Maternal Microbiota Modulate a Fragile X-like Syndrome in Offspring Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. Microbial Treatments
2.4. Phenotyping Mice
2.5. Caesarian Rederivation of Offspring
2.6. Histopathology
2.7. FMRP Expression
2.8. Inflammatory Cytokines
2.9. Statistics
3. Results
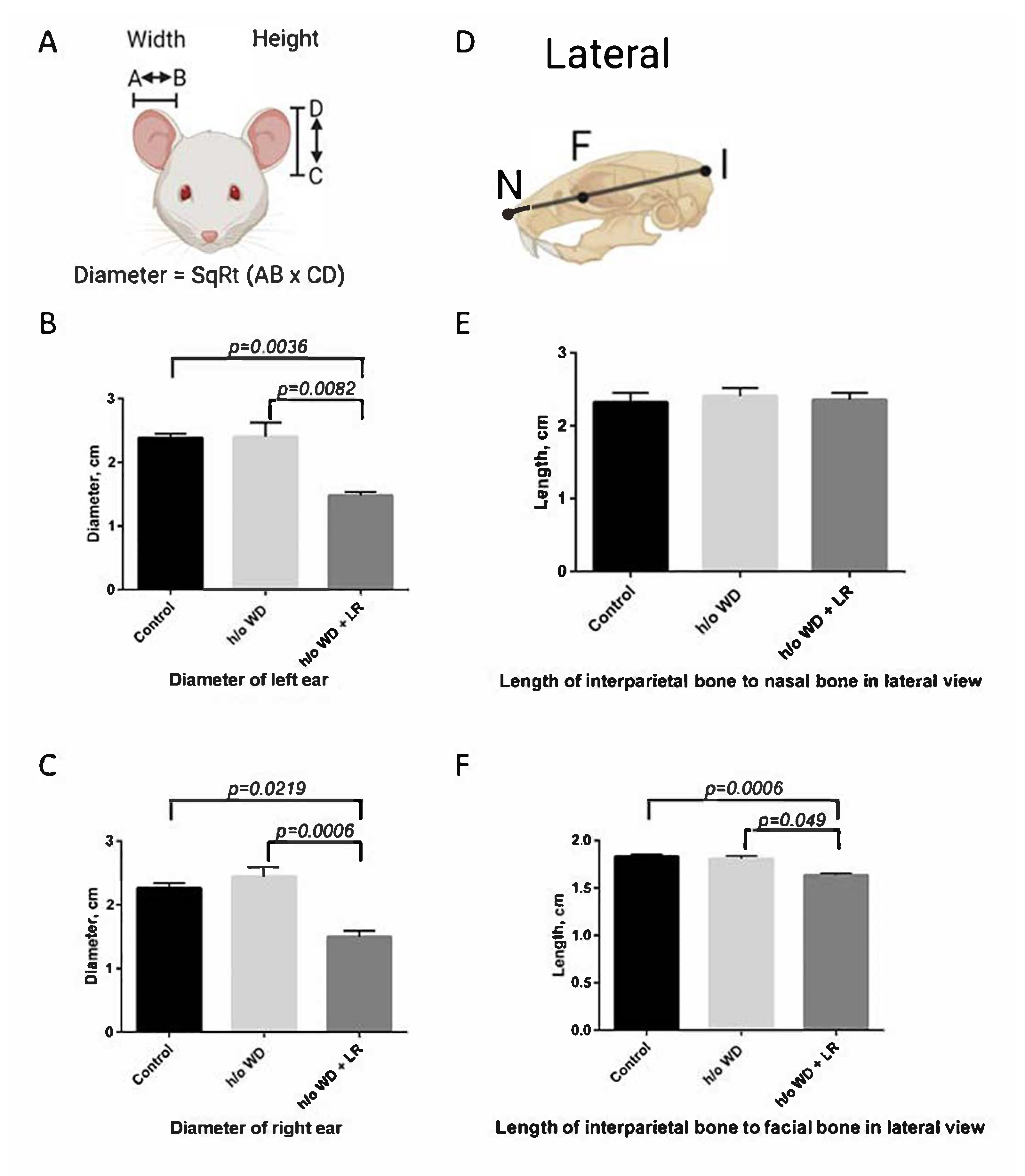
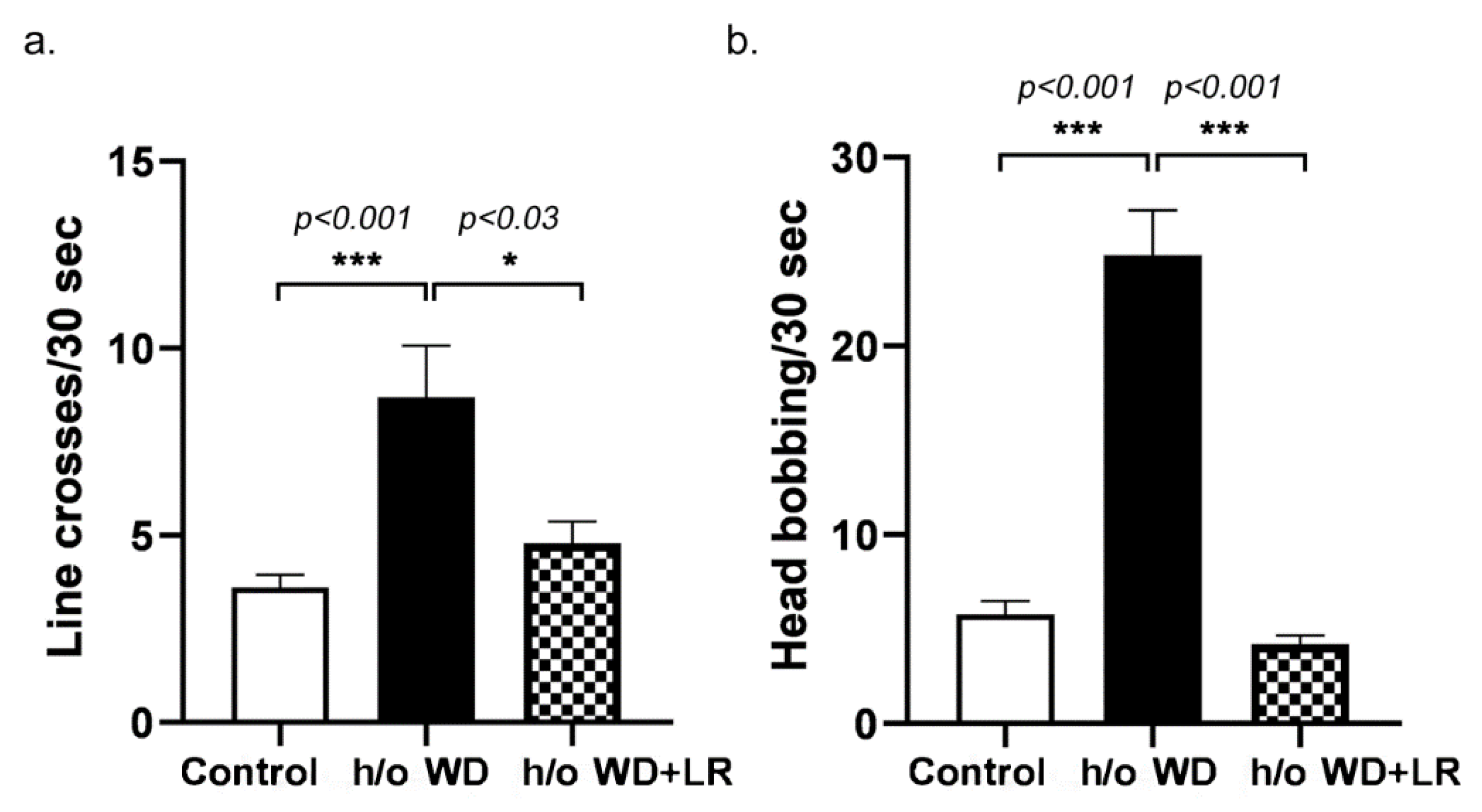
3.1. Offspring Mice Exhibit a Fragile X Syndrome (FXS)-like Phenotype including Elongated Head, Enlarged Ears, Head Bobbing, and Hyperactivity
3.2. In Utero L. reuteri Supplementation in Drinking Water Counteracts FXS-like Phenotype in Offspring Mice
3.3. Pregnant Mice Supplemented with L. reuteri Exhibit Lower Circulating Levels of Pro-Inflammatory Cytokine Interleukin -17A
3.4. Fragile X-like Phenotype in Mice Is Retained after Caesarian-Section Rederivation
3.5. In Utero Microbial Rescue with L. reuteri Blunts Methylation of FMRP
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, S.M.; Vale, W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006, 8, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Rautava, S.; Aakko, J.; Isolauri, E.; Salminen, A. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci. Rep. 2016, 6, 23129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunn, A.B.; Jordan, S.; Baker, B.J.; Carlson, N.S. The Maternal Infant Microbiome. MCN Am. J. Matern./Child Nurs. 2017, 42, 318–325. [Google Scholar] [CrossRef]
- Li, Y.; Toothaker, J.M.; Ben-Simon, S.; Ozeri, L.; Schweitzer, R.; McCourt, B.T.; McCourt, C.C.; Werner, L.; Snapper, S.B.; Shouval, D.S.; et al. In utero human intestine harbors unique metabolome, including bacterial metabolites. JCI Insight 2020, 5, e138751. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Prince, A.L.; Bader, D.; Hu, M.; Ganu, R.; Baquero, K.; Blundell, P.; Alan Harris, R.; Frias, A.E.; Grove, K.L.; et al. High-fat maternal diet during pregnancy persistently alters the offspring microbiome in a primate model. Nat. Commun. 2014, 5, 3889. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poutahidis, T.; Varian, B.J.; Levkovich, T.; Lakritz, J.R.; Mirabal, S.; Kwok, C.; Ibrahim, Y.M.; Kearney, S.M.; Chatzigiagkos, A.; Alm, E.J.; et al. Dietary microbes modulate transgenerational cancer risk. Cancer Res. 2015, 75, 1197–1204. [Google Scholar] [CrossRef] [Green Version]
- D’Hulst, C.; Heulens, I.; Brouwer, J.R.; Willemsen, R.; De Geest, N.; Reeve, S.P.; De Deyn, P.P.; Hassan, B.A.; Kooy, R.F. Expression of the GABAergic system in animal models for fragile X syndrome and fragile X associated tremor/ataxia syndrome (FXTAS). Brain Res. 2009, 1253, 176–183. [Google Scholar] [CrossRef]
- Buffington, S.A.; Di Prisco, G.V.; Auchtung, T.A.; Ajami, N.J.; Petrosino, J.F.; Costa-Mattioli, M. Microbial Reconstitution Reverses Maternal Diet-Induced Social and Synaptic Deficits in Offspring. Cell 2016, 165, 1762–1775. [Google Scholar] [CrossRef] [Green Version]
- Erdman, S.E.; Poutahidis, T. Microbes and Oxytocin: Benefits for Host Physiology and Behavior. Int. Rev. Neurobiol. 2016, 131, 91–126. [Google Scholar] [CrossRef]
- Hagerman, R.J.; Berry-Kravis, E.; Hazlett, H.C.; Bailey, D.B., Jr.; Moine, H.; Kooy, R.F.; Tassone, F.; Gantois, I.; Sonenberg, N.; Mandel, J.L.; et al. Fragile X syndrome. Nat. Rev. Dis. Primers 2017, 3, 17065. [Google Scholar] [CrossRef] [PubMed]
- Darnell, J.C.; Klann, E. The translation of translational control by FMRP: Therapeutic targets for FXS. Nat. Neurosci. 2013, 16, 1530–1536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, J.L.; Jones, K.L.; Yeo, G.W. Repeat RNA expansion disorders of the nervous system: Post-transcriptional mechanisms and therapeutic strategies. Crit. Rev. Biochem. Mol. Biol. 2021, 56, 31–53. [Google Scholar] [CrossRef]
- Nobile, V.; Pucci, C.; Chiurazzi, P.; Neri, G.; Tabolacci, E. DNA Methylation, Mechanisms of FMR1 Inactivation and Therapeutic Perspectives for Fragile X Syndrome. Biomolecules 2021, 11, 296. [Google Scholar] [CrossRef]
- Verkerk, A.J.; Pieretti, M.; Sutcliffe, J.S.; Fu, Y.H.; Kuhl, D.P.; Pizzuti, A.; Reiner, O.; Richards, S.; Victoria, M.F.; Zhang, F.P.; et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 1991, 65, 905–914. [Google Scholar] [CrossRef]
- Bassell, G.J.; Warren, S.T. Fragile X syndrome: Loss of local mRNA regulation alters synaptic development and function. Neuron 2008, 60, 201–214. [Google Scholar] [CrossRef] [Green Version]
- Kaufmann, W.E.; Kidd, S.A.; Andrews, H.F.; Budimirovic, D.B.; Esler, A.; Haas-Givler, B.; Stackhouse, T.; Riley, C.; Peacock, G.; Sherman, S.L.; et al. Autism Spectrum Disorder in Fragile X Syndrome: Cooccurring Conditions and Current Treatment. Pediatrics 2017, 139, S194–S206. [Google Scholar] [CrossRef] [Green Version]
- Rude, K.M.; Pusceddu, M.M.; Keogh, C.E.; Sladek, J.A.; Rabasa, G.; Miller, E.N.; Sethi, S.; Keil, K.P.; Pessah, I.N.; Lein, P.J.; et al. Developmental exposure to polychlorinated biphenyls (PCBs) in the maternal diet causes host-microbe defects in weanling offspring mice. Environ. Pollut. 2019, 253, 708–721. [Google Scholar] [CrossRef] [Green Version]
- Muhle, R.; Trentacoste, S.V.; Rapin, I. The genetics of autism. Pediatrics 2004, 113, e472–e486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry-Kravis, E. Epilepsy in fragile X syndrome. Dev. Med. Child Neurol. 2002, 44, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Boyle, L.; Kaufmann, W.E. The behavioral phenotype of FMR1 mutations. Am. J. Med. Genet. C Semin. Med. Genet. 2010, 154, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, D.; Oranje, B.; Razak, K.A.; Siegel, S.J.; Schmid, S. Sensory processing in autism spectrum disorders and Fragile X syndrome-From the clinic to animal models. Neurosci. Biobehav. Rev. 2017, 76, 235–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagerman, R.J. Lessons from fragile X regarding neurobiology, autism, and neurodegeneration. J. Dev. Behav. Pediatr. 2006, 27, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.W.; Hessl, D.; Goodlin-Jones, B.; Ferranti, J.; Bacalman, S.; Barbato, I.; Tassone, F.; Hagerman, P.J.; Herman, H.; Hagerman, R.J. Autism profiles of males with fragile X syndrome. Am. J. Ment. Retard. 2008, 113, 427–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maltman, N.; Friedman, L.; Lorang, E.; Sterling, A. Brief Report: Linguistic Mazes and Perseverations in School-Age Boys with Fragile X Syndrome and Autism Spectrum Disorder and Relationships with Maternal Maze Use. J. Autism Dev. Disord. 2021, 52, 897–907. [Google Scholar] [CrossRef]
- Kaufmann, W.E.; Cortell, R.; Kau, A.S.; Bukelis, I.; Tierney, E.; Gray, R.M.; Cox, C.; Capone, G.T.; Stanard, P. Autism spectrum disorder in fragile X syndrome: Communication, social interaction, and specific behaviors. Am. J. Med. Genet. A 2004, 129, 225–234. [Google Scholar] [CrossRef]
- Dolen, G.; Osterweil, E.; Rao, B.S.; Smith, G.B.; Auerbach, B.D.; Chattarji, S.; Bear, M.F. Correction of fragile X syndrome in mice. Neuron 2007, 56, 955–962. [Google Scholar] [CrossRef] [Green Version]
- D’Hulst, C.; De Geest, N.; Reeve, S.P.; Van Dam, D.; De Deyn, P.P.; Hassan, B.A.; Kooy, R.F. Decreased expression of the GABAA receptor in fragile X syndrome. Brain Res. 2006, 1121, 238–245. [Google Scholar] [CrossRef]
- Gibson, J.R.; Bartley, A.F.; Hays, S.A.; Huber, K.M. Imbalance of neocortical excitation and inhibition and altered UP states reflect network hyperexcitability in the mouse model of fragile X syndrome. J. Neurophysiol. 2008, 100, 2615–2626. [Google Scholar] [CrossRef]
- Anbuhl, K.L.; Benichoux, V.; Greene, N.T.; Brown, A.D.; Tollin, D.J. Development of the head, pinnae, and acoustical cues to sound location in a precocial species, the guinea pig (Cavia porcellus). Hear. Res. 2017, 356, 35–50. [Google Scholar] [CrossRef]
- McCullagh, E.A.; Poleg, S.; Greene, N.T.; Huntsman, M.M.; Tollin, D.J.; Klug, A. Characterization of Auditory and Binaural Spatial Hearing in a Fragile X Syndrome Mouse Model. eNeuro 2020, 7. [Google Scholar] [CrossRef] [Green Version]
- Simon, Y.; Marchadier, A.; Riviere, M.K.; Vandamme, K.; Koenig, F.; Lezot, F.; Trouve, A.; Benhamou, C.L.; Saffar, J.L.; Berdal, A.; et al. Cephalometric assessment of craniofacial dysmorphologies in relation with Msx2 mutations in mouse. Orthod. Craniofac. Res. 2014, 17, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, M.; Yamamura, K. Cranial bone morphometric study among mouse strains. BMC Evol. Biol. 2008, 8, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poutahidis, T.; Kleinewietfeld, M.; Smillie, C.; Levkovich, T.; Perrotta, A.; Bhela, S.; Varian, B.J.; Ibrahim, Y.M.; Lakritz, J.R.; Kearney, S.M.; et al. Microbial reprogramming inhibits Western diet-associated obesity. PLoS ONE 2013, 8, e68596. [Google Scholar] [CrossRef] [Green Version]
- Budimirovic, D.B.; Schlageter, A.; Filipovic-Sadic, S.; Protic, D.D.; Bram, E.; Mahone, E.M.; Nicholson, K.; Culp, K.; Javanmardi, K.; Kemppainen, J.; et al. A Genotype-Phenotype Study of High-Resolution FMR1 Nucleic Acid and Protein Analyses in Fragile X Patients with Neurobehavioral Assessments. Brain Sci. 2020, 10, 694. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Folsom, T.D. The role of fragile X mental retardation protein in major mental disorders. Neuropharmacology 2011, 60, 1221–1226. [Google Scholar] [CrossRef] [Green Version]
- Baker, E.K.; Arpone, M.; Aliaga, S.M.; Bretherton, L.; Kraan, C.M.; Bui, M.; Slater, H.R.; Ling, L.; Francis, D.; Hunter, M.F.; et al. Incomplete silencing of full mutation alleles in males with fragile X syndrome is associated with autistic features. Mol. Autism. 2019, 10, 21. [Google Scholar] [CrossRef] [Green Version]
- Goo, N.; Bae, H.J.; Park, K.; Kim, J.; Jeong, Y.; Cai, M.; Cho, K.; Jung, S.Y.; Kim, D.H.; Ryu, J.H. The effect of fecal microbiota transplantation on autistic-like behaviors in Fmr1 KO mice. Life Sci. 2020, 262, 118497. [Google Scholar] [CrossRef]
- Altimiras, F.; Garcia, J.A.; Palacios-Garcia, I.; Hurley, M.J.; Deacon, R.; Gonzalez, B.; Cogram, P. Altered Gut Microbiota in a Fragile X Syndrome Mouse Model. Front. Neurosci. 2021, 15, 653120. [Google Scholar] [CrossRef] [PubMed]
- Tabouy, L.; Getselter, D.; Ziv, O.; Karpuj, M.; Tabouy, T.; Lukic, I.; Maayouf, R.; Werbner, N.; Ben-Amram, H.; Nuriel-Ohayon, M.; et al. Dysbiosis of microbiome and probiotic treatment in a genetic model of autism spectrum disorders. Brain Behav. Immun. 2018, 73, 310–319. [Google Scholar] [CrossRef]
- Del Hoyo Soriano, L.; Thurman, A.J.; Harvey, D.J.; Ted Brown, W.; Abbeduto, L. Genetic and maternal predictors of cognitive and behavioral trajectories in females with fragile X syndrome. J. Neurodev. Disord. 2018, 10, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerdo, T.; Garcia-Valdes, L.; Altmae, S.; Ruiz, A.; Suarez, A.; Campoy, C. Role of microbiota function during early life on child’s neurodevelopment. Trends Food Sci. Tech. 2016, 57, 273–288. [Google Scholar] [CrossRef]
- Laker, R.C.; Wlodek, M.E.; Connelly, J.J.; Yan, Z. Epigenetic origins of metabolic disease: The impact of the maternal condition to the offspring epigenome and later health consequences. Food Sci. Hum. Wellness 2013, 2, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Borre, Y.E.; O’Keeffe, G.W.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Microbiota and neurodevelopmental windows: Implications for brain disorders. Trends Mol. Med. 2014, 20, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Varian, B.J.; Poutahidis, T.; DiBenedictis, B.T.; Levkovich, T.; Ibrahim, Y.; Didyk, E.; Shikhman, L.; Cheung, H.K.; Hardas, A.; Ricciardi, C.E.; et al. Microbial lysate upregulates host oxytocin. Brain Behav. Immun. 2017, 61, 36–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, G.B.; Yim, Y.S.; Wong, H.; Kim, S.; Kim, H.; Kim, S.V.; Hoeffer, C.A.; Littman, D.R.; Huh, J.R. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 2016, 351, 933–939. [Google Scholar] [CrossRef] [Green Version]
- Al Olaby, R.R.; Zafarullah, M.; Barboza, M.; Solakyildirim, K.; Peng, G.; Alvarez, M.R.; Erdman, S.E.; Lebrilla, C.; Tassone, F. Differenital methylation profile in Fragile X syndrome-prone offspring mice after in utero exposure to Lactobacillus reuteri. Genes 2022, 13, 1300. [Google Scholar] [CrossRef]
- Soden, M.E.; Chen, L. Fragile X Protein FMRP Is Required for Homeostatic Plasticity and Regulation of Synaptic Strength by Retinoic Acid. J. Neurosci. 2010, 30, 16910–16921. [Google Scholar] [CrossRef] [Green Version]
- Lessard, M.; Chouiali, A.; Drouin, R.; Sebire, G.; Corbin, F. Quantitative measurement of FMRP in blood platelets as a new screening test for fragile X syndrome. Clin. Genet. 2012, 82, 472–477. [Google Scholar] [CrossRef]
- Kim, K.; Hessl, D.; Randol, J.L.; Espinal, G.M.; Schneider, A.; Protic, D.; Aydin, E.Y.; Hagerman, R.J.; Hagerman, P.J. Association between IQ and FMR1 protein (FMRP) across the spectrum of CGG repeat expansions. PLoS ONE 2019, 14, e0226811. [Google Scholar] [CrossRef] [Green Version]
- Boggs, A.E.; Schmitt, L.M.; McLane, R.D.; Adayev, T.; LaFauci, G.; Horn, P.S.; Dominick, K.C.; Gross, C.; Erickson, C.A. Optimization, validation and initial clinical implications of a Luminex-based immunoassay for the quantification of Fragile X Protein from dried blood spots. Sci. Rep. 2022, 12, 5617. [Google Scholar] [CrossRef]
- LaFauci, G.; Adayev, T.; Kascsak, R.; Kascsak, R.; Nolin, S.; Mehta, P.; Brown, W.T.; Dobkin, C. Fragile X screening by quantification of FMRP in dried blood spots by a Luminex immunoassay. J. Mol. Diagn. 2013, 15, 508–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schachtle, M.A.; Rosshart, S.P. The Microbiota-Gut-Brain Axis in Health and Disease and Its Implications for Translational Research. Front. Cell Neurosci. 2021, 15, 698172. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Sivamaruthi, B.S.; Suganthy, N.; Kesika, P.; Chaiyasut, C. The Role of Microbiome, Dietary Supplements, and Probiotics in Autism Spectrum Disorder. Int. J. Environ. Res. Public Health 2020, 17, 2647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castle, J.C.; Zhang, C.; Shah, J.K.; Kulkarni, A.V.; Kalsotra, A.; Cooper, T.A.; Johnson, J.M. Expression of 24,426 human alternative splicing events and predicted cis regulation in 48 tissues and cell lines. Nat. Genet. 2008, 40, 1416–1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, V.W. The expanding genomic landscape of autism: Discovering the ‘forest’ beyond the ‘trees’. Future Neurol. 2013, 8, 29–42. [Google Scholar] [CrossRef] [Green Version]
- Simmons, D. Epigenetic influence and disease. Nat. Educ. 2008, 1, 6. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varian, B.J.; Weber, K.T.; Kim, L.J.; Chavarria, T.E.; Carrasco, S.E.; Muthupalani, S.; Poutahidis, T.; Zafarullah, M.; Al Olaby, R.R.; Barboza, M.; et al. Maternal Microbiota Modulate a Fragile X-like Syndrome in Offspring Mice. Genes 2022, 13, 1409. https://doi.org/10.3390/genes13081409
Varian BJ, Weber KT, Kim LJ, Chavarria TE, Carrasco SE, Muthupalani S, Poutahidis T, Zafarullah M, Al Olaby RR, Barboza M, et al. Maternal Microbiota Modulate a Fragile X-like Syndrome in Offspring Mice. Genes. 2022; 13(8):1409. https://doi.org/10.3390/genes13081409
Chicago/Turabian StyleVarian, Bernard J., Katherine T. Weber, Lily J. Kim, Tony E. Chavarria, Sebastian E. Carrasco, Sureshkumar Muthupalani, Theofilos Poutahidis, Marwa Zafarullah, Reem R. Al Olaby, Mariana Barboza, and et al. 2022. "Maternal Microbiota Modulate a Fragile X-like Syndrome in Offspring Mice" Genes 13, no. 8: 1409. https://doi.org/10.3390/genes13081409
APA StyleVarian, B. J., Weber, K. T., Kim, L. J., Chavarria, T. E., Carrasco, S. E., Muthupalani, S., Poutahidis, T., Zafarullah, M., Al Olaby, R. R., Barboza, M., Solakyildirim, K., Lebrilla, C., Tassone, F., Wu, F., Alm, E. J., & Erdman, S. E. (2022). Maternal Microbiota Modulate a Fragile X-like Syndrome in Offspring Mice. Genes, 13(8), 1409. https://doi.org/10.3390/genes13081409






