Transcriptome and Metabonomic Analysis of Tamarix ramosissima Potassium (K+) Channels and Transporters in Response to NaCl Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Plant Material Treatment
2.3. Transcriptome Sequencing and Differentially Expressed Genes (DEGs) Screening
2.4. Metabolic Extraction, Detection and Differential Metabolite Screening
2.5. Quantitative Real-Time PCR (qRT-PCR) Validation
3. Results
3.1. Transcriptional Sequencing Quality Analysis of T. ramosissima Roots under NaCl Stress with Exogenous Potassium
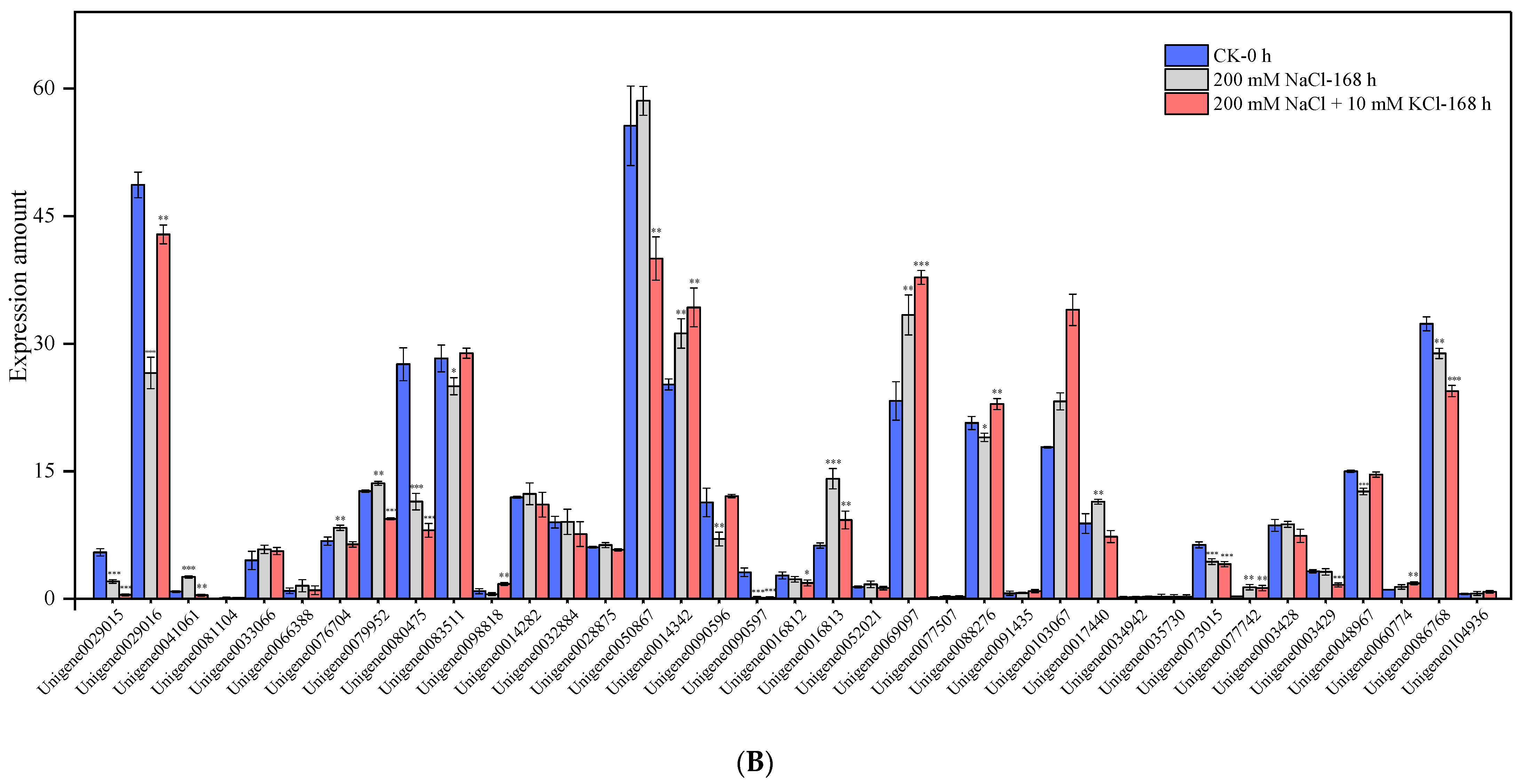
3.2. Mining and Expression Level Analysis of K+ Channel and Transporter-Related Genes
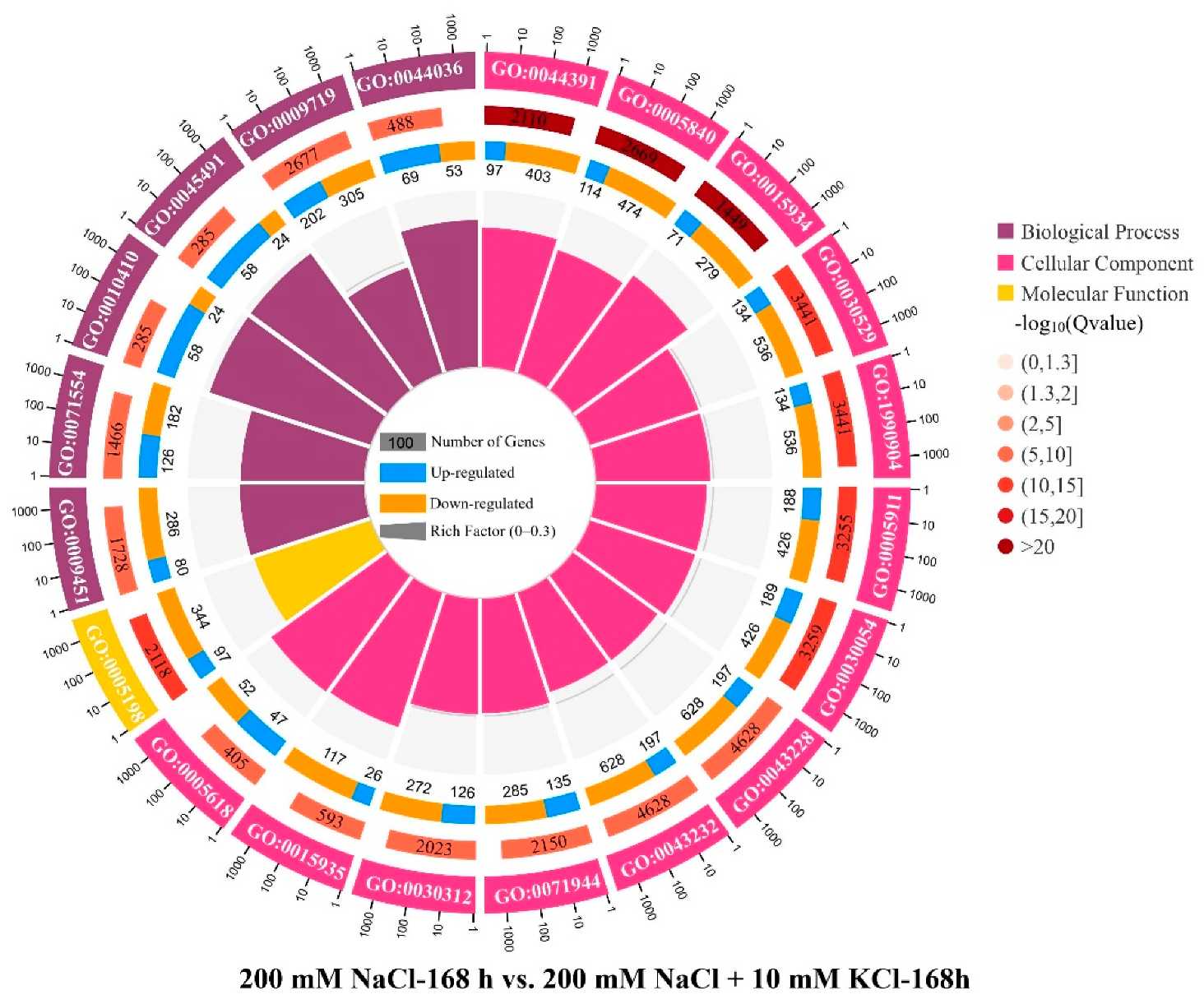
3.3. GO Enrichment and Expression Changes of DEGs in K+ Channels and Transporters
3.4. OPLS-DA Model Analysis
3.5. Correlation Analysis of DEGs and Metabolites in K+ Channels and Transporters
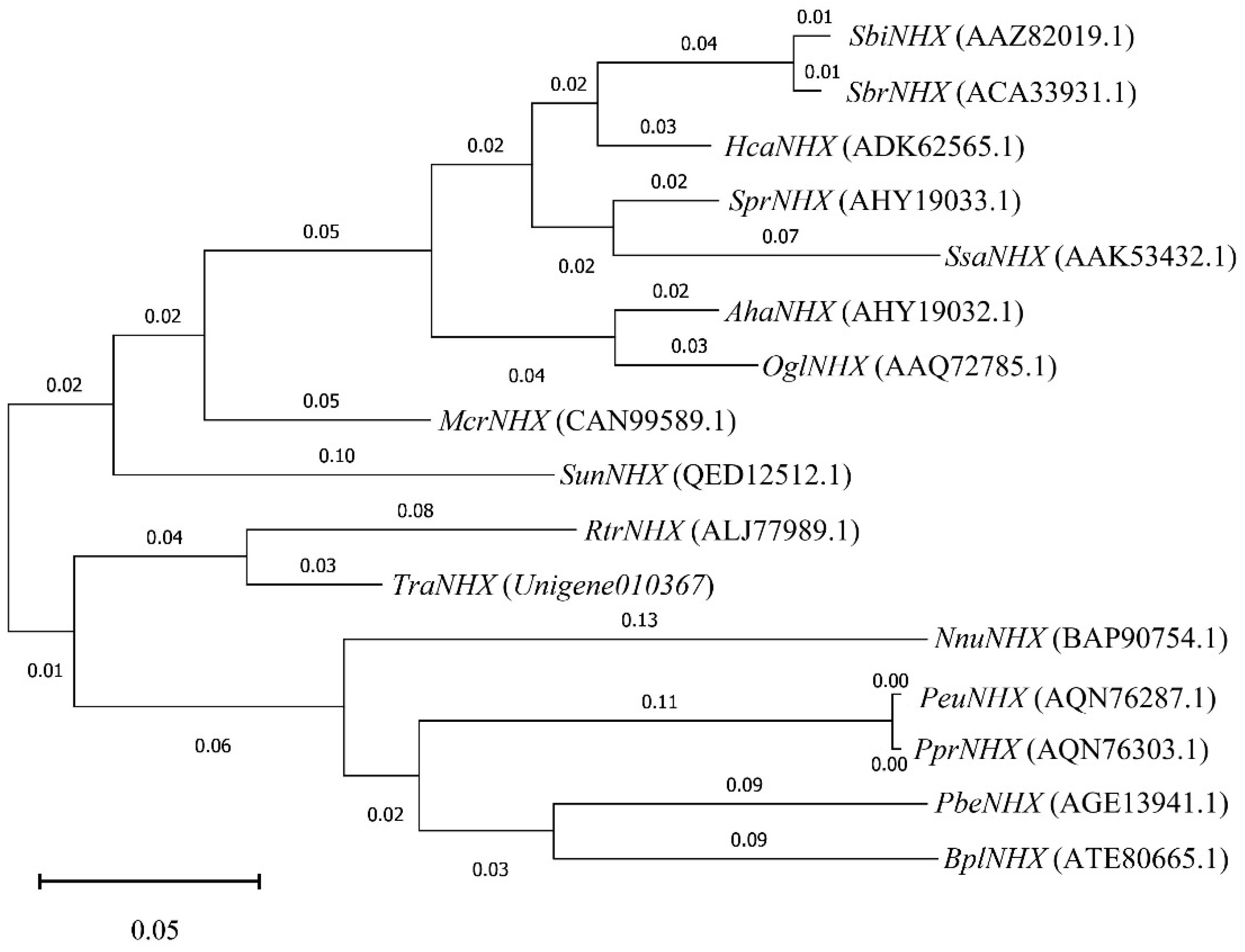
3.6. Phylogenetic Tree Analysis of Key DEGs
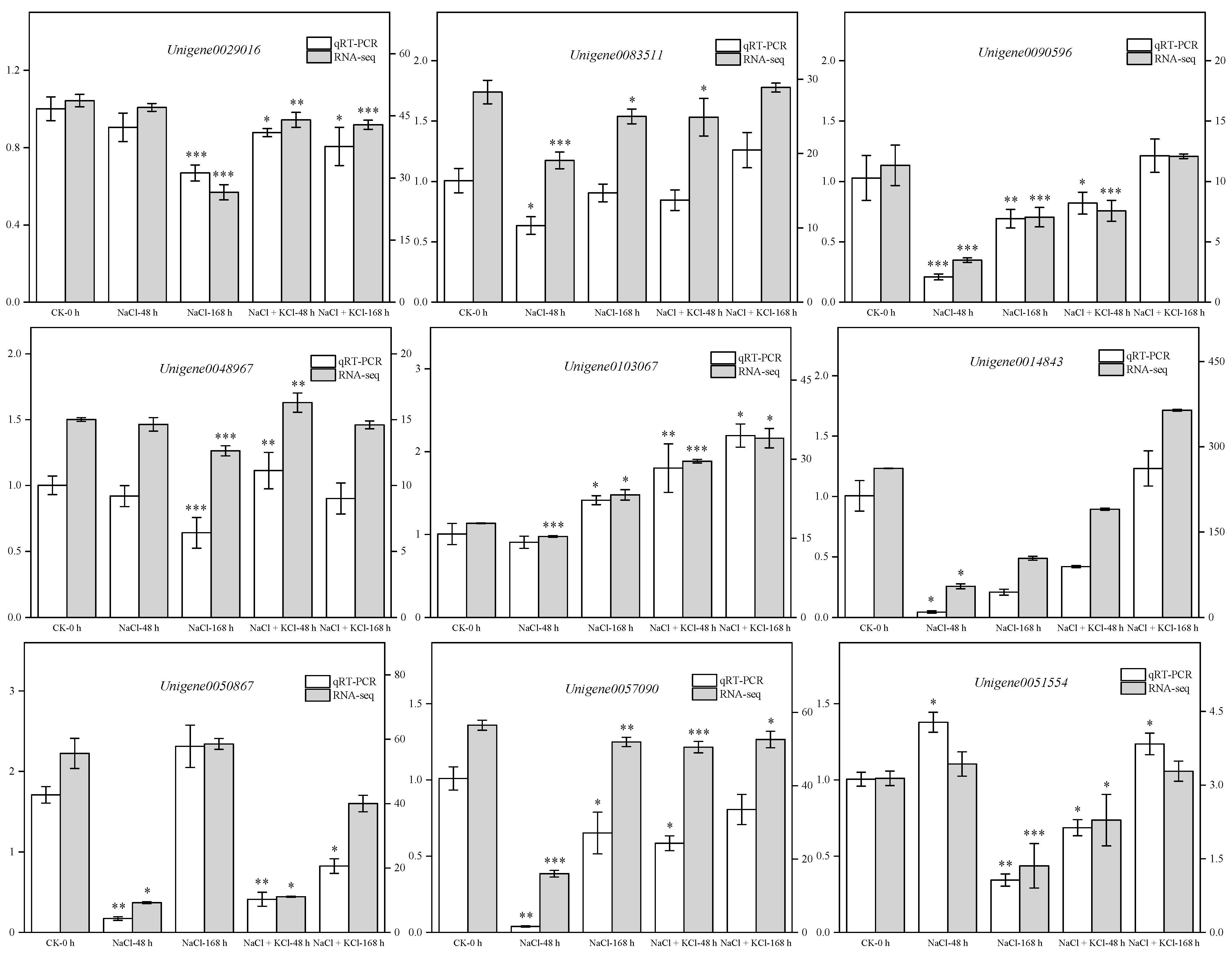
3.7. Quantitative Real-Time PCR (qRT-PCR) Validation of DEGs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leigh, R.A.; Jones, R.G.W. A hypothesis relating critical potassium concentrations for growth to the distribution and functions of this ion in the plant cell. New Phytol. 1984, 97, 1–13. [Google Scholar] [CrossRef]
- Zörb, C.; Senbayram, M.; Peiter, E. Potassium in agriculture-Status and perspectives. J. Plant Physiol. 2014, 171, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Kollist, H.; Nuhkat, M.; Roelfsema, M.R. Closing gaps: Linking elements that control stomatal movement. New Phytol. 2014, 203, 44–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, W.H. Potassium transport and signaling in higher plants. Annu. Rev. Plant Biol. 2013, 64, 451–476. [Google Scholar] [CrossRef] [Green Version]
- Amtmann, A.; Troufflard, S.; Armengaud, P. The effect of potassium nutrition on pest and disease resistance in plants. Physiol. Plant 2008, 133, 682–691. [Google Scholar] [CrossRef]
- Shabala, S.; Cuin, T.A. Potassium transport and plant salt tolerance. Physiol. Plant. 2008, 133, 651–669. [Google Scholar] [CrossRef]
- Wang, X.; Mohamed, I.; Ali, M.; Abbas, M.H.H.; Shah, G.M.; Chen, F. Potassium distribution in root and non-root zones of two cotton genotypes and its accumulation in their organs as affected by drought and potassium stress conditions. J. Plant Nutr. Soil Sci. 2019, 182, 72–81. [Google Scholar] [CrossRef] [Green Version]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wu, W.H. Regulation of potassium transport and signaling in plants. Curr. Opin. Plant Biol. 2017, 39, 123–128. [Google Scholar] [CrossRef]
- Epstein, E.; Rains, D.W.; Elzam, O.E. Resolution of dual mechanisms of potassium absorption by barley roots. Proc. Natl. Acad. Sci. USA 1963, 49, 684–692. [Google Scholar] [CrossRef] [Green Version]
- Glass, A.D.; Dunlop, J. The influence of potassium content on the kinetics of potassium influx into excised ryegrass and barley roots. Planta 1978, 141, 117–119. [Google Scholar] [CrossRef]
- Schroeder, J.I.; Ward, J.M.; Gassmann, W. Perspectives on the physiology and structure of inward-rectifying K+ channels in higher plants: Biophysical implications for K+ uptake. Annu. Rev. Biophys. Biomol. Struct. 1994, 23, 441–471. [Google Scholar] [CrossRef]
- Maathuis, F.J.; Sanders, D. Regulation of K+ absorption in plant root cells by external K+: Interplay of different plasma membrane K+ transporters. J. Exp. Bot. 1997, 48, 451–458. [Google Scholar] [CrossRef]
- Cherel, I.; Lefoulon, C.; Boeglin, M.; Sentenac, H. Molecular mechanisms involved in plant adaptation to low K(+) availability. J. Exp. Bot. 2014, 65, 833–848. [Google Scholar] [CrossRef]
- White, P.J. Improving potassium acquisition and utilisation by crop plants. J. Plant Nutr. Soil Sci. 2013, 176, 305–316. [Google Scholar] [CrossRef]
- Siddiqi, M.Y.; Glass, A.D.M. A model for the regulation of K+ influx, and tissue potassium concentrations by negative feedback effects upon plasmalemma influx. Plant Physiol. 1986, 81, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Lebaudy, A.; Very, A.A.; Sentenac, H. K+ channel activity in plants: Genes, regulations and functions. FEBS Lett. 2007, 581, 2357–2366. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Li, M.; Oh, S.; Kronzucker, H.J. Capacity and plasticity of potassium channels and high-affinity transporters in roots of barley and Arabidopsis. Plant Physiol. 2013, 162, 496–511. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.; Hu, J.; Zhou, X.R.; Yuan, H.J.; Kumar, T.; Luan, S.; Wang, S.M. ZxAKT1 is essential for K+-uptake and K+/Na+ homeostasis in the succulent xerophyte Zygophyllum xanthoxylum. Plant J. 2017, 90, 48–60. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Justice Afriyie, A.; Li, R.; Dominic, K.; Li, L.; Li, R.; Zhao, W. Molecular cloning of potassium transporter gene, MaHAK5 of mulberry (Morus alba L.) and gene expression and biochemistry analysis under potassium stress. J. Hortic. Sci. Biotechnol. 2019, 94, 130–136. [Google Scholar] [CrossRef]
- Rus, A.; Yokoi, S.; Sharkhuu, A.; Reddy, M.; Lee, B.H.; Matsumoto, T.K.; Koiwa, H.; Zhu, J.K.; Bressan, R.A.; Hasegawa, P.M. AtHKT1 is a salt tolerance determinant that controls Na(+) entry into plant roots. Proc. Natl. Acad. Sci. USA 2001, 98, 14150–14155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, P.; Katschnig, D.; de Boer, A.H. HKT transporters—State of the art. Int. J. Mol. Sci. 2013, 14, 20359–20385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horie, T.; Hauser, F.; Schroeder, J.I. HKT transporter-mediated salinity resistance mechanisms in Arabidopsis and monocot crop plants. Trends Plant Sci. 2009, 14, 660–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Hui, H.; Yu, D. Overexpression of a novel soybean gene modulating Na and K transport enhances salt tolerance in transgenic tobacco plants. Physiol. Plant. 2011, 141, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Ping, L.Z.F.Y. Research Progress of HKT Transporter Genes in Plants. Agric. Biotechnol. 2016, 5, 12–15. [Google Scholar] [CrossRef]
- Fu, L.; Shen, Q.; Kuang, L.; Yu, J.; Wu, D.; Zhang, G. Metabolite profiling and gene expression of Na/K transporter analyses reveal mechanisms of the difference in salt tolerance between barley and rice. Plant Physiol. Biochem. 2018, 130, 248–257. [Google Scholar] [CrossRef]
- Khan, I.U.; Ali, A.; Yun, D. Arabidopsis NHX transporters: Sodium and potassium antiport mythology and sequestration during ionic stress. J. Plant Biol. 2018, 61, 292–300. [Google Scholar] [CrossRef]
- Yang, L.; Liu, H.; Fu, S.M.; Ge, H.M.; Tang, R.J.; Yang, Y.; Wang, H.H.; Zhang, H.X. Na+/H+ and K+/H+ antiporters AtNHX1 and AtNHX3 from Arabidopsis improve salt and drought tolerance in transgenic poplar. Biol. Plant. 2017, 61, 641–650. [Google Scholar] [CrossRef]
- Song, Z.Z.; Su, Y.H. Distinctive potassium-accumulation capability of alligatorweed (Alternanthera philoxeroides) links to high-affinity potassium transport facilitated by K+-uptake systems. Weed Sci. 2012, 61, 77–84. [Google Scholar] [CrossRef]
- Song, Z.Z.; Yang, S.; Zhu, H.; Jin, M.; Su, Y.H. Heterologous expression of an alligatorweed high-affinity potassium transporter gene enhances salinity tolerance in Arabidopsis thaliana. Am. J. Bot. 2014, 101, 840–850. [Google Scholar] [CrossRef]
- Wang, Z.; He, Z.; Xu, X.; Shi, X.; Ji, X.; Wang, Y. Revealing the salt tolerance mechanism of Tamarix hispida by large-scale identification of genes conferring salt tolerance. Tree Physiol. 2021, 41, 2153–2170. [Google Scholar] [CrossRef]
- Garthwaite, A.J.; von Bothmer, R.; Colmer, T.D. Salt tolerance in wild Hordeum species is associated with restricted entry of Na+ and Cl- into the shoots. J. Exp. Bot. 2005, 56, 2365–2378. [Google Scholar] [CrossRef]
- Lu, Y.; Lei, J.Q.; Zeng, F.J.; Xu, L.S.; Peng, S.L.; Gao, H.H.; Liu, G.J. Effects of NaCl treatment on growth and Ec-ophysiology Characteristics of Tamarix ramossisma. J. Des. Res. 2014, 34, 1509–1515. (In Chinese) [Google Scholar] [CrossRef]
- Chen, Y.; Wang, G.; Zhang, H.; Zhang, N.; Jiang, J.; Song, Z. Transcriptome analysis of Tamarix ramosissima leaves in response to NaCl stress. PLoS ONE 2022, 17, e265653. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Conesa, A.; Gotz, S.; Garcia-Gomez, J.M.; Terol, J.; Talon, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [Green Version]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Saccenti, E.; Hoefsloot, H.C.J.; Smilde, A.K.; Westerhuis, J.A.; Heniks, M.M.W.B. Reflections on univariate and multivariate analysis of metabolomics data. Metabolomics 2014, 10, 361–374. [Google Scholar] [CrossRef]
- Zhang, J.; Flowers, T.J.; Wang, S. Mechanisms of sodium uptake by roots of higher plants. Plant Soil 2010, 326, 45–60. [Google Scholar] [CrossRef]
- Shabala, S.; Cuin, T.A.; Pottosin, I. Polyamines prevent NaCl-induced K+ efflux from pea mesophyll by blocking non-selective cation channels. FEBS Lett. 2007, 581, 1993–1999. [Google Scholar] [CrossRef] [Green Version]
- Çolpan, E.; Zengin, M.; Özbahçe, A. The effects of potassium on the yield and fruit quality components of stick tomato. Hortic. Environ. Biotechnol. 2013, 54, 20–28. [Google Scholar] [CrossRef]
- Ardie, S.W.; Xie, L.; Takahashi, R.; Liu, S.; Takano, T. Cloning of a high-affinity K+ transporter gene PutHKT2;1 from Puccinellia tenuiflora and its functional comparison with OsHKT2;1 from rice in yeast and Arabidopsis. J. Exp. Bot. 2009, 60, 3491–3502. [Google Scholar] [CrossRef] [Green Version]
- Carden, D.E.; Walker, D.J.; Flowers, T.J.; Miller, A.J. Single-cell measurements of the contributions of cytosolic Na⁺ and K⁺ to salt tolerance. Plant Physiol. 2003, 131, 676–683. [Google Scholar] [CrossRef] [Green Version]
- Kaya, C.; Kirnak, H.; Higgs, D. Enhancement of growth and normal growth parameters by foliar application of potassium and phosphorus in tomato cultivars grown at high (NaCl) salinity. J. Plant Nutr. 2001, 24, 357–367. [Google Scholar] [CrossRef]
- Chartzoulakis, K.; Psarras, G.; Vemmos, S.; Loupassaki, M.; Bertaki, M. Response of two olive cultivars to salt stress and potassium supplement. J. Plant Nutr. 2006, 29, 2063–2078. [Google Scholar] [CrossRef]
- Kumar, S.; Beena, A.S.; Awana, M.; Singh, A. Physiological, biochemical, epigenetic and molecular analyses of wheat (Triticum aestivum) genotypes with contrasting salt tolerance. Front. Plant Sci. 2017, 8, 1151. [Google Scholar] [CrossRef] [Green Version]
- Marriboina, S.; Sengupta, D.; Kumar, S.; Reddy, A.R. Physiological and molecular insights into the high salinity tolerance of Pongamia pinnata (L.) pierre, a potential biofuel tree species. Plant Sci. 2017, 258, 102–111. [Google Scholar] [CrossRef]
- Sandhu, D.; Cornacchione, M.V.; Ferreira, J.F.; Suarez, D.L. Variable salinity responses of 12 alfalfa genotypes and comparative expression analyses of salt-response genes. Sci. Rep. 2017, 7, 42958. [Google Scholar] [CrossRef]
- Ali, Z.; Park, H.C.; Bressan, R.A.; Bohnert, H.J.; Lee, S.Y.; Yun, D.; Ali, A.; Oh, D.; Aman, R.; Kropornicka, A.; et al. TsHKT1;2, a HKT1 homolog from the extremophile Arabidopsis relative Thellungiella salsuginea, shows K+ specificity in the presence of NaCl. Plant Physiol. 2012, 158, 1463–1474. [Google Scholar] [CrossRef] [Green Version]
- Munns, R.; James, R.A.; Xu, B.; Athman, A.; Conn, S.J.; Jordans, C.; Byrt, C.S.; Hare, R.A.; Tyerman, S.D.; Tester, M.; et al. Wheat grain yield on saline soils is improved by an ancestral Na(+) transporter gene. Nat. Biotechnol. 2012, 30, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Liu, Y.; Kang, D.; Fan, K.; Wang, C.; Wang, G.; Liu, Y. Two alternative splicing variants of maize HKT1;1 confer salt tolerance in transgenic tobacco plants. Plant Cell Tissue Organ Cult. 2015, 123, 569–578. [Google Scholar] [CrossRef]
- Rubio, F.; Gassmann, W.; Schroeder, J.I. Sodium-driven potassium uptake by the plant potassium transporter HKT1 and mutations conferring salt tolerance. Science 1995, 270, 1660–1663. [Google Scholar] [CrossRef] [PubMed]
- Gierth, M.; Maser, P.; Schroeder, J.I. The potassium transporter AtHAK5 functions in K⁺ deprivation-induced high-affinity K⁺ uptake and AKT1 K⁺ channel contribution to K⁺ uptake kinetics in Arabidopsis roots. Plant Physiol. 2005, 137, 1105–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santa-Maria, G.E.; Rubio, F.; Dubcovsky, J.; Rodriguez-Navarro, A. The HAK1 gene of barley is a member of a large gene family and encodes a high-affinity potassium transporter. Plant Cell 1997, 9, 2281–2289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, H.; Tang, Q.; Cai, J.; Xu, B.; Xu, G.; Yu, L. Rice OsHAK16 functions in potassium uptake and translocation in shoot, maintaining potassium homeostasis and salt tolerance. Planta 2019, 250, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Apse, M.P.; Sottosanto, J.B.; Blumwald, E. Vacuolar cation/H+ exchange, ion homeostasis, and leaf development are altered in a T-DNA insertional mutant of AtNHX1, the Arabidopsis vacuolar Na+/H+ antiporter. Plant J. 2003, 36, 229–239. [Google Scholar] [CrossRef]
- Bassil, E.; Coku, A.; Blumwald, E. Cellular ion homeostasis: Emerging roles of intracellular NHX Na+/H+ antiporters in plant growth and development. J. Exp. Bot. 2012, 63, 5727–5740. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Leidi, E.O.; Pardo, J.M. How do vacuolar NHX exchangers function in plant salt tolerance? Plant Signal. Behav. 2010, 5, 792–795. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Q.S. Plant and yeast NHX antiporters: Roles in membrane trafficking. J. Integr. Plant Biol. 2012, 54, 66–72. [Google Scholar] [CrossRef]


| ID | Primer Name | Primer Sequence (5′-3′) |
|---|---|---|
| 1 | Unigene0029016 | F:CTCGCTGTTTGGTGTGATGT |
| R:CCGCCGTCTTCAACCACAAC | ||
| 2 | Unigene0083511 | F:GGAATCTGGCAAAATGGGTG |
| R:GTCCTTCTCCGATACTTTCC | ||
| 3 | Unigene0090596 | F:CTGCGAAAGAAGATTGAAAC |
| R:AGAGTTTCCACGCTTTTCCT | ||
| 4 | Unigene0048967 | F:TAAGTCGTGCCTCCAATCTC |
| R:CTCAATCTGTGTGCCGCTTT | ||
| 5 | Unigene0103067 | F:GTTACTTTCAGGCAGCAGAT |
| R:GTTATCATTATCGCATTCCC | ||
| 6 | Unigene0014843 | F:GATGGGGATGTTCACTTCTG |
| R:CACCCTGATTCCCCGTCTTA | ||
| 7 | Unigene0057090 | F:GGTAGATTCCCTCCTTGGTG |
| R:GTAACCGCCAAAGCCACTAT | ||
| 8 | Unigene0050867 | F:TATTGAAGAGGTAGGCGGCG |
| R:CACTTCGCTTTCGCCCATTA | ||
| 9 | Unigene0051554 | F:ATCATCGGGGCTGTTTCTGC |
| R:CTCAGCCACAGCACCCTCAA | ||
| 10 | Tubulin | F:GCTGAGATTACAACCGCTG |
| R:CTGTTCGTTTGGTCTTGATT |
| Gene ID | Description |
|---|---|
| Shaker | |
| Unigene0029015 | Potassium channel AKT1-like |
| Unigene0029016 | Predicted: potassium channel AKT1 |
| Unigene0041061 | Potassium channel AKT2/3 isoform X2 |
| Unigene0081104 | Predicted: potassium channel AKT1 |
| TPK | |
| Unigene0033066 | Predicted: two-pore potassium channel 1-like |
| Unigene0066388 | Two pore potassium channel c |
| Unigene0076704 | Two-pore potassium channel 5-like isoform X2 |
| Unigene0079952 | Two-pore potassium channel 3-like |
| Unigene0080475 | Predicted: two-pore potassium channel 1 isoform X2 |
| Unigene0083511 | two-pore potassium channel 3 |
| Unigene0098818 | Predicted: two-pore potassium channel 1 isoform X3 |
| HAK/KUP/KT | |
| Unigene0014282 | Low affinity potassium transport system protein kup isoform 1 |
| Unigene0032884 | Potassium transport system protein kup |
| Unigene0028875 | Potassium transporter |
| Unigene0050867 | High affinity H+/K+ symporter |
| Unigene0014342 | Predicted: probable potassium transporter 13 |
| HKT | |
| Unigene0090596 | Sodium transporter HKT1 |
| Unigene0090597 | Sodium transporter HKT1 |
| CPAs | |
| Unigene0016812 | Predicted: sodium/hydrogen exchanger 1 |
| Unigene0016813 | Predicted: sodium/hydrogen exchanger 1-like |
| Unigene0052021 | Sodium/hydrogen exchanger 4 |
| Unigene0069097 | Vacuolar membrane Na+/H+ antiporter |
| Unigene0077507 | Na+/H+ exchanger 3, partial |
| Unigene0088276 | Sodium/hydrogen exchanger 6 like, partial |
| Unigene0091435 | Sodium/hydrogen exchanger 2-like |
| Unigene0103067 | Vacuolar membrane Na+/H+ antiporter |
| Unigene0017440 | Predicted: cation/H+ antiporter 20 |
| Unigene0034942 | Cation/H+ antiporter 15-like isoform X1 |
| Unigene0035730 | Predicted: cation/H+ antiporter 2-like |
| Unigene0073015 | Cation/H+ antiporter 15-like |
| Unigene0077742 | Cation/H+ antiporter like |
| Unigene0003428 | K+ efflux antiporter 4-like isoform X1 |
| Unigene0003429 | K+ efflux antiporter 4-like isoform X2 |
| Unigene0048967 | Predicted: K+ efflux antiporter 5 |
| Unigene0060774 | K+ efflux antiporter 3, chloroplastic isoform X3 |
| Unigene0086768 | Predicted: K+ efflux antiporter 2, chloroplastic |
| Unigene0104936 | K+ efflux antiporter 5-like |
| Gene ID | GO Cellular Component | GO Molecular Function | GO Biological Process |
|---|---|---|---|
| Shaker | |||
| Unigene0029015 | - | - | - |
| Unigene0029016 | - | - | - |
| Unigene0041061 | - | - | - |
| Unigene0081104 | GO:0031224; GO:0043231 | GO:0000166; GO:0005249; GO:0005515 | GO:0000041; GO:0000904; GO:0001101; GO:0006970; GO:0007015; GO:0009267; GO:0009933; GO:0010053; GO:0010119; GO:0015698; GO:0022610; GO:0045229; GO:0048588; GO:0071805 |
| TPK | |||
| Unigene0033066 | GO:0005774; GO:0031224 | GO:0005249; GO:0046872 | GO:0005976; GO:0006875; GO:0006970; GO:0006996; GO:0009845; GO:0034220; GO:0050794; GO:0051259; GO:0070838 |
| Unigene0066388 | GO:0005774 | GO:0005249 | GO:0006811 |
| Unigene0076704 | - | - | - |
| Unigene0079952 | - | - | - |
| Unigene0080475 | GO:0005774; GO:0031224 | GO:0005249; GO:0046872 | GO:0006875; GO:0016043; GO:0034220; GO:0050789; GO:0070838 |
| Unigene0083511 | - | - | - |
| Unigene0098818 | GO:0005774; GO:0031224 | GO:0005249; GO:0046872 | GO:0005976; GO:0006875; GO:0006970; GO:0006996; GO:0009845; GO:0034220; GO:0050794; GO:0051259; GO:0070838 |
| HAK/KUP/KT | |||
| Unigene0014282 | GO:0016020 | - | GO:0006807; GO:0044237; GO:0044238; GO:0044763; GO:0071704 |
| Unigene0032884 | GO:0043231 | - | - |
| Unigene0028875 | GO:0009536; GO:0031224 | GO:0046873 | GO:0030001; GO:0034220 |
| Unigene0050867 | GO:0031224 | GO:0022820 | GO:0030001; GO:0034220 |
| Unigene0014342 | GO:0016020 | GO:0000166; GO:0005249; GO:0005515 | GO:0000041; GO:0000904; GO:0001101; GO:0006970; GO:0007015; GO:0009267; GO:0009933; GO:0010053; GO:0010119; GO:0015698; GO:0022610; GO:0045229; GO:0048588; GO:0071805 |
| HKT | |||
| Unigene0090596 | - | - | - |
| Unigene0090597 | - | - | - |
| CPAs | |||
| Unigene0016812 | GO:0031224 | GO:0005451 | GO:0006814; GO:0006970; GO:0015992; GO:0044763; GO:0055065; GO:0055067 |
| Unigene0016813 | GO:0016020 | GO:0005451; GO:0046873 | GO:0006814; GO:0010119; GO:0055065; GO:0055067; GO:0071805 |
| Unigene0052021 | GO:0016020; GO:0043231; GO:0044444 | GO:0015299; GO:0046873 | GO:0015672; GO:0030001; GO:0065007; GO:0098662 |
| Unigene0069097 | GO:0005773; GO:0031090; GO:0031224 | GO:0005451; GO:0005515; GO:0046873 | GO:0006625; GO:0006814; GO:0006970; GO:0010119; GO:0015992; GO:0048193; GO:0048827; GO:0055065; GO:0055067 |
| Unigene0077507 | GO:0016020 | GO:0005451; GO:0046873 | GO:0006814; GO:0044763; GO:0055065; GO:0055067 |
| Unigene0088276 | GO:0031224 | GO:0005451 | GO:0006814; GO:0015992; GO:0044763; GO:0055067 |
| Unigene0091435 | GO:0031090; GO:0031224 | GO:0005451 | GO:0006814; GO:0006970; GO:0015992; GO:0044763; GO:0055065; GO:0055067 |
| Unigene0103067 | GO:0005773; GO:0031090; GO:0031224 | GO:0005451; GO:0005515; GO:0046873 | GO:0006625; GO:0006814; GO:0006970; GO:0010119; GO:0015992; GO:0048193; GO:0048827; GO:0055065; GO:0055067 |
| Unigene0017440 | GO:0009536; GO:0031224 | GO:0005451 | GO:0006605; GO:0006814; GO:0006875; GO:0015992; GO:0055067 |
| Unigene0034942 | - | - | GO:0006812; GO:0009987 |
| Unigene0035730 | GO:0031224 | GO:0005451 | GO:0000904; GO:0006814; GO:0009664; GO:0009814; GO:0015992; GO:0033554 |
| Unigene0073015 | - | GO:0015297 GO:0007275 | GO:0015672; GO:0044763 |
| Unigene0077742 | GO:0016020 | GO:0015299 | GO:0015672; GO:0016043; GO:0030001; GO:0044763; GO:0055067 |
| Unigene0003428 | GO:0031224 | GO:0005451; GO:0046873; GO:0046914 | GO:0015992; GO:0030001; GO:0044763 |
| Unigene0003429 | GO:0031224 | GO:0005451; GO:0046873; GO:0046914 | GO:0015992; GO:0030001; GO:0044763 |
| Unigene0048967 | GO:0031224 | GO:0005451; GO:0046873 | GO:0015992; GO:0030001; GO:0044763 |
| Unigene0060774 | GO:0031224; GO:0042170 | GO:0005451; GO:0046873 | GO:0006468; GO:0015992; GO:0030001; GO:0043269; GO:0044763 |
| Unigene0086768 | GO:0009526; GO:0031224 | GO:0005451; GO:0046873 | GO:0015992; GO:0030001; GO:0044763 |
| Unigene0104936 | GO:0031224 | GO:0005451; GO:0046873 | GO:0030001 |
| Gene ID | Log2Fold Change | |
|---|---|---|
| 200 mM NaCl-48 h vs. 200 mM NaCl + 10 mM KCl-48 h | 200 mM NaCl-168 h vs. 200 mM NaCl + 10 mM KCl-168 h | |
| Shaker | ||
| Unigene0029015 | 0.72 | −2.13 |
| Unigene0029016 | −0.10 | 0.69 |
| Unigene0041061 | −2.90 | −2.56 |
| Unigene0081104 | −2.73 | 0.19 |
| TPK | ||
| Unigene0033066 | 1.04 | −0.05 |
| Unigene0066388 | −0.38 | −0.59 |
| Unigene0076704 | 0.12 | −0.38 |
| Unigene0079952 | 0.30 | −0.53 |
| Unigene0080475 | 1.02 | −0.51 |
| Unigene0083511 | 0.38 | 0.21 |
| Unigene0098818 | 0.96 | 1.68 |
| HAK/KUP/KT | ||
| Unigene0014282 | −0.79 | −0.16 |
| Unigene0032884 | −0.70 | −0.25 |
| Unigene0028875 | −1.09 | −0.13 |
| Unigene0050867 | 0.26 | −0.55 |
| Unigene0014342 | 1.07 | 0.13 |
| HKT | ||
| Unigene0090596 | 1.12 | 0.78 |
| Unigene0090597 | −0.41 | 0.07 |
| CPAs | ||
| Unigene0016812 | −1.03 | −0.30 |
| Unigene0016813 | −0.67 | −0.61 |
| Unigene0052021 | 0.39 | −0.43 |
| Unigene0069097 | 0.98 | 0.18 |
| Unigene0077507 | 1.28 | 0.08 |
| Unigene0088276 | −0.36 | 0.27 |
| Unigene0091435 | −1.07 | 0.40 |
| Unigene0103067 | 0.95 | 0.55 |
| Unigene0017440 | 1.31 | −0.64 |
| Unigene0034942 | 0.24 | 0.06 |
| Unigene0035730 | −0.74 | 0.10 |
| Unigene0073015 | −0.68 | −0.10 |
| Unigene0077742 | 2.47 | −0.11 |
| Unigene0003428 | −0.39 | −0.24 |
| Unigene0003429 | −1.86 | −0.95 |
| Unigene0048967 | 0.15 | 0.21 |
| Unigene0060774 | −0.99 | 0.39 |
| Unigene0086768 | 0.18 | −0.24 |
| Unigene0104936 | −4.12 | 0.38 |
| Family | Species | Description | Gene | Protein ID | CDS (bp) | ORF Length (aa) |
|---|---|---|---|---|---|---|
| Tamaricaceae | Reaumuria trigyna | vacuolar membrane Na+/H+ antiporter | RtrNHX | ALJ77989.1 | 1662 | 553 |
| Amaranthaceae | Suaeda pruinosa | Na+/H+ antiporter | SprNHX | AHY19033.1 | 1662 | 553 |
| Amaranthaceae | Atriplex halimus | Na+/H+ antiporter | AhaNHX | AHY19032.1 | 1668 | 555 |
| Amaranthaceae | Salicornia bigelovii | Na+/H+ antiporter | SbiNHX | AAZ82019.1 | 1683 | 560 |
| Amaranthaceae | Halostachys caspica | Na+/H+ antiporter | HcaNHX | ADK62565.1 | 1656 | 551 |
| Amaranthaceae | Oxybasis glauca | Na+/H+ antiporter | OglNHX | AAQ72785.1 | 1656 | 551 |
| Amaranthaceae | Salicornia brachiata | Na+/H+ antiporter | SbrNHX | ACA33931.1 | 1683 | 560 |
| Amaranthaceae | Suaeda salsa | Na+/H+ antiporter | SsaNHX | AAK53432.1 | 1671 | 556 |
| Salicaceae | Populus pruinosa | Na+/H+ antiporter | PprNHX | AQN76303.1 | 1635 | 544 |
| Betulaceae | Betula platyphylla | Na+/H+ antiporter | BplNHX | ATE80665.1 | 1626 | 541 |
| Aizoaceae | Mesembryanthemum crystallinum | vacuolar Na+/H+ antiporter | McrNHX | CAN99589.1 | 1671 | 556 |
| Cactaceae | Selenicereus undatus | Na+/H+ antiporter | SunNHX | QED12512.1 | 1662 | 553 |
| Salicaceae | Populus euphratica | Na+/H+ antiporter | PeuNHX | AQN76287.1 | 1635 | 544 |
| Rosaceae | Pyrus betulifolia | vacuolar Na+/H+ antiporter | PbeNHX | AGE13941.1 | 1629 | 542 |
| Nelumbonaceae | Nelumbo nucifera | vacuolar Na+/H+ antiporter | NnuNHX | BAP90754.1 | 1617 | 538 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Zhang, S.; Du, S.; Jiang, J.; Wang, G. Transcriptome and Metabonomic Analysis of Tamarix ramosissima Potassium (K+) Channels and Transporters in Response to NaCl Stress. Genes 2022, 13, 1313. https://doi.org/10.3390/genes13081313
Chen Y, Zhang S, Du S, Jiang J, Wang G. Transcriptome and Metabonomic Analysis of Tamarix ramosissima Potassium (K+) Channels and Transporters in Response to NaCl Stress. Genes. 2022; 13(8):1313. https://doi.org/10.3390/genes13081313
Chicago/Turabian StyleChen, Yahui, Shiyang Zhang, Shanfeng Du, Jiang Jiang, and Guangyu Wang. 2022. "Transcriptome and Metabonomic Analysis of Tamarix ramosissima Potassium (K+) Channels and Transporters in Response to NaCl Stress" Genes 13, no. 8: 1313. https://doi.org/10.3390/genes13081313
APA StyleChen, Y., Zhang, S., Du, S., Jiang, J., & Wang, G. (2022). Transcriptome and Metabonomic Analysis of Tamarix ramosissima Potassium (K+) Channels and Transporters in Response to NaCl Stress. Genes, 13(8), 1313. https://doi.org/10.3390/genes13081313







