Investigation of Linear Amplification Using Abasic Site-Containing Primers Coupled to Routine STR Typing for LT-DNA Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample, DNA Polymerase and Primer Design
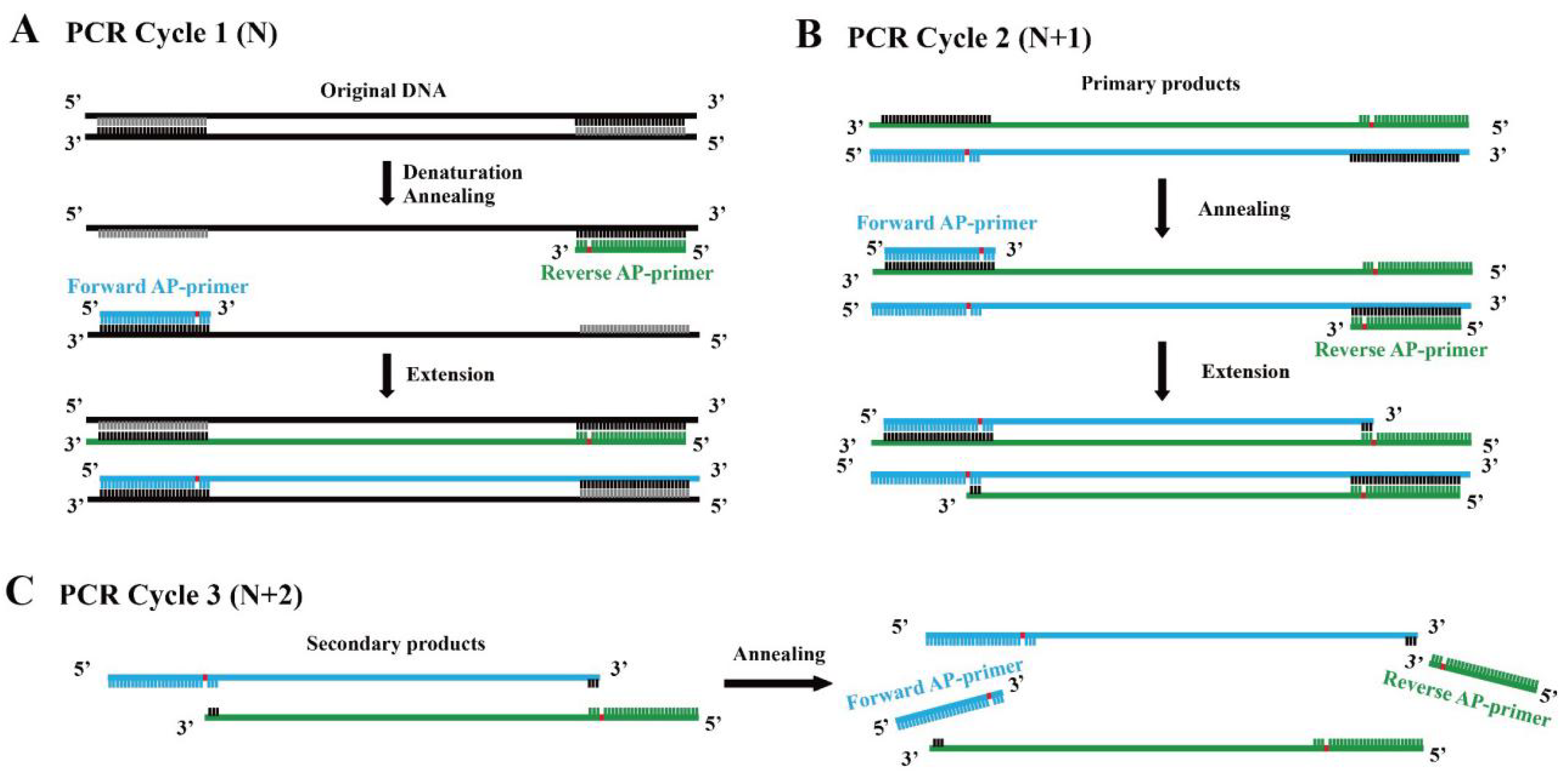
2.2. The Development of the abLAFD Method
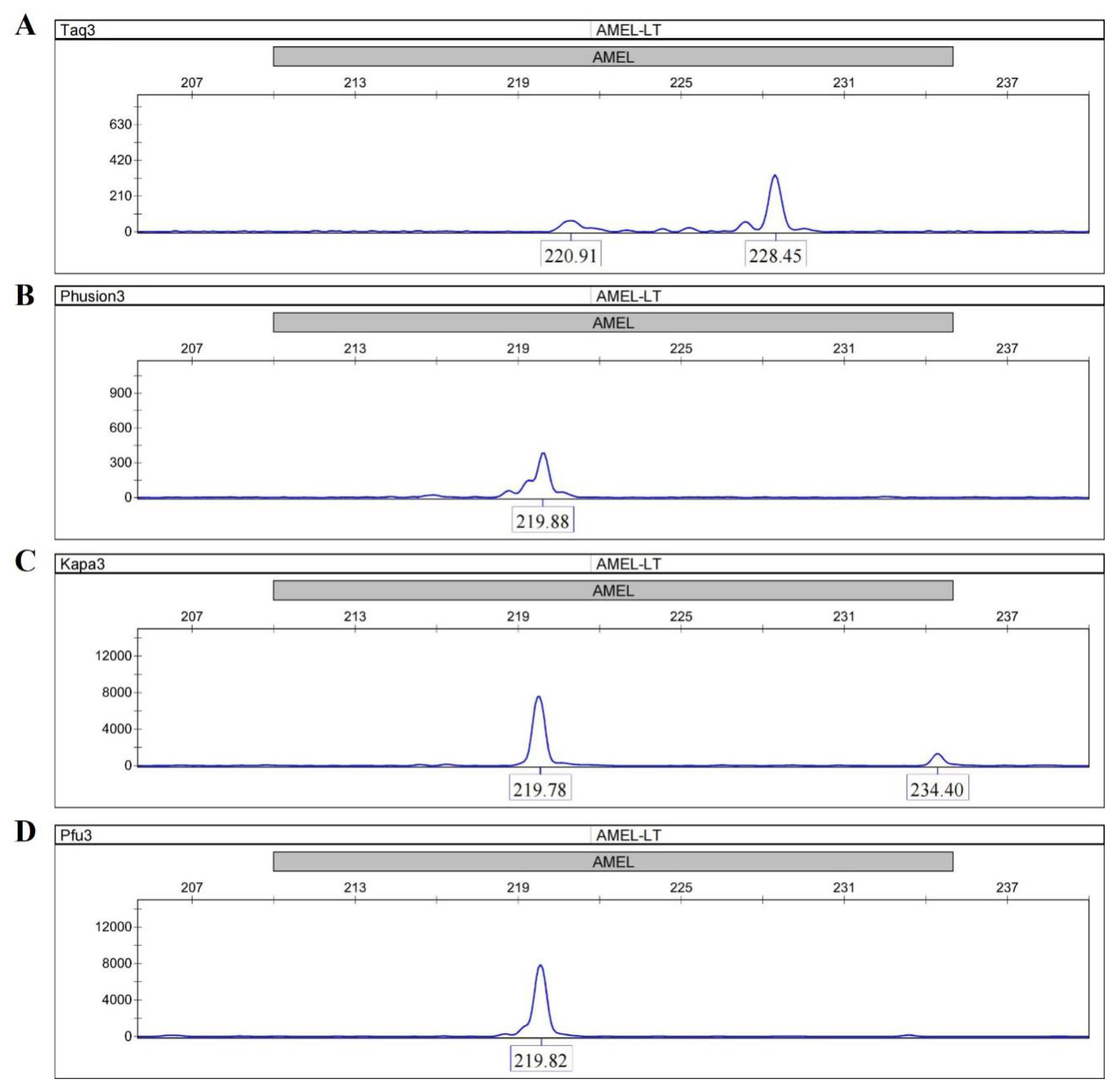
2.2.1. DNA Polymerase Screening—The Assay of DNA Polymerase Bypass Activity
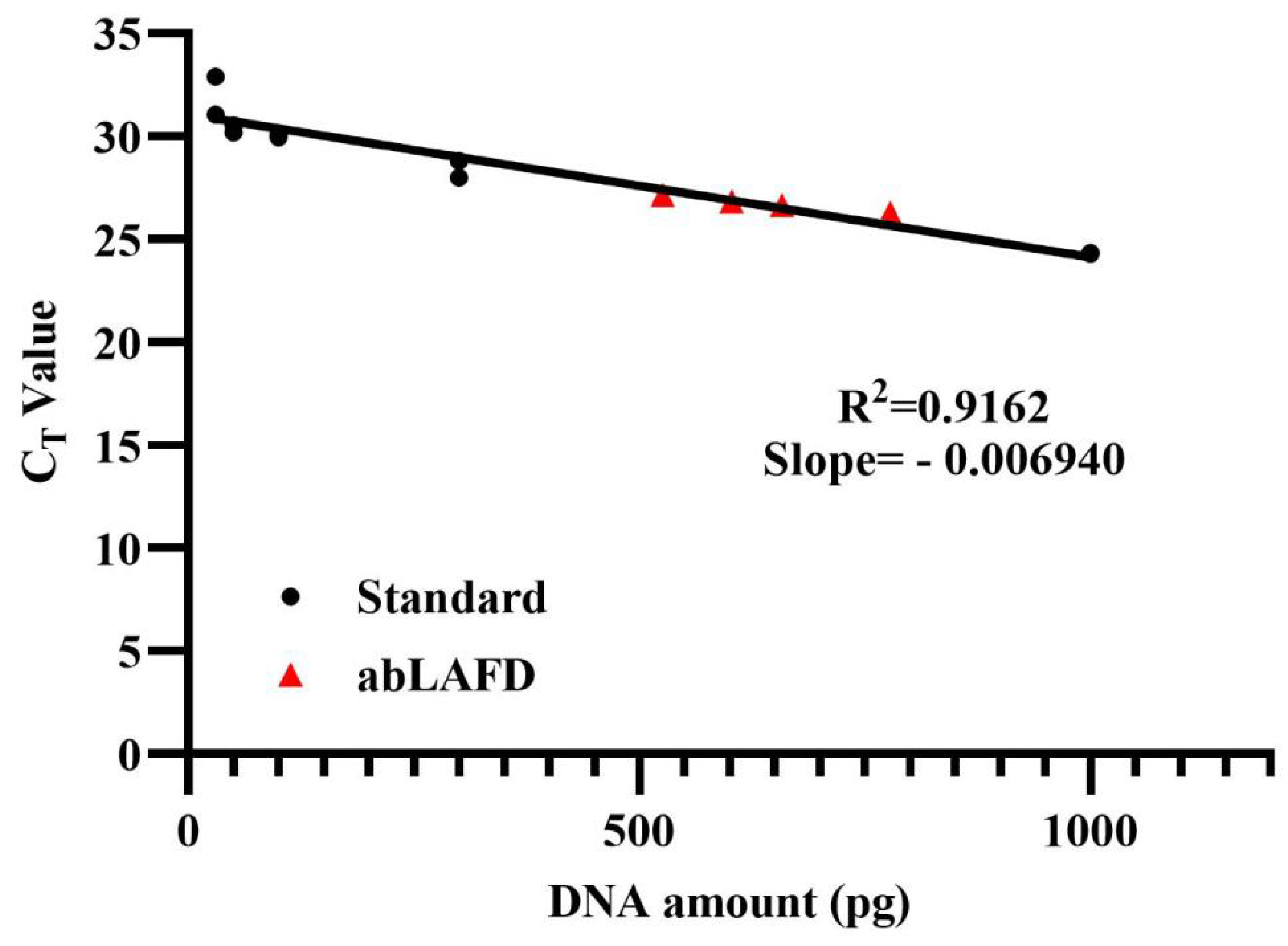
2.2.2. Assessment of the Amplification Efficiency of the abLAFD Method
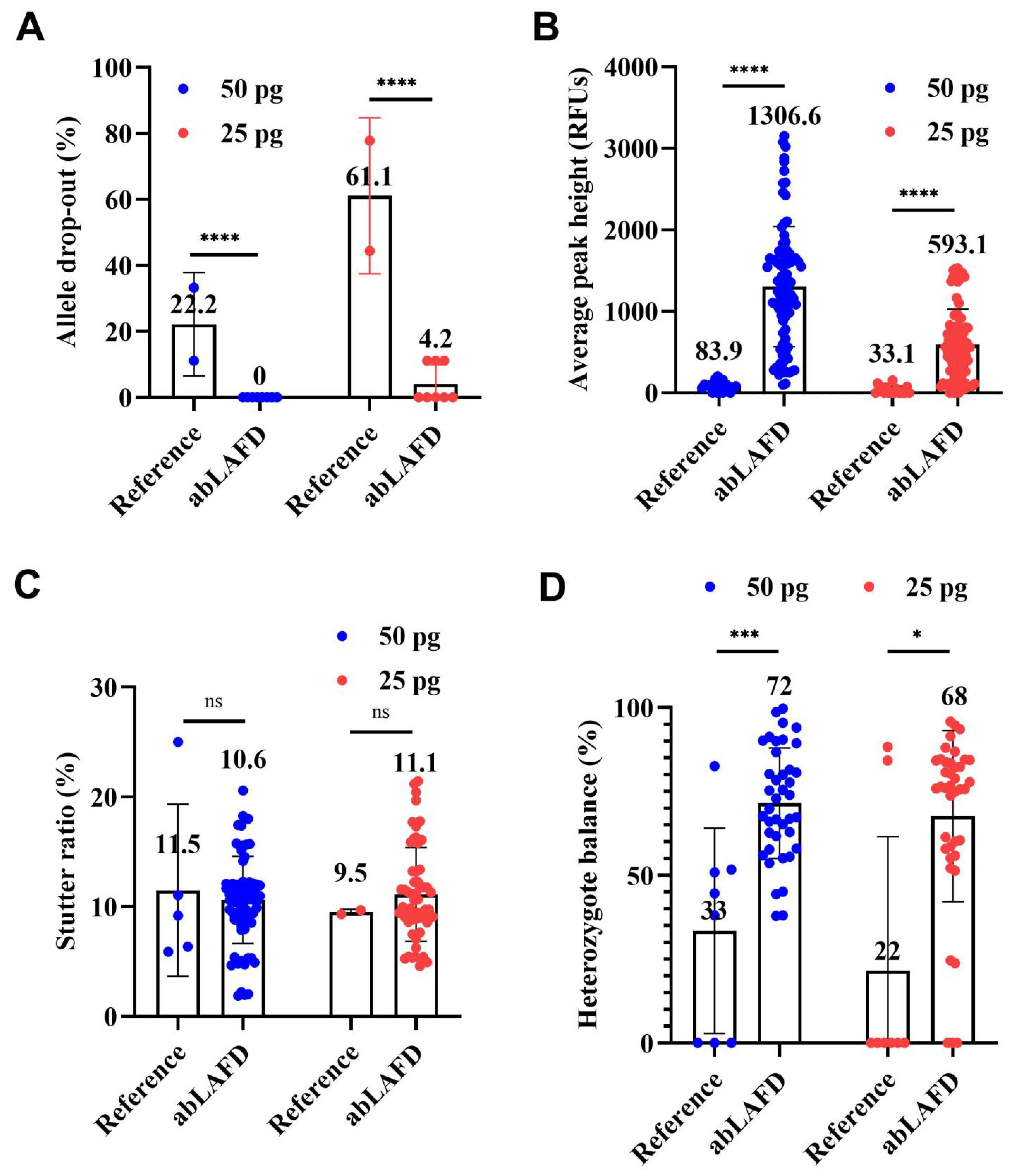
2.3. Application of abLAFD Method to STR Typing
2.4. Data Analysis
3. Results
3.1. The Assay of Bypass Activity for DNA Polymerases
3.2. Amplification Efficiency of the abLAFD Method
3.3. Evaluation of the Performance of abLAFD Method in STR Typing
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alessandrini, F.; Cecati, M.; Pesaresi, M.; Turchi, C.; Carle, F.; Tagliabracci, A. Fingerprints as evidence for a genetic profile: Morphological study on fingerprints and analysis of exogenous and individual factors affecting DNA typing. J. Forensic Sci. 2003, 48, 586–592. [Google Scholar] [CrossRef] [Green Version]
- Van Oorschot, R.A.; Ballantyne, K.N.; Mitchell, R.J. Forensic trace DNA: A review. Investig. Genet. 2010, 1, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinones, I.; Daniel, B. Cell free DNA as a component of forensic evidence recovered from touched surfaces. Forensic Sci. Int. Genet. 2012, 6, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.; Blackie, R.; Taylor, D.; Linacre, A. DNA profiles generated from a range of touched sample types. Forensic Sci. Int. Genet. 2018, 36, 13–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitaker, J.; Cotton, E.; Gill, P. A comparison of the characteristics of profiles produced with the AMPFlSTR® SGM Plus™ multiplex system for both standard and low copy number (LCN) STR DNA analysis. Forensic Sci. Int. 2001, 123, 215–223. [Google Scholar] [CrossRef]
- Harbison, S.; Fallow, M.; Bushell, D. An analysis of the success rate of 908 trace DNA samples submitted to the Crime Sample Database Unit in New Zealand. Aust. J. Forensic Sci. 2008, 40, 49–53. [Google Scholar] [CrossRef]
- Gill, P.; Whitaker, J.; Flaxman, C.; Brown, N.; Buckleton, J. An investigation of the rigor of interpretation rules for STRs derived from less than 100 pg of DNA. Forensic Sci. Int. 2000, 112, 17–40. [Google Scholar] [CrossRef]
- Smith, P.J.; Ballantyne, J. Simplified Low-Copy-Number DNA Analysis by Post-PCR Purification. J. Forensic Sci. 2007, 52, 820–829. [Google Scholar] [CrossRef]
- Forster, L.; Thomson, J.; Kutranov, S. Direct comparison of post-28-cycle PCR purification and modified capillary electrophoresis methods with the 34-cycle “low copy number” (LCN) method for analysis of trace forensic DNA samples. Forensic Sci. Int. Genet. 2008, 2, 318–328. [Google Scholar] [CrossRef]
- Westen, A.; Nagel, J.H.A.; Benschop, C.C.G.; Weiler, N.E.C.; De Jong, B.J.; Sijen, T. Higher Capillary Electrophoresis Injection Settings as an Efficient Approach to Increase the Sensitivity of STR Typing. J. Forensic Sci. 2009, 54, 591–598. [Google Scholar] [CrossRef]
- Weiler, N.E.; Matai, A.S.; Sijen, T. Extended PCR conditions to reduce drop-out frequencies in low template STR typing including unequal mixtures. Forensic Sci. Int. Genet. 2012, 6, 102–107. [Google Scholar] [CrossRef]
- McNevin, D.; Edson, J.; Robertson, J.; Austin, J.J. Reduced reaction volumes and increased Taq DNA polymerase concentration improve STR profiling outcomes from a real-world low template DNA source: Telogen hairs. Forensic Sci. Med. Pathol. 2015, 11, 326–338. [Google Scholar] [CrossRef]
- Nagy, M.; Rascon, J.; Massenkeil, G.; Ebell, W.; Roewer, L. Evaluation of whole-genome amplification of low-copy-number DNA in chimerism analysis after allogeneic stem cell transplantation using STR marker typing. Electrophoresis 2006, 27, 3028–3037. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Wang, Z.; Kong, Q.; Wang, X.; Huang, A.; Li, C.; Liu, X. Evaluating the effects of whole genome amplification strategies for amplifying trace DNA using capillary electrophoresis and massive parallel sequencing. Forensic Sci. Int. Genet. 2022, 56, 102599. [Google Scholar] [CrossRef] [PubMed]
- Van der Plaetsen, A.S.; Deleye, L.; Cornelis, S.; Tilleman, L.; Van Nieuwerburgh, F.; Deforce, D. STR profiling and Copy Number Variation analysis on single, preserved cells using current Whole Genome Amplification methods. Sci. Rep. 2017, 7, 17189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grisedale, K.; van Daal, A. Linear amplification of target prior to PCR for improved low template DNA results. BioTechniques 2014, 56, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, C.; Sanadai, S.; Hata, Y.; Okuto, H.; Noskov, V.N.; Loakes, D.; Negishi, K. Difference between deoxyribose- and tetrahydrofuran-type abasic sites in the in vivo mutagenic responses in yeast. Nucleic Acids Res. 2002, 30, 5129–5135. [Google Scholar] [CrossRef]
- Gal, J.; Schnell, R.; Szekeres, S.; Kalman, M. Directional cloning of native PCR products with preformed sticky ends (Autosticky PCR). Mol. Gen. Genet. 1999, 260, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, J.; Frohme, M.; Glokler, J. Hinge-initiated Primer-dependent Amplification of Nucleic Acids (HIP)—A New Versatile Isothermal Amplification Method. Sci. Rep. 2017, 7, 7683. [Google Scholar] [CrossRef] [Green Version]
- Belousova, E.A.; Rechkunova, N.I.; Lavrik, O.I. Thermostable DNA polymerases can perform translesion synthesis using 8-oxoguanine and tetrahydrofuran-containing DNA templates. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2006, 1764, 97–104. [Google Scholar] [CrossRef]
- Takagi, M.; Nishioka, M.; Kakihara, H.; Kitabayashi, M.; Inoue, H.; Kawakami, B.; Oka, M.; Imanaka, T. Characterization of DNA polymerase from Pyrococcus sp. strain KOD1 and its application to PCR. Appl. Environ. Microbiol. 1997, 63, 4504–4510. [Google Scholar] [CrossRef] [Green Version]
- Johansson, E.; Dixon, N. Replicative DNA Polymerases. Cold Spring Harb. Perspect. Biol. 2013, 5, a012799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, J.M. Forensic DNA Typing Protocols; Carracedo, A., Ed.; Methods in Molecular Biology Series; Spinger: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Loeb, L.A.; Preston, B.D. Mutagenesis by Apurinic Apyrimidinic Sites. Annu. Rev. Genet. 1986, 20, 201–230. [Google Scholar] [CrossRef] [PubMed]
- Hogg, M.; Wallace, S.S.; Doublié, S. Crystallographic snapshots of a replicative DNA polymerase encountering an abasic site. EMBO J. 2004, 23, 1483–1493. [Google Scholar] [CrossRef]
- Berjón-Otero, M.; Villar, L.; de Vega, M.; Salas, M.; Redrejo-Rodríguez, M. DNA polymerase from temperate phage Bam35 is endowed with processive polymerization and abasic sites translesion synthesis capacity. Proc. Natl. Acad. Sci. USA 2015, 112, E3476–E3484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Gac, N.T.; Delagoutte, E.; Germain, M.; Villani, G. Inactivation of the 3′-5′ Exonuclease of the Replicative T4 DNA Polymerase Allows Translesion DNA Synthesis at an Abasic Site. J. Mol. Biol. 2004, 336, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Song, L.; Stroud, J.; Parris, D.S. Mechanisms by which herpes simplex virus DNA polymerase limits translesion synthesis through abasic sites. DNA Repair 2008, 7, 95–107. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Zhou, Y.; Yao, Y.; Qian, J.; Liu, B.; Yang, Q.; Shao, C.; Li, H.; Sun, K.; Tang, Q.; et al. A 16-plex Y-SNP typing system based on allele-specific PCR for the genotyping of Chinese Y-chromosomal haplogroups. Leg. Med. 2020, 46, 101720. [Google Scholar] [CrossRef]
- Zhang, R.; Tan, Y.; Jian, H.; Qu, S.; Liu, Y.; Zhu, J.; Wang, L.; Lv, M.; Liao, M.; Zhang, L.; et al. A new approach to detect a set of SNP-SNP markers: Combining ARMS-PCR with SNaPshot technology. Electrophoresis 2020, 41, 1189–1197. [Google Scholar] [CrossRef]
| Primer | Primer Sequence (5′–3′) |
|---|---|
| C-primer | F: FAM-CACCTCATCCTGGGCACCC R: GGCTTGAGGCCAACCATCAG |
| TC-primer | F: FAM-GTGTCTTCACCTCATCCTGGGCACCC R: GTGTCTTGGCTTGAGGCCAACCATCAG |
| TaC-primer | F: FAM-GTGTCTT/idSp/CACCTCATCCTGGGCACCC R: GTGTCTT/idSp/GGCTTGAGGCCAACCATCAG |
| ab-primer | F: CTACCACCTCATCCTGGGC/idSp/CCCTG R: GAGGACAGACTGAGTCAGAG/idSp/GGCCAG |
| Q-primer | F: CCTGGGCTCTGTAAAGAATAGTG R: CAGAGCTTAAACTGGGAAGCTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, X.; Li, Z.; Zhou, Z.; Qian, J.; Yao, Y.; Shao, C.; Tang, Q.; Xie, J. Investigation of Linear Amplification Using Abasic Site-Containing Primers Coupled to Routine STR Typing for LT-DNA Analysis. Genes 2022, 13, 1386. https://doi.org/10.3390/genes13081386
Qian X, Li Z, Zhou Z, Qian J, Yao Y, Shao C, Tang Q, Xie J. Investigation of Linear Amplification Using Abasic Site-Containing Primers Coupled to Routine STR Typing for LT-DNA Analysis. Genes. 2022; 13(8):1386. https://doi.org/10.3390/genes13081386
Chicago/Turabian StyleQian, Xiaoqin, Zhimin Li, Zhihan Zhou, Jinglei Qian, Yining Yao, Chengchen Shao, Qiqun Tang, and Jianhui Xie. 2022. "Investigation of Linear Amplification Using Abasic Site-Containing Primers Coupled to Routine STR Typing for LT-DNA Analysis" Genes 13, no. 8: 1386. https://doi.org/10.3390/genes13081386
APA StyleQian, X., Li, Z., Zhou, Z., Qian, J., Yao, Y., Shao, C., Tang, Q., & Xie, J. (2022). Investigation of Linear Amplification Using Abasic Site-Containing Primers Coupled to Routine STR Typing for LT-DNA Analysis. Genes, 13(8), 1386. https://doi.org/10.3390/genes13081386





