HSPA8 Single-Nucleotide Polymorphism Is Associated with Serum HSC70 Concentration and Carotid Artery Atherosclerosis in Nonalcoholic Fatty Liver Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Physical Examination and Serum Biochemical Measurements
2.3. Liver Biopsy and Histological Assessment
2.4. Intima–Media Thickness Measurements
2.5. DNA Extraction and HSPA8 Genotyping
2.6. Statistics
3. Results
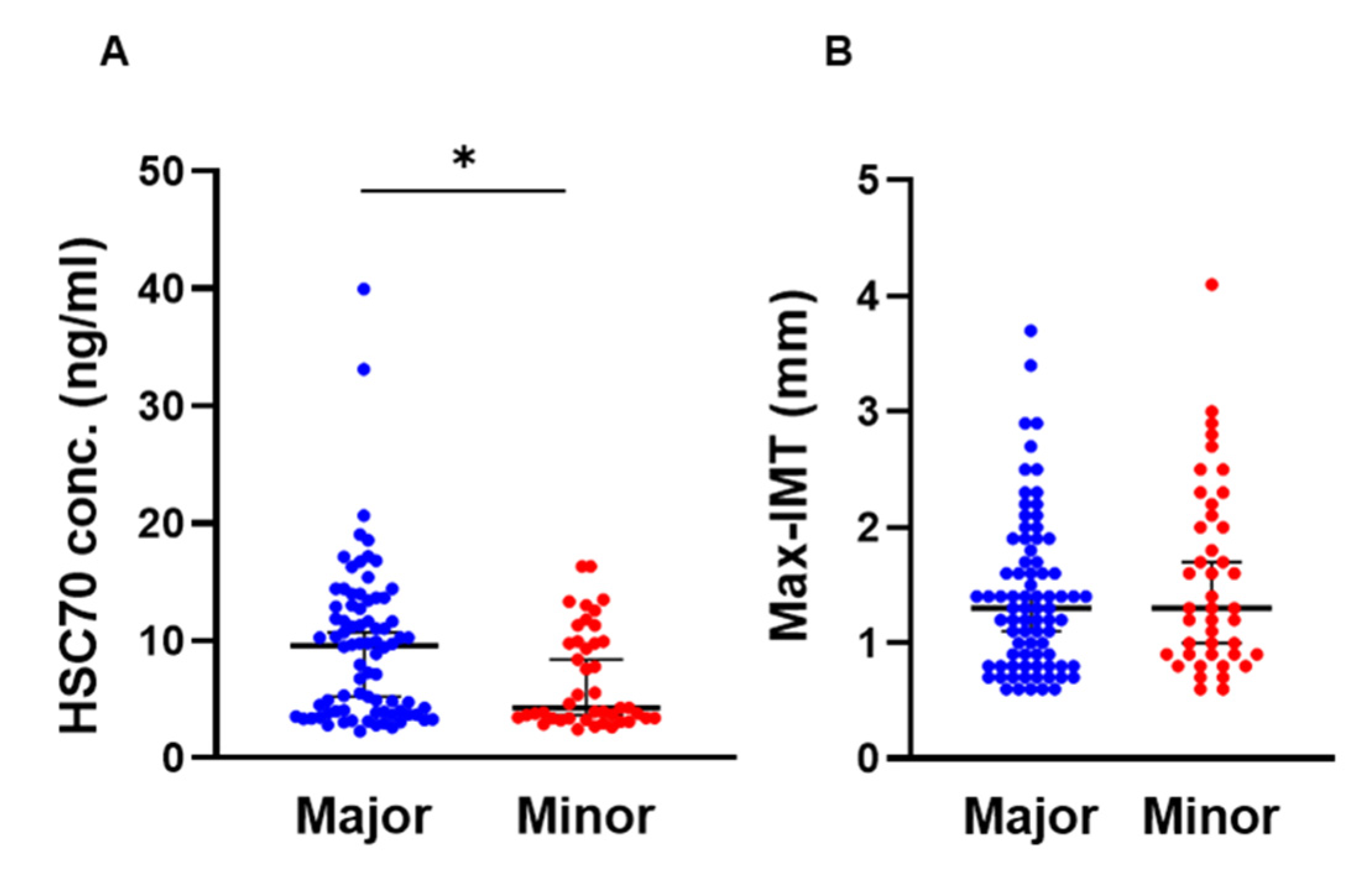
3.1. Comparison of HSPA8 Genotype Characteristics
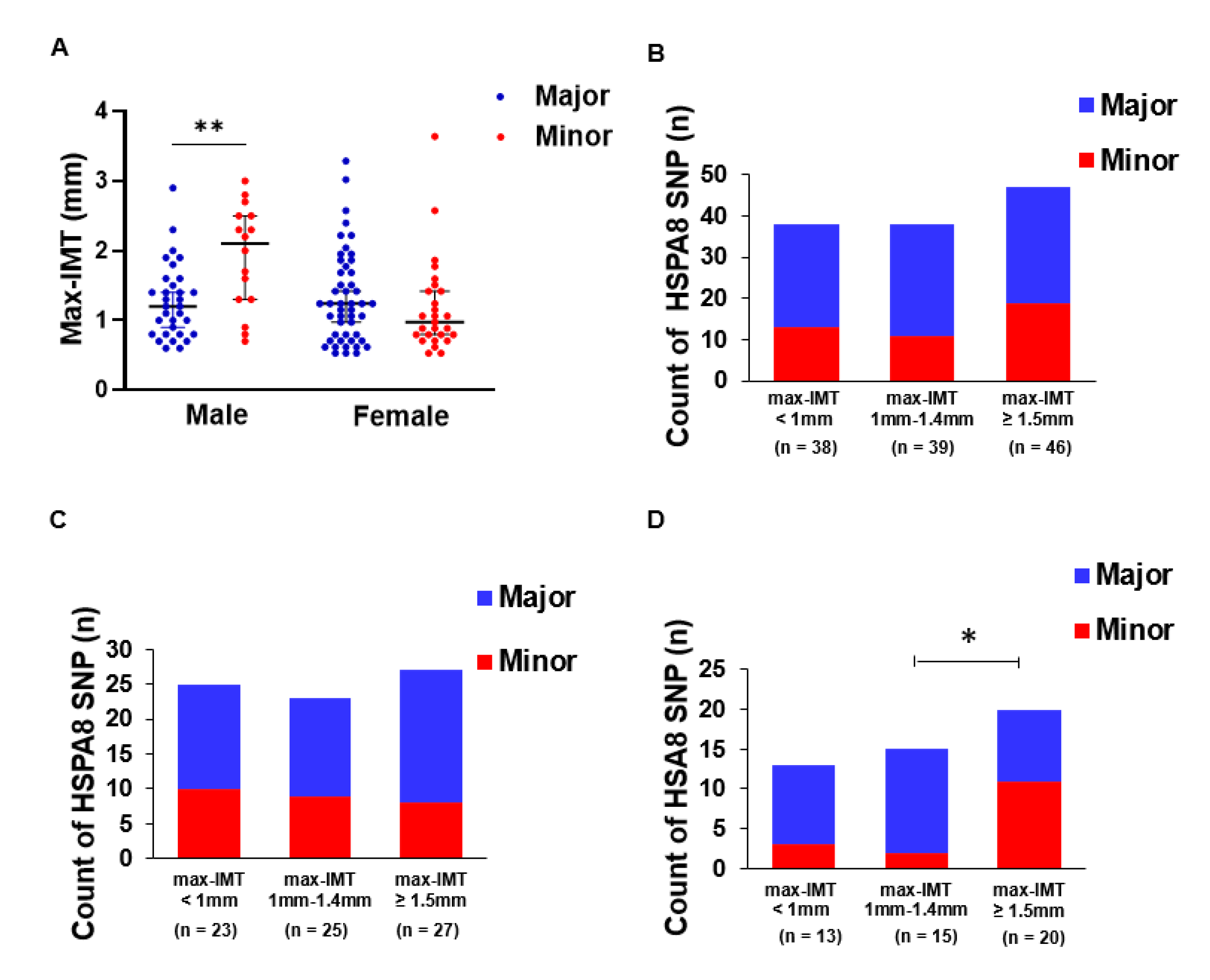
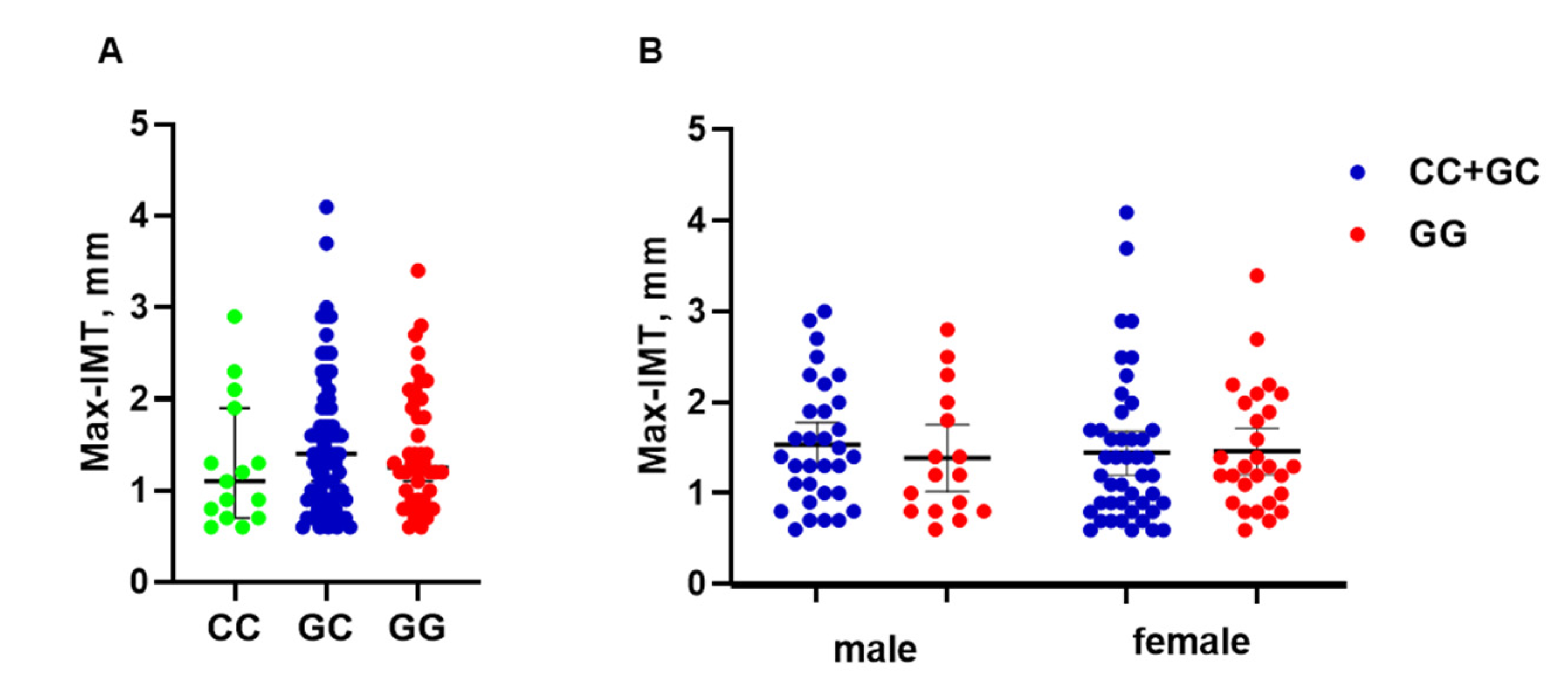
3.2. HSPA8 Genotype Is Associated with Max-IMT in Men
3.3. PNPLA3 Genotype Is Not Associated with Max-IMT
3.4. Relationship between Hepatic Fibrosis and Arteriosclerosis
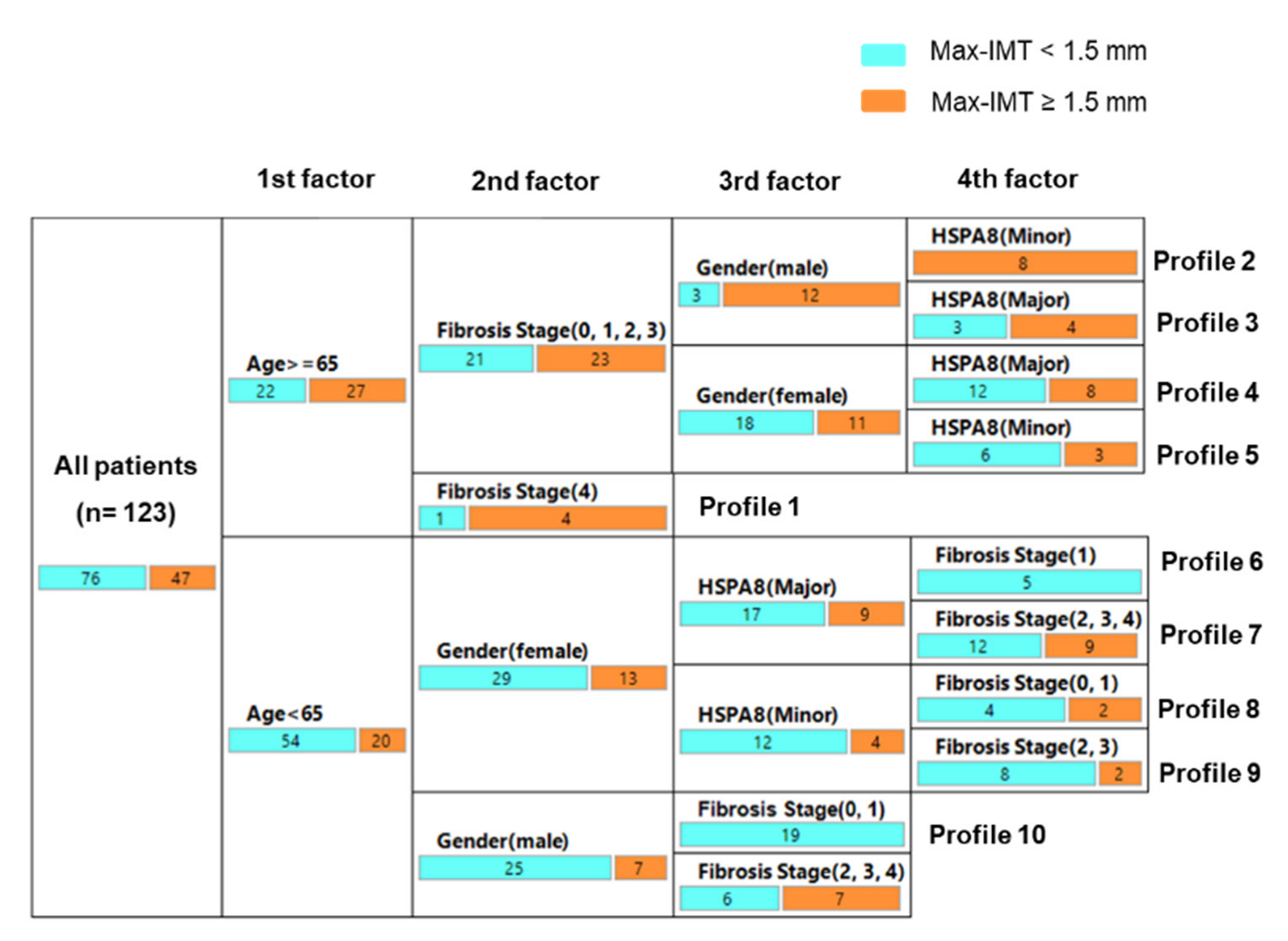
3.5. Decision-Tree Algorithm for Profiles of Significant Arteriosclerosis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef]
- Noureddin, M.; Vipani, A.; Bresee, C.; Todo, T.; Kim, I.K.; Alkhouri, N.; Setiawan, V.; Tran, T.; Ayoub, W.S.; Lu, S.C.; et al. NASH leading cause of liver transplant in women: Updated analysis of indications for liver transplant and ethnic and gender variances. Am. J. Gastroenterol. 2018, 113, 1649–1659. [Google Scholar] [CrossRef]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef] [Green Version]
- Targher, G.; Corey, K.E.; Byrne, C.D. NAFLD, and cardiovascular and cardiac diseases: Factors influencing risk, prediction and treatment. Diabetes Metab. 2021, 47, 101215. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Van Natta, M.L.; Clark, J.; Neuschwander-Tetri, B.A.; Diehl, A.; Dasarathy, S.; Loomba, R.; Chalasani, N.; Kowdley, K.; Hameed, B.; et al. Prospective Study of Outcomes in Adults with Nonalcoholic Fatty Liver Disease. N. Engl. J. Med. 2021, 385, 1559–1569. [Google Scholar] [CrossRef]
- Fujii, H.; Iwaki, M.; Hayashi, H.; Toyoda, H.; Oeda, S.; Hyogo, H.; Kawanaka, M.; Morishita, A.; Munekage, K.; Kawata, K.; et al. Clinical Outcomes in Biopsy-Proven Nonalcoholic Fatty Liver Disease Patients: A Multicenter Registry-based Cohort Study. Clin. Gastroenterol. Hepatol. 2022, S1542-3565(22)00008-8. [Google Scholar] [CrossRef]
- Simon, T.G.; Roelstraete, B.; Hagström, H.; Sundström, J.; Ludvigsson, J.F. Non-alcoholic fatty liver disease and incident major adverse cardiovascular events: Results from a nationwide histology cohort. Gut 2021, 6, 34489307. [Google Scholar] [CrossRef]
- Stols-Gonçalves, D.; Hovingh, G.K.; Nieuwdorp, M.; Holleboom, A.G. NAFLD and Atherosclerosis: Two Sides of the Same Dysmetabolic Coin? Trends Endocrinol. Metab. 2019, 30, 891–902. [Google Scholar] [CrossRef]
- Stahl, E.P.; Dhindsa, D.S.; Lee, S.K.; Sandesara, P.B.; Chalasani, N.P.; Sperling, L.S. Nonalcoholic Fatty Liver Disease and the Heart JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 948–963. [Google Scholar] [CrossRef]
- Grobbee, D.E.; Bots, M.L. Carotid artery intima-media thickness as an indicator of generalized atherosclerosis. J. Intern. Med. 1994, 236, 567–573. [Google Scholar] [CrossRef]
- Howard, G.; Sharrett, A.R.; Heiss, G.; Evans, G.W.; Chamb-less, L.E.; Riley, W.A.; Burke, G.L. Carotid artery intimal-medial thickness distribution in general populations as evaluated by B-mode ultrasound. ARIC Investigators. Stroke 1993, 24, 1297–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenz, M.W.; Markus, H.S.; Bots, M.L.; Rosvall, M.; Sitzer, M. Prediction of clinical cardiovascular events with carotidintima-media thickness: A systematic review and meta-analysis. Circulation 2007, 115, 459–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nezu, T.; Hosomi, N.; Aoki, S.; Matsumoto, M. Carotid Intima-Media Thickness for Atherosclerosis. J. Atheroscler. Thrombosis 2016, 23, 18–31. [Google Scholar] [CrossRef] [Green Version]
- Sookoian, S.; Pirola, C.J. Non-alcoholic fatty liver disease is strongly associated with carotid atherosclerosis: A systematic review. J. Hepatol. 2008, 49, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Ampuero, J.; Gallego-Durán, R.; Romero-Gómez, M. Association of NAFLD with subclinical atherosclerosis and coronary-artery disease: Meta-analysis. Rev. Esp. Enferm. Dig. 2015, 107, 10–16. [Google Scholar]
- Sinn, D.H.; Kang, D.; Chang, Y.; Ryu, S.; Gu, S.; Kim, H.; Seong, D.; Cho, S.J.; Yi, B.K.; Park, H.D.; et al. Non-alcoholic fatty liver disease and progression of coronary artery calcium score: A retrospective cohort study. Gut 2017, 66, 323–329. [Google Scholar] [CrossRef]
- Anstee, Q.M.; Darlay, R.; Cockell, S.; Meroni, M.; Govaere, O.; Tiniakos, D.; Burt, A.D.; Bedossa, P.; Palmer, J.; Liu, Y.L.; et al. Genome-wide association study of non-alcoholic fatty liver and steatohepatitis in a histologically characterised cohort. J. Hepatol. 2020, 73, 505–515. [Google Scholar] [CrossRef]
- Liu, Y.L.; Patman, G.L.; Leathart, J.B.; Piguet, A.C.; Burt, A.D.; Dufour, J.F.; Day, C.P.; Daly, A.K.; Reeves, H.L.; Anstee, Q.M. Carriage of the PNPLA3 rs738409 C > G polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J. Hepatol. 2014, 61, 75–81. [Google Scholar] [CrossRef]
- Petta, S.; Valenti, L.; Marchesini, G.; Marco, V.D.; Licata, A.; Cammà, C.; Barcellona, M.R.; Cabibi, D.; Donati, B.; Fracanzani, A.; et al. PNPLA3 GG genotype and carotid atherosclerosis in patients with non-alcoholic fatty liver disease. PLoS ONE 2013, 8, e74089. [Google Scholar]
- Costanzo, A.D.; D’Erasmo, L.; Polimeni, L.; Baratta, F.; Coletta, P.; Martino, M.D.; Loffredo, L.; Perri, L.; Ceci, F.; Montali, A.; et al. Non-alcoholic fatty liver disease and subclinical atherosclerosis: A comparison of metabolically—Versus genetically-driven excess fat hepatic storage. Atherosclerosis 2017, 257, 232–239. [Google Scholar] [CrossRef]
- Unalp-Arida, A.; Ruhl, C.E. Patatin-Like Phospholipase Domain-Containing Protein 3 I148M and Liver Fat and Fibrosis Scores Predict Liver Disease Mortality in the U.S. Population. Hepatology 2020, 71, 820–834. [Google Scholar] [CrossRef] [PubMed]
- Stricher, F.; Macri, C.; Ruff, M.; Muller, S. HSPA8/HSC70 chaperone protein Structure, function, and chemical targeting. Autophagy 2013, 9, 1937–1954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glazier, A.A.; Hafeez, N.; Mellacheruvu, D.; Basrur, V.; Nesvizhskii, A.I.; Lee, L.M.; Shao, H.; Tang, V.; Yob, J.M.; Gestwicki, J.E.; et al. HSC70 is a chaperone for wild-type and mutant cardiac myosin binding protein C. JCI Insight 2018, 3, e99319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, M.; Guo, H.; Yang, X.; Zhou, L.; Zhang, X.; Cheng, L.; Zeng, H.; Hu, F.B.; Tanguay, R.M.; Wu, T. Genetic Variations in HSPA8 Gene Associated with Coronary Heart Disease Risk in a Chinese Population. PLoS ONE 2010, 5, e9684. [Google Scholar] [CrossRef]
- Horio, M.; Imai, E.; Yasuda, Y.; Watanabe, T.; Matsuo, S. Performance of GFR equations in Japanese subjects. Clin. Exp. Nephrol. 2013, 17, 352–358. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Natta, M.V.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Brunt, E.M.; Janney, C.G.; Di Bisceglie, A.M.; Neuschwander-Tetri, B.A.; Bacon, B.R. Nonalcoholic steatohepatitis: A proposal for grading and staging the histological lesions. Am. J. Gastroenterol. 1999, 94, 2467–2474. [Google Scholar] [CrossRef]
- Nascimbeni, F.; Bedossa, P.; Fedchuk, L.; Pais, R.; Charlotte, F.; Lebray, P.; Poynard, T.; Ratziu, V. Clinical validation of the FLIP algorithm and the SAF score in patients with non-alcoholic fatty liver disease. J. Hepatol. 2020, 72, 828–838. [Google Scholar] [CrossRef]
- Hensley, B.; Huang, C.; Martinez, C.V.C.; Shokoohi, H.; Liteplo, A. Ultrasound Measurement of Carotid Intima-Media Thickness and Plaques in Predicting Coronary Artery Disease. Ultrasound Med. Biol. 2020, 46, 1608–1613. [Google Scholar] [CrossRef]
- Terminology and Diagnostic Criteria Committee; Japan Society of Ultrasonics in Medicine. Standard method for ultrasound evaluation of carotid artery lesions. J. Med. Ultrason (2001) 2009, 36, 219–226. [Google Scholar]
- Baldassarre, D.; Hamsten, A.; Veglia, F.; de Faire, U.; Humphries, S.E.; Smit, A.J.; Giral, P.; Kurl, S.; Rauramaa, R.; Mannarino, E.; et al. IMPROVE Study Group. Measurements of carotid intima-media thickness and of interadventitia common carotid diameter improve prediction of cardiovascular events: Results of the IMPROVE (Carotid Intima Media Thickness [IMT] and IMT-Progression as Predictors of Vascular Events in a High Risk European Population) study. J. Am. Coll. Cardiol. 2012, 60, 1489–1499. [Google Scholar] [PubMed] [Green Version]
- Romeo, S.; Kozlitina, J.; Xing, C.; Pertsemlidis, A.; Cox, D.; Pennacchio, L.A.; Boerwinkle, E.; Cohen, J.C.; Hobbs, H.H. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2008, 40, 1461–1465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eslam, M.; Valenti, L.; Romeo, S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. J. Hepatol. 2018, 68, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; Seth, D.; Day, C.P. Genetic factors that affect risk of alcoholic and nonalcoholic fatty liver disease. Gastroenterology 2016, 150, 1728–1744. [Google Scholar] [CrossRef] [PubMed]
- Abul-Husn, N.S.; Cheng, X.; Li, A.H.; Xin, Y.; Schurmann, C.; Stevis, P.; Liu, Y.; Kozlitina, J.; Stender, S.; Wood, G.C.; et al. A protein-truncatingHSD17B13 variant and protection from chronic liver disease. N. Engl. J. Med. 2018, 378, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Rotman, Y.; Koh, C.; Zmuda, J.M.; Kleiner, D.E.; Liang, T.J.; NASH CRN. The association of genetic variability in patatin-like phospholipase domain-containing protein 3 (PNPLA3) with histological severity of nonalcoholic fatty liver disease. Hepatology 2010, 52, 894–903. [Google Scholar] [CrossRef] [Green Version]
- Kawaguchi, T.; Sumida, Y.; Umemura, A.; Matsuo, K.; Takahashi, M.; Takamura, T.; Yasui, K.; Saibara, T.; Hashimoto, E.; Kawanaka, M. Japan Study Group of Nonalcoholic Fatty Liver Disease. Genetic polymorphisms of the human PNPLA3 gene are strongly associated with severity of non-alcoholic fatty liver disease in Japanese. PLoS ONE 2012, 7, e38322. [Google Scholar] [CrossRef] [Green Version]
- Nikpay, M.; Goel, A.; Won, H.H.; Hall, L.M.; Willenborg, C.; Kanoni, S.; Saleheen, D.; Kyriakou, T.; Nelson, C.P.; Hopewell, J.C.; et al. A comprehensive 1000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat. Genet. 2015, 47, 1121–1130. [Google Scholar]
- Koyama, S.; Ito, K.; Terao, C.; Akiyama, M.; Horikoshi, M.; Momozawa, Y.; Matsunaga, H.; Ieki, H.; Ozaki, K.; Onouchi, Y.; et al. Population-specific and trans-ancestry genome-wide analyses identify distinct and shared genetic risk loci for coronary artery disease. Nat. Genet. 2020, 52, 1169–1177. [Google Scholar] [CrossRef]
- Bis, J.C.; Kavousi, M.; Franceschini, N.; Isaacs, A.; Abecasis, G.R.; Schminke, U.; Post, S.W.; Smith, A.V.; Cupples, L.A.; Markus, H.S.; et al. Meta-analysis of genome-wide association studies from the CHARGE consortium identifies common variants associated with carotid intima media thickness and plaque. Nat. Genet. 2011, 43, 940–947. [Google Scholar] [CrossRef] [Green Version]
- Matsukura, M.; Ozaki, K.; Takahashi, A.; Onouchi, Y.; Morizono, T.; Komai, H.; Shigematsu, H.; Kudo, T.; Inoue, Y.; Kimura, H.; et al. Genome-Wide Association Study of Peripheral Arterial Disease in a Japanese Population. PLoS ONE 2015, 10, e0139262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farzanegi, P.; Dana, A.; Ebrahimpoor, Z.; Asadi, M.; Azarbayjani, M.A. Mechanisms of beneficial effects of exercise training on non-alcoholic fatty liver disease (NAFLD): Roles of oxidative stress and inflammation. Eur. J. Sport Sci. 2019, 19, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Xia, N.; Li, H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.H.; Chang, Y.J.; Lin, S.; Yang, W.Y. Hsc70/Stub1 promotes the removal of individual oxidatively stressed peroxisomes. Nat. Commun. 2020, 11, 5267. [Google Scholar] [CrossRef] [PubMed]
- Kander, M.C.; Cui, Y.; Liu, Z. Gender difference in oxidative stress: A new look at the mechanisms for cardiovascular diseases. J. Cell. Mol. Med. 2017, 21, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, U.; Kaushik, S.; Varticovski, L.; Cuervo, A.M. The chaperone-mediated autophagy receptor organizes in dynamic protein complexes at the lysosomal membrane. Mol. Cell. Biol. 2008, 28, 5747–5763. [Google Scholar] [CrossRef] [Green Version]
- Filali-Mouncef, Y.; Hunter, C.; Roccio, F.; Zagkou, S.; Dupont, N.; Primard, C.; Proikas-Cezanne, T.; Reggiori, F. The ménage à trois of autophagy, lipid droplets and liver disease. Autophagy 2022, 18, 50–72. [Google Scholar] [CrossRef]
- Madrigal-Matute, J.; de Bruijn, J.; van Kuijk, K.; Riascos-Bernal, D.F.; Diaz, A.; Tasset, I.; Martín-Segura, A.; Gijbels, M.J.J.; Sander, B.; Kaushik, S.; et al. Protective role of chaperone-mediated autophagy against atherosclerosis. Proc. Natl. Acad. Sci. USA 2022, 119, e2121133119. [Google Scholar] [CrossRef]
- Kaushik, S.; Cuervo, A.M. Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis. Nat. Cell Biol. 2015, 17, 759–770. [Google Scholar] [CrossRef] [Green Version]
- Greenberg, A.S.; Coleman, R.A.; Kraemer, F.B.; McManaman, J.L.; Obin, M.S.; Puri, V.; Yan, Q.W.; Miyoshi, H.; Mashek, D.G. The role of lipid droplets in metabolic disease in rodents and humans. J. Clin. Investig. 2011, 121, 2102–2110. [Google Scholar] [CrossRef] [Green Version]

| Major Allele | Minor Allele | p Value | |
|---|---|---|---|
| Characteristics | n = 80 | n = 43 | |
| Gender, Male/Female, n (%) | 32 (40)/48 (60) | 16 (37.2)/27 (62.8) | 0.762 |
| Age, Years | 60 (47–69) | 64 (56–68) | 0.173 |
| Height, cm | 157.5 (151.2–165.3) | 156.8 (150.9–163.5) | 0.392 |
| Weight, kg | 72.2 (60.8–78.5) | 73.5 (63.2–83.7) | 0.282 |
| BMI, kg/m2 * | 27.5 (25.6–32) | 30 (28–34.1) | 0.031 |
| Hypertension, n (%) * | 43 (53.8) | 33(76.7) | 0.012 |
| Dyslipidemia, n (%) | 63 (78.8) | 36 (83.7) | 0.507 |
| Diabetes, n (%) | 49 (61.3) | 31 (72.1) | 0.229 |
| Hyperuricemia, n (%) * | 26 (32.5) | 22 (51.2) | 0.043 |
| Platelet count, ×103/µL | 19.9 (16.2–23.5) | 17.2 (14.9–21.3) | 0.015 |
| Albumin, g/dL | 4.1 (3.8–4.3) | 4 (3.8–4.2) | 0.512 |
| AST, U/L | 56.5 (38–77.8) | 50 (29–78) | 0.442 |
| ALT, U/L * | 63 (40.8–103.5) | 47 (31–72) | 0.015 |
| GGT, U/L | 63.5 (43.3–111.5) | 65 (39–93) | 0.384 |
| ALP, U/L | 242 (191.8–301) | 224 (190–263) | 0.176 |
| Total cholesterol, mg/dL | 187 (162–212.8) | 175 (160–198) | 0.231 |
| HDL-C, mg/dL | 47 (39.3–60.8) | 47 (40–57) | 0.554 |
| LDL-C, mg/dL | 116.5 (94–135.3) | 104 (93–127) | 0.283 |
| Triglyceride, mg/dL | 145.5 (106.5–178) | 151 (113–187) | 0.494 |
| BUN, mg/dL | 13.3 (11.2–16.4) | 14.5 (11.7–16.9) | 0.323 |
| Cr, mg/dL | 0.69 (0.6–0.9) | 0.69 (0.6–0.9) | 0.775 |
| eGFR, ml/min | 75.1 (63.2–89.7) | 67.3 (57.2–91.1) | 0.259 |
| FPG, mg/dL | 109 (97–134) | 112 (95–130) | 0.920 |
| HbA1c, % | 6.2 (5.6–7.3) | 6.1 (5.7–6.6) | 0.696 |
| HOMA-IR | 4.3 (2.8–7.1) | 5 (3.2–6.9) | 0.392 |
| HSC70 (ng/mL) * | 9.53 (3.9–13) | 4.2 (3.3–9.9) | 0.013 |
| Max-IMT, mm | 1.3 (0.8–1.8) | 1.3 (0.9–2.1) | 0.392 |
| NAFL/NASH, n | 18/62 | 13/30 | 0.346 |
| Steatosis Score, n (0/1/2/3) | 0/52/18/10 | 0/30/9/4 | 0.828 |
| Inflammation Score, n (0/1/2/3) | 2/42/30/6 | 2/25/13/3 | 0.803 |
| Ballooning Score, n (0/1/2) | 17/35/28 | 14/16/13 | 0.387 |
| Fibrosis Stage, n (0/1/2/3/4) | 3/29/20/24/4 | 5/9/12/14/3 | 0.274 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Mori, H.; Tomiga, Y.; Tanaka, K.; Perveen, R.; Mine, K.; Inadomi, C.; Yoshioka, W.; Kubotsu, Y.; Isoda, H.; et al. HSPA8 Single-Nucleotide Polymorphism Is Associated with Serum HSC70 Concentration and Carotid Artery Atherosclerosis in Nonalcoholic Fatty Liver Disease. Genes 2022, 13, 1265. https://doi.org/10.3390/genes13071265
Zhao W, Mori H, Tomiga Y, Tanaka K, Perveen R, Mine K, Inadomi C, Yoshioka W, Kubotsu Y, Isoda H, et al. HSPA8 Single-Nucleotide Polymorphism Is Associated with Serum HSC70 Concentration and Carotid Artery Atherosclerosis in Nonalcoholic Fatty Liver Disease. Genes. 2022; 13(7):1265. https://doi.org/10.3390/genes13071265
Chicago/Turabian StyleZhao, Wenli, Hitoe Mori, Yuki Tomiga, Kenichi Tanaka, Rasheda Perveen, Keiichiro Mine, Chika Inadomi, Wataru Yoshioka, Yoshihito Kubotsu, Hiroshi Isoda, and et al. 2022. "HSPA8 Single-Nucleotide Polymorphism Is Associated with Serum HSC70 Concentration and Carotid Artery Atherosclerosis in Nonalcoholic Fatty Liver Disease" Genes 13, no. 7: 1265. https://doi.org/10.3390/genes13071265
APA StyleZhao, W., Mori, H., Tomiga, Y., Tanaka, K., Perveen, R., Mine, K., Inadomi, C., Yoshioka, W., Kubotsu, Y., Isoda, H., Kuwashiro, T., Oeda, S., Akiyama, T., Zhao, Y., Ozaki, I., Nagafuchi, S., Kawaguchi, A., Aishima, S., Anzai, K., & Takahashi, H. (2022). HSPA8 Single-Nucleotide Polymorphism Is Associated with Serum HSC70 Concentration and Carotid Artery Atherosclerosis in Nonalcoholic Fatty Liver Disease. Genes, 13(7), 1265. https://doi.org/10.3390/genes13071265








