Prevention of Blindness in Stickler Syndrome
Abstract
:1. Introduction
2. Methods
3. Is Prevention of Retinal Detachment Required?
4. Which Stickler Syndrome Patients Should Receive Prophylaxis?
5. How Should Prophylactic Treatment Be Performed in Stickler Syndrome?
6. Laser vs. Cryotherapy Retinopexy
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- UK Department of Health and Social Care. Prevention Is Better Than Cure: Our Vision to Help you Live Well for Longer; The Health Foundation: London, UK, 2018. [Google Scholar]
- van Leeuwen, J.S. Cure is better than prevention. BMJ (Clin. Res. Ed.) 2004, 328, 350. [Google Scholar] [CrossRef] [Green Version]
- Dye, C. The Great Health Dilemma: Is Prevention Better Than Cure? Oxford University Press: Oxford, UK, 2021. [Google Scholar]
- Mitry, D.; Awan, M.A.; Borooah, S.; Siddiqui, M.A.; Brogan, K.; Fleck, B.W.; Wright, A.; Campbell, H.; Singh, J.; Charteris, D.G.; et al. Surgical outcome and risk stratification for primary retinal detachment repair: Results from the Scottish Retinal Detachment study. Br. J. Ophthalmol. 2012, 96, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Jackson, T.L.; Donachie, P.H.; Sallam, A.; Sparrow, J.M.; Johnston, R.L. United Kingdom National Ophthalmology Database study of vitreoretinal surgery: Report 3, retinal detachment. Ophthalmology 2014, 121, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Kiew, G.; Poulson, A.V.; Newman, D.K.; Alexander, P.; Snead, M.P. Montgomery and informed consent during COVID-19: Pneumatic retinopexy versus pars plana vitrectomy or scleral buckling for retinal detachment repair. Med. Leg. J. 2021, 89, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.M.; Macky, T.A. Pediatric rhegmatogenous retinal detachment. Int. Ophthalmol. Clin. 2011, 51, 147–171. [Google Scholar] [CrossRef]
- Abeysiri, P.; Bunce, C.; da Cruz, L. Outcomes of surgery for retinal detachment in patients with Stickler syndrome: A comparison of two sequential 20-year cohorts. Graefe’s Arch. Clin. Exp. Ophthalmol. Albrecht Von Graefes Arch. Fur Klin. Und Exp. Ophthalmol. 2007, 245, 1633–1638. [Google Scholar] [CrossRef]
- Lee, A.C.; Greaves, G.H.; Rosenblatt, B.J.; Deramo, V.A.; Shakin, E.P.; Fastenberg, D.M.; Ferrone, P.J. Long-Term Follow-Up of Retinal Detachment Repair in Patients With Stickler Syndrome. Ophthalmic Surg. Lasers Imaging Retin. 2020, 51, 612–616. [Google Scholar] [CrossRef]
- Read, S.P.; Aziz, H.A.; Kuriyan, A.; Kothari, N.; Davis, J.L.; Smiddy, W.E.; Flynn, H.W., Jr.; Murray, T.G.; Berrocal, A. Retinal Detachment Surgery in a Pediatric Population: Visual and Anatomic Outcomes. Retina 2018, 38, 1393–1402. [Google Scholar] [CrossRef]
- Wubben, T.J.; Branham, K.H.; Besirli, C.G.; Bohnsack, B.L. Retinal detachment and infantile-onset glaucoma in Stickler syndrome associated with known and novel COL2A1 mutations. Ophthalmic Genet. 2018, 39, 615–618. [Google Scholar] [CrossRef]
- Fincham, G.S.; Pasea, L.; Carroll, C.; McNinch, A.M.; Poulson, A.V.; Richards, A.J.; Scott, J.D.; Snead, M.P. Prevention of retinal detachment in Stickler syndrome: The Cambridge prophylactic cryotherapy protocol. Ophthalmology 2014, 121, 1588–1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monin, C.; Allagui, M.; Larricart, P.; Ameline, B.; Haut, J. Prevention of non-traumatic retinal detachment by surgical cerclage. Apropos of 20 cases. J. Fr. Ophtalmol. 1993, 16, 247–253. [Google Scholar] [PubMed]
- Monin, C.; Van Effenterre, G.; Andre-Sereys, P.; Haut, J. Prevention of retinal detachment in Wagner-Stickler disease. Comparative study of different methods. Apropos of 22 cases. J. Fr. Ophtalmol. 1994, 17, 167–174. [Google Scholar] [PubMed]
- Billington, B.M.; Leaver, P.K.; McLeod, D. Management of retinal detachment in the Wagner-Stickler syndrome. Trans. Ophthalmol. Soc. U. K. 1985, 104 Pt 8, 875–879. [Google Scholar]
- Snead, M.P.; McNinch, A.M.; Poulson, A.V.; Bearcroft, P.; Silverman, B.; Gomersall, P.; Parfect, V.; Richards, A.J. Stickler syndrome, ocular-only variants and a key diagnostic role for the ophthalmologist. Eye (Lond. Engl.) 2011, 25, 1389–1400. [Google Scholar] [CrossRef] [Green Version]
- Leiba, H.; Oliver, M.; Pollack, A. Prophylactic laser photocoagulation in stickler syndrome. Eye 1996, 10, 701–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ang, A.; Poulson, A.V.; Goodburn, S.F.; Richards, A.J.; Scott, J.D.; Snead, M.P. Retinal detachment and prophylaxis in type 1 Stickler syndrome. Ophthalmology 2008, 115, 164–168. [Google Scholar] [CrossRef]
- Ripandelli, G.; Rossi, T.; Pesci, F.R.; Cecere, M.; Stirpe, M. The Prophylaxis of Fellow-Eye Retinal Detachment in Stickler Syndrome: A Retrospective Series. Retina 2022, 42, 250–255. [Google Scholar] [CrossRef]
- Poulson, A.V.; Hooymans, J.M.; Richards, A.J.; Bearcroft, P.; Murthy, R.; Baguley, D.M.; Scott, J.D.; Snead, M.P. Clinical features of type 2 Stickler syndrome. J. Med. Genet. 2004, 41, e107. [Google Scholar] [CrossRef] [Green Version]
- Morris, R.E.; Parma, E.S.; Robin, N.H.; Sapp, M.R.; Oltmanns, M.H.; West, M.R.; Fletcher, D.C.; Schuchard, R.A.; Kuhn, F. Stickler syndrome (SS): Laser prophylaxis for retinal detachment (modified ora secunda cerclage, osc/ss). Clin. Ophthalmol. 2021, 15, 19–29. [Google Scholar] [CrossRef]
- Alshahrani, S.T.; Ghazi, N.G.; Al-Rashaed, S. Rhegmatogenous retinal detachments associated to Stickler syndrome in a tertiary eye care center in Saudi Arabia. Clin. Ophthalmol. (Auckl. N.Z.) 2016, 10, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Glaser, B.M.; Vidaurri-Leal, J.; Michels, R.G.; Campochiaro, P.A. Cryotherapy during surgery for giant retinal tears and intravitreal dispersion of viable retinal pigment epithelial cells. Ophthalmology 1993, 100, 466–470. [Google Scholar] [CrossRef]
- Shapiro, M.J.; Blair, M.P.; Solinski, M.A.; Zhang, D.L.; Jabbehdari, S. The importance of early diagnosis of Stickler syndrome: Finding opportunities for preventing blindness. Taiwan J. Ophthalmol. 2018, 8, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Jaccoma, E.H.; Conway, B.P.; Campochiaro, P.A. Cryotherapy causes extensive breakdown of the blood-retinal barrier. A comparison with argon laser photocoagulation. Arch. Ophthalmol. 1985, 103, 1728–1730. [Google Scholar] [CrossRef] [PubMed]
- Veckeneer, M.; Van Overdam, K.; Bouwens, D.; Feron, E.; Mertens, D.; Peperkamp, E.; Ringens, P.; Mulder, P.; Van Meurs, J. Randomized clinical trial of cryotherapy versus laser photocoagulation for retinopexy in conventional retinal detachment surgery. Am. J. Ophthalmol. 2001, 132, 343–347. [Google Scholar] [CrossRef]
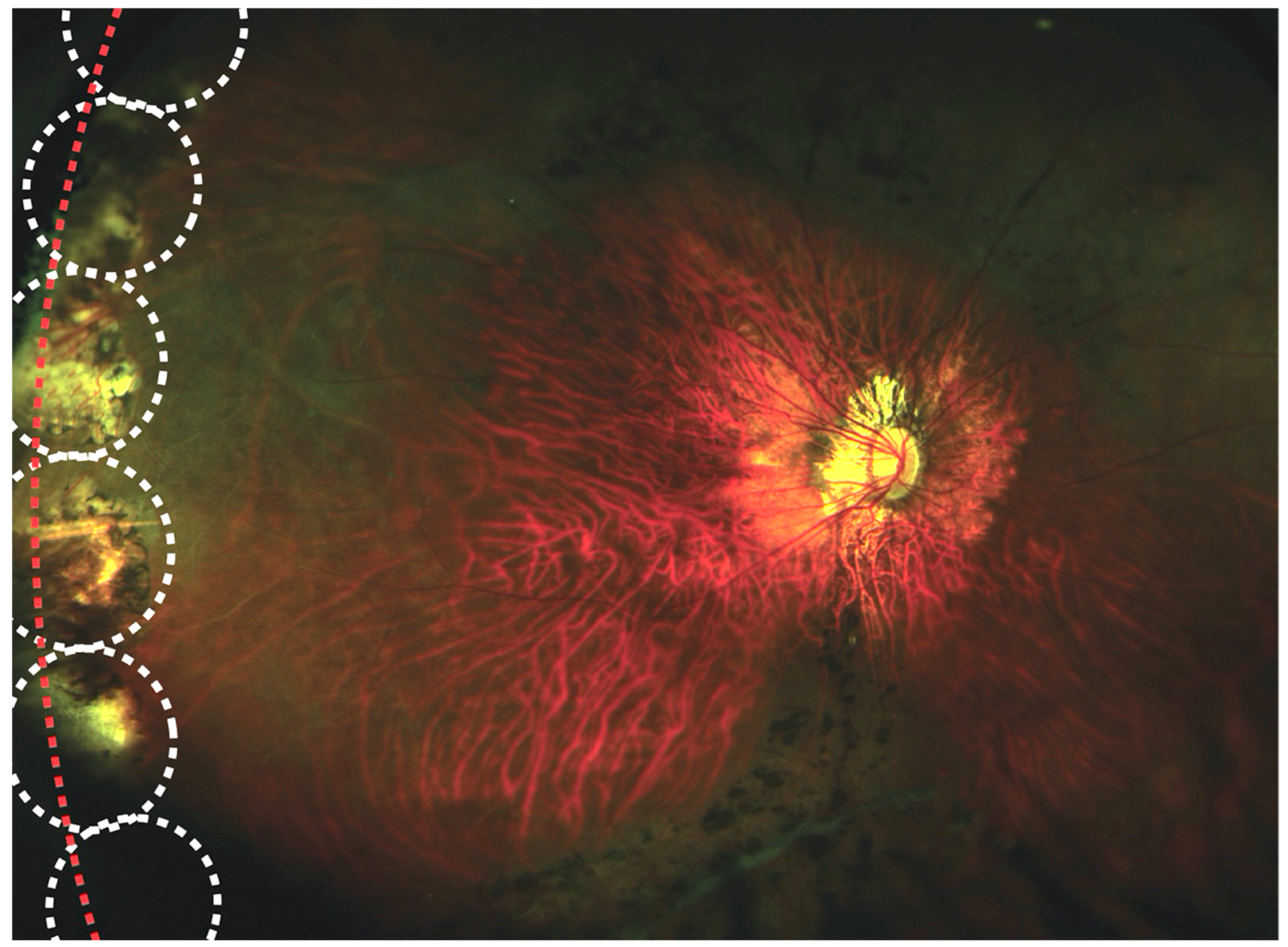
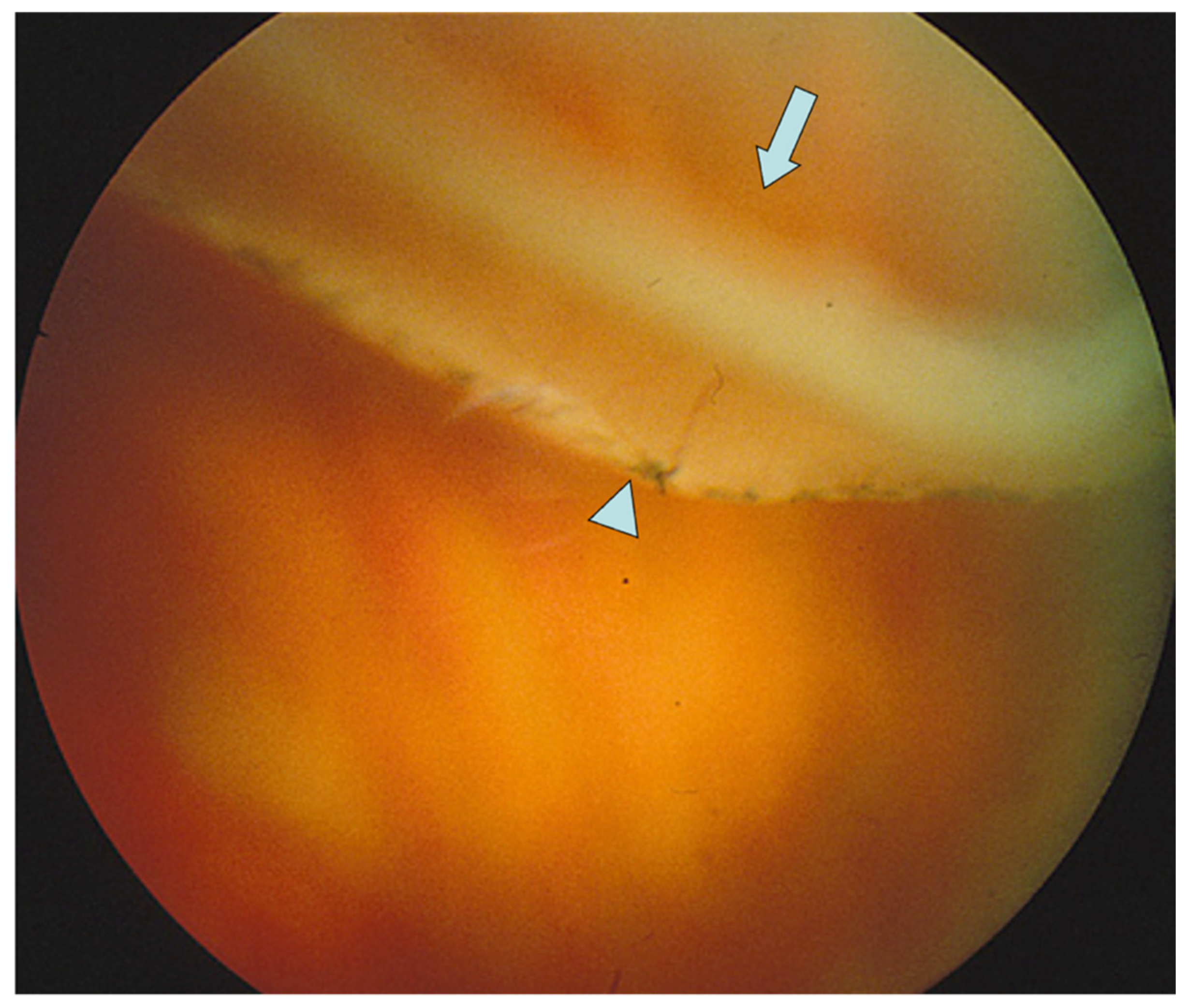

| Author | Stickler Type (n) | Laser/Cryotherapy/Buckle | Type of Study | Follow Up | Results |
|---|---|---|---|---|---|
| Monin et al., 1994, Paris, France [14] | 22 patients with Wagner–Stickler syndrome | Laser photocoagulation, or encircling scleral buckle in fellow eyes of patients with RD in the first eye | Retrospective case series (no control group) | Up to 5.5 years | 50% of patients receiving laser treatment developed RD. None of the scleral buckle patients developed RD. |
| Leiba et al., 1996, Rehovat, Israel [17] | 10 patients from a single family with genetically confirmed Type 1 Stickler syndrome. Untreated family members were used as controls during the study follow-up | Primary prophylactic laser photocoagulation, either (a) circumferentially, at the posterior border of retinal lesions, or (b) around areas of abnormal retina | Retrospective study | 1–15 years | 10% of lasered eyes developed retinal detachment, compared to 44% of non-lasered eyes |
| Ang et al., 2008, Cambridge, UK [18] | 93 patients (155 eyes) with genetically confirmed Type 1 Stickler syndrome and 111 control patients (222 eyes) who did not receive any intervention | 360-degree cryotherapy of the juxtaoral retina, for prevention of giant retinal tear | Retrospective comparative case series | Up to 33 years | With no retinopexy, 73% of the patients suffered RD, and 48% were bilateral. Of those receiving retinopexy, 8% developed RD, but none were bilateral. |
| Fincham et al., 2014, Cambridge, UK [12] | 293 patients with genetically confirmed Type 1 Stickler syndrome and 194 control patients who did not receive any intervention | Cambridge Prophylactic Cryotherapy Protocol: 360-degree cryotherapy of the juxtaoral retina, for prevention of giant retinal tear | Retrospective comparative case series, matched for age and follow-up duration | 1–36 years | The bilateral and unilateral control group had a 5.0-fold and 8.4-fold, respectively, increased risk compared to eyes receiving prophylaxis |
| Al-Shahrani et al., 2015, Riyadh, Saudi Arabia, [22] | 70 eyes of patients with Stickler syndrome. Genetic testing not specified. No control group. | Details of prophylactic laser retinopexy not specified | Retrospective case series | 1 week to 10 years | No genetic confirmation, no control group and no details of type of laser prophylaxis, so impossible to assess prophylaxis efficacy from this study. |
| Wubben et al., 2018, Ann Arbor, Michigan, USA [11] | 15 patients with genetically confirmed Type 1 Stickler syndrome; of these, 20 eyes had prophylactic laser retinopexy | Laser (details not reported) | Retrospective comparative case series | 4 months -16 years | 5% risk of RD with prophylaxis; 50% risk of RD without prophylaxis |
| Morris et al., 2021, Birmingham, Alabama, USA [21] | 5 eyes of 4 patients from a single family with confirmed Type 2 Stickler syndrome | Encircling grid laser (Modified Ora Secunda Cerclage) | Retrospective case series | 3–12 years | 0/5 eyes developed retinal tear or retinal detachment. |
| Ripandelli et al. (2022), Rome, Italy [19] | Fellow eyes of patients with genetically confirmed Type 1 Stickler syndrome who had had unilateral retinal detachment. | All eyes received a 6 mm-wide encircling band. Cryoretinopexy was performed on any retinal tears, holes or lattice degeneration | Retrospective case series | Mean 15.6 years, all >12 years | Scleral buckle without cryo: 5/13 developed RDScleral buckle with cryo: 0/39 developed RD |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexander, P.; Snead, M.P. Prevention of Blindness in Stickler Syndrome. Genes 2022, 13, 1150. https://doi.org/10.3390/genes13071150
Alexander P, Snead MP. Prevention of Blindness in Stickler Syndrome. Genes. 2022; 13(7):1150. https://doi.org/10.3390/genes13071150
Chicago/Turabian StyleAlexander, Philip, and Martin P. Snead. 2022. "Prevention of Blindness in Stickler Syndrome" Genes 13, no. 7: 1150. https://doi.org/10.3390/genes13071150
APA StyleAlexander, P., & Snead, M. P. (2022). Prevention of Blindness in Stickler Syndrome. Genes, 13(7), 1150. https://doi.org/10.3390/genes13071150






