Photosynthetic, Respirational, and Growth Responses of Six Benthic Diatoms from the Antarctic Peninsula as Functions of Salinity and Temperature Variations
Abstract
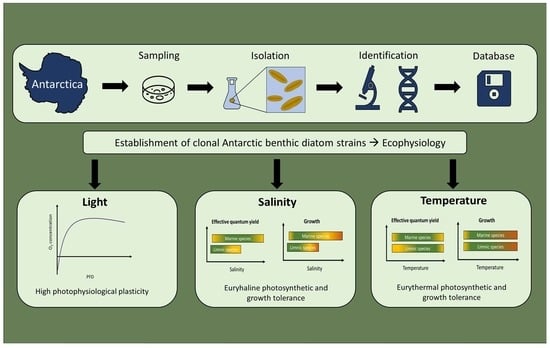
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Culture Establishment and Maintenance Conditions
2.3. Acquisition and Identification of Morphometric Data
2.4. DNA Extraction, Amplification, and Sequencing
2.5. Data Curation
2.6. Photosynthetic Efficiency
2.7. Light Irradiance Curves (P–I Curves)
2.8. Temperature-Dependent Photosynthesis and Respiration
2.9. Growth Rates
2.10. Statistical Analysis
3. Results
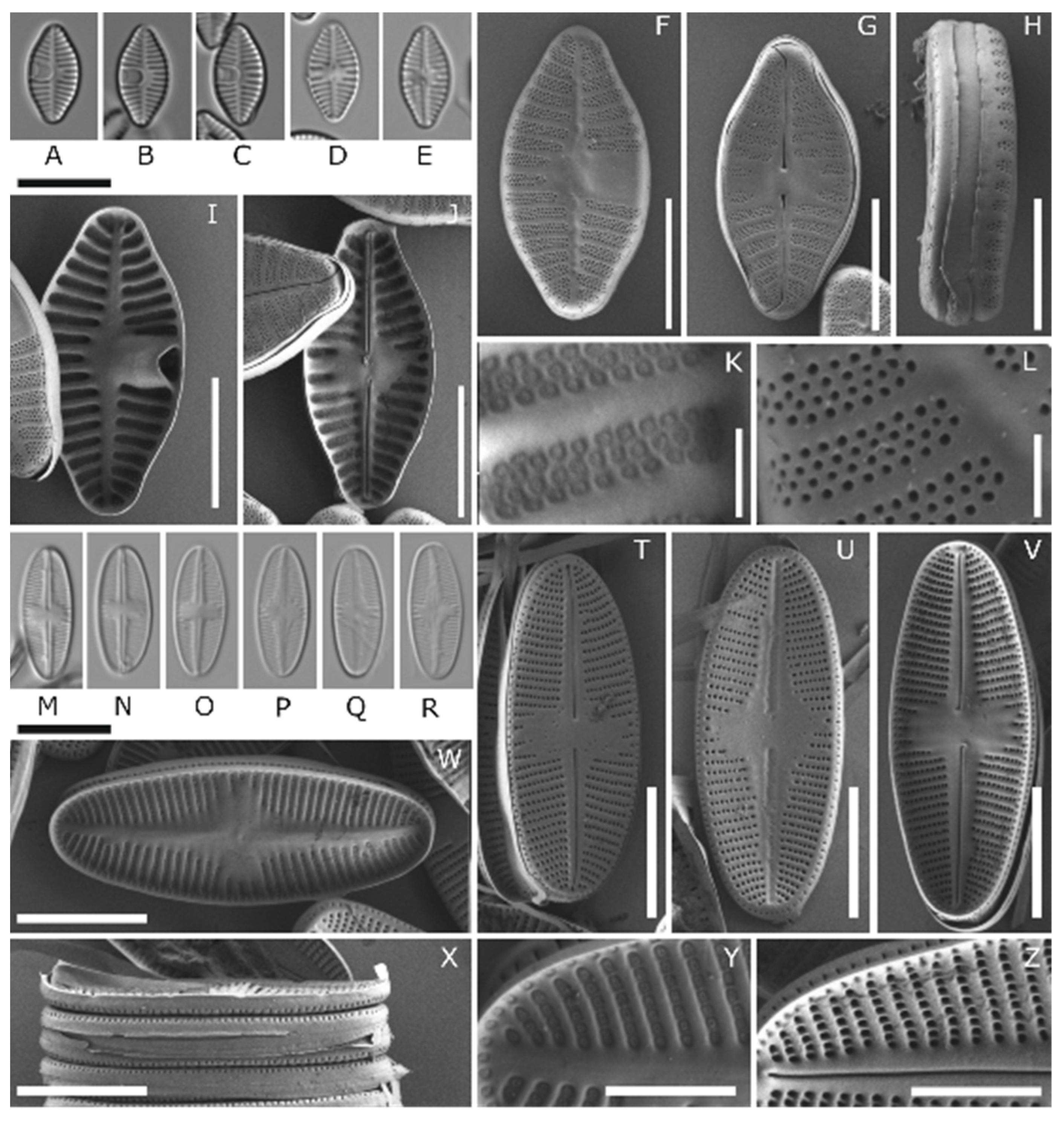
3.1. Species Identification
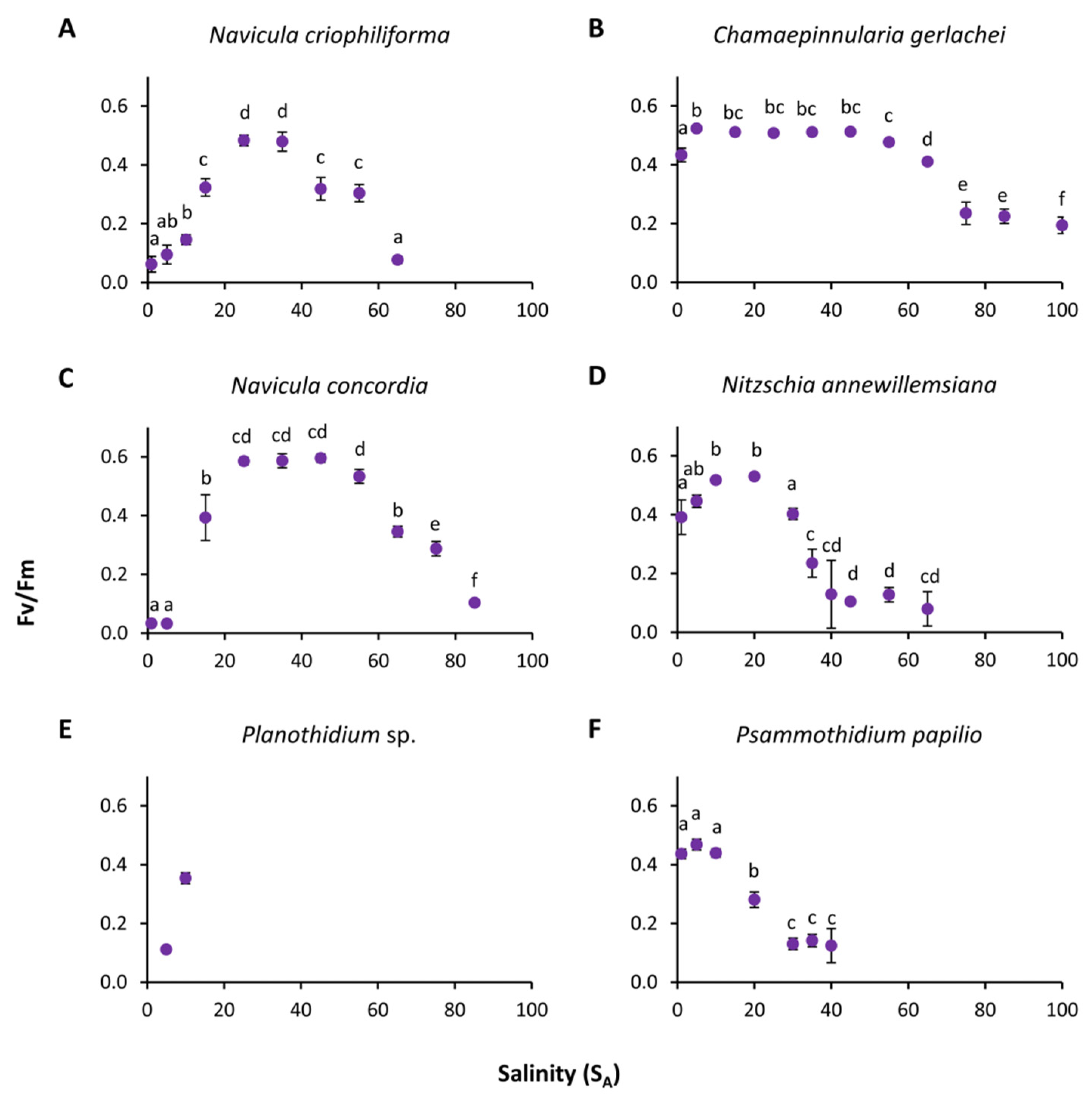
3.2. Photosynthetic Potential
3.3. Light-Dependent Photosynthesis
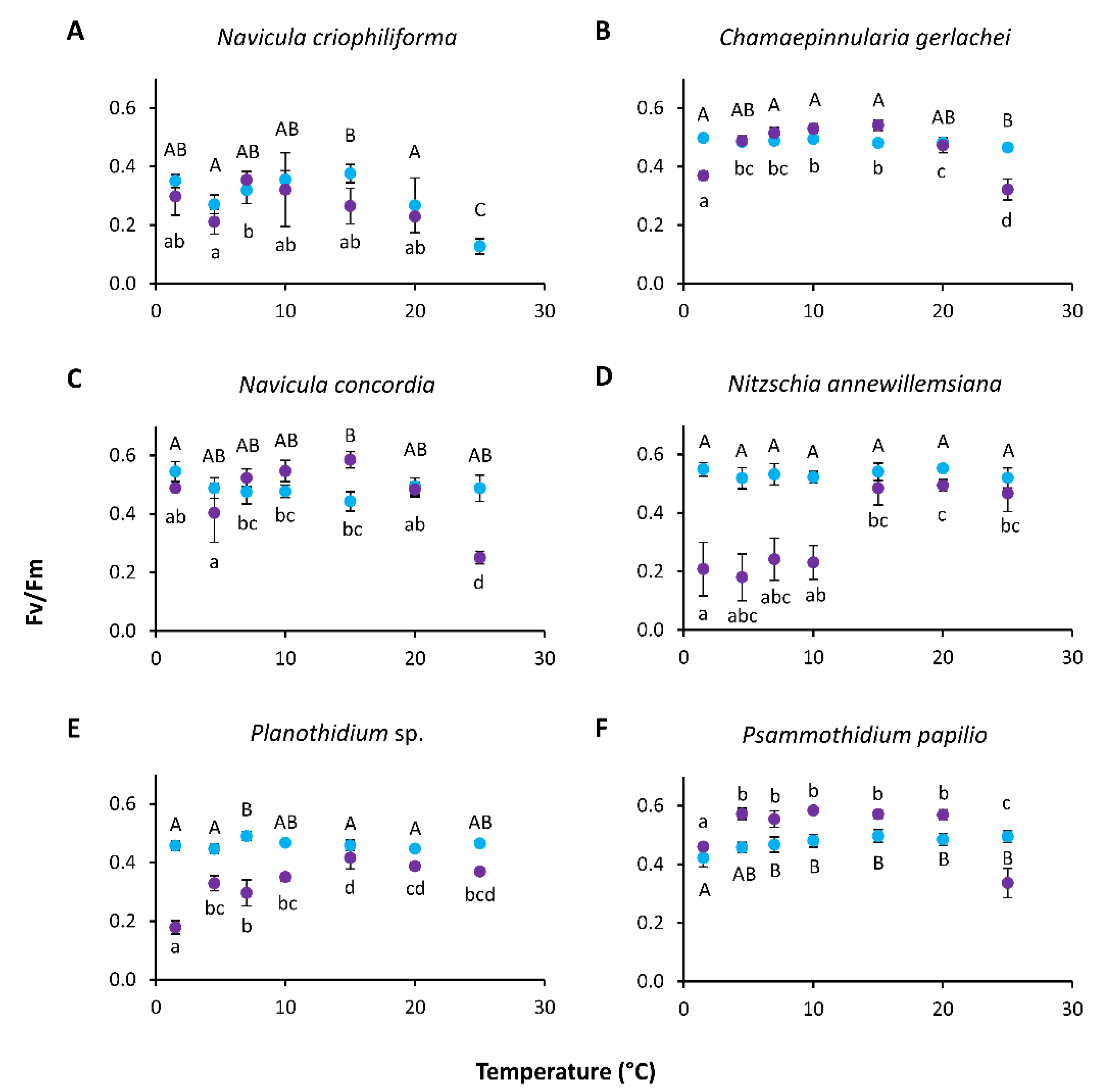
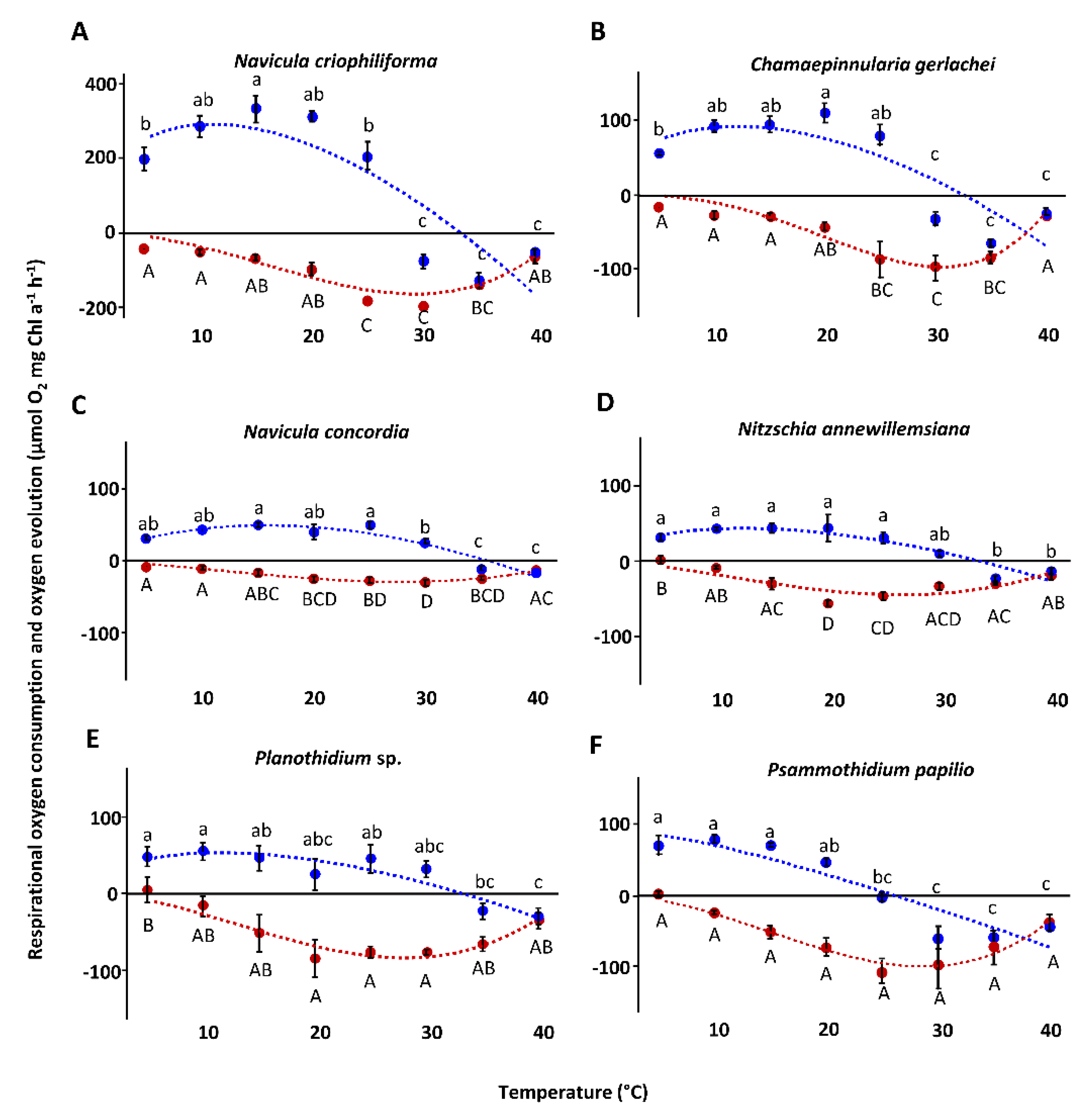
3.4. Temperature-Dependent Photosynthesis and Respiration
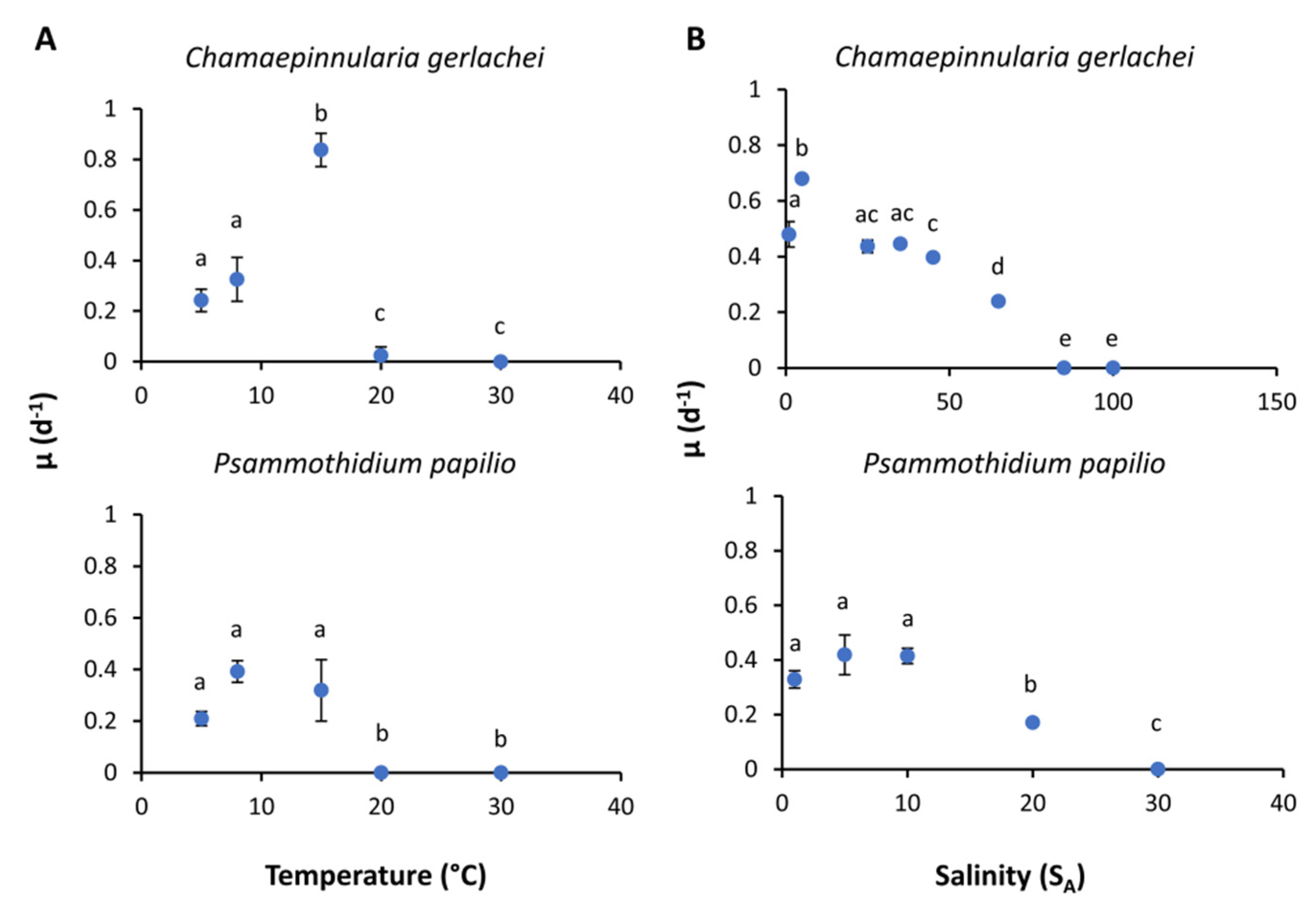
3.5. Growth Rates
4. Discussion
4.1. Light
4.2. Temperature
4.3. Salinity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Summary for Policymaker: Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Field, C.B., Barros, V.R., Dokken, D.J., Mach, K.J., Mastrandrea, M.D., Bilir, T.E., Chatterjee, M., Ebi, K.L., Estrada, Y.O., Genova, R.C., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; pp. 1–32. [Google Scholar]
- Overland, J.E.; Wang, M.; Walsh, J.E.; Stroeve, J.C. Future Arctic climate changes: Adaptation and mitigation time scales. Earth’s Future 2014, 2, 68–74. [Google Scholar] [CrossRef]
- Winterfeld, M.; Mollenhauer, G.; Dummann, W.; Köhler, P.; Lembke-Jene, L.; Meyer, V.; Hefter, J.; McIntyre, C.; Wacker, L.; Kokfelt, U.; et al. Deglacial mobilization of pre-aged terrestrial carbon from degrading permafrost. Nat. Commun. 2018, 9, 3666. [Google Scholar] [CrossRef]
- Cahoon, L.B. The role of benthic microalgae in neritic ecosystems. Oceanogr. Mar. Biol. Annu. Rev. 1999, 37, 47–86. [Google Scholar]
- Risgaard-Petersen, N.; Rysgaard, S.; Nielsen, L.P.; Revsbech, N.P. Diurnal variation of denitrification and nitrification in sediments colonized by benthic microphytes. Limnol. Oceanogr. 1994, 39, 573–579. [Google Scholar] [CrossRef]
- Beninger, P.G.; Cuadrado, D.; van de Koppel, J. Sedimentary and biological patterns on mudflats. In Mudflat Ecology; Beninger, P., Ed.; Springer: Cham, Switzerland, 2018; Volume 7. [Google Scholar]
- Mann, D.G. The species concept in diatoms. Phycologia 1999, 38, 437–495. [Google Scholar] [CrossRef]
- Glud, R.N.; Kühl, M.; Wenzhöfer, F.; Rysgaard, S. Benthic diatoms of a high Arctic fjord (Young Sound, NE Greenland): Importance for ecosystem primary production. Mar. Ecol. Prog. Ser. 2002, 238, 15–29. [Google Scholar] [CrossRef]
- Woelfel, J.; Schumann, R.; Peine, F.; Flohr, A.; Kruss, A.; Tegwoski, J.; Blondel, P.; Wiencke, C.; Karsten, U. Microphytobenthos of Arctic Kongsfjorden (Svalbard, Norway): Biomass and potential primary production along the shoreline. Polar Biol. 2010, 33, 1239–1253. [Google Scholar] [CrossRef][Green Version]
- Woelfel, J.; Eggert, A.; Karsten, U. Global warming could stimulate Arctic microphytobenthos primary production in Kongsfjorden (Svalbard, Norway)—in situ measurements and modelled changes. Mar. Ecol. Prog. Ser. 2014, 501, 25–40. [Google Scholar] [CrossRef]
- Sevilgen, D.S.; de Beer, D.; Al-Handal, A.Y.; Brey, T.; Polerecky, L. Oxygen budgets in subtidal arctic (Kongsfjorden, Svalbard) and temperate (Helgoland, North Sea) microphytobenthic communities. Mar. Ecol. Prog. Ser. 2014, 504, 27–42. [Google Scholar] [CrossRef][Green Version]
- Glud, R.N.; Woelfel, J.; Karsten, U.; Kühl, M.; Rysgaard, S. Benthic microalgal production in the Arctic: Applied methods and status of the current database. Bot. Mar. 2009, 52, 559–571. [Google Scholar] [CrossRef]
- Gilbertson, R.; Langan, E.; Mock, T. Diatoms and their microbiomes in complex and changing polar oceans. Front. Microbiol. 2022, 13, 786764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Qu, C.; Zhang, K.; He, Y.; Zhao, X.; Yang, L.; Zheng, Z.; Ma, X.; Wang, X.; Wang, W.; et al. Adaptation to extreme Antarctic environments revealed by the genome of a sea ice green alga. Curr. Biol. 2020, 30, 3330–3341. [Google Scholar] [CrossRef] [PubMed]
- Hüner, N.P.A.; Smith, D.R.; Cvetkovska, M.; Zhang, X.; Ivanov, A.G.; Szyszka-Mroz, B.; Kalra, I.; Morgan-Kiss, R. Photosynthetic adaptation to polar life: Energy balance, photoprotection and genetic redundancy. J. Plant Physiol. 2022, 268, 153557. [Google Scholar] [CrossRef] [PubMed]
- Morgan-Kiss, R.M.; Ivanov, A.G.; Modla, S.; Czymmek, K.; Hüner, N.P.A.; Priscu, J.C.; Lisle, J.T.; Hanson, T.E. Identity and physiology of a new psychrophilic eukaryotic green alga, Chlorella sp., strain BI, isolated from a transitory pond near Bratina Island, Antarctica. Extremophiles 2008, 12, 701–711. [Google Scholar] [CrossRef]
- Maykut, G.A.; Grenfell, T.C. The spectral distribution of light beneath first-year sea ice in the Arctic Ocean. Limnol. Oceanogr. 1975, 20, 554–563. [Google Scholar] [CrossRef]
- Lazzara, L.; Nardello, I.; Ermanni, C.; Mangoni, O.; Saggiomo, V. Light environment and seasonal dynamics of microalgae in the annual sea ice at Terra Nova Bay, Ross Sea, Antarctica. Antarct. Sci. 2007, 19, 83–92. [Google Scholar] [CrossRef]
- Post, A.L.; Meijers, A.J.S.; Fraser, A.D.; Meiners, K.M.; Ayers, J.; Bindoff, N.L.; Bindoff, H.J.; Griffiths, A.P.; Van de Putte, P.E.; O’Brien, K.M.; et al. Chapter 4 Environmental Setting. In Biogeographic Atlas of the Southern Ocean; de Broyer, C., Koubbi, P., Griffith, H.J., Raymond, B., Ude-kem d’Acoz, C., Van de Putte, A.P., Danis, B., David, B., Grant, S., Gutt, J., et al., Eds.; The Scientific Committee on Antarctic Research: Cambridge, UK, 2014; pp. 46–64. [Google Scholar]
- Kanz, B.; Büdel, B.; Jung, P.; Karsten, U.; Printzen, C. Leben zwischen Eis und Felsen. Biol. Unserer Zeit 2020, 50, 122–133. [Google Scholar] [CrossRef]
- Wulff, A.; Roleda, M.Y.; Zacher, K.; Wiencke, C. Exposure to sudden light burst after prolonged darkness—A case study on benthic diatoms in Antarctica. Diatom Res. 2008, 23, 519–532. [Google Scholar] [CrossRef]
- Wulff, A.; Iken, K.; Quartino, M.L.; Al-Handal, A.; Wiencke, C.; Clayton, M.N. Biodiversity, biogeography and zonation of benthic micro- and macroalgae in the Arctic and Antarctic. Bot. Mar. 2009, 52, 491–507. [Google Scholar] [CrossRef]
- Palmisano, A.C.; SooHoo, J.B.; White, D.C.; Smith, G.A.; Stanton, G.R.; Burckle, L.H. Shade adapted benthic diatoms beneath Antarctic Sea Ice. J. Phycol. 1985, 21, 664–667. [Google Scholar] [CrossRef]
- Wilhelm, C.; Büchel, C.; Fisahn, J.; Goss, R.; Jakob, T.; LaRoche, J.; Lavaud, J.; Lohr, M.; Riebesell, U.; Stehfest, K.; et al. The regulation of carbon and nutrient assimilation in diatoms is significantly different from green algae. Protist 2006, 157, 91–124. [Google Scholar] [CrossRef] [PubMed]
- Harper, M.A. Movement and migration of diatoms on sand grains. Br. Phycol. J. 1969, 4, 97–103. [Google Scholar] [CrossRef]
- Schlie, C.; Karsten, U. Microphytobenthic diatoms isolated from sediments of the Adventfjorden (Svalbard): Growth as function of temperature. Polar Biol. 2017, 40, 1043–1051. [Google Scholar] [CrossRef]
- Karsten, U.; Schaub, I.; Woelfel, J.; Sevilgen, D.; Schlie, C.; Becker, B.; Wulff, A.; Graeve, M.; Wagner, H. Living on cold substrata: New insights and approaches to study microphytobenthos ecophysiology and ecology in Kongsfjorden. In The Ecosystem of Kongsfjorden, Svalbard. Advances in Polar Ecology; Hop, H., Wiencke, C., Eds.; Springer Nature Switzerland AG: Basle, Switzerland, 2019; Volume 2, pp. 303–330. [Google Scholar]
- Longhi, M.L.; Schloss, I.R.; Wiencke, C. Effect of Irradiance and Temperature on Photosynthesis and Growth of Two Antarctic Benthic Diatoms, Gyrosigma subsalinum and Odontella litigiosa. Bot. Mar. 2003, 46, 276–284. [Google Scholar] [CrossRef]
- Morita, R.Y. Psychrophilic bacteria. Bacteriol. Rev. 1975, 39, 144–167. [Google Scholar] [CrossRef]
- Blanc, G.; Agarkova, I.; Grimwood, J.; Kuo, A.; Brueggeman, A.; Dunigan, D.D.; Gurnon, J.; Ladunga, I.; Lindquist, E.; Lucas, S.; et al. The genome of the polar eukaryotic microalga Coccomyxa subellipsoidea reveals traits of cold adaptation. Genome Biol. 2012, 13, R39. [Google Scholar] [CrossRef]
- Wiencke, C.; Tom Dieck, I. Temperature requirements for growth and survival of macroalgae from Antarctica and southern Chile. Mar. Ecol. Prog. Ser. 1990, 59, 157–170. [Google Scholar] [CrossRef]
- Sabbe, K.; Verleyen, E.; Hodgson, D.A.; Vanhoutte, K.; Vyverman, W. Benthic diatom flora of freshwater and saline lakes in the Larsemann Hills and Rauer Islands, East Antarctica. Antarct. Sci. 2003, 15, 227–248. [Google Scholar] [CrossRef]
- Gómez, I.; Wulff, A.; Roleda, M.Y.; Huovinen, P.; Karsten, U.; Quartino, M.L.; Dunton, K.; Wiencke, C. Light and temperature demands of marine benthic microalgae and seaweeds in polar regions. In Biology of Polar Benthic Algae; Wiencke, C., Ed.; Walter de Gruyter: Berlin, Germany, 2009; pp. 195–220. [Google Scholar]
- Verleyen, E.; Van de Vijver, B.; Tytgat, B.; Pinseel, E.; Hodgson, D.A.; Kopalová, K.; Chown, S.L.; Van Ranst, E.; Imura, S.; Kudoh, S.; et al. Diatoms define a novel freshwater biogeography of the Antarctic. Ecography 2021, 44, 548–560. [Google Scholar] [CrossRef]
- Kirst, G.O.; Wiencke, C. Ecophysiology of polar algae. Oceanogr. Lit. Rev. 1995, 12, 1094. [Google Scholar] [CrossRef]
- Glaser, K.; Karsten, U. Salinity tolerance in biogeographically different strains of the marine benthic diatom Cylindrotheca closterium (Bacillariophyceae). J. Appl. Phycol. 2020, 32, 3809–3816. [Google Scholar] [CrossRef]
- Klöser, H.; Ferreyra, G.A.; Schloss, I.R.; Mercuri, G.; Laturnus, F.; Curtosi, A. Hydrography of Potter Cove, a Small Fjord-like Inlet on King George Island (South Shetlands). Estuar. Coast. Shelf Sci. 1994, 38, 523–537. [Google Scholar] [CrossRef]
- Hernández, E.A.; Lopez, J.L.; Piquet, A.M.-T.; Mac Cormack, W.P.; Buma, A.G.J. Changes in salinity and temperature drive marine bacterial communities’ structure at Potter Cove, Antarctica. Polar Biol. 2019, 42, 2177–2191. [Google Scholar] [CrossRef]
- Guillard, R.R.L.; Ryther, J.H. Studies or marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea Cleve. Canandian J. Microbiol. 1962, 8, 229–239. [Google Scholar]
- Guillard, R.R.L. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals; Smith, W.L., Chanley, M.H., Eds.; Plenum Press: New York, NY, USA, 1975; pp. 26–60. [Google Scholar]
- Walne, P.R. Studies on the food value of nineteen genera of algae to juvenile bivalves of the genera Ostrea, Crassostrea, Mercenaria and Mytilus. Fish. Investig. Ser. 1970, 2, 26. [Google Scholar]
- Gemeinholzer, B.; Droege, G.; Zetzsche, H.; Haszprunar, G.; Klenk, H.-P.; Güntsch, A.; Berendsohn, W.; Wägele, J.W. The DNA Bank Network: The start from a German initiative. Biopreserv. Biobank. 2011, 9, 51–55. [Google Scholar] [CrossRef]
- Zimmermann, J.; Jahn, R.; Gemeinholzer, B. Barcoding diatoms: Evaluation of the V4 subregion on the 18S rRNA gene, including new primers and protocols. Org. Divers. Evol. 2011, 11, 173. [Google Scholar] [CrossRef]
- Jahn, R.; Abarca, N.; Gemeinholzer, B.; Mora, D.; Skibbe, O.; Kulikovskiy, M.; Gusev, E.; Kusber, W.H.; Zimmermann, J. Planothidium lanceolatum and Planothidium frequentissimum reinvestigated with molecular methods and morphology: Four new species and the taxonomic importance of the sinus and cavum. Diatom Res. 2017, 32, 75–107. [Google Scholar] [CrossRef]
- Abarca, N.; Jahn, R.; Zimmermann, J.; Enke, N. Does the cosmopolitan diatom Gomphonema parvulum (Kützing) Kützing have a biogeography? PLoS ONE 2014, 9, e086885. [Google Scholar] [CrossRef]
- Droege, G.; Barker, K.; Seberg, O.; Coddington, J.; Benson, E.; Berendsohn, W.G.; Bunk, B.; Butler, C.; Cawsey, E.M.; Deck, J.; et al. The Global Genome Biodiversity Network (GGBN) Data Standard specification. Database 2016, 2016, baw125. [Google Scholar] [CrossRef]
- Gruenstaeudl, M. Annonex2embl: Automatic preparation of annotated DNA sequences for bulk submissions to ENA. Bioinformatics 2019, 36, 3841–3848. [Google Scholar] [CrossRef] [PubMed]
- Prelle, L.R.; Graiff, A.; Gründling-Pfaff, S.; Sommer, V.; Kuriyama, K.; Karsten, U. Photosynthesis and respiration of Baltic Sea benthic diatoms to changing environmental conditions and growth responses of selected species as affected by an adjacent peatland (Hütelmoor). Front. Microbiol. 2019, 10, 1500. [Google Scholar] [CrossRef] [PubMed]
- HELCOM (2015). Annex C-4. Phytoplankton Chlorophyll a, in HELCOM Combine, (Helsinki: HELCOM), 257-263. Available online: https://helcom.fi/media/publications/Manual-for-Marine-Monitoring-in-the-COMBINE-Programme-of-HELCOM.pdf (accessed on 30 November 2021).
- Walsby, A.E. Numerical integration of phytoplankton photosynthesis through time and depth in a water column. New Phytol. 1997, 136, 189–209. [Google Scholar] [CrossRef]
- Karsten, U.; Lütz, C.; Holzinger, A. Ecophysiological performance of the aeroterrestrial green alga Klebsormidium crenulatum (Charophyceae, Streptophyta) isolated from an alpine soil crust with an emphasis on desiccation stress. Phycologia 2010, 46, 1187–1197. [Google Scholar] [CrossRef]
- Karsten, U.; Klimant, I.; Holst, G. A new in vivo fluorimetric technique to measure growth of adhering phototrophic microorganisms. Appl. Environ. Microbiol. 1996, 62, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Gustavs, L.; Schumann, R.; Eggert, A.; Karsten, U. In vivo growth fluorometry: Accuracy and limits of microalgal growth rate measurements in ecophysiological investigations. Aquat. Microb. Ecol. 2009, 55, 95–104. [Google Scholar] [CrossRef]
- Prelle, L.R.; Albrecht, M.; Karsten, U.; Damer, P.; Giese, T.; Jähns, J.; Müller, S.; Schulz, L.; Viertel, L.; Glaser, K.; et al. Ecophysiological and Cell Biological Traits of Benthic Diatoms From Coastal Wetlands of the Southern Baltic Sea. Front. Microbiol. 2021, 12, 796. [Google Scholar] [CrossRef]
- Yan, W.; Hunt, L.A. An equation for modelling the temperature response of plants using only the cardinal temperatures. Ann. Bot. 1999, 84, 607–614. [Google Scholar] [CrossRef]
- Van de Vijver, B.; Sterken, M.; Vyverman, W.; Mataloni, G.; Nedbalová, L.; Kopalová, K.; Elster, J.; Verleyen, E.; Sabbe, K. Four new non-marine diatom taxa from the subantarctic and Antarctic regions. Diatom Res. 2010, 25, 431–443. [Google Scholar] [CrossRef]
- Kopalová, K.; Veselá, J.; Elster, J.; Nedbalová, L.; Komárek, J.; Van de Vijver, B. Benthic diatoms (Bacillariophyta) from seepages and streams on James Ross Island (NW Weddell Sea, Antarctica). Plant Ecol. Evol. 2012, 145, 190–208. [Google Scholar] [CrossRef]
- Sterken, M.; Verleyen, E.; Jones, V.J.; Hodgson, D.A.; Vyverman, W.; Sabbe, K.; Van de Vijver, B. An illustrated and annotated checklist of freshwater diatoms (Bacillariophyta) from Livingston, Signy and Beak Island (Maritime Antarctic Region). Plant Ecol. Evol. 2015, 148, 431–455. [Google Scholar] [CrossRef]
- Zidarova, R.; Kopalová, K.; Van de Vijver, B. Diatoms from the Antarctic Region. I: Maritime Antarctica; Koeltz Botanical Books: Oberreifenberg, Germany, 2016; Volume 24. [Google Scholar]
- Witkowski, A.; Riaux-Gobin, C.; Daniszewska-Kowalczyk, G. New marine diatom (Bacillariophyta) Species described from Kerguelen Islands coastal area and pertaining to Navícula S.S. with some remarks on morphological variation of the genus. Vie et Milieu 2010, 60, 117–133. [Google Scholar]
- Zidarova, R.; Ivanov, P.; Hineva, E.; Dzhembekova, N. Diversity and habitat preferences of benthic diatoms from South Bay (Livingston Island, Antarctica). Plant Ecol. Evol. 2022, 155, 70–106. [Google Scholar] [CrossRef]
- Hamsher, S.; Kateřina, K.; Kociolek, P.; Zidarova, R.; Van de Vijver, B. The genus Nitzschia on the South Shetland Islands and James Ross Island. J. Czech Phycol. Soc. 2016, 16, 79–102. [Google Scholar] [CrossRef]
- Kellogg, D.E.; Stuiver, M.; Kellogg, T.B.; Denton, G.H. Non-marine diatoms from late Wisconsin perched deltas in Taylor Valley, Antarctica. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1980, 30, 157–189. [Google Scholar] [CrossRef]
- Silva, J.F.; Oliveira, M.A.; Alves, R.P.; Cassol, A.P.V.; Anunciação, R.R.; Silva, E.P.; Luis Schünemann, A.; Batista Pereira, A. Geographic distribution of epilithic diatoms (Bacillariophyceae) in Antarctic lakes, South Shetland Islands, Maritime Antarctica Region. Check List 2019, 15, 797–809. [Google Scholar] [CrossRef]
- Smetacek, V. Diatoms and the Ocean Carbon Cycle. Protist 1999, 150, 25–32. [Google Scholar] [CrossRef]
- Jones, J. The diversity, distribution and ecology of diatoms from Antarctic inland waters. Biodivers. Conserv. 1996, 5, 1433–1449. [Google Scholar] [CrossRef]
- Karsten, U.; Schumann, R.; Rothe, S.; Jung, I.; Medlin, L. Temperature and light requirements for growth of two diatom species (Bacillariophyceae) isolated from an Arctic macroalga. Polar Biol. 2006, 29, 476–486. [Google Scholar] [CrossRef]
- Drew, E.A.; Hastings, R.M. A year-round ecophysiological study of Himantothallus grandifolius (Desmarestiales, Phaeophyta) at Signy Island, Antarctica. Phycologia 1992, 31, 262–277. [Google Scholar] [CrossRef]
- Hoffmann, R.; Al-Handal, A.Y.; Wulff, A.; Deregibus, D.; Zacher, K.; Quartino, M.L.; Wenzhöfer, F.; Braeckman, U. Implications of Glacial Melt-Related Processes on the Potential Primary Production of a Microphytobenthic Community in Potter Cove (Antarctica). Front. Mar. Sci. 2019, 6, 655. [Google Scholar] [CrossRef]
- Blommaert, L.; Lavaud, J.; Vyverman, W.; Sabbe, K. Behavioural versus physiological photoprotection in epipelic and epipsammic benthic diatoms. Eur. J. Phycol. 2018, 53, 146–155. [Google Scholar] [CrossRef]
- Pavlov, A.K.; Leu, E.; Hanelt, D.; Bartsch, I.; Karsten, U.; Hudson, S.R.; Gallet, J.-C.; Cottier, F.; Cohen, J.H.; Berge, J.; et al. The underwater light climate in Kongsfjorden and its ecological implications. In The Ecosystem of Kongsfjorden, Svalbard. Advances in Polar Ecology; Hop, H., Wiencke, C., Eds.; Springer Nature Switzerland AG: Basle, Switzerland, 2019; Volume 2, pp. 137–170. [Google Scholar]
- Malerba, M.E.; Palacios, M.M.; Palacios Delgado, Y.M.; Beardall, J.; Marshall, D.J. Cell size, photosynthesis and the package effect: An artificial selection approach. New Phytol. 2018, 219, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Serôdio, J.; Vieira, S.; Cruz, S. Photosynthetic activity, photoprotection and photoinhibition in intertidal microphytobenthos as studied in situ using variable chlorophyll fluorescence. Cont. Shelf Res. 2008, 28, 1363–1375. [Google Scholar] [CrossRef]
- Han, B.-P. Effect of photoinhibition on algal photosynthesis: A dynamic model. J. Plankton Res. 2000, 22, 865–885. [Google Scholar] [CrossRef]
- Young, J.N.; Goldman, J.A.; Kranz, S.A.; Torell, P.D.; Morel, F.M. Slow carboxylation of Rubisco constrains the maximum rate of carbon fixation during Antarctic phytoplankton blooms. New Phytol. 2014, 205, 172–181. [Google Scholar] [CrossRef]
- Atkin, O.K.; Tjoelker, M.G. Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci. 2003, 8, 343–351. [Google Scholar] [CrossRef]
- Karsten, U.; Herburger, K.; Holzinger, A. Photosynthetic plasticity in the green algal species Klebsormidium flaccidum (Streptophyta) from a terrestrial and a freshwater habitat. Phycologia 2016, 56, 213–220. [Google Scholar] [CrossRef]
- Gleich, S.J.; Plough, L.V.; Gilbert, P.M. Photosynthetic efficiency and nutrient physiology of the diatom Thalassiosira pseudonana at three growth temperatures. Mar. Biol. 2020, 167, 124. [Google Scholar] [CrossRef]
- Bailleul, B.; Berne, N.; Murik, O.; Petroutsos, D.; Prihoda, J.; Tanaka, A.; Villanova, V.; Bligny, R.; Flori, S.; Falconet, D.; et al. Energetic coupling between plastids and mitochondria drives CO2 assimilation in diatoms. Nature 2015, 524, 366–369. [Google Scholar] [CrossRef]
- Eggert, A.; van Hasselt, P.R.; Breeman, A.M. Chilling-induced photoinhibition in nine isolates of Valonia utricularis (Chlorophyta) from different climate regions. J. Plant Physiol. 2003, 160, 881–891. [Google Scholar] [PubMed]
- Graiff, A.; Liessner, D.; Karsten, U.; Bartsch, I. Temperature tolerance of western Baltic Sea Fucus vesiculosus—Growth, photosynthesis and survival. J. Exp. Mar. Biol. Ecol. 2015, 471, 8–16. [Google Scholar] [CrossRef]
- Fiala, M.; Oriol, L. Light-temperature interactions on the growth of Antarctic diatoms. Polar Biol. 1989, 10, 629–636. [Google Scholar] [CrossRef]
- Gilstad, M.; Sakshaug, E. Growth rates of ten diatom species from the Barents Sea at different irradiances and day lengths. Mar. Ecol. Prog. Ser. 1990, 64, 169–173. [Google Scholar] [CrossRef]
- Abele, D.; Vazquez, S.; Buma, A.G.; Hernandez, E.; Quiroga, C.; Held, C.; Frickenhaus, S.; Harms, L.; Lopez, J.; Helmke, E.; et al. Pelagic and benthic communities of the Antarctic ecosystem of Potter Cove: Genomics and ecological implications. Mar. Genom. 2017, 33, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Holland, O.; Shaw, J.; Stark, J.S.; Wilson, K.W. Hull fouling marine invasive species pose a very low, but plausible, risk of introduction to East Antarctica in climate change scenarios. Divers. Distrib. 2021, 27, 973–988. [Google Scholar] [CrossRef]
- Palmisano, A.C.; SooHoo, J.B.; Moe, R.L.; Sullivan, C.W. Sea ice microbial communities. VII. Changes in under-ice spectral irradiance during the development of Antarctic Sea ice microalgal communities. Mar. Ecol. Prog. Ser. 1987, 35, 165–173. [Google Scholar] [CrossRef]
- Ryan, K.G.; Ralph, P.; McMinn, A. Acclimation of Antarctic bottom-ice algal communities to lowered salinities during melting. Polar Biol. 2004, 27, 679–686. [Google Scholar] [CrossRef]
- Ralph, P.J.; Ryan, K.G.; Martin, A.; Fenton, G. Melting out of sea ice causes greater photosynthetic stress in algae than freezing. J. Phycol. 2007, 43, 948–956. [Google Scholar] [CrossRef]
- Petrou, K.; Doblin, M.A.; Ralph, P.J. Heterogeneity in the photoprotective capacity of three Antarctic diatoms during short-term changes in salinity and temperature. Mar. Biol. 2011, 158, 1029–1041. [Google Scholar] [CrossRef]
- Ludwiczak, A.; Osiak, M.; Cárdenas-Pérez, S.; Lubińska-Mielińska, S.; Piernik, A. Osmotic Stress or Ionic Composition: Which Affects the Early Growth of Crop Species More? Agronomy 2021, 11, 435. [Google Scholar] [CrossRef]
- Karsten, U.; Wiencke, C.; Kirst, G.O. The Effect of Salinity Changes upon the Physiology of Eulittoral Green Macroalgae from Antarctica and Southern Chile. I. Cell Viability, Growth, Photosynthesis and Dark Respiration. J. Plant Physiol. 1991, 138, 667–673. [Google Scholar] [CrossRef]
- Karsten, U.; Schlie, C.; Woelfel, J.; Becker, B. Benthic diatoms in Arctic Seas-ecological functions and adaptions. Polarforschung 2012, 81, 77–84. [Google Scholar]
- Admiraal, W. Salinity tolerance of benthic estuarine diatoms as tested with a rapid polarographic measurement of photosynthesis. Mar. Biol. 1977, 39, 11–18. [Google Scholar] [CrossRef]
- Scholz, B.; Liebezeit, G. Growth responses of 25 benthic marine Wadden Sea diatoms isolated from the Solthörn tidal flat (southern North Sea) in relation to varying culture conditions. Diatom Res. 2012, 27, 65–73. [Google Scholar] [CrossRef]
- Woelfel, J.; Schoknecht, A.; Schaub, I.; Enke, N.; Schumann, R.; Karsten, U. Growth and photosynthesis characteristics of three benthic diatoms from the brackish southern Baltic Sea in relation to varying environmental conditions. Phycologia 2014, 53, 639–651. [Google Scholar] [CrossRef]
- Thomas, D.N.; Dieckmann, G.S. Antarctic Sea ice—A habitat for extremophiles. Science 2002, 295, 641–644. [Google Scholar] [CrossRef]





| Sample Location | Site | Sample Origin | Altitude/ Water Depth | Date of Sampling | Collector | Georeference |
|---|---|---|---|---|---|---|
| APC06 | Potter Cove, coast at Penon 0 | Marine | 0 m | 29 January 2020 | J. Zimmermann | S 62°14′30.55″, W 58°40′54.96″ |
| APC12 | Potter Cove, coast east of Carlini Station | Brackish | 0 m | 30 January 2020 | J. Zimmermann | S 62°14′07.78″, W 58°39′27.91″ |
| APC14 | Potter Cove, Island A4 | Marine | 15 m depth | 31 January 2020 | J. Zimmermann, G.L. Campana, Diver Team | S 62°13′43.61″, W 58°39′49.36″ |
| APC18 | Potter Cove, drinking water reservoir | Freshwater | 51 m a.s.l. | 1 February 2020 | J. Zimmermann | S 62°14′16.30″, W 58°39′44.10″ |
| APC28 | Potter Cove, coast at Penon de Pesca | Marine | 5 m depth | 7 February 2020 | J. Zimmermann, G.L. Campana, Diver Team | S 62°14′16.5″, W 58°42′44.2” |
| Strain | Scientific Name | Marine/ Freshwater | Length (µm) | Width (µm) | Striae in 10 µm | Marker Genes |
|---|---|---|---|---|---|---|
| APC14 D296_001 | Chamaepinnularia gerlachei | Marine | 17.1–20.6 | 4.1–5.4 | 18–20 | whole 18 S, rbcL |
| APC06 D288_003 | Navicula criophiliforma | Marine | 24.2–52.4 | 5.8–8.5 | 11–12 | 18 SV4, rbcL |
| APC28 D310_004 | Navicula concordia | Marine | 29.5–30.5 | 4.7–5.3 | 13–14 | 18 SV4, rbcL |
| APC18 D300_012 | Nitzschia annewillemsiana | Freshwater | 15.2–17.1 | 3.6–4.1 | 25–26 | 18 SV4, rbcL |
| APC18 D300_015 | Planothidium sp. | Freshwater | 10.9–11.3 | 5.6–6.1 | 16–18 (RV) 17–18 (SV) | 18 SV4, rbcL |
| APC18 D300_023 | Psammothidium papilio | Freshwater | 13.8–14.7 | 5.4–5.9 | 28–30 (RV) 26–30 (SV) | 18 SV4, rbcL |
| Species | NPPmax (µmol O2 mg−1 Chl a h−1) | Respiration (µmol O2 mg−1 Chl a h−1) | α (µmol O2 mg−1 Chl a h−1) (µmol Photons m−2 s−1)−1 | β (µmol O2 mg−1 Chl a h−1) (µmol Photons m−2 s−1)−1 | Ik (µmol Photons m−2 s−1) | Ic (µmol Photons m−2 s−1) | NPPmax: Respiration |
|---|---|---|---|---|---|---|---|
| Navicula criophiliforma | 202.3 ± 45.4 a | −47 ± 8.9 a | 3.9 ± 0.4 a | −0.03 ± 0.02 a | 64 ± 11.5 a | 13.4 ± 1.4 ab | 4.3 ± 0.9 a |
| Chamaepinnularia gerlachei | 90.3 ± 4.1 b | −26.2 ± 0.8 b | 2 ± 0.1 b | −0.01 ± 0.00 bc | 59.8 ± 1.7 a | 15.3 ± 0.2 a | 3.5 ± 0.1 ab |
| Navicula concordia | 42 ± 14.5 bc | −10.5 ± 3.1 c | 2 ± 0.6 b | −0.02 ± 0.01 ac | 25.9 ± 2.7 bc | 5.8 ± 1 c | 4 ± 0.4 a |
| Nitzschia annewillemsiana | 36.6 ± 5.4 bc | −25.9 ± 3.7 b | 3.8 ± 0.69 a | −0.01 ± 0.00 bc | 16.3 ± 3.9 b | 8.7 ± 0.3 cd | 1.4 ± 0 c |
| Planothidium sp. | 30.7 ± 0.5 c | −16.0 ± 6.2 bc | 1.1 ± 0.3 b | 0.0 ± 0.0 b | 23.6 ± 8.7 bc | 17.5 ± 3 a | 1.9 ± 0.4 c |
| Psammothidium papilio | 52.2 ± 5 bc | −19.2 ± 1.7 bc | 2.1 ± 0.2 b | −0.00 ± 0.00 bc | 33.7 ± 1.8 c | 10.6 ± 0.2 bd | 2.7 ± 0.1 b |
| Navicula criophiliforma | Chamaepinnularia gerlachei | Navicula concordia | Nitzschia annewillemsiana | Planothidium sp. | Psammothidium papilio | |||
|---|---|---|---|---|---|---|---|---|
| Growth (salinity) | Maximal growth rate | - | 0.58 | - | - | - | 0.42 | |
| Optimal salinity | - | 6.53 | - | - | - | 5.28 | ||
| Maximal salinity | - | 93.69 | - | - | - | 29.26 | ||
| Residual sum of squares | - | 0.0884 | - | - | - | 0.03172 | ||
| Salinity range for | Optimal growth (80% growth rate) | - | 0.13–31.79 | - | - | - | 0.90–13.71 | |
| Growth (20% growth rate) | - | 0.00–79.23 | - | - | - | 0.00–25 | ||
| Growth (temperature) | Maximal growth rate | - | 0.44 | - | - | - | 0.30 | |
| Optimal temperature | - | 12.96 | - | - | - | 6.48 | ||
| Maximal temperature | - | 28.85 | - | - | - | 6.53 | ||
| Residual sum of squares | - | 0.9345 | - | - | - | 5.28 | ||
| Temperature range for | Optimal growth (80% growth rate) | - | 6.48–19.89 | - | - | - | 1.56–14.47 | |
| Growth (20% growth rate) | - | 0.90–27.11 | - | - | - | 0.00–25.10 | ||
| Photosynthesis | Maximal photosynthetic rate | 292.59 | 91.37 | 48.93 | 43.46 | 53.49 | 85.82 | |
| Optimal temperature | 11.12 | 12.08 | 15.66 | 12.48 | 11.52 | 2.99 | ||
| Maximal temperature | 33.35 | 32.47 | 35.63 | 33.49 | 30.30 | 26.03 | ||
| Residual sum of squares | 199,464 | 31,639 | 2012 | 4575 | 13,940 | 10,575 | ||
| Temperature range for | Optimal photosynthesis (80% photosynthetic rate) | 4.11–20.14 | 5.01–20.56 | 7.67–24.32 | 5.19–21.23 | 4.44–20.43 | 0.2–10.05 | |
| Photosynthesis (20% photosynthetic rate) | 0.2–30.68 | 0.37–30.1 | 0.99–33.34 | 0.38–31.04 | 0.25–30.71 | 0–22.48 | ||
| Respiration | Maximal respirational rate | −185.99 | −97.77 | −28.98 | −45.46 | −84.06 | −100.19 | |
| Optimal temperature | 29.67 | 30.59 | 27.65 | 26.61 | 28.04 | 28.66 | ||
| Maximal temperature | 41.9 | 41.17 | 44.44 | 42.88 | 42.97 | 42.49 | ||
| Residual sum of squares | 24,352 | 8086 | 343.9 | 2815 | 13,143 | 8103 | ||
| Temperature range for | Optimal respiration (80% respirational rate) | 22.26–35.62 | 23.91–35.79 | 18.57–35.57 | 17.83–34.28 | 19.62–35.16 | 20.64–35.31 | |
| Respiration (20% respirational rate) | 10.34–40.77 | 12.4–40.21 | 6.33–42.8 | 6.03–41.3 | 7.51–41.54 | 8.55–41.18 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prelle, L.R.; Schmidt, I.; Schimani, K.; Zimmermann, J.; Abarca, N.; Skibbe, O.; Juchem, D.; Karsten, U. Photosynthetic, Respirational, and Growth Responses of Six Benthic Diatoms from the Antarctic Peninsula as Functions of Salinity and Temperature Variations. Genes 2022, 13, 1264. https://doi.org/10.3390/genes13071264
Prelle LR, Schmidt I, Schimani K, Zimmermann J, Abarca N, Skibbe O, Juchem D, Karsten U. Photosynthetic, Respirational, and Growth Responses of Six Benthic Diatoms from the Antarctic Peninsula as Functions of Salinity and Temperature Variations. Genes. 2022; 13(7):1264. https://doi.org/10.3390/genes13071264
Chicago/Turabian StylePrelle, Lara R., Ina Schmidt, Katherina Schimani, Jonas Zimmermann, Nelida Abarca, Oliver Skibbe, Desiree Juchem, and Ulf Karsten. 2022. "Photosynthetic, Respirational, and Growth Responses of Six Benthic Diatoms from the Antarctic Peninsula as Functions of Salinity and Temperature Variations" Genes 13, no. 7: 1264. https://doi.org/10.3390/genes13071264
APA StylePrelle, L. R., Schmidt, I., Schimani, K., Zimmermann, J., Abarca, N., Skibbe, O., Juchem, D., & Karsten, U. (2022). Photosynthetic, Respirational, and Growth Responses of Six Benthic Diatoms from the Antarctic Peninsula as Functions of Salinity and Temperature Variations. Genes, 13(7), 1264. https://doi.org/10.3390/genes13071264