How Our Microbiome Influences the Pathogenesis of Alopecia Areata
Abstract
1. Introduction
2. Pathogenesis of Alopecia Areata
2.1. Hair Cycle Disruption
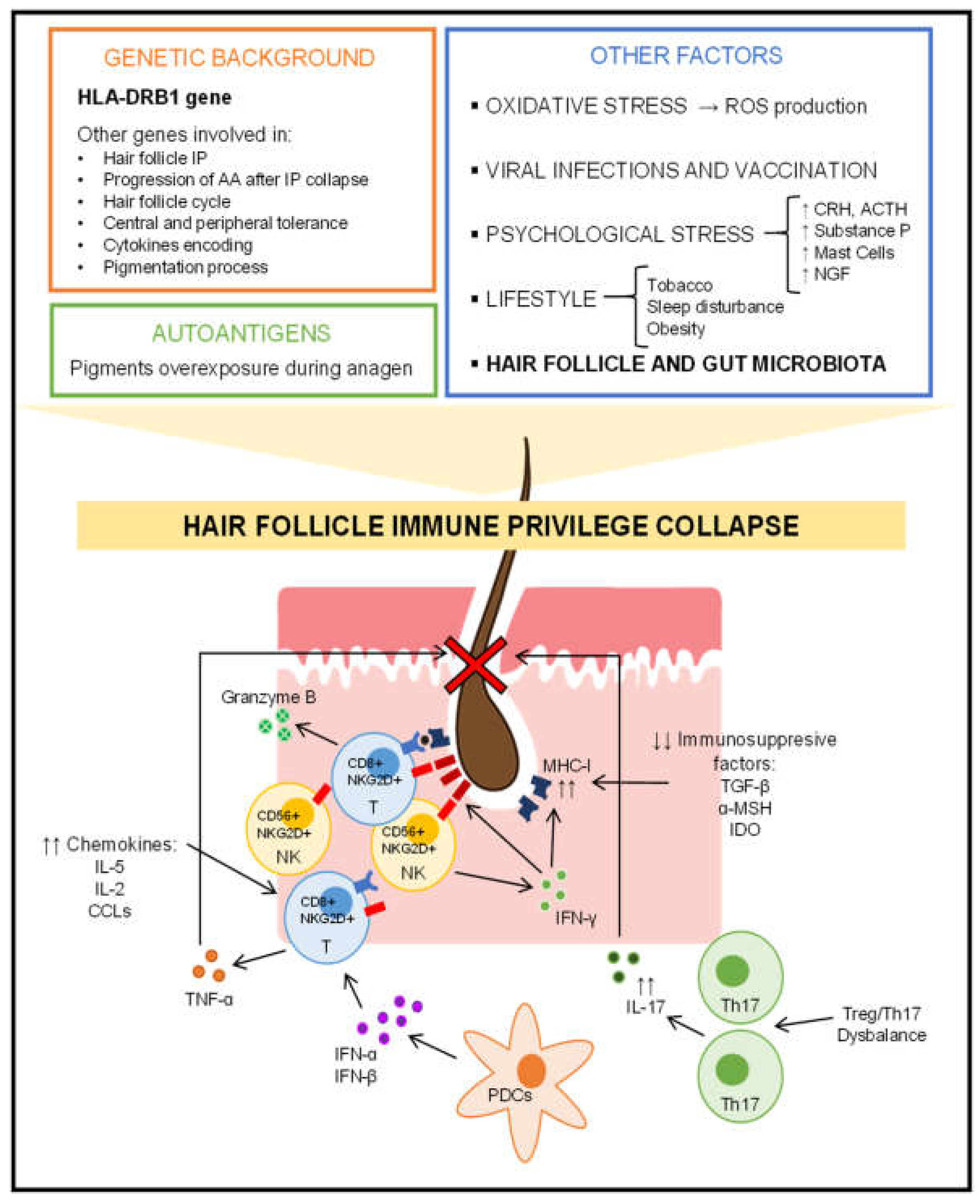
2.2. Hair Follicle Immune Privilege Collapse and Autoimmune Response
2.3. Role of Genetics in Alopecia Areata
2.4. Additional Influential Factors in the Development of Alopecia Areata
3. Alopecia Areata and Microbiota
3.1. Hair Follicle/Scalp Microbiota and Alopecia Areata
3.1.1. Role of the Hair Follicle/Scalp Microbiota in the Pathogenesis of Alopecia Areata
3.1.2. Analysis of the Hair Follicle/Scalp Microbiota in Alopecia Areata Patients
3.2. Gut Microbiota and Alopecia Areata
3.2.1. Role of the Gut Microbiota in the Pathogenesis of Alopecia Areata
3.2.2. Analysis of the Gut Microbiota in Alopecia Areata Patients
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simakou, T.; Butcher, J.P.; Reid, S.; Henriquez, F.L. Alopecia areata: A multifactorial autoimmune condition. J. Autoimmun. 2019, 98, 74–85. [Google Scholar] [CrossRef]
- Ghanaat, M. Types of hair loss and treatment options, including the novel low-level light therapy and its proposed mechanism. South Med. J. 2010, 103, 917–921. [Google Scholar] [CrossRef]
- Strazzulla, L.C.; Wang, E.H.C.; Avila, L.; Lo Sicco, K.; Brinster, N.; Christiano, A.M.; Shapiro, J. Alopecia areata: Disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J. Am. Acad. Dermatol. 2018, 78, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Li, X.; Wang, C.; Zhang, J. Alopecia Areata: An Update on Etiopathogenesis, Diagnosis, and Management. Clin. Rev. Allergy Immunol. 2021, 61, 403–423. [Google Scholar] [CrossRef]
- Pratt, C.H.; King, L.E., Jr.; Messenger, A.G.; Christiano, A.M.; Sundberg, J.P. Alopecia areata. Nat. Rev. Dis. Primers 2017, 3, 17011. [Google Scholar] [CrossRef]
- Lew, B.L.; Shin, M.K.; Sim, W.Y. Acute diffuse and total alopecia: A new subtype of alopecia areata with a favorable prognosis. J. Am. Acad. Dermatol. 2009, 60, 85–93. [Google Scholar] [CrossRef]
- Asz-Sigall, D.; Ortega-Springall, M.F.; Smith-Pliego, M.; Rodríguez-Lobato, E.; Martinez-Velasco, M.A.; Arenas, R.; Vincenzi, C.; Tosti, A. White hair in alopecia areata: Clinical forms and proposed physiopathological mechanisms. J. Am. Acad. Dermatol. 2019; online ahead of print. [Google Scholar] [CrossRef]
- Meah, N.; Wall, D.; York, K.; Bhoyrul, B.; Bokhari, L.; Asz-Sigall, D.; Bergfeld, W.F.; Betz, R.C.; Blume-Peytavi, U.; Callender, V.; et al. The Alopecia Areata Consensus of Experts (ACE) study part II: Results of an international expert opinion on diagnosis and laboratory evaluation for alopecia areata. J. Am. Acad. Dermatol. 2021, 84, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- García-Hernández, M.J.; Rodríguez-Pichardo, A. Multivariate analysis in alopecia areata: Risk factors and validity of clinical forms. Arch. Dermatol. 1999, 135, 998–999. [Google Scholar] [CrossRef]
- Chelidze, K.; Lipner, S.R. Nail changes in alopecia areata: An update and review. Int. J. Dermatol. 2018, 57, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Gwillim, E.; Patel, K.R.; Hua, T.; Rastogi, S.; Ibler, E.; Silverberg, J.I. Epidemiology of alopecia areata, ophiasis, totalis, and universalis: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2020, 82, 675–682. [Google Scholar] [CrossRef]
- Villasante Fricke, A.C.; Miteva, M. Epidemiology and burden of alopecia areata: A systematic review. Clin. Cosmet. Investig. Dermatol. 2015, 8, 397–403. [Google Scholar] [CrossRef]
- Lee, S.; Lee, H.; Lee, C.H.; Lee, W.S. Comorbidities in alopecia areata: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2019, 80, 466–477.e16. [Google Scholar] [CrossRef]
- Rossi, A.; Muscianese, M.; Piraccini, B.M.; Starace, M.; Carlesimo, M.; Mandel, V.D.; Alessandrini, A.; Calvieri, S.; Caro, G.; D'arino, A.; et al. Italian Guidelines in diagnosis and treatment of alopecia areata. G. Ital. Dermatol. Venereol. 2019, 154, 609–623. [Google Scholar] [CrossRef]
- Delamere, F.M.; Sladden, M.M.; Dobbins, H.M.; Leonardi-Bee, J. Interventions for alopecia areata. Cochrane Database Syst. Rev. 2008, 2, CD004413. [Google Scholar] [CrossRef]
- Mancuso, G.; Balducci, A.; Casadio, C.; Farina, P.; Staffa, M.; Valenti, L.; Milani, M. Efficacy of betamethasone valerate foam formulation in comparison with betamethasone dipropionate lotion in the treatment of mild-to-moderate alopecia areata: A multicenter, prospective, randomized, controlled, investigator-blinded trial. Int. J. Dermatol. 2003, 42, 572–575. [Google Scholar] [CrossRef]
- Meah, N.; Wall, D.; York, K.; Bhoyrul, B.; Bokhari, L.; Sigall, D.A.; Bergfeld, W.F.; Betz, R.C.; Blume-Peytavi, U.; Callender, V.; et al. The Alopecia Areata Consensus of Experts (ACE) study: Results of an international expert opinion on treatments for alopecia areata. J. Am. Acad. Dermatol. 2020, 83, 123–130. [Google Scholar] [CrossRef]
- Fukumoto, T.; Fukumoto, R.; Magno, E.; Oka, M.; Nishigori, C.; Horita, N. Treatments for alopecia areata: A systematic review and network meta-analysis. Dermatol. Ther. 2021, 34, e14916. [Google Scholar] [CrossRef]
- King, B.; Ohyama, M.; Kwon, O.; Zlotogorski, A.; Ko, J.; Mesinkovska, N.A.; Hordinsky, M.; Dutronc, Y.; Wu, W.S.; McCollam, J.; et al. Two Phase 3 Trials of Baricitinib for Alopecia Areata. N. Engl. J. Med. 2022, 386, 1687–1699. [Google Scholar] [CrossRef]
- Sánchez-Díaz, M.; Montero-Vilchez, T.; Bueno-Rodriguez, A.; Molina-Leyva, A.; Arias-Santiago, S. Alopecia Areata and Dexamethasone Mini-Pulse Therapy, A Prospective Cohort: Real World Evidence and Factors Related to Successful Response. J. Clin. Med. 2022, 11, 1694. [Google Scholar] [CrossRef]
- Rajabi, F.; Drake, L.A.; Senna, M.M.; Rezaei, N. Alopecia areata: A review of disease pathogenesis. Br. J. Dermatol. 2018, 179, 1033–1048. [Google Scholar] [CrossRef]
- Suchonwanit, P.; Kositkuljorn, C.; Pomsoong, C. Alopecia Areata: An Autoimmune Disease of Multiple Players. Immunotargets Ther. 2021, 10, 299–312. [Google Scholar] [CrossRef]
- Minokawa, Y.; Sawada, Y.; Nakamura, M. Lifestyle Factors Involved in the Pathogenesis of Alopecia Areata. Int. J. Mol. Sci. 2022, 23, 1038. [Google Scholar] [CrossRef]
- Miyauchi, E.; Shimokawa, C.; Steimle, A.; Desai, M.S.; Ohno, H. The impact of the gut microbiome on extra-intestinal autoimmune diseases. Nat. Rev. Immunol. 2022. online ahead of print. [Google Scholar] [CrossRef]
- De Pessemier, B.; Grine, L.; Debaere, M.; Maes, A.; Paetzold, B.; Callewaert, C. Gut-Skin Axis: Current Knowledge of the Interrelationship between Microbial Dysbiosis and Skin Conditions. Microorganisms 2021, 9, 353. [Google Scholar] [CrossRef]
- Navarro-López, V.; Núñez-Delegido, E.; Ruzafa-Costas, B.; Sánchez-Pellicer, P.; Agüera-Santos, J.; Navarro-Moratalla, L. Probiotics in the Therapeutic Arsenal of Dermatologists. Microorganisms 2021, 9, 1513. [Google Scholar] [CrossRef]
- Sánchez-Pellicer, P.; Navarro-Moratalla, L.; Núñez-Delegido, E.; Ruzafa-Costas, B.; Agüera-Santos, J.; Navarro-López, V. Acne, Microbiome, and Probiotics: The Gut–Skin Axis. Microorganisms 2022, 10, 1303. [Google Scholar] [CrossRef]
- Carmona-Cruz, S.; Orozco-Covarrubias, L.; Sáez-de-Ocariz, M. The Human Skin Microbiome in Selected Cutaneous Diseases. Front. Cell. Infect. Microbiol. 2022, 12, 834135. [Google Scholar] [CrossRef]
- Buffoli, B.; Rinaldi, F.; Labanca, M.; Sorbellini, E.; Trink, A.; Guanziroli, E.; Rezzani, R.; Rodella, L.F. The human hair: From anatomy to physiology. Int. J. Dermatol. 2014, 53, 331–341. [Google Scholar] [CrossRef]
- Anzai, A.; Wang, E.H.C.; Lee, E.Y.; Aoki, V.; Christiano, A.M. Pathomechanisms of immune-mediated alopecia. Int. Immunol. 2019, 31, 439–447. [Google Scholar] [CrossRef]
- Lin, X.; Zhu, L.; He, J. Morphogenesis, Growth Cycle and Molecular Regulation of Hair Follicles. Front. Cell. Dev. Biol. 2022, 10, 899095. [Google Scholar] [CrossRef] [PubMed]
- Peckham, S.J.; Sloan, S.B.; Elston, D.M. Histologic features of alopecia areata other than peribulbar lymphocytic infiltrates. J. Am. Acad. Dermatol. 2011, 65, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Bernárdez, C.; Molina-Ruiz, A.M.; Requena, L. Histologic features of alopecias-part I: Nonscarring alopecias. Actas Dermosifiliogr. 2015, 106, 158–467. [Google Scholar] [CrossRef] [PubMed]
- Watson, V.E.; Faniel, M.L.; Kamili, N.A.; Krueger, L.D.; Zhu, C. Immune-mediated alopecias and their mechanobiological aspects. Cells Dev. 2022, 170, 203793. [Google Scholar] [CrossRef] [PubMed]
- Paus, R.; Ito, N.; Takigawa, M.; Ito, T. The hair follicle and immune privilege. J. Investig. Dermatol. Symp. Proc. 2003, 8, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Paus, R.; Bulfone-Paus, S.; Bertolini, M. Hair Follicle Immune Privilege Revisited: The Key to Alopecia Areata Management. J. Investig. Dermatol. Symp. Proc. 2018, 19, S12–S17. [Google Scholar] [CrossRef]
- Ito, T.; Ito, N.; Bettermann, A.; Tokura, Y.; Takigawa, M.; Paus, R. Collapse and restoration of MHC class-I-dependent immune privilege: Exploiting the human hair follicle as a model. Am. J. Pathol. 2004, 164, 623–634. [Google Scholar] [CrossRef]
- Bertolini, M.; McElwee, K.; Gilhar, A.; Bulfone-Paus, S.; Paus, R. Hair follicle immune privilege and its collapse in alopecia areata. Exp. Dermatol. 2020, 29, 703–725. [Google Scholar] [CrossRef]
- Rosenblum, M.D.; Yancey, K.B.; Olasz, E.B.; Truitt, R.L. CD200, a "no danger" signal for hair follicles. J. Dermatol. Sci. 2006, 41, 165–174. [Google Scholar] [CrossRef]
- Kotwica-Mojzych, K.; Jodłowska-Jędrych, B.; Mojzych, M. CD200:CD200R Interactions and Their Importance in Immunoregulation. Int. J. Mol. Sci. 2021, 22, 1602. [Google Scholar] [CrossRef]
- Trautman, S.; Thompson, M.; Roberts, J.; Thompson, C.T. Melanocytes: A possible autoimmune target in alopecia areata. J. Am. Acad. Dermatol. 2009, 61, 529–530. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, M.; Rossi, A.; Paus, R. Cover Image: Are melanocyte-associated peptides the elusive autoantigens in alopecia areata? Br. J. Dermatol. 2017, 176, 1106. [Google Scholar] [CrossRef] [PubMed]
- Petukhova, L.; Duvic, M.; Hordinsky, M.; Norris, D.; Price, V.; Shimomura, Y.; Kim, H.; Singh, P.; Lee, A.; Chen, W.V.; et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature 2010, 466, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Dai, Z.; Jabbari, A.; Cerise, J.E.; Higgins, C.A.; Gong, W.; de Jong, A.; Harel, S.; DeStefano, G.M.; Rothman, L.; et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat. Med. 2014, 20, 1043–1049. [Google Scholar] [CrossRef]
- Ghoreishi, M.; Martinka, M.; Dutz, J.P. Type 1 interferon signature in the scalp lesions of alopecia areata. Br. J. Dermatol. 2010, 163, 57–62. [Google Scholar] [CrossRef]
- Ito, T.; Ito, N.; Saatoff, M.; Hashizume, H.; Fukamizu, H.; Nickoloff, B.J.; Takigawa, M.; Paus, R. Maintenance of hair follicle immune privilege is linked to prevention of NK cell attack. J. Investig. Dermatol. 2008, 128, 1196–1206. [Google Scholar] [CrossRef]
- Gilhar, A.; Keren, A.; Shemer, A.; d'Ovidio, R.; Ullmann, Y.; Paus, R. Autoimmune disease induction in a healthy human organ: A humanized mouse model of alopecia areata. J. Investig. Dermatol. 2013, 133, 844–847. [Google Scholar] [CrossRef]
- Gilhar, A.; Kam, Y.; Assy, B.; Kalish, R.S. Alopecia areata induced in C3H/HeJ mice by interferon-gamma: Evidence for loss of immune privilege. J. Investig. Dermatol. 2005, 124, 288–289. [Google Scholar] [CrossRef]
- Freyschmidt-Paul, P.; McElwee, K.J.; Hoffmann, R.; Sundberg, J.P.; Vitacolonna, M.; Kissling, S.; Zöller, M. Interferon-gamma-deficient mice are resistant to the development of alopecia areata. Br. J. Dermatol. 2006, 155, 515–521. [Google Scholar] [CrossRef]
- Ito, T.; Suzuki, T.; Sakabe, J.I.; Funakoshi, A.; Fujiyama, T.; Tokura, Y. Plasmacytoid dendritic cells as a possible key player to initiate alopecia areata in the C3H/HeJ mouse. Allergol. Int. 2020, 69, 121–131. [Google Scholar] [CrossRef]
- Saadeh, D.; Kurban, M.; Abbas, O. Update on the role of plasmacytoid dendritic cells in inflammatory/autoimmune skin diseases. Exp. Dermatol. 2016, 25, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Tokura, Y. Alopecia areata triggered or exacerbated by swine flu virus infection. J. Dermatol. 2012, 39, 863–864. [Google Scholar] [CrossRef] [PubMed]
- Tanemura, A.; Oiso, N.; Nakano, M.; Itoi, S.; Kawada, A.; Katayama, I. Alopecia areata: Infiltration of Th17 cells in the dermis, particularly around hair follicles. Dermatology 2013, 226, 333–336. [Google Scholar] [CrossRef]
- Lee, G.R. The Balance of Th17 versus Treg Cells in Autoimmunity. Int. J. Mol. Sci. 2018, 19, 730. [Google Scholar] [CrossRef] [PubMed]
- Loh, S.H.; Moon, H.N.; Lew, B.L.; Sim, W.Y. Role of T helper 17 cells and T regulatory cells in alopecia areata: Comparison of lesion and serum cytokine between controls and patients. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Kasumagic-Halilovic, E.; Prohic, A.; Cavaljuga, S. Tumor necrosis factor-alpha in patients with alopecia areata. Indian. J. Dermatol. 2011, 56, 494–496. [Google Scholar] [CrossRef]
- Abdel Halim, D.; Abu Zeid, O.M.; Rashed, L.; Saleh, M.A. Alteration of serum and tissue tumor necrosis factor alpha levels: A possible mechanism of action of oral pulse steroids in the treatment of alopecia areata. J. Cosmet. Dermatol. 2019, 18, 1128–1132. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, M.; Zilio, F.; Rossi, A.; Kleditzsch, P.; Emelianov, V.E.; Gilhar, A.; Keren, A.; Meyer, K.C.; Wang, E.; Funk, W.; et al. Abnormal interactions between perifollicular mast cells and CD8+ T-cells may contribute to the pathogenesis of alopecia areata. PLoS One 2014, 9, e94260. [Google Scholar] [CrossRef]
- Blaumeiser, B.; van der Goot, I.; Fimmers, R.; Hanneken, S.; Ritzmann, S.; Seymons, K.; Betz, R.C.; Ruzicka, T.; Wienker, T.F.; De Weert, J.; et al. Familial aggregation of alopecia areata. J. Am. Acad. Dermatol. 2006, 54, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Petukhova, L.; Christiano, A.M. The genetic architecture of alopecia areata. J. Investig. Dermatol. Symp. Proc. 2013, 16, S16–S22. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Liu, S.; Zhu, K.; Luo, H.; Li, Q.; Zhang, Y.; Huang, S.; Chen, Q.; Cao, Y. HLA-DRB1 polymorphisms and alopecia areata disease risk: A systematic review and meta-analysis. Medicine 2018, 97, e11790. [Google Scholar] [CrossRef] [PubMed]
- Arango, M.T.; Perricone, C.; Kivity, S.; Cipriano, E.; Ceccarelli, F.; Valesini, G.; Shoenfeld, Y. HLA-DRB1 the notorious gene in the mosaic of autoimmunity. Immunol. Res. 2017, 65, 82–98. [Google Scholar] [CrossRef]
- Zuo, J.; Willcox, B.E.; Moss, P. ULBPs: Regulators of human lymphocyte stress recognition. Oncotarget 2017, 8, 106157–106158. [Google Scholar] [CrossRef] [PubMed]
- Seok, H.; Jeon, H.S.; Park, H.J.; Kim, S.K.; Choi, J.H.; Lew, B.L.; Chung, J.H.; Sim, W.Y. Association of HSPA1B SNP rs6457452 with Alopecia Areata in the Korean population. Immunol. Investig. 2014, 43, 212–223. [Google Scholar] [CrossRef]
- Betz, R.C.; Petukhova, L.; Ripke, S.; Huang, H.; Menelaou, A.; Redler, S.; Becker, T.; Heilmann, S.; Yamany, T.; Duvic, M.; et al. Genome-wide meta-analysis in alopecia areata resolves HLA associations and reveals two new susceptibility loci. Nat. Commun. 2015, 6, 5966. [Google Scholar] [CrossRef]
- Rajabi, F.; Abdollahimajd, F.; Jabalameli, N.; Nassiri Kashani, M.; Firooz, A. The Immunogenetics of Alopecia areata. Adv. Exp. Med. Biol. 2022, 1367, 19–59. [Google Scholar] [CrossRef] [PubMed]
- Wengraf, D.A.; McDonagh, A.J.; Lovewell, T.R.; Vasilopoulos, Y.; Macdonald-Hull, S.P.; Cork, M.J.; Messenger, A.G.; Tazi-Ahnini, R. Genetic analysis of autoimmune regulator haplotypes in alopecia areata. Tissue. Antigens. 2008, 71, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Conteduca, G.; Rossi, A.; Megiorni, F.; Parodi, A.; Ferrera, F.; Tardito, S.; Battaglia, F.; Kalli, F.; Negrini, S.; Pizzuti, A.; et al. Single nucleotide polymorphisms in the promoter regions of Foxp3 and ICOSLG genes are associated with Alopecia areata. Clin. Exp. Med. 2014, 14, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.X.; Chen, W.J.; Liang, J.Q.; Wang, Y.J.; Jin, L.; Xu, C.; Kang, X.J. The association between rs2476601 polymorphism in PTPN22 gene and risk of alopecia areata: A meta-analysis of case-control studies. Medicine 2019, 98, e15448. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Lee, H.; Lew, B.L.; Sim, W.Y.; Kim, Y.O.; Lee, S.W.; Lee, S.; Cho, I.K.; Kwon, J.T.; Kim, H.J. Association between TAP1 gene polymorphisms and alopecia areata in a Korean population. Genet Mol. Res. 2015, 14, 18820–18827. [Google Scholar] [CrossRef] [PubMed]
- Seleit, I.; Bakry, O.A.; Gayed, E.A.E.; Gawad, A.E.D. Polymorphism of FAS and FAS Ligand Genes in Alopecia Areata: A Case-control Study in Egyptian Population. Indian J. Dermatol. 2018, 63, 220–226. [Google Scholar] [CrossRef]
- AlFadhli, S.; Nanda, A. Genetic evidence for the involvement of NOTCH4 in rheumatoid arthritis and alopecia areata. Immunol. Lett. 2013, 150, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Chung, J.H.; Park, H.J.; Kang, S.W.; Lim, D.J.; Byun, S.H.; Baek, D.G.; Ko, H.Y.; Lew, B.L.; Baik, H.H.; et al. Polymorphisms in the promoter regions of the CXCL1 and CXCL2 genes contribute to increased risk of alopecia areata in the Korean population. Genet Mol. Res. 2015, 14, 9667–9674. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Degenhardt, F.; Hofmann, A.; Redler, S.; Basmanav, F.B.; Heilmann-Heimbach, S.; Hanneken, S.; Giehl, K.A.; Wolff, H.; Moebus, S.; et al. Genomewide analysis of copy number variants in alopecia areata in a Central European cohort reveals association with MCHR2. Exp. Dermatol. 2017, 26, 536–541. [Google Scholar] [CrossRef]
- Petukhova, L.; Patel, A.V.; Rigo, R.K.; Bian, L.; Verbitsky, M.; Sanna-Cherchi, S.; Erjavec, S.O.; Abdelaziz, A.R.; Cerise, J.E.; Jabbari, A.; et al. Integrative analysis of rare copy number variants and gene expression data in alopecia areata implicates an aetiological role for autophagy. Exp. Dermatol. 2020, 29, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Abdel Fattah, N.S.; Ebrahim, A.A.; El Okda, E.S. Lipid peroxidation/antioxidant activity in patients with alopecia areata. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 403–408. [Google Scholar] [CrossRef]
- Akar, A.; Arca, E.; Erbil, H.; Akay, C.; Sayal, A.; Gür, A.R. Antioxidant enzymes and lipid peroxidation in the scalp of patients with alopecia areata. J. Dermatol. Sci. 2002, 29, 85–90. [Google Scholar] [CrossRef]
- Acharya, P.; Mathur, M.C. Oxidative stress in alopecia areata: A systematic review and meta-analysis. Int. J. Dermatol. 2020, 59, 434–440. [Google Scholar] [CrossRef]
- Sachdeva, S.; Khurana, A.; Goyal, P.; Sardana, K. Does oxidative stress correlate with disease activity and severity in alopecia areata? An analytical study. J. Cosmet. Dermatol. 2022, 21, 1629–1634. [Google Scholar] [CrossRef]
- Fokam, D.; Hoskin, D. Instrumental role for reactive oxygen species in the inflammatory response. Front. Biosci. (Landmark Ed) 2020, 25, 1110–1119. [Google Scholar] [CrossRef]
- Jain, U.; Saxena, K.; Chauhan, N. Helicobacter pylori induced reactive oxygen Species: A new and developing platform for detection. Helicobacter 2021, 26, e12796. [Google Scholar] [CrossRef]
- Paus, R.; Arck, P. Neuroendocrine perspectives in alopecia areata: Does stress play a role? J. Investig. Dermatol. 2009, 129, 1324–1326. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Ito, T.; Kromminga, A.; Bettermann, A.; Takigawa, M.; Kees, F.; Straub, R.H.; Paus, R. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. FASEB J. 2005, 19, 1332–1334. [Google Scholar] [CrossRef] [PubMed]
- Arck, P.C.; Handjiski, B.; Peters, E.M.; Peter, A.S.; Hagen, E.; Fischer, A.; Klapp, B.F.; Paus, R. Stress inhibits hair growth in mice by induction of premature catagen development and deleterious perifollicular inflammatory events via neuropeptide substance P-dependent pathways. Am. J. Pathol. 2003, 162, 803–814. [Google Scholar] [CrossRef]
- Azzawi, S.; Penzi, L.R.; Senna, M.M. Immune Privilege Collapse and Alopecia Development: Is Stress a Factor. Skin Appendage. Disord. 2018, 4, 236–244. [Google Scholar] [CrossRef]
- Ito, N.; Sugawara, K.; Bodó, E.; Takigawa, M.; van Beek, N.; Ito, T.; Paus, R. Corticotropin-releasing hormone stimulates the in situ generation of mast cells from precursors in the human hair follicle mesenchyme. J. Investig. Dermatol. 2010, 130, 995–1004. [Google Scholar] [CrossRef]
- Alexopoulos, A.; Chrousos, G.P. Stress-related skin disorders. Rev. Endocr. Metab. Disord. 2016, 17, 295–304. [Google Scholar] [CrossRef]
- Smatti, M.K.; Cyprian, F.S.; Nasrallah, G.K.; Al Thani, A.A.; Almishal, R.O.; Yassine, H.M. Viruses and Autoimmunity: A Review on the Potential Interaction and Molecular Mechanisms. Viruses 2019, 11, 762. [Google Scholar] [CrossRef]
- Fukuyama, M.; Ito, T.; Ohyama, M. Alopecia areata: Current understanding of the pathophysiology and update on therapeutic approaches, featuring the Japanese Dermatological Association guidelines. J. Dermatol. 2022, 49, 19–36. [Google Scholar] [CrossRef]
- Nguyen, B.; Tosti, A. Alopecia in patients with COVID-19: A systematic review and meta-analysis. JAAD Int. 2022, 7, 67–77. [Google Scholar] [CrossRef]
- May Lee, M.; Bertolani, M.; Pierobon, E.; Lotti, T.; Feliciani, C.; Satolli, F. Alopecia areata following COVID-19 vaccination: Vaccine-induced autoimmunity? Int. J. Dermatol. 2022, 61, 634–635. [Google Scholar] [CrossRef]
- Dai, Y.X.; Yeh, F.Y.; Shen, Y.J.; Tai, Y.H.; Chou, Y.J.; Chang, Y.T.; Chen, T.J.; Li, C.P.; Wu, C.Y. Cigarette Smoking, Alcohol Consumption, and Risk of Alopecia Areata: A Population-Based Cohort Study in Taiwan. Am. J. Clin. Dermatol. 2020, 21, 901–911. [Google Scholar] [CrossRef]
- Melnik, B.C.; John, S.M.; Chen, W.; Plewig, G. T helper 17 cell/regulatory T-cell imbalance in hidradenitis suppurativa/acne inversa: The link to hair follicle dissection, obesity, smoking and autoimmune comorbidities. Br. J. Dermatol. 2018, 179, 260–272. [Google Scholar] [CrossRef]
- Dai, Y.X.; Tai, Y.H.; Chen, C.C.; Chang, Y.T.; Chen, T.J.; Chen, M.H. Bidirectional association between alopecia areata and sleep disorders: A population-based cohort study in Taiwan. Sleep Med. 2020, 75, 112–116. [Google Scholar] [CrossRef]
- Inui, S.; Hamasaki, T.; Itami, S. Sleep quality in patients with alopecia areata: Questionnaire-based study. Int. J. Dermatol. 2014, 53, e39–e41. [Google Scholar] [CrossRef]
- Besedovsky, L.; Lange, T.; Haack, M. The Sleep-Immune Crosstalk in Health and Disease. Physiol. Rev. 2019, 99, 1325–1380. [Google Scholar] [CrossRef]
- Hagino, T.; Okazaki, S.; Serizawa, N.; Suzuki, K.; Kaga, M.; Otsuka, Y.; Mikami, E.; Hoashi, T.; Saeki, H.; Matsuda, H.; et al. Dietary Habits in Japanese Patients with Alopecia Areata. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1579–1591. [Google Scholar] [CrossRef]
- Nakamizo, S.; Honda, T.; Adachi, A.; Nagatake, T.; Kunisawa, J.; Kitoh, A.; Otsuka, A.; Dainichi, T.; Nomura, T.; Ginhoux, F.; et al. High fat diet exacerbates murine psoriatic dermatitis by increasing the number of IL-17-producing γδ T cells. Sci. Rep. 2017, 7, 14076. [Google Scholar] [CrossRef]
- Taylor, E.B. The complex role of adipokines in obesity, inflammation, and autoimmunity. Clin. Sci. 2021, 135, 731–752. [Google Scholar] [CrossRef]
- Nakatsuji, T.; Chiang, H.I.; Jiang, S.B.; Nagarajan, H.; Zengler, K.; Gallo, R.L. The microbiome extends to subepidermal compartments of normal skin. Nat. Commun. 2013, 4, 431. [Google Scholar] [CrossRef]
- Watanabe, K.; Nishi, E.; Tashiro, Y.; Sakai, K. Mode and Structure of the Bacterial Community on Human Scalp Hair. Microbes. Environ. 2019, 34, 252–259. [Google Scholar] [CrossRef]
- Matard, B.; Meylheuc, T.; Briandet, R.; Casin, I.; Assouly, P.; Cavelier-balloy, B.; Reygagne, P. First evidence of bacterial biofilms in the anaerobe part of scalp hair follicles: A pilot comparative study in folliculitis decalvans. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 853–860. [Google Scholar] [CrossRef]
- Lousada, M.B.; Lachnit, T.; Edelkamp, J.; Rouillé, T.; Ajdic, D.; Uchida, Y.; Di Nardo, A.; Bosch, T.C.G.; Paus, R. Exploring the human hair follicle microbiome. Br. J. Dermatol. 2021, 184, 802–815. [Google Scholar] [CrossRef]
- Polak-Witka, K.; Rudnicka, L.; Blume-Peytavi, U.; Vogt, A. The role of the microbiome in scalp hair follicle biology and disease. Exp. Dermatol. 2020, 29, 286–294. [Google Scholar] [CrossRef]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- Constantinou, A.; Kanti, V.; Polak-Witka, K.; Blume-Peytavi, U.; Spyrou, G.M.; Vogt, A. The Potential Relevance of the Microbiome to Hair Physiology and Regeneration: The Emerging Role of Metagenomics. Biomedicines 2021, 9, 236. [Google Scholar] [CrossRef]
- Reithmayer, K.; Meyer, K.C.; Kleditzsch, P.; Tiede, S.; Uppalapati, S.K.; Gläser, R.; Harder, J.; Schröder, J.M.; Paus, R. Human hair follicle epithelium has an antimicrobial defence system that includes the inducible antimicrobial peptide psoriasin (S100A7) and RNase 7. Br. J. Dermatol. 2009, 161, 78–89. [Google Scholar] [CrossRef]
- Chen, X.; Niyonsaba, F.; Ushio, H.; Okuda, D.; Nagaoka, I.; Ikeda, S.; Okumura, K.; Ogawa, H. Synergistic effect of antibacterial agents human beta-defensins, cathelicidin LL-37 and lysozyme against Staphylococcus aureus and Escherichia coli. J. Dermatol. Sci. 2005, 40, 123–132. [Google Scholar] [CrossRef]
- Wang, Z.; Mascarenhas, N.; Eckmann, L.; Miyamoto, Y.; Sun, X.; Kawakami, T.; Di Nardo, A. Skin microbiome promotes mast cell maturation by triggering stem cell factor production in keratinocytes. J. Allergy Clin. Immunol. 2017, 139, 1205–1216.e6. [Google Scholar] [CrossRef]
- Sobiepanek, A.; Kuryk, Ł.; Garofalo, M.; Kumar, S.; Baran, J.; Musolf, P.; Siebenhaar, F.; Fluhr, J.W.; Kobiela, T.; Plasenzotti, R.; et al. The Multifaceted Roles of Mast Cells in Immune Homeostasis, Infections and Cancers. Int. J. Mol. Sci. 2022, 23, 2249. [Google Scholar] [CrossRef]
- Kashem, S.W.; Haniffa, M.; Kaplan, D.H. Antigen-Presenting Cells in the Skin. Annu. Rev. Immunol. 2017, 35, 469–499. [Google Scholar] [CrossRef]
- Hall, J.B.; Cong, Z.; Imamura-Kawasawa, Y.; Kidd, B.A.; Dudley, J.T.; Thiboutot, D.M.; Nelson, A.M. Isolation and Identification of the Follicular Microbiome: Implications for Acne Research. J. Investig. Dermatol. 2018, 138, 2033–2040. [Google Scholar] [CrossRef]
- Lai, Y.; Di Nardo, A.; Nakatsuji, T.; Leichtle, A.; Yang, Y.; Cogen, A.L.; Wu, Z.R.; Hooper, L.V.; Schmidt, R.R.; von Aulock, S.; et al. Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat. Med. 2009, 15, 1377–1382. [Google Scholar] [CrossRef]
- Mohan, G.C.; Silverberg, J.I. Association of Vitiligo and Alopecia Areata With Atopic Dermatitis: A Systematic Review and Meta-analysis. JAMA Dermatol. 2015, 151, 522–528. [Google Scholar] [CrossRef]
- Edslev, S.M.; Agner, T.; Andersen, P.S. Skin Microbiome in Atopic Dermatitis. Acta Derm. Venereol. 2020, 100, adv00164. [Google Scholar] [CrossRef]
- Malik, K.; Guttman-Yassky, E. Cytokine Targeted Therapeutics for Alopecia Areata: Lessons from Atopic Dermatitis and Other Inflammatory Skin Diseases. J. Investig. Dermatol. Symp. Proc. 2018, 19, S62–S64. [Google Scholar] [CrossRef]
- Pinto, D.; Sorbellini, E.; Marzani, B.; Rucco, M.; Giuliani, G.; Rinaldi, F. Scalp bacterial shift in Alopecia areata. PLoS One 2019, 14, e0215206. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef]
- Clavaud, C.; Jourdain, R.; Bar-Hen, A.; Tichit, M.; Bouchier, C.; Pouradier, F.; El Rawadi, C.; Guillot, J.; Ménard-Szczebara, F.; Breton, L.; et al. Dandruff is associated with disequilibrium in the proportion of the major bacterial and fungal populations colonizing the scalp. PLoS One 2013, 8, e58203. [Google Scholar] [CrossRef]
- Wang, E.; Lee, J.S.; Hee, T.H. Is propionibacterium acnes associated with hair casts and alopecia? Int. J. Trichology 2012, 4, 93–97. [Google Scholar] [CrossRef]
- Pinto, D.; Calabrese, F.M.; De Angelis, M.; Celano, G.; Giuliani, G.; Gobbetti, M.; Rinaldi, F. Predictive Metagenomic Profiling, Urine Metabolomics, and Human Marker Gene Expression as an Integrated Approach to Study Alopecia Areata. Front. Cell. Infect. Microbiol. 2020, 10, 146. [Google Scholar] [CrossRef]
- Fyhrquist, N.; Muirhead, G.; Prast-Nielsen, S.; Jeanmougin, M.; Olah, P.; Skoog, T.; Jules-Clement, G.; Feld, M.; Barrientos-Somarribas, M.; Sinkko, H.; et al. Microbe-host interplay in atopic dermatitis and psoriasis. Nat. Commun. 2019, 10, 4703. [Google Scholar] [CrossRef]
- Wang, E.H.C.; Yu, M.; Breitkopf, T.; Akhoundsadegh, N.; Wang, X.; Shi, F.T.; Leung, G.; Dutz, J.P.; Shapiro, J.; McElwee, K.J. Identification of Autoantigen Epitopes in Alopecia Areata. J. Investig. Dermatol. 2016, 136, 1617–1626. [Google Scholar] [CrossRef]
- Juhasz, M.; Chen, S.; Khosrovi–Eghbal, A.; Ekelem, C.; Landaverde, Y.; Baldi, P.; Mesinkovska, N.A. Characterizing the Skin and Gut Microbiome of Alopecia Areata Patients. SKIN J. Cutan. Med. 2020, 4, 23–30. [Google Scholar] [CrossRef]
- Won, E.J.; Jang, H.H.; Park, H.; Kim, S.J. A Potential Predictive Role of the Scalp Microbiome Profiling in Patients with Alopecia Areata: Staphylococcus caprae, Corynebacterium, and Cutibacterium Species. Microorganisms 2022, 10, 864. [Google Scholar] [CrossRef]
- Ho, B.S.; Ho, E.X.P.; Chu, C.W.; Ramasamy, S.; Bigliardi-Qi, M.; de Sessions, P.F. Bigliardi, P.L. Microbiome in the hair follicle of androgenetic alopecia patients. PLoS One 2019, 14, e0216330. [Google Scholar] [CrossRef]
- Rinaldi, F.; Trink, A.; Pinto, D. Efficacy of Postbiotics in a PRP-Like Cosmetic Product for the Treatment of Alopecia Area Celsi: A Randomized Double-Blinded Parallel-Group Study. Dermatol. Ther. 2020, 10, 483–493. [Google Scholar] [CrossRef]
- Cruciani, M.; Masiello, F.; Pati, I.; Marano, G.; Pupella, S.; De Angelis, V. Platelet-rich plasma for the treatment of alopecia: A systematic review and meta-analysis. Blood Transfus. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics-A Step Beyond Pre- and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef]
- Gill, S.R.; Pop, M.; Deboy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic analysis of the human distal gut microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef]
- Shanahan, F.; Ghosh, T.S.; O'Toole, P.W. The Healthy Microbiome-What Is the Definition of a Healthy Gut Microbiome? Gastroenterology 2021, 160, 483–494. [Google Scholar] [CrossRef]
- Moya, A.; Ferrer, M. Functional Redundancy-Induced Stability of Gut Microbiota Subjected to Disturbance. Trends Microbiol. 2016, 24, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Takiishi, T.; Fenero, C.I.M.; Câmara, N.O.S. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers 2017, 5, e1373208. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Wu, L.; Huntington, N.D.; Zhang, X. Crosstalk Between Gut Microbiota and Innate Immunity and Its Implication in Autoimmune Diseases. Front. Immunol. 2020, 11, 282. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Naik, S. Compartmentalized and systemic control of tissue immunity by commensals. Nat. Immunol. 2013, 14, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Lin, G.; Ferenczi, K. The skin microbiome and the gut-skin axis. Clin. Dermatol. 2021, 39, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Lee, C.H.; Chi, C.C. Association of Psoriasis With Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. JAMA Dermatol. 2018, 154, 1417–1423. [Google Scholar] [CrossRef]
- Ramírez-Boscá, A.; Navarro-López, V.; Martínez-Andrés, A.; Such, J.; Francés, R.; Horga de la Parte, J.; Asín-Llorca, M. Identification of Bacterial DNA in the Peripheral Blood of Patients With Active Psoriasis. JAMA Dermatol. 2015, 151, 670–671. [Google Scholar] [CrossRef] [PubMed]
- Kinashi, Y.; Hase, K. Partners in Leaky Gut Syndrome: Intestinal Dysbiosis and Autoimmunity. Front. Immunol. 2021, 12, 673708. [Google Scholar] [CrossRef]
- Clemente, J.C.; Manasson, J.; Scher, J.U. The role of the gut microbiome in systemic inflammatory disease. BMJ 2018, 360, j5145. [Google Scholar] [CrossRef]
- Borde, A.; Åstrand, A. Alopecia areata and the gut-the link opens up for novel therapeutic interventions. Expert. Opin. Ther. Targets 2018, 22, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Gilhar, A.; Schrum, A.G.; Etzioni, A.; Waldmann, H.; Paus, R. Alopecia areata: Animal models illuminate autoimmune pathogenesis and novel immunotherapeutic strategies. Autoimmun. Rev. 2016, 15, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Sundberg, J.P.; Elson, C.O.; Bedigian, H.; Birkenmeier, E.H. Spontaneous, heritable colitis in a new substrain of C3H/HeJ mice. Gastroenterology 1994, 107, 1726–1735. [Google Scholar] [CrossRef]
- Maghfour, J.; Olson, J.; Conic, R.R.Z.; Mesinkovska, N.A. The Association between Alopecia and Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Dermatology 2021, 237, 658–672. [Google Scholar] [CrossRef] [PubMed]
- Glassner, K.L.; Abraham, B.P.; Quigley, E.M.M. The microbiome and inflammatory bowel disease. J. Allergy Clin. Immunol. 2020, 145, 16–27. [Google Scholar] [CrossRef] [PubMed]
- McElwee, K.J.; Niiyama, S.; Freyschmidt-Paul, P.; Wenzel, E.; Kissling, S.; Sundberg, J.P.; Hoffmann, R. Dietary soy oil content and soy-derived phytoestrogen genistein increase resistance to alopecia areata onset in C3H/HeJ mice. Exp. Dermatol. 2003, 12, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.; Dai, Z.; Christiano, A.M. Gut microbiota plays a role in the development of alopecia areata. J. Investig. Dermatol. 2017, 137, S112. [Google Scholar] [CrossRef]
- Raugh, A.; Allard, D.; Bettini, M. Nature vs. nurture: FOXP3, genetics, and tissue environment shape Treg function. Front. Immunol. 2022, 13, 911151. [Google Scholar] [CrossRef]
- Sanchez Rodriguez, R.; Pauli, M.L.; Neuhaus, I.M.; Yu, S.S.; Arron, S.T.; Harris, H.W.; Yang, S.H.; Anthony, B.A.; Sverdrup, F.M.; Krow-Lucal, E.; et al. Memory regulatory T cells reside in human skin. J. Clin. Invest. 2014, 124, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Scharschmidt, T.C.; Vasquez, K.S.; Pauli, M.L.; Leitner, E.G.; Chu, K.; Truong, H.A.; Lowe, M.M.; Sanchez Rodriguez, R.; Ali, N.; Laszik, Z.G.; et al. Commensal Microbes and Hair Follicle Morphogenesis Coordinately Drive Treg Migration into Neonatal Skin. Cell Host Microbe 2017, 21, 467–477.e5. [Google Scholar] [CrossRef] [PubMed]
- McElwee, K.J.; Freyschmidt-Paul, P.; Hoffmann, R.; Kissling, S.; Hummel, S.; Vitacolonna, M.; Zöller, M. Transfer of CD8(+) cells induces localized hair loss whereas CD4(+)/CD25(-) cells promote systemic alopecia areata and CD4(+)/CD25(+) cells blockade disease onset in the C3H/HeJ mouse model. J. Investig. Dermatol. 2005, 124, 947–957. [Google Scholar] [CrossRef]
- Ali, N.; Zirak, B.; Rodriguez, R.S.; Pauli, M.L.; Truong, H.A.; Lai, K.; Ahn, R.; Corbin, K.; Lowe, M.M.; Scharschmidt, T.C. Regulatory T Cells in Skin Facilitate Epithelial Stem Cell Differentiation. Cell 2017, 169, 1119–1129.e11. [Google Scholar] [CrossRef]
- Hamed, F.N.; Åstrand, A.; Bertolini, M.; Rossi, A.; Maleki-Dizaji, A.; Messenger, A.G.; McDonagh, A.J.G.; Tazi-Ahnini, R. Alopecia areata patients show deficiency of FOXP3+CD39+ T regulatory cells and clonotypic restriction of Treg TCRβ-chain, which highlights the immunopathological aspect of the disease. PLoS One 2019, 14, e0210308. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Kirby, J.; Reilly, C.M.; Luo, X.M. Leaky Gut As a Danger Signal for Autoimmune Diseases. Front. Immunol. 2017, 8, 598. [Google Scholar] [CrossRef]
- Hacınecipoğlu, F.; Gönül, M.; Özdemir, Ş.; Demir, Ö.F. Is there a link between alopecia areata and gut? J. Cosmet. Dermatol. 2022. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Rebello, D.; Wang, E.; Yen, E.; Lio, P.A.; Kelly, C.R. Hair Growth in Two Alopecia Patients after Fecal Microbiota Transplant. ACG Case Rep. J. 2017, 4, e107. [Google Scholar] [CrossRef]
- Xie, W.R.; Yang, X.Y.; Xia, H.H.; Wu, L.H.; He, X.X. Hair regrowth following fecal microbiota transplantation in an elderly patient with alopecia areata: A case report and review of the literature. World J. Clin. Cases 2019, 7, 3074–3081. [Google Scholar] [CrossRef]
- Moreno-Arrones, O.M.; Serrano-Villar, S.; Perez-Brocal, V.; Saceda-Corralo, D.; Morales-Raya, C.; Rodrigues-Barata, R.; Moya, A.; Jaen-Olasolo, P.; Vano-Galvan, S. Analysis of the gut microbiota in alopecia areata: Identification of bacterial biomarkers. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 400–405. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, P.; Hu, R.; Qi, S.; Zhao, Y.; Miao, Y.; Han, Y.; Zhou, L.; Yang, Q. Gut microbiota characterization in Chinese patients with alopecia areata. J. Dermatol. Sci. 2021, 102, 109–115. [Google Scholar] [CrossRef]
- Rangu, S.; Lee, J.J.; Hu, W.; Bittinger, K.; Castelo-Soccio, L. Understanding the Gut Microbiota in Pediatric Patients with Alopecia Areata and their Siblings: A Pilot Study. JID Innov. 2021, 1, 100051. [Google Scholar] [CrossRef]
- Huang, E.Y.; Inoue, T.; Leone, V.A.; Dalal, S.; Touw, K.; Wang, Y.; Much, M.W.; Theriault, B.; Higuchi, K.; Donovan, S.; et al. Using corticosteroids to reshape the gut microbiome: Implications for inflammatory bowel diseases. Inflamm. Bowel. Dis. 2015, 21, 963–972. [Google Scholar] [CrossRef]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef]
| Reference | Methodology and Study Population | Key Results |
|---|---|---|
| Pinto 2019 [117] | 15 patients with alopecia areata and 15 healthy controls. Study of the microbiota of the scalp surface of alopecia areata lesions by swab procedure. NGS 16S rRNA. qPCR of Cutibacterium acnes, Staphylococcus aureus and Staphylococcus epidermidis. | Increase in α-diversity in alopecia areata patients. Actinobacteria y Firmicutes are de the two main phyla in the scalp microbiota in both study groups (similar abundances). C. acnes/S. epidermidis and C. acnes/S. aureus ratios significantly were increased in alopecia areata patients. |
| Pinto 2020 [121] | 47 patients with alopecia areata and 47 healthy controls. Study of the microbiota of the scalp surface of alopecia areata lesions by swab procedure. Study of the microbiota of the dermis, epidermis, and hypodermis of the scalp by biopsy in four patients with alopecia areata and four healthy controls. NGS 16S rRNA. qPCR of genes involved in genetic susceptibility to alopecia areata. | Biopsies: In alopecia areata patients at the dermis level it was observed a decrease in Candidatus Aquiluna, Staphylococcus and 2 OTUs representing Microthrixaceae and ACK-M1 families, and an increase in Acinetobacter. In alopecia areata patients at the epidermis level, an increase in Anaerococcus and Neisseria was observed, and an absence of SMB53 genus (Clostridiacea), and a decrease in Staphylococcus. No significant differences were reported at the hypodermis level between the two study groups. No differences in α-diversity between the skin layers. PICRUSt, KEGG: In alopecia areata patients it was observed an increase in some functional profiles such as the environmental information processing and the cellular antigen pathway. Anaerococcus, Neisseria and Acinetobacter correlated negatively, in alopecia areata patients, with FAS and SOD2 genes but positively with the NOD2 gene. |
| Juhasz 2020 [124] | 25 patients with alopecia areata, alopecia universalis or alopecia totalis and 15 healthy controls. Study of the microbiota of the scalp surface of alopecia areata lesions by swab procedure. NGS 16S rRNA. | No significant differences were detected in α-diversity between the two study groups. Β-diversity analysis did not show any differences between the two study groups. Significant decrease in Clostridia in alopecia areata patients. |
| Wong 2022 [125] | 33 patients with alopecia areata (26 and 7 with moderate and severe symptoms, respectively) and 12 healthy controls. Study of the microbiota of the scalp surface of alopecia areata lesions by swab procedure. NGS 16S rRNA. | Increase in α-diversity in alopecia areata patients. A-diversity did not discriminate against patients with alopecia areata according to their severity. PCoA using Bray-Curtis distance did not exhibit significant differences between the study groups (and severity groups in alopecia areata patients). Decrease in Staphylococcaceae and Burkholceriaceae in alopecia areata patients and in severe versus moderate forms. Strong decrease in Staphylococcus caprae in patients with severe alopecia areata. Cutibacterium species/S. caprae ratio was increased from healthy controls (0.97) to moderate alopecia areata (2.13) and severe alopecia areata (16.01). |
| Rinaldi 2020 [127] | Randomised, double-bind, placebo-controlled, clinical trial. Evaluation of the efficacy of a gel containing biomimetic peptides, Tropaeoleum majus extract and postbiotics such as plantaricin A and Lactobacillus kunkei bee bread in 160 alopecia areata patients with a SALT score between S2 and S5. | In the postbiotic-enriched gel group, were reported a “complete regression” in 47% of patients, a “partial regression” in 14% of patients and a “no respond” in 6% of patients. In the placebo group, only 5% of patients achieved “complete regression”. |
| Reference | Methodology and Study Population | Key Results |
|---|---|---|
| Rebello 2017 [161] | Case Report. two patients with Clostridiodes difficile infection and treated with FMT. Moreover, both patients presented alopecia areata. | This report highlights two patients with coexisting alopecia areata and recurrent CDI who experienced hair regrowth after FMT. This could suggest a strong immunological response to FMT. |
| Xie 2019 [162] | Case Report. A patient diagnosed with noninfectious diarrhea, depressive disorder, and patchy alopecia areata. The patient was treated with six rounds of FMT. | It was observed new hair growth on the affected region of his scalp without taking any other therapies for alopecia areata before and after FMT. |
| Moreno-Arrones 2020 [163] | 15 patients with alopecia universalis and 15 healthy controls. Stool samples. NGS 16S rRNA. | No significant differences in α-diversity between the two study groups. β-diversity analysis did not show any differences between the two study groups. Using the LefSe tool it was observed characteristic increases in alopecia areata patients in Erysipelotrichaceae, Lachnospiraceae and Eggerthellaceae families, and Holdemania filiformis, Parabacteroides johnsonii, Clostridiales vadin BB60 group, Bacteroides eggerthii, and Parabacteroides distasonis genera. The highest diagnostic efficacy was reported for P. distasonis and Clostridiales vadin BB60 group with AUC > 0.7. These bacteria were subjected to a multivariate regression model predictive of alopecia areata status and a combined AUC = 0.804 was obtained. This model indicated that a 25% increase in the abundance of P. distatonis and Clostridiales vadin BB60 group increased the risk of developing alopecia universalis by 9.4% and in the case of Clostridiales vadin BB60 by 11.4%. |
| Lu 2021 [164] | 33 patients with alopecia areata and 30 healthy controls. Stool samples. NGS 16S rRNA. | No significant differences in α-diversity between the two study groups. ADONIS tool using UniFrac distance exhibit significant differences between the study groups. Using the LefSe tool it was observed characteristic increases in alopecia areata patients in Blautia, Phyllobacterium, Dorea, Anaerostipes, Megasphaera, Collinsella, Sphingomonas, and Pseudomonas genera, and in Erysipelotrichaceae family. Achromobacter, Megasphaera and Lachnospiraceae Incertae Sedis were identified by a random forest model as biomarker bacteria for alopecia areata status. |
| Rangu 2021 [165] | 21 children with alopecia areata aged 4 to 17 years and their siblings (as a control group) aged 4 to 17 years. Stool samples. Shotgun Metagenomics. | No significant differences in α-diversity between the two study groups. β-diversity analysis using the Bray–Curtis distance did not show any differences between the two study groups. A linear mixed model revealed that Ruminicoccus bicirculans exhibited lower relative abundance in alopecia areata patients. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Pellicer, P.; Navarro-Moratalla, L.; Núñez-Delegido, E.; Agüera-Santos, J.; Navarro-López, V. How Our Microbiome Influences the Pathogenesis of Alopecia Areata. Genes 2022, 13, 1860. https://doi.org/10.3390/genes13101860
Sánchez-Pellicer P, Navarro-Moratalla L, Núñez-Delegido E, Agüera-Santos J, Navarro-López V. How Our Microbiome Influences the Pathogenesis of Alopecia Areata. Genes. 2022; 13(10):1860. https://doi.org/10.3390/genes13101860
Chicago/Turabian StyleSánchez-Pellicer, Pedro, Laura Navarro-Moratalla, Eva Núñez-Delegido, Juan Agüera-Santos, and Vicente Navarro-López. 2022. "How Our Microbiome Influences the Pathogenesis of Alopecia Areata" Genes 13, no. 10: 1860. https://doi.org/10.3390/genes13101860
APA StyleSánchez-Pellicer, P., Navarro-Moratalla, L., Núñez-Delegido, E., Agüera-Santos, J., & Navarro-López, V. (2022). How Our Microbiome Influences the Pathogenesis of Alopecia Areata. Genes, 13(10), 1860. https://doi.org/10.3390/genes13101860





