a minimum of two source samples. The portion of unknown sources for the oxic sediment samples was lower (55.8 and 56.2%) than for the anoxic samples (81 and 70%; Figure 9a). For the oxic samples, the initial sediment accounted for 3.6% (after 240 h) and 1.5% (after 720 h) of the communities, whereas this source was negligeable for the anoxic samples (less than 10−4). Furthermore, for the oxic incubations, the source of the communities was the community from the previous time point (40.1% after 240 h and 36.8% after 720 h). For the anoxic ones, the source was mainly the community from the previous time point after 240 h (S12H_ax contributed 18.2% to S240H_ax), and after 720 h the source originated from both previous time points’ communities (S12H_ax contributed 6.6% and S240H_ax 23% to S720H_ax).
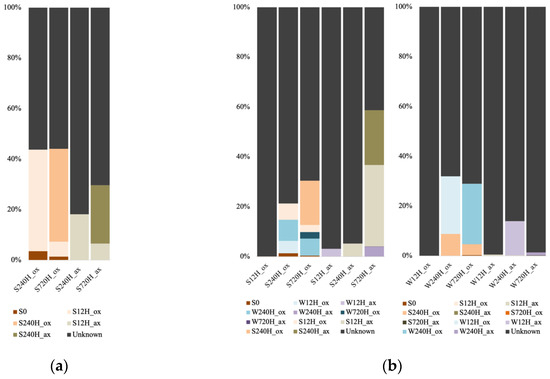
Figure 9.
Microbial source tracking of (a) archaeal sediment communities using the initial sediment (S0) and the previous sediment incubations time points (S) as sources; (b) bacterial sediment and leachate communities, using the initial sediment (S0) and the previous sediment (S) and water (W) incubation timepoints as sources.
For the bacterial samples, we were able to run potential sources for the 12 h samples, since we used the leachate samples as a source for the sediment samples and vice-versa. In oxic conditions, a vast majority of the sediment community source was unknown after 12 h incubation (99.9%; Figure 9b). Source for the 240 h samples were the initial sediment, and both leachate samples from the previous and same timepoints. We observed the same for the 720 h, with the major source being the sediment sample from the previous timepoint (17.6%). In anoxic conditions, a small source of sediment community for the 12 h incubation was the leachate sample from the same timepoint (3.1%). After 240 h incubation, a small source was the sediment sample from the previous timepoint (5.1%). And after 720 h, a small source was the water sample from the previous timepoint (W240H_ax contributed 5%), but the major source was the sediment samples from the previous timepoints (S12H_ax contributed 32.6% and S240H_ax 21.7%). For the leachate samples after 12 h whether in oxic or anoxic conditions, most of the sources were unknown (99.9 and 99.4%). For the oxic incubations, after 240 h and 720 h, the 240 h sediment community contributed 8.8% and 4.3% to the community, whereas leachate samples from the previous timepoints were the major sources (W12H_ox contributed 23.1% to W240H_ox, and W240H_ox contributed 24.3% to W720H_ox). For the anoxic incubations, most of the sources remained unknown, with a small source from the previous leachate timepoints (W12H_ax contributed 13.7% to W240H_ax, and W240H_ax contributed 1.3% to W720H_ax).
4. Discussion
4.1. Organic Matter Degradation and Mineral Alteration
During the leaching experiments, both mineral dissolution/precipitation and organic matter degradation occurred. Oxic conditions favoured the dissolution of minerals such as sulphides and the degradation of organic matter. In contrast, anoxic conditions mainly favoured the dissolution of oxyhydroxide phases and the degradation of organic matter.
In order to evaluate POM degradation, several proxies such as DOC, TDN, P-PO4, and potentially S-SO4 and protons can be used. DOC concentrations should increase as POM is degraded. However, part of this dissolved organic carbon can be oxidized to CO2, leading to a decrease of DOC, and thus, DOC cannot be used as a conservative parameter. Under oxic conditions, TDN can be considered as a good tracer of POM degradation as nitrate cannot be reduced to N2 in the presence of dissolved oxygen. Under anoxic conditions, nitrogen originating from POM degradation can exist in its N(-III) form, the only species accounting for DON, and it can be partly oxidized to nitrate by MnO2 [46,47] or by an anammox reaction mediated by bacteria [48,49]. In both cases, nitrogen can then be reduced to N2. Hence, the TDN concentration cannot be considered conservative under anoxic conditions. Therefore, variations of all nutrients have to be taken into account.
In our study under oxic conditions, pathways of organic matter degradation led to its partial mineralization in CO2 as DOC decreased and nitrate formation was still greater than consumption, but a significant consumption of phosphorous limited its input.
Under anoxic conditions different and less mineralized by-products of organic matter degradation were observed. TDN reached a plateau after 10 days. Nitrate consumption may have caused a slowdown in organic matter degradation. Under these conditions, phosphorous was not limiting. DOC production was much greater, whereas less TDN was produced. Anoxic leachates emitted a putrid odour, possibly caused by organosulphur molecules. No phosphorus consumption was clearly evidenced under anoxic conditions (i.e., consumption was lower than production). Hence, under both conditions, sulphate release was recorded, but it was greater under oxic conditions. Under the latter condition, sulphate may have originated from sulphide oxidation as framboidal pyrites were observed in sediment, and this explains why proton input was much greater under oxic conditions.
4.2. Microbial Community Changes in the Sediment
During both the oxic and anoxic incubations, bacterial diversity increased with time (from 12 to 240 and 720 h), whereas archaeal diversity was relatively stable, suggesting a higher capacity of the Archaea to acclimatize and respond to the resuspension event mimicked during our incubations. This could also indicate that Bacteria were more vulnerable to the change in their abiotic environment compared to the Archaea, leading to the settling of new additional taxa. These observations could be linked to potential exchanges of nutrients and microbial cells from water to sediment. Indeed, bacterial water communities were a non-negligeable source for the bacterial sediment communities over the three timepoints, likely contributing to the community change and diversity increase. On the other hand, since very few Archaea seem to inhabit the dam lake water (as attested by both the leaching experiments, and the sequencing of the lake water itself), the only source to the archaeal sediment communities were the communities actually or previously present. Therefore, less community and diversity change occurred during the resuspension event.
Dynamic hydraulic events caused, for instance, by dam maintenance, can lead to the flow of oxygenated water from the lake epilimnion, but also anoxic waters when organic matter concentrations are high [24]. For this reason, we investigated the consequences of resuspension in oxic or anoxic conditions. When looking at sediment and water samples separately, the presence/absence of oxygen did not significantly influence archaeal and bacterial diversity indices. However, the presence/absence of oxygen did account for some of the variation observed in the bacterial mesocosm communities. Clustering of bacterial samples showed that after 12 h, both oxic and anoxic samples clustered together, whereas all samples after 12 h did not cluster at all, indicating major community shifts occurring after 12 h when faced with a resuspension event. This suggests that if the hydraulic events have short durations, the water characteristic in terms of oxygen content will not affect the bacterial community. In oxic conditions, the initial sediment used to set up the incubation (S0) only explained a small proportion of the communities for both the archaeal and bacterial sediment incubations. The latter was not observed in anoxic conditions, suggesting that during a resuspension event, when faced with oxygenated water, a portion of the bacterial populations present before the event is able to survive and contribute to the community even after 720 h of resuspension activity. It seems likely that mixing with anoxic waters led to a more significant change in environmental conditions, explaining the more drastic change in microbial community composition.
4.3. Potential Microbial Metabolisms in the Sediment
Most of the dominant bacterial and archaeal groups detected at each time point were not the dominant groups in the initial sediments, further showing that the changes caused by the resuspension event favored some taxa over others. For the oxic experiments, apart from Bacillus at 12 h, the same dominant bacterial genera were detected at each time point (Pseudoarthrobacter, Massilia, and Sphingomonas), with different relative abundances. Members of these genera are all aerobic chemoorganotrophs using saccharides and organic acids as energy and carbon sources, ultimately leading to CO2 emission [50,51,52]. The result of these heterotrophic activities can explain the observed decrease of organic matter, as well as the decrease of pH, which occurred throughout the incubations. At the 240 h and 720 h timepoints, we observed the presence of Massilia, containing strains able to survive in environments with heavy metal concentrations [51], which might be correlated to the increase of heavy metals detected in the water samples, indicative of carbonate dissolution.
During the anoxic incubations, Bacillus was detected at all time points but was dominant after 12 h incubation. All bacterial groups detected after 240 h incubation were mainly fermenters (Clostridium, Desulfitobacterium, unclassified Prolixibacteraceae) and chemoorganotrophs (Lutispora, unclassified Caloramatoraceae) producing ethanol, acetate, lactate, or propionate [53,54,55,56]. In anaerobic conditions, fermentation is much less effective in terms of energy production than respiration, and also produces less CO2. Combined with a slower growth rate, this could explain why organic matter degradation reached a plateau at this time point as well. After 240 h incubation, we detected Desulfitobacterium, which is able to reduce sulfite, thiosulfate, or nitrate for use as electron acceptors [53]. Sulfate was present in the water samples and reached a peak at 240 h, most likely available to act as an electron acceptor.
Methanogens dominated both the oxic and anoxic incubations, probably explaining why the presence/absence of oxygen did not significantly impact the archaeal diversity indices. Methanogens can use three types of substrates (acetate, methylamines, and bicarbonate) to produce methane, and we detected genera able to use all of them (Methanosaeta, Methanosarcina, Methanoregula, Methanocella) [57,58,59,60]. Methanogenesis is the final step in the mineralization of organic matter in soils and sediments. It is highly likely that they were stimulated by the production of acetate, H2, and CO2 by the chemoorganotrophic bacteria to further degrade organic matter to methane and CO2 (except for the autotrophic methanogens, which only produce methane). Thus, methanogenic activities in the sediments could also account for the decrease of organic matter that was observed in the water over time. In return, bacteria that ferment fatty acids benefit from these activities, since the autotrophic methanogens remove H2, which inhibits their activities [61]. Methanogens are typically viewed as strict anaerobes because of their oxygen-sensitive enzymes. However, there is evidence that some methanogens contain defense and repair mechanisms that allow them to survive in suboxic environments [62], which could explain why they dominated the aerobic incubations. It is also possible that methanogens locally inhabited sediment pores that were depleted in oxygen. The Methanoregula genus was relatively more abundant in the the oxic incubations after 240 h, which can be correlated to the lower pH measured in these mesocosms compared to the anoxic ones (5.5 to 4.4 in the oxic mesocosms, and 6.5 in the anoxic ones). Indeed, this methanogen contains one strain adapted to oligotrophic and acidic (pH 4.5 to 5.5) environments [60].
Bathyarchaeota have been suggested to degrade extracellular recalcitrant molecules such as aromatic compounds, peptidoglycan, lignin, and cellulose [42,63], which are all compounds typically found in lake sediments such as the Villerest dam lake [32,33]. In addition, strains of the bacterial genus Dyella dominant after 240 h are also able to hydrolyze cellulose [64]. The presence of microorganisms able to hydrolyze complex polymers into smaller, more usable molecules most likely stimulated the activities of chemoorganotrophic bacteria during the incubations and participated in the overall decrease of organic matter to CO2.
4.4. Leaching of Microbial Communities in the Water
The initial water used to set up the incubations was sterile and contained no organisms, hence, the populations that thrived in the leachate samples necessarily stemmed from the sediments collected in the Villerest dam, using nutrients leaching from the sediments. Bacterial diversity increased with time, indicating that some of the benthic bacteria leached into the water and were able to grow and acclimate to a planktonic lifestyle. However, the diversity indices were significantly lower in the leachate samples than in the sediments, suggesting that most of the benthic bacteria could not acclimatize to an aquatic niche. It is also likely that many organisms were missing nutrients, carbon, and energy sources to survive. This is further supported by the fact that the environment type (water/sediment) explained part of the bacterial mesocosm community variance, and that the lefse analysis indicated that a big number of genera were significantly higher in the sediment samples compared to the leachate samples. Variation was smaller inside the water sample group compared to the sediment sample group, but this could also be due to the fact that we did not add nutrients to the water during the leaching experiments.
Bacterial source tracking showed that the water communities stemmed from water communities from previous timepoints. Sediment communities contributed to the water communities mainly in the oxic incubations, suggesting that leaching of bacterial populations from the sediment to the water column would be mostly favorable when resuspension events were caused by oxygenated waters.
The extremely low number of archaeal reads recovered in the leachate samples could be explained by the high number of methanogenic sequences found in the sediments. The methanogens might have endured some protected exposure to oxygen in the sediments, but as soon as they leached in the oxic water it is most likely most did not survive. These results also suggest that benthic archaea do not settle well in an aquatic environment, which is supported by the low number of reads recovered from the dam lake water.
4.5. Potential Microbial Metabolisms in the Water
In oxic conditions, most dominant water bacterial species were also dominating the sediments (Sphingomonas, Pseudoarthrobacter, and Dyella). As observed in the sediments, these bacterial groups are chemoorganotrophs assimilating saccharides, organic acids, or long chain alkanes, and producing CO2. These chemoorganotrophs probably benefited from the leaching of organic matter from the sediments (due to abiotic or biotic reactions) to the water, and also contributed to decreasing the pH. Moreover, Sphingomonas and Dyella are acido-tolerant [65,66], allowing them to grow in the waters where the pH decreased overtime (5.5 to 4.4 in the oxic mesocosms).
The presence of the methane oxidizing Methylocella after 12 h incubation, and not after, likely indicates that methanogenic activities were highest in the sediments within the first 12 h [67]. Also, this bacterium can fix N2, which could partially account for the increase of total dissolved nitrogen (TDN) in the oxic water samples. After 720 h incubation in oxic conditions, there was a high relative abundance of Rhodanobacter, which contains strains able to break down lindane [68]. This aromatic compound is a component of insecticides and suggests that appearance of a bacterial population able to use more complex carbohydrates when all simpler ones are depleted. Bacterial degradation of lindane leads to organic acids, which can be used by the other chemoorganotrophic bacteria.
Sphingomonas, Pseudoarthrobacter, and unclassified Burkholderiaceae were identified after 12 and 240 h incubation in anoxic conditions. These bacteria are chemoorganotrophs that use nitrate as electron acceptor when oxygen is not available. A metal-reducing microbe (Magnetospirillum) was identified at the 240 h timepoint [69]. This dissimilatory metal-reducer completely oxidizes short-chain carboxylic acids probably produced by the other chemoorganotrophic bacterial communities. Only chemoorganotrophs composed the dominant bacterial groups after 720 h incubation. Two of the distinct genera detected show evidence of adaptation to oligotrophic growth, suggesting that most nutrients were depleted. Indeed, Cellulomonas can use complex recalcitrant polymers derived from plant and/or fungal walls [70], while Caulobacter has a unique reproductive mode that reflects adaptation to nutrient-limited environments [71].
Most of the few archaeal reads recovered in the oxic waters belonged to the Bathy_6. Although no isolated cultures of Bathyarchaeota exist, it is thought they are mostly benthic organisms, which would also explain why leaching of members of this group did not survive well in the aquatic environment. Archaeal ammonia oxidizers were detected in the waters and could have supplied the Bacteria and some archaea with nitrate. Indeed, we detected sequences affiliated with the methane oxidizing denitrifying cand. Methanoperendens [72]. This archaeon could have benefited from the activity of the ammonia oxidizing Nitrososphaeraceae producing nitrate [73]. It could also have contributed to the observed dissolved organic nitrogen (DON) increase by producing N2.
5. Conclusions
Our controlled leaching experiments highlighted mineral dissolution/precipitation and organic matter degradation in both oxic and anoxic conditions. Most of the microorganisms identified during the incubations were either chemoorganotrophs or fermenters, likely acidifying their environment, and participating in carbonate dissolution and trace element release. This has been previously documented using dredged sediments [28]. Resuspension events seemed to favor the activities of microbes involved in organic matter degradation, with a chain of degradation using complex polymers down to C1-type compounds, suggesting a vast microbial network cooperation.
The leaching experiment influenced microbial diversity and community composition in the sediment samples. Archaeal was stable over time, compared to bacterial diversity, indicating a higher vulnerability of Bacteria to fluctuating conditions. We did not detect many Archaea in the leachate water, and bacterial diversity was lower in the leachate samples, suggesting that many of the benthic microbial populations cannot settle or colonize the water column following a resuspension event.
The type of water (oxygenated or anoxic) that causes hydraulic events played a major role in selecting for distinct microbial communities, which, in turn, influenced organic matter degradation and the release of trace elements, probably due to a slower metabolism of anaerobic microbes. It is likely, however, that experimental conditions biased the observed results [74], and future studies based on metagenomic or metatranscriptomic analyses of in situ samples before and after resuspension events will help support our findings.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes13081416/s1, Table S1: Relative abundance of the major archaeal taxonomic groups, in the oxic (ox) and anoxic (ax) incubations, and in the sediment (S) and water (W) samples; Table S2: Relative abundance of the major bacterial taxonomic groups, in the oxic (ox) and anoxic (ax) incubations, and in the sediment (S) and water (W) samples.
Author Contributions
Conceptualization, C.S.L., N.G. and C.G.; methodology, A.D. and C.S.L.; software, A.D. and C.S.L.; validation, C.S.L., N.G. and C.G.; formal analysis, A.D., C.S.L.; investigation, A.D.; resources, C.S.L.; data curation, A.D. and C.S.L.; visualization, A.D. and C.S.L.; supervision, C.S.L.; project administration, C.S.L.; funding acquisition, C.S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Canada Research Chair in Aquatic Environmental Genomics, and a Natural Sciences and Engineering Research Council discovery grant RGPIN-2019-06670 both awarded to C.S.L.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The obtained sequences were deposited in the National Center for Biotechnology Information (NCBI) under the BioProject ID: PRJNA805122.
Acknowledgments
We thank the Interuniversity Research Group in Limnology (GRIL) and their funders, the Fonds de recherche—nature et technologie (FRQNT, Québec). The authors would also like to thank Laura Zeppetelli-Bédard for her help with the DNA extractions. We thank Mykyta Shumskykh for the leaching experiments.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Uroz, S.; Calvaruso, C.; Turpault, M.P.; Frey-Klett, P. Mineral weathering by bacteria: Ecology, actors and mechanisms. Trends Microbiol. 2009, 17, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Wild, B.; Daval, D.; Beaulieu, E.; Pierret, M.C.; Viville, D.; Imfelda, G. In-situ dissolution rates of silicate minerals and associated bacterial communities in the critical zone (Strengbach catchment, France). Geochim. Cosmochim. Acta 2019, 249, 95–120. [Google Scholar] [CrossRef]
- Ullman, W.J.; Kirchman, D.L.; Welch, S.A.; Vandevivere, P. Laboratory evidence for microbially mediated silicate mineral dissolution in nature. Chem. Geol. 1996, 132, 11–17. [Google Scholar] [CrossRef]
- Barker, W.W.; Welch, S.A.; Chu, S.; Banfield, J.F. Experimental observations of the effects of bacteria on aluminosilicate weathering. Am. Mineral. 1998, 83, 1551–1563. [Google Scholar] [CrossRef]
- Liermann, L.J.; Kalinowski, B.E.; Brantley, S.L.; Ferry, J.G. Role of bacterial siderophores in dissolution of hornblende. Geochim. Cosmochim. 2000, 64, 587–602. [Google Scholar] [CrossRef]
- Norris, P.R. Acidophilic bacteria and their activity in mineral sulfide oxidation. In Microbial Mineral Recovery; Erlich, H.L., Brierler, C., Eds.; McGraw-Hill: New York, NK, USA, 1990. [Google Scholar]
- Mueller, B. Experimental interactions between clay minerals and bacteria: A review. Pedosphere 2015, 25, 799–810. [Google Scholar] [CrossRef]
- Rogers, J.R.; Bennett, P.C.; Choi, W.J. Feldspars as a source of nutrients for microorganisms. Am. Mineral. 1998, 83, 1532–1540. [Google Scholar] [CrossRef]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial phosphorus solubilization and its potential use in sustainable agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef]
- Watteau, F.; Berthelin, J. Microbial dissolution of iron and aluminum from soil minerals: Efficiency and specificity of hydrowamate siderophores compared to aliphatic acids. Eur. J. Soil. Biol. 1994, 30, 1–9. [Google Scholar]
- Kalinowski, B.E.; Liermann, L.J.; Givens, S.; Brantley, S.L. Rates of bacteria-promoted solubilization of Fe from minerals, a review of problems and approaches. Chem. Geol. 2000, 169, 357–370. [Google Scholar] [CrossRef]
- Schippers, A.; Sand, W. Bacterial leaching of metal sulfides proceeds by two indirect mechanisms via thiosulfate or via polysulfides and sulfur. Appl. Environ. Microbiol. 1999, 65, 319–321. [Google Scholar] [CrossRef] [PubMed]
- Bosecker, K. Bioleaching, metal solubilization by microorganisms. FEMS Microbiol. Rev. 1997, 20, 591–604. [Google Scholar] [CrossRef]
- Benzerara, K.; Menguy, N.; Guyot, F.; Chaïrat, C.; Oelkers, E.H.; McEldoney, S. Experimental colonization and weathering of orthopyroxenes by the pleomorphic bacteria Ramlibacter tatahouinensis. Geomicrobiol. J. 2004, 21, 341–349. [Google Scholar] [CrossRef]
- Welch, S.A.; Barker, W.W.; Banfield, J.F. Microbial extracellular polysaccharides and plagioclase dissolution. Geochim. Cosmochim. Acta 1999, 63, 1405–1419. [Google Scholar] [CrossRef]
- Zwirglmaier, K.; Keiz, K.; Engel, M.; Geist, J.; Geist, J. Seasonal and spatial patterns of microbial diversity along a trophic gradient in the interconnected lakes of Osterseen Lake District, Bavaria. Front. Microbiol. 2015, 6, 1168. [Google Scholar] [CrossRef] [PubMed]
- Wurzbacher, C.; Fuchs, A.; Attermeyer, K.; Frindte, K.; Grossart, H.-P.; Hupfer, M.; Casper, P.; Monaghan, M.T. Shifts among Eukaryota, Bacteria and Archaea define the vertical organization of a lake sediment. Microbiome 2017, 5, 41. [Google Scholar] [CrossRef]
- Gallon, C.; Tessier, A.; Gobeil, C.; La Torre, A.; Catalina, M. Modeling diagenesis of lead in sediments of a Canadian Shield lake. Geochim. Cosmochim. Acta 2004, 68, 3531–3545. [Google Scholar] [CrossRef]
- Chappaz, A.; Gobeil, C.; Tessier, A. Geochemical and anthropogenic enrichments of Mo in sediments from perennially oxic and seasonally anoxic lakes in Eastern Canada. Geochim. Cosmochim. Acta 2008, 72, 170–184. [Google Scholar] [CrossRef]
- Couture, R.M.; Gobeil, C.; Tessier, A. Arsenic, iron and sulfur co-diagenesis in lake sediments. Geochim. Cosmochim. Acta 2010, 74, 1238–1255. [Google Scholar] [CrossRef]
- Cooke, G.D.; Welch, E.B.; Peterson, S.; Nichols, S.A. Restoration and Management of Lakes and Reservoirs, 2nd ed.; Lewis Publishers: Boca Raton, FL, USA, 1993. [Google Scholar]
- Carr, M.K.; Sadeghian, A.; Lindenschmidt, K.A.; Rinke, K.; Morales-Marin, L. Impacts of varying dam outflow elevations on water temperature, dissolved oxygen, and nutrient distribution in a large prairie reservoir. Environ. Eng. Sci. 2020, 37, 78–97. [Google Scholar] [CrossRef]
- Gassama, N.; Cocirta, C.; Kasper, H.U. Use of major and selected trace elements to describe mixing processes in water reservoir. Comptes-Rendus Géosci. 2012, 344, 25–32. [Google Scholar] [CrossRef]
- Mishra, A.; Alnahit, A.; Campbell, B. Impact of land uses, drought, flood, wildfire, and cascading events on water quality and microbial communities, a review and analysis. J. Hydrol. 2021, 596, 125707. [Google Scholar] [CrossRef]
- Espa, P.; Castelli, E.; Crosa, G.; Gentili, G. Environmental effects of storage preservation practices: Controlled flushing of fine sediment deom a small hydropower reservoir. Environ. Manag. 2013, 52, 261–276. [Google Scholar] [CrossRef]
- Frémion, F.; Courtin-Nomade, A.; Bordas, F.; Lenain, J.F.; Jugé, P.; Kestens, T.; Mourier, B. Impact of sediments resuspension on metal solubilisation and water quality during recurrent reservoir sluicing management. Sci. Total Environ. 2016, 562, 201–215. [Google Scholar] [CrossRef]
- Newcombe, C.P.; MacDonald, D.D. Effects of suspended sediments on aquatic ecosystems. N. Am. J. Fish. Manag. 1991, 11, 72–82. [Google Scholar] [CrossRef]
- Lors, C.; Tiffreau, C.; Laboudigue, A. Effects of bacterial activities on the releases of heavy metals from contaminated dredged sediments. Chemosphere 2004, 56, 619–630. [Google Scholar] [CrossRef]
- Fang, W.; Wei, Y.; Liu, J.; Kosson, D.S.; van der Sloot, H.A.; Zhang, P. Effects of aerobic and anaerobic biological processes on leaching of heavy metals from soil amended with sewage sludge compost. Waste Manag. 2016, 58, 324–334. [Google Scholar] [CrossRef]
- Wu, L.; Jacobson, A.D.; Chen, H.-C.; Hausner, M. Characterization of elemental release during microbe-basalt interaction at T = 28 °C. Geochim. Cosmochim. Acta 2007, 71, 2224–2239. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, S.; Ma, X.; Mao, X.; He, L.; Sheng, X. Distinct mineral weathering effectiveness and metabolic activity between mineral-weathering bacteria Burkholderia metallica F22 and Burkholderia phytofirmans G34. Chem. Geol. 2018, 489, 38–45. [Google Scholar] [CrossRef]
- Dhivert, E.; Grosbois, C.; Coynel, A.; Lefèvre, I.; Desmet, M. Influences of major flood sediment inputs on sedimentary and geochemical signals archived in a reservoir core (Upper Loire Basin, France). Catena 2015, 126, 75–85. [Google Scholar] [CrossRef]
- Dhivert, E.; Grosbois, C.; Courtin-Nomade, A.; Bourrain, M.D. Dynamics of metallic contaminants at a basin scale—Spatial and temporal reconstruction from four sediment cores (Loire fluvial system, France). Sci. Total Environ. 2016, 541, 1504–1515. [Google Scholar] [CrossRef]
- Liu, S.; Yu, H.; Yu, Y.; Huang, J.; Zhou, Z.; Zeng, J.; Chen, P.; Xiao, F.; He, Z.; Yan, Q. Ecological stability of microbial communities in lake Donghue regulated by keystone taxa. Ecol. Indic. 2022, 136, 108695. [Google Scholar] [CrossRef]
- Gantner, S.; Andersson, A.F.; Alonso-Saez, L.; Bertilsson, S. Novel primers for 16S rRNA-based archaeal community analyses in environmental samples. J. Microbiol. Methods 2011, 84, 8–12. [Google Scholar] [CrossRef]
- Stahl, D.A.; Amann, R. Development and application of nucleic acid probes in bacterial systematics. In Nucleic Acid Techniques in Bacterial Systematics; Stackebrandt, E., Goodfellow, M., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 1991; pp. 205–248. [Google Scholar]
- Muyzer, G.; de Waal, E.C.; Uitterlinden, A.G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analaysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Schloss, P. Reintroducing mothur, 10 years later. Appl. Environ. Microbiol. 2020, 86, e02343-19. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNE gene database project, improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Liu, X.; Li, M.; Castelle, C.J.; Probst, A.J.; Zhou, Z.P.; Banfield, J.F.; Gu, J. Insights into the ecology, evolution, and metabolism of the widespread Woesearchaeotal lineages. Microbiome 2018, 6, 102. [Google Scholar] [CrossRef]
- Zhou, Z.; Pan, J.; Wang, F.; Gu, J.; Li, M. Bathyarchaeota, globally distributed metabolic generalists in anoxic Environments. FEMS Microbiol. Rev. 2018, 42, 639–655. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 1 August 2020).
- Suzuki, R.; Shimodaira, H. Pvclust: An R package for assessing the uncertainty in hierarchical clustering. Bioinform 2006, 22, 1540–1542. [Google Scholar] [CrossRef]
- Shenhav, L.; Thompson, M.; Joseph, T.A.; Briscoe, L.; Furman, O.; Bogumil, D.; Mizrahi, I.; Péer, I.; Halperin, E. FEAST, fast expectation-maximization for microbial source tracking. Nat. Methods 2019, 16, 627–632. [Google Scholar] [CrossRef]
- Javanaud, C.; Michotey, V.; Guasco, S.; Garcia, N.; Anschutz, P.; Canton, M.; Bonin, P. Anaerobic ammonium oxidation mediated by Mn-oxides: From sediment to strain level. Res. Microbiol. 2011, 162, 848–857. [Google Scholar] [CrossRef]
- Fernandes, S.O.; Javanaud, C.; Aigle, A.; Garcia, N.; Anschutz, P.; Canton, M.; Bonin, P. Anaerobic nitrification-denitrification mediated by Mn-oxides in meso-tidal sediments: Implications for N2 and N2O production. J. Mar. Syst. 2015, 144, 1–8. [Google Scholar] [CrossRef]
- Jetten, M.S.M.; Strous, M.; van de Pas-Schooten, K.T.; Schalk, J.; van Dongen, U.G.J.M.; van de Graaf, A.A.; Logemann, S.; Muyzer, G.; van Loosdrecht, M.C.M.; Kuenen, J.G. The anaerobic oxidation of ammonium. FEMS Microbiol. Rev. 1998, 22, 421–437. [Google Scholar] [CrossRef]
- Dalsgaard, T.; Thamdrup, B.; Canfield, D.E. Anaerobic ammonium oxidation (anammox) in the marine environment. Res. Microbiol. 2005, 156, 457–464. [Google Scholar] [CrossRef]
- Yabuuchi, E.; Yano, I.; Oyaizu, H.; Hashimoto, Y.; Ezaki, T.; Yamamoto, H. Proposals of Sphingomonas paucimobilis gen. nov. and comb. Nov., Sphingomonas paracpaucimobilis sp. Nov., Sphingomonas yanoikuyae sp. Nov., Sphingomonas adhaesiva sp. Nov., Sphingomonas capsulate comb. Nov., and two genospecies of the genus Sphingomonas. Microbiol. Immunol. 1990, 34, 99–119. [Google Scholar] [CrossRef]
- Du, Y.; Yu, X.; Wang, G. Massilia tieshanensis sp. Nov., isolated from mining soil. Int. J. Syst. Evol. 2012, 62, 2356–2362. [Google Scholar] [CrossRef]
- Busse, H.J. Review of the taxonomy of the genus Arthrobacter, emendation of the genus Athrobacter sensus lato, proposal to reclassify selected species of the genus Arthrobacter in the novel genera Glutamicibacter gen. nov., Paeniglutamicibacter gen. nov. and Pseudoarthrobacter gen. nov., and emended description of Arthrobacter roseus. Int. J. Syst. Evol. 2016, 66, 9–37. [Google Scholar]
- Utkin, I.; Woese, K.; Wiegel, J. Isolation and characterization of Desulfitobacterium dehalogenans gen. nov., sp. nov., an anaerobic bacterium which reductively dechlorinates chlorophenolic compounds. Int. J. Syst. Evol. 1994, 44, 612–619. [Google Scholar] [CrossRef]
- Holmes, D.E.; Nevin, K.P.; Woodard, T.L.; Peacock, A.D.; Lovley, D.R. Prolixibacter bellariivorans gen. nov., sp. nov., a sugar-fermenting, psychrotolerant anaerobe of the phylum Bacteroidetes, isolated from a marine-sediment fuel cell. Int. J. Syst. Evol. 2007, 57, 701–707. [Google Scholar] [CrossRef]
- Shiratori, H.; Ohiwa, H.; Ikeno, H.; Ayame, S.; Kataoka, N.; Miya, A.; Beppu, T.; Ueda, K. Lutispora thermophila gen. nov., sp. nov., a thermophilic, spore-forming bacterium isolated from a thermophilic methanogenic bioreactor digesting municipal solid wastes. Int. J. Syst. Evol. 2008, 58, 964–969. [Google Scholar] [CrossRef]
- Ogg, C.D.; Patel, B.K.C. Caloramator australicus sp. nov., a thermophilic, anaerobic bacterium from the Great Artesian Basin of Australia. Int. J. Syst. Evol. 2009, 59, 95–101. [Google Scholar] [CrossRef]
- Sowers, K.R.; Baron, S.F.; Ferry, J.G. Methanosarcina acetivorans sp. nov., an acetotrophic methane-producing bacterium isolated from marine sediments. Appl. Environ. Microbiol. 1984, 47, 971–978. [Google Scholar] [CrossRef]
- Patel, G.B.; Sprott, G.D. Methanosaeta concilii gen. nov., sp. nov. (“Methanothrix concilii”) and Methanosaeta thermoacetophila nom. rev., com. nov. Int. J. Syst. Evol. 1990, 40, 78–82. [Google Scholar]
- Sakai, S.; Imachi, H.; Hanada, S.; Ohashi, A.; Harada, H.; Kamagata, Y. Methanocella paludicola gen. nov., sp. nov., a methane-producing archaeon, the first isolate of the lineage ‘Rice Cluster I’, and proposal of the new archaeal order Methanocellales ord. nov. Int. J. Syst. Evol. 2008, 58, 929–936. [Google Scholar] [CrossRef]
- Bräuer, S.L.; Cadillo-Quiroz, H.; Ward, R.J.; Yavitt, J.B.; Zinde, S.H. Methanoregula boonei gen. nov., sp. nov., an acidiphilic methanogen isolated from an acidic peat bog. Int. J. Syst. Evol. 2011, 61, 45–52. [Google Scholar] [CrossRef]
- Conrad, R.; Erkel, C.; Liesack, W. Rice Cluster I methanogens, an important group of Archaea producing greenhouse gas in soil. Curr. Opin. 2006, 17, 262–267. [Google Scholar] [CrossRef]
- Guerrero-Cruz, S.; Cremers, G.; van Alen, T.A.; Den Camp, H.J.M.O.; Jetten, M.S.M. Response of the anaerobic methanotroph “Candidatus Methanoperedens nitroreducens” to oxygen stress. Appl. Environ. Microbiol. 2018, 84, e01832-18. [Google Scholar] [CrossRef]
- Yu, T.; Wu, W.; Liang, W.; Wang, F. Growth of sedimentary Bathyarchaeota on lignin as an energy source. Proc. Natl. Acad. Sci. USA 2018, 115, 6022–6027. [Google Scholar] [CrossRef]
- Too, C.C.; Ong, K.S.; Lee, S.M.; Yule, C.M.; Keller, A. Draft genome sequence of Dyella sp. strain C9, isolated from a Malaysian tropical peal swamp forest. ASM 2018, 7, e01083-18. [Google Scholar]
- Hashidoko, Y.; Kitagawa, E.; Iwahashi, H. Design of Shingomonad-detecting probes for DNA array, and its application to investigate the behavior, distribution, and source of rhizospherous Sphingomonas and other Sphingomonads inhabiting an acid sulfate soil paddock in Kalimantan, Indonesia. Biosci. Biotechnol. Biochem. 2007, 71, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Weon, H.Y.; Anandham, R.; Kim, B.Y.; Hong, S.B.; Jeon, Y.A.; Kwon, S.W. Dyella soli sp. and Dyella terrae sp. nov., isolated from soil. Int. J. Syst. Evol. 2009, 59, 1685–1690. [Google Scholar] [CrossRef] [PubMed]
- Dedysh, S.N.; Liesack, W.; Khelenina, V.N.; Suzina, N.E.; Trotsenko, Y.A.; Semrau, J.D.; Bares, A.M.; Panikov, N.S.; Tiedje, M.J. Methylocella palutris gen. nov., sp. nov., a new methane-oxidizing acidophilic bacterium from peat bogs, representing a novel subtype of serine-pathway methanotrophs. Int. J. Syst. Evol. 2000, 50, 955–969. [Google Scholar] [CrossRef]
- Nalin, R.; Simonet, P.; Vogel, T.M.; Normand, P. Rhodanobacter lindaniclastus gen. nov., sp. nov., a lindane-degrading bacterium. Int. J. Syst. Evol. 1999, 49, 19–23. [Google Scholar] [CrossRef]
- Dziuba, M.; Koziaeva, V.; Grouzdev, D.; Burganskaya, E.; Baslerov, R.; Kolganova, T.; Chernyadyev, A.; Osipov, G.; Andrianova, E.; Gorlenko, V. Magnetospirillum caucaseum sp. nov., Magnetospirillum marisnigri sp. nov. and Magnetospirillum moscoviense sp. nov., freshwater magnetotactic bacteria isolated from three distinct geographical locations in European Russia. Int. J. Syst. Evol. 2016, 66, 2069–2077. [Google Scholar] [CrossRef]
- Bagnara, C.; Toci, R.; Gaudin, C.; Belaich, J.P. Isolation and characterization of a cellulolytic microorganism, Cellulomonas fermentans sp. nov. Int. J. Syst. Evol. 1985, 35, 502–507. [Google Scholar]
- Abraham, W.R.; Strömpl, C.; Meyer, H.; Lindholst, S.; Moore, E.; Christ, R.; Vancanneyt, M.; Tindall, B.; Bennasar, A.; Smit, J.; et al. Phylogeny and polyphasic taxonomy of Caulobacter species. Proposal of Maricaulis gen. nov. with Maricaulis maris (Poindexter) comb. nov. as the type species, and emended description of the genera Brevundimonas and Caulobacter. Int. J. Syst. Evol. 1999, 49, 1053–1073. [Google Scholar] [CrossRef]
- Vaksmaa, A.; Guerrero-Cruz, S.; van Alen, T.A.; Cremers, G.; Ettwig, K.F.; Claudia Lüke, M.S.M.J. Enrichment of anaerobic nitrate-dependant methanotrophic ‘Candidatus Methanoperedens nitroreducens’ archaea from an Italian paddy field soil. Appl. Microbiol. Biotechnol. 2017, 101, 7075–7084. [Google Scholar] [CrossRef]
- Spang, A.; Poehlin, A.; Offre, P.; Zumbrägel, S.; Haider, S.; Rychlik, N.; Nowka, B.; Schmeisser, C.; Lebedeva, E.V.; TRattei, H.; et al. The genome of the ammonia-oxidizing Candidatus Nitrososphaera gargensis, insights into metabolic versatility and environmental adaptations. Environ. Microbiol. 2012, 14, 3122–3145. [Google Scholar] [CrossRef]
- Adamczuk, M. Do experimental conditions bias plankton responses to increased concentration of dissolved organic matter (DOM)? A meta-analytical synthesis of the published results. Sci. Total Environ. 2021, 787, 147590. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).