Abstract
The polygenic scores (PGSs) are developed to help clinicians in distinguishing individuals at high risk of developing disease outcomes from the general population. Clear cell renal cell carcinoma (ccRCC) is a complex disorder that involves numerous biological pathways, one of the most important of which is responsible for the microRNA biogenesis machinery. Here, we defined the biological-pathway-specific PGS in a case-control study of ccRCC in the Volga-Ural region of the Eurasia continent. We evaluated 28 DNA SNP variants, located in microRNA biogenesis genes, in 464 individuals with clinically diagnosed ccRCC and 1042 individuals without the disease. Individual genetic risks were defined using the SNP-variant effects derived from the ccRCC association analysis. The final weighted and unweighted PGS models were based on 21 SNPs, and 7 SNPs were excluded due to high LD. In our dataset, microRNA-machinery-weighted PGS revealed 1.69-fold higher odds (95% CI [1.51–1.91]) for ccRCC risk in individuals with ccRCC compared with controls with a p-value of 2.0 × 10−16. The microRNA biogenesis pathway weighted PGS predicted the risk of ccRCC with an area under the curve (AUC) = 0.642 (95%nCI [0.61–0.67]). Our findings indicate that DNA variants of microRNA machinery genes modulate the risk of ccRCC in Volga-Ural populations. Moreover, larger powerful genome-wide association studies are needed to reveal a wider range of genetic variants affecting microRNA processing. Biological-pathway-based PGSs will advance the development of innovative screening systems for future stratified medicine approaches in ccRCC.
1. Introduction
Renal cell carcinoma (RCC) is a malignancy of the kidney, accounting for approximately 90% of all kidney cancers. It is the tenth most common form of cancer in the world [1]. According to statistical data, the incidence of renal cancer is steadily increasing [2], with more than 179,000 deaths from renal cell cancer registered solely in 2020 [3].
The major histologic subtype of RCC is clear cell RCC (ccRCC), accounting for ~75–80% [4]. CcRCC is highly aggressive, with approximately 30% of individuals showing metastasis at the time of diagnosis and poor prognosis. The issue of obtaining better knowledge about ccRCC predisposition is related to a number of clinical and research areas such as screening, risk stratification, diagnostics, disease severity, prognosis, and clinical trials of new drugs. The identification of specific genetic markers affecting the risk of ccRCC involves clarifying the diagnosis, adjusting the methods of treatment, and developing better prognosis approaches and tools that are sensitive enough for early identification of ccRCC pathogenesis. Recent studies demonstrated the potential of PGS as a useful instrument in determining the risk of diverse diseases, including various cancers [5,6,7]. PGSs combine the effects of disease-associated single-nucleotide polymorphisms (SNPs), providing marked cancer risk stratification in the general population [8].
MicroRNAs play an important role in ccRCC [9,10,11,12,13]. MicroRNAs (miRNAs) are a very important part of the post-transcriptional mechanism of gene expression and are involved in carcinogenesis. SNPs in genes of microRNA biogenesis pathways can affect mature microRNA levels and lead to the modulation of a wide range of processes regulated by them [14].
Because miRNAs cover a wide range of regulated genes, SNPs in miRNA genes and target sites can function as modifiers of the effects of microRNA on phenotypes and disease susceptibility [15]. Moreover, SNPs located in biogenesis and miRNA precursor genes can have complex effects, influencing miRNA maturation, functional strand selection, and target mRNA definition. The presence of SNPs either in the genomic miRNA sequences or in the 3′UTR of cancer-associated genes can influence miRNA-dependent regulation, thus changing tumor susceptibility [16].
The identification of germline genetic variants that predict ccRCC risk and that may serve as additional markers to somatic alterations is a promising approach. Although genetic polymorphisms of miRNA biogenesis machinery genes are widely implicated in cancer development [17,18], polygenic risk scales involving miRNA biogenesis genes have not been previously evaluated. The current literature on PGS in ccRCC is scarce compared with the vast number of reported SNPs associated with the development of the disease. Most models have been constructed in individuals of European origin, and ethnic-specific PGS is required to account for genetic variations in different populations.
Here, we evaluated the effects of 28 SNPs located in miRNA machinery genes on ccRCC risk in three ethnic groups living in the Volga-Ural region and implemented a polygenic risk score (PGS) approach to evaluate the cumulative effects of these DNA variants.
2. Materials and Methods
2.1. Study Sample
All genotyped individuals with ccRCC and controls without the disease were residents of the Volga-Ural region of Eurasia. The study was performed according to the ethical standards of the Bioethics Committee who developed the Declaration of Helsinki of the World Association which governs “the ethical principles of medical research involving human subjects” [19] and with the ethical standards of the Research Ethics Committee of the Institute of Biochemistry and Genetics, a subdivision of the Ufa Federal Research Centre of the Russian Academy of Sciences. Informed written consent was obtained from each participant included in this study. Overall, 464 individuals with nonfamilial ccRCC (cases) were included in the study. All individuals with ccRCC underwent radical nephrectomy at Bashkir State Medical University Clinic. The inclusion criterion for the study was histologically confirmed ccRCC, and such individuals were not prescribed chemotherapy or radiotherapy prior to the collections of blood samples. There were no age, sex, ethnicity, or cancer stage restrictions for participation in the study. The control group comprised 1042 unrelated individuals from the general population residing in the same region and without oncological diseases in their family history, and corresponded to the group of cases in terms of clinical and demographic characteristics.
2.2. Blood Sample Collection, DNA Extraction, and Genotyping Procedures
The selection of 28 SNPs in microRNA biogenesis pathway genes and pre-miRNAs for genotyping in study samples was performed using the databases of the International HapMap Project, dbSNP, and miRBase registry and Ensembl [20,21,22,23]. All SNPs have a reported minor allele frequency (MAF) of >0.01 in Europeans.
Peripheral blood samples were collected from each subject and placed into 7 mL vacutainer tubes containing EDTA. Genomic DNA was extracted from blood leukocytes by using conventional proteinase K digestion and phenol-chloroform extraction methods. DNA concentration was measured with a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Fitchburg, WI, USA). Genomic DNA samples were normalized at 50 ng/μL. Genotyping of SNP variants of biogenesis genes and miRNA precursors was carried out using OpenArray technology on a Quant Studio™12K Flex Real-Time PCR System (Applied Biosystems, CA, USA). Samples were analyzed on special slide chips containing a total of 3072 through holes for individual reactions at 33 ηL. A 3 μL reaction mixture (TaqMan® OpenArray® Genotyping MasterMix, Applied Biosystems, Foster City, CA, USA) was mixed with 3 μL of DNA samples in a 384-well plate and then loaded into a custom-designed OpenArray plate preloaded with the genotyping primers and probe for the selected SNPs by using a QuantStudio 12 K Flex AccuFill system. PCR was performed under the following conditions: initial denaturation at 95 °C for 10 min; denaturation at 92 °C for 15 s, and annealing/elongation at 60 °C for 1 min (40 cycles). The genotyping data analysis was performed using the software package TaqMan Genotyper Software v.1.3.
2.3. Quality Control (QC) and Association Analysis
For quality control purposes, we evaluated the Hardy–Weinberg equilibrium by comparing the genotype distribution for each SNP variant genotyped with the expectation calculated from the allele frequencies. Overall, the average call rate for all SNPs was 98%. All calculations were performed using PLINK 1.9 [24] and the R 4.1.1 software tool and environment [25]. For each SNP, allelic ORs for ccRCC with 95% confidence intervals were calculated using logistic regression and assuming a log-additive genetic model. First, the individual genetic risks were determined using PGS based on 28 SNPs located in miRNA biogenesis genes (Table S2 in Supplementary File_3.docx). Further sensitivity analysis was performed and SNPs with r2 >0.2 were excluded due to high LD (Supplementary Materials File_4.docx). Final weighted and unweighted PGS models were based on 21 SNPs (Table 2). Given the unavailability of a training sample from the genome-wide association studies (GWAS) data, an analysis of the logistic regression model was carried out, and the values of the effects (OR, odds ratio) of the studied polymorphic variants were obtained (Table 2). Statistical significance for PGS was defined as p < 0.05. We also applied the Bonferroni correction method to the single-variant association analysis for multiple tests, for a total of 21 independent tests (PBonferroni < 0.05/21), providing us with the study-wide threshold of PBonferroni < 0.0023.
2.4. PGS Calculation
The PGS was calculated for each individual using the risk allele effect weighted sum of all studied polymorphic loci. The risk allele count for each SNP was weighted by its effect size based on its association with ccRCC under a log-additive genetic model implemented within a logistic regression analysis with adjustments for age, sex, and ethnicity. We used PLINK 1.9 [24] for logistic regression analysis. For variants with OR <1, we used the inverse OR and 1 (the reported effect allele frequency) so that the association was in the direction of ccRCC risk for all SNPs. Thus, the effect size obtained for our data was used as weights. Additionally, the impact of unweighted PGS on the ccRCC was assessed for sensitivity purposes. As a result, two models of polygenic risk, weighted and unweighted, were compared.
2.5. Receiver Operating Characteristic (ROC) Analysis
The distribution of the individual PGSs was compared between the cases and controls, and their discriminatory capacities were evaluated using receiver operating characteristic (ROC) curves in the R environment with the pROC package [26]. The strength of model to predict ccRCC against controls was assessed by comparing the area under the curve (AUC) of the respective ROC curves, which compares the true-positive rate against the false-positive rate.
3. Results
3.1. Association Analysis
We used a ccRCC study from the Volga-Ural region, including 464 nonfamilial ccRCC cases and 1042 unrelated population-based controls without cancer, which are described in Table 1.

Table 1.
Characteristics of study population.
The analysis of 21 SNPs, located in miRNA biogenesis genes, highlighted the strongest association for rs1057035 in DICER1 (OR [95% CI] = 1.85 [1.58–2.18], p-value = 4.05 × 10−14, Table 2). After Bonferroni correction for multiple testing, three SNPs showed significant effect on ccRCC risk in our study (Table 2). These included rs11060845 at PIWIL1, rs3809142 at RAN gene, and rs1057035 in DICER1. Moreover, five additional variants reached nominal significance within ccRCC association analysis, namely, rs595055 at AGO1, rs13078 in DICER1, rs6505162 at NSRP1, rs1991401 at DDX5, and rs720012 in DGCR8.

Table 2.
Association between reduced set of 21 DNA variants in miRNA biogenesis genes and ccRCC in Volga-Ural populations.
Table 2.
Association between reduced set of 21 DNA variants in miRNA biogenesis genes and ccRCC in Volga-Ural populations.
| Gene Name | Chromosome | Position, (GRCh37) | rsID | Risk Allele/Non-Risk Allele | Effect Allele Frequency Cases/Controls | OR (95% CI) | p-Value |
|---|---|---|---|---|---|---|---|
| AGO1 | 1 | 36,380,133 | rs595055 | T/C | 0.26/0.30 | 1.20 (0.71–0.99) | 0.04 |
| DDX20 | 1 | 112,308,953 | rs197412 | T/C | 0.47/0.49 | 1.11 (0.77–1.06) | 0.21 |
| DROSHA | 5 | 31,435,627 | rs4867329 | A/C | 0.46/0.49 | 1.11 (0.77–1.05) | 0.19 |
| C5orf22 | 5 | 31,532,789 | rs17409893 | A/G | 0.31/0.32 | 1.08 (0.78–1.10) | 0.37 |
| XPO5 | 6 | 43,492,578 | rs2257082 | G/A | 0.33/0.33 | 1.00 (0.84–1.18) | 0.98 |
| AGO2 | 8 | 141,555,862 | rs3864659 | A/C | 0.12/0.14 | 1.15 (0.69–1.10) | 0.24 |
| AGO2 | 8 | 141,594,460 | rs7005286 | T/C | 0.23/0.21 | 1.11 (0.93–1.33) | 0.26 |
| MIR196A2 | 12 | 54,385,599 | rs11614913 | T/C | 0.38/0.37 | 1.06 (0.91–1.25) | 0.45 |
| PIWIL1 | 12 | 130,852,174 | rs11060845 | G/T | 0.07/0.11 | 1.69 (0.44–0.79) | 4.32 × 10−4 |
| RAN | 12 | 131,355,546 | rs3809142 | C/T | 0.11/0.16 | 1.57 (0.50–0.81) | 3.05 × 10−4 |
| DICER1 | 14 | 95,554,142 | rs1057035 | C/T | 0.44/0.29 | 1.85 (1.58-2.18) | 4.05 × 10−14 |
| DICER1 | 14 | 95,556,747 | rs13078 | T/A | 0.13/0.17 | 1.28 (0.62–0.98) | 0.03 |
| GEMIN4 | 17 | 649,232 | rs3744741 | C/T | 0.15/0.18 | 1.19 (0.68–1.04) | 0.11 |
| GEMIN4 | 17 | 649,505 | rs4968104 | T/A | 0.21/0.22 | 1.06 (0.78–1.14) | 0.55 |
| GEMIN4 | 17 | 649,935 | rs2740348 | G/C | 0.19/0.17 | 1.20 (0.98–1.47) | 0.07 |
| microRNA-423 (NSRP1) | 17 | 28,444,183 | rs6505162 | A/C | 0.50/0.46 | 1.17 (1.00–1.37) | 0.04 |
| DDX5 | 17 | 62,502,435 | rs1991401 | G/A | 0.44/0.38 | 1.25 (1.07–1.47) | 4.99 × 10−3 |
| MIR27A | 19 | 13,947,292 | rs895819 | T/C | 0.34/0.35 | 1.06 (0.80–1.11) | 0.45 |
| DGCR8 | 22 | 20,098,544 | rs417309 | G/A | 0.08/0.10 | 1.17 (0.65–1.12) | 0.26 |
| DGCR8 | 22 | 20,098,582 | rs720012 | A/G | 0.24/0.20 | 1.24 (1.03–1.49) | 0.02 |
| DGCR8 | 22 | 20,098,882 | rs720014 | C/T | 0.24/0.21 | 1.15 (0.95–1.40) | 0.14 |
Abbreviations: OR—odds ratio; CI—confidence interval. Association test statistics for three variants surviving the multiple testing correction (see Methods) is highlighted in bold characters.
As a result of weighted PGS analysis, 1.69 times higher chances (95% CI [1.51–1.91]) of the ccRCC risk were revealed in cases compared with controls, with a statistically significant p-value of 2.0 × 10−16. Unweighted PGS models showed a lower odds ratio OR (95% CI) of 1.60 (1.42–1.80) with a less significant p-value of 2.95 × 10−14 (Supplementary Materials File_1.docx).
3.2. Receiver Operator Characteristic Analysis
We compared the discriminative ability of two PGS models for the case/control status by developing the ROC curves. The microRNA biogenesis pathway weighted PGS predicted the risk of developing ccRCC with an AUC of 0.642 (95% CI [0.61–0.67], sensitivity of 0.71, and a specificity of 0.50 (Figure 1). For the unweighted PGS model, see Figure S1 in Supplementary File_1.docx.
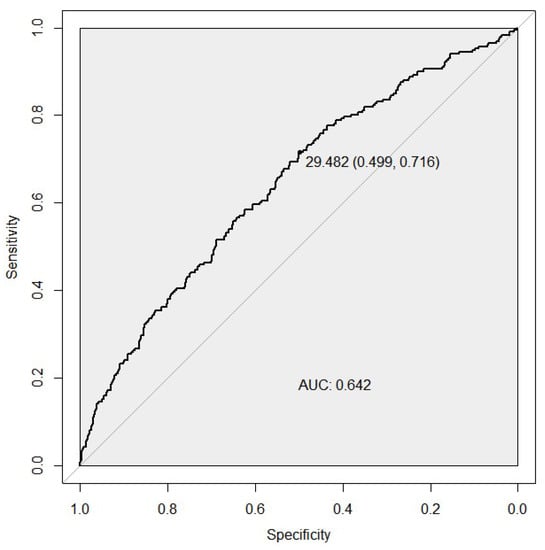
Figure 1.
ROC curves assessing the discriminative power of the weighted PGS model for the ccRCC risk. The best predictive point is shown with the ideal cut-off for the PGS and with estimates for specificity and sensitivity at that point. AUC, area under the curve.
4. Discussion
In this study, we evaluated the ability of DNA variants to predict the risk of ccRCC in microRNA biogenesis pathway loci. We found suggestive evidence of the combined effects of the weighted PGS on ccRCC risks in the Volga-Ural region of Eurasia. We included 28 SNPs of microRNA biogenesis pathway genes that recently showed an association with the development of diverse types of cancers, including clear cell carcinoma. As a result, we found the combination of 21 microRNA biogenesis SNPs could predict 1.69 times higher chances (95% CI [1.51–1.91]) of ccRCC. We also evaluated the PheWAS data of the studied SNPs and found a correlation with several traits including urological cancers, in particular bladder cancer and other types of malignances (Supplementary File_2.docx).
Recently, it was described that a gene pathway-based PGS approach for genetic risk prediction for human phenotypes may shed light on disease biology and identify core gene networks that contribute the most risk to a polygenic disorder [27]. Today, the most popular procedure used to investigate genetic variants is GWAS, which allows for the simultaneous analysis of millions of DNA variants. As a result of this approach, we can identify hundreds of disease susceptibility loci containing low-risk variants, and only a few of them will be the most significantly associated with disease risk. At the same time, in RCC, with population prevalence of 4.91/100,000 [3], the assessment of the influence of certain genotypes and alleles was traditionally most often carried out by identifying the association of individual polymorphisms with the risk of developing the disease.
Direct evidence of the inherited predisposition to RCC is provided by a number of rare cancer syndromes with defined germline mutations in 11 genes (BAP1, FLCN, FH, MET, PTEN, SDHB, SDHC, SDHD, TSC1, TSC2, and VHL), associated with the development of different RCC subtypes [28]. Nevertheless, all of these genes can explain only a two-fold increased risk of RCC in first-degree relatives of individuals with RCC [29]. According to estimates of previous GWAS, the established RCC risk loci account for only about 10% of the familial risk of disease [30].
Growing evidence suggests that SNPs in core components of miRNA biogenesis may impair or enhance miRNA processing efficiency or function, which can function as an oncogene or tumor suppressor [31]. Evidence from published reports highlights that SNPs in miRNAs, which encode their biogenesis pathway and target binding sites, may affect the regulatory capacity of miRNAs by affecting miRNA processing or miRNA–mRNA interactions [32]. To date, most of the studies in this field have had a case-control design and have been based on a candidate gene approach [15]. For instance, in one study of renal cancer 41 SNPs in 11 miRNA biogenesis genes were analyzed [33]. Two SNPs in the GEMIN4 gene were significantly associated with the renal carcinoma risk. Moreover, the common GEMIN4 H3 haplotype (wmmwww, where w is the wild-type allele, and m is the minor allele), consisting of six nonsynonymous SNPs in the order rs910924, rs2740348, rs7813, rs3744741, rs1062923, and rs4968104, was protective of developing RCC (OR = 0.66, 95% CI: 0.45–0.97) in the respective haplotype analysis [33].
The number of studies using PGS in RCC is rather limited. Thus, in one study, PGS analysis of 13 GWAS-established ccRCC SNPs was recently performed for tumor molecular subtypes: ccA, characterized by overexpression of genes associated with hypoxia, angiogenesis, and fatty and organic acid metabolism; and the poorer-prognosis ccB, overexpressing genes regulating epithelial-to-mesenchymal transition, the cell cycle, and wound healing. This GWAS-based PGS signal was associated with both ccA and, in particular, ccB tumors (90th vs. 10th percentile: OR (95% CI) = 1.82 (1.11–2.99), p-value = 0.02 and OR (95% CI) = 2.87 (1.64–5.01), p-value = 2 × 10−4, respectively) [34]. In addition, the genetic risk score based on leukocyte telomere-length-associated SNPs was connected with the risk of recurrence in individuals with renal cell carcinoma [35]. Another study showed a relatively low AUC (95% CI) of 0.567 (0.54–0.59) for PGS of the 15 genetic variants identified by previous GWAS in association with the risk of renal cancer [36].
5. Conclusions
Despite the investigation of molecular-pathway-based SNPs allowing for a determination of significant associations with RCC, GWAS is urgently needed to discover new uncommon and rare variants that explain a vital group of the variation in complex characteristics.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes13071281/s1, (Supplementary File_1) Figure S1: ROC curves assessing the discriminative power of the weighted PRS model for the ccRCC risk; (Supplementary File_2) Table S1: PheWAS data of studied SNPs in microRNA biogenesis pathway genes; (Supplementary File_3) Table S2: Association between 28 DNA variants in miRNA biogenesis genes and ccRCC in Volga-Ural populations; Figures S2 and S3. ROC curves assessing the discriminative power of the unweighted PRS model for the ccRCC risk; (Supplementary Materials File_4) Table S3: Linkage disequilibium using the European+Asian populations LD estimates from the 1000 Genomes Project (www.ldlink.nci.nih.gov) between SNPs located within 500 kb from each other.
Author Contributions
E.K. and A.K. designed the clinical and genetic RCC study; I.G. designed the current study; V.P. and A.I. collected phenotypic information and blood samples; G.G. extracted DNA and performed genotyping; E.I. performed quality control of genotyping data and statistical analysis; I.P. overviewed the statistical analysis; A.K., I.G., E.I. and I.P. wrote the manuscript, all authors evaluated the analytical results and contributed to the manuscript contents. All authors have read and agreed to the published version of the manuscript.
Funding
This work was in part funded by the Ministry of Science and Higher Education of Russian Federation (075-15-2021-595). In the study, DNA samples from the Collection of Biological Materials of Human Beings of the IBG UFRC RAS were used, supported by the Program of Bioresource Collections of the FASO of Russia (agreement no. 007-030164/).
Institutional Review Board Statement
The study was conducted in accordance with Declaration of Helsinki, and approved by the Ethic Committee of Institute of Biochemistry and Genetics—Subdivision of the Ufa Federal Research Centre of the Russian Academy of Sciences (protocol code 11 and date of approval 27 October 2014).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Acknowledgments
We would like to thank the participants of the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Singh, D. Current updates and future perspectives on the management of renal cell carcinoma. Life Sci. 2020, 264, 118632. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Xu, F.; Tian, J.; Wang, Y.; Guo, N.; Wan, Z.; He, M.; Gao, M.; Gao, K.; Chong, T. Prognostic value of circulating tumor cells and immune-inflammatory cells in patients with renal cell carcinoma. Urol. Oncol. 2022, 40, e21–e167. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2020; Available online: https://gco.iarc.fr/today (accessed on 22 March 2022).
- Hsieh, J.J.; Purdue, M.P.; Signoretti, S.; Swanton, C.; Albiges, L.; Schmidinger, M.; Heng, D.Y.; Larkin, J.; Ficarra, V. Renal cell carcinoma. Nat. Rev. Dis. Prim. 2017, 3, 17009. [Google Scholar] [CrossRef] [PubMed]
- Maher, E.R. Hereditary renal cell carcinoma syndromes: Diagnosis, surveillance and management. World J. Urol. 2018, 36, 1891–1898. [Google Scholar] [CrossRef] [Green Version]
- Nicolas, E.; Demidova, E.V.; Iqbal, W.; Serebriiskii, I.G.; Vlasenkova, R.; Ghatalia, P.; Zhou, Y.; Rainey, K.; Forman, A.F.; Dunbrack, R.L.; et al. Interaction of germline variants in a family with a history of early-onset clear cell renal cell carcinoma. Mol. Genet. Genomic Med. 2019, 7, e556. [Google Scholar] [CrossRef]
- Wong, E.C.L.; Breau, R.H.; Mallick, R.; Wood, L.; Pouliot, F.; Basappa, N.S.; Tanguay, S.; Soulières, D.; So, A.; Heng, D.; et al. Renal Cell Carcinoma in the Canadian Indigenous Population. Curr. Oncol. 2019, 26, 367–371. [Google Scholar] [CrossRef] [Green Version]
- Mavaddat, N.; Pharoah, P.D.P.; Michailidou, K.; Tyrer, J.; Brook, M.; Bolla, M.K.; Wang, Q.; Dennis, J.; Dunning, A.M.; Shah, M.; et al. Prediction of Breast Cancer Risk Based on Profiling with Common Genetic Variants. J. Natl. Cancer Inst. 2015, 107, djv036. [Google Scholar] [CrossRef]
- Gao, B.; Wang, L.; Zhang, Y.; Zhang, N.; Han, M.; Liu, H.; Sun, D.; Liu, Y. MiR-532-3p suppresses cell viability, migration and invasion of clear cell renal cell carcinoma through targeting TROAP. Cell Cycle 2021, 20, 1578–1588. [Google Scholar] [CrossRef]
- Kalantzakos, T.J.; Sullivan, T.B.; Gloria, T.; Canes, D.; Moinzadeh, A.; Rieger-Christ, K.M. MiRNA-424-5p Suppresses Proliferation, Migration, and Invasion of Clear Cell Renal Cell Carcinoma and Attenuates Expression of O-GlcNAc-Transferase. Cancers 2021, 13, 5160. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, J.; Hu, X.; Ge, Q.; Xiao, J.; Ginting, C.N. Expression of miR-410 in peripheral blood of patients with clear cell renal cell carcinoma and its effect on proliferation and invasion of Caki-2 cells. J. BUON 2021, 26, 2059–2066. Available online: https://pubmed.ncbi.nlm.nih.gov/34761617/ (accessed on 3 June 2022).
- Shi, L.; Wang, M.; Li, H.; You, P. MicroRNAs in Body Fluids: A More Promising Biomarker for Clear Cell Renal Cell Carcinoma. Cancer Manag. Res. 2021, 13, 7663–7675. [Google Scholar] [CrossRef] [PubMed]
- Chalbatani, G.M.; Momeni, S.A.; Hadloo, M.H.M.; Karimi, Z.; Hadizadeh, M.; Jalali, S.A.; Miri, S.R.; Memari, F.; Hamblin, M.R. Comprehensive analysis of ceRNA networks to determine genes related to prognosis, overall survival, and immune infiltration in clear cell renal carcinoma. Comput. Biol. Med. 2021, 141, 105043. [Google Scholar] [CrossRef]
- Lee, S.S.; Min, H.; Ha, J.Y.; Kim, B.H.; Choi, M.S.; Kim, S. Dysregulation of the miRNA biogenesis components DICER1, DROSHA, DGCR8 and AGO2 in clear cell renal cell carcinoma in both a Korean cohort and the cancer genome atlas kidney clear cell carcinoma cohort. Oncol. Lett. 2019, 18, 4337–4345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, B.M.; Robles, A.I.; Harris, C.C. Genetic variation in microRNA networks: The implications for cancer research. Nat. Rev. Cancer 2010, 10, 389–402. [Google Scholar] [CrossRef]
- Fabbri, M.; Valeri, N.; Calin, G.A. MicroRNAs and genomic variations: From Proteus tricks to Prometheus gift. Carcinogenesis 2009, 30, 912–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero-Cordoba, S.L.; Salido-Guadarrama, I.; Rodriguez-Dorantes, M.; Hidalgo-Miranda, A. miRNA biogenesis: Biological impact in the development of cancer. Cancer Biol. Ther. 2014, 15, 1444–1455. [Google Scholar] [CrossRef] [Green Version]
- Hata, A.; Kashima, R. Dysregulation of microRNA biogenesis machinery in cancer. Crit. Rev. Biochem. Mol. Biol. 2015, 51, 121–134. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [Green Version]
- Sherry, S.T.; Ward, M.; Sirotkin, K. dbSNP—Database for Single Nucleotide Polymorphisms and Other Classes of Minor Genetic Variation. Genome Res. 1999, 9, 677–679. [Google Scholar] [CrossRef]
- Gibbs, R.A.; Belmont, J.W.; Hardenbol, P.; Willis, T.D.; Yu, F.L.; Yang, H.M.; Ch’ang, L.Y.; Huang, W.; Liu, B.; Shen, Y.; et al. The international HapMap project. Nature 2003, 426, 789–796. [Google Scholar] [CrossRef] [Green Version]
- Griffiths-Jones, S.; Grocock, R.J.; Van Dongen, S.; Bateman, A.; Enright, A.J. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006, 34, D140–D144. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.L.; Achuthan, P.; Allen, J.; Allen, J.; Alvarez-Jarreta, J.; Amode, M.R.; Armean, I.M.; Azov, A.G.; Bennett, R.; Bhai, J.; et al. Ensembl 2021. Nucleic Acids Res. 2021, 49, D884–D891. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: www.R-project.org (accessed on 22 March 2022).
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.-C.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef]
- Baker, E.; Schmidt, K.M.; Sims, R.; O’Donovan, M.C.; Williams, J.; Holmans, P.; Escott-Price, V.; GERAD Consortium. POLARIS: Polygenic LD-adjusted risk score approach for set-based analysis of GWAS data. Genet. Epidemiol. 2018, 42, 366–377. [Google Scholar] [CrossRef] [Green Version]
- Mucci, L.A.; Hjelmborg, J.B.; Harris, J.R.; Czene, K.; Havelick, D.J.; Scheike, T.; Graff, R.E.; Holst, K.; Möller, S.; Unger, R.H.; et al. Familial Risk and Heritability of Cancer among Twins in Nordic Countries. JAMA J. Am. Med. Assoc. 2016, 315, 68–76. [Google Scholar] [CrossRef] [Green Version]
- Haas, N.B.; Nathanson, K.L. Hereditary Kidney Cancer Syndromes. Adv. Chronic Kidney Dis. 2014, 21, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Scelo, G.; Purdue, M.P.; Brown, K.M.; Johansson, M.; Wang, Z.; Eckel-Passow, J.E.; Ye, Y.; Hofmann, J.; Choi, J.; Foll, M.; et al. Genome-wide association study identifies multiple risk loci for renal cell carcinoma. Nat. Commun. 2017, 8, 15724. [Google Scholar] [CrossRef]
- Guo, Z.; Shu, Y.; Zhou, H.; Zhang, W. Identification of diagnostic and prognostic biomarkers for cancer: Focusing on genetic variations in microRNA regulatory pathways. Mol. Med. Rep. 2016, 13, 1943–1952. [Google Scholar] [CrossRef] [Green Version]
- Mishra, P.J.; Mishra, P.J.; Banerjee, D.; Bertino, J.R. MiRSNPs or MiR-polymorphisms, new players in microRNA mediated regulation of the cell: Introducing microRNA pharmacogenomics. Cell Cycle 2008, 7, 853–858. [Google Scholar] [CrossRef] [Green Version]
- Horikawa, Y.; Wood, C.G.; Yang, H.; Zhao, H.; Ye, Y.; Gu, J.; Lin, J.; Habuchi, T.; Wu, X. Single Nucleotide Polymorphisms of microRNA Machinery Genes Modify the Risk of Renal Cell Carcinoma. Clin. Cancer Res. 2008, 14, 7956–7962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purdue, M.P.; Rhee, J.; Moore, L.; Gao, X.; Sun, X.; Kirk, E.; Bencko, V.; Janout, V.; Mates, D.; Zaridze, D.; et al. Differences in risk factors for molecular subtypes of clear cell renal cell carcinoma. Int. J. Cancer 2021, 149, 1448–1454. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Tsai, C.-W.; Chang, W.-S.; Xu, J.; Xu, Y.; Bau, D.-T.; Gu, J. Prognostic value of leukocyte telomere length in renal cell carcinoma patients. Am. J. Cancer Res. 2020, 10, 3428–3439. Available online: https://pubmed.ncbi.nlm.nih.gov/33163281/ (accessed on 3 June 2022). [PubMed]
- Jia, G.; Lu, Y.; Wen, W.; Long, J.; Liu, Y.; Tao, R.; Li, B.; Denny, J.C.; Shu, X.-O.; Zheng, W. Evaluating the Utility of Polygenic Risk Scores in Identifying High-Risk Individuals for Eight Common Cancers. JNCI Cancer Spectr. 2020, 4, pkaa021. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).