A Brief History of Drosophila (Female) Meiosis
Abstract
1. Introduction
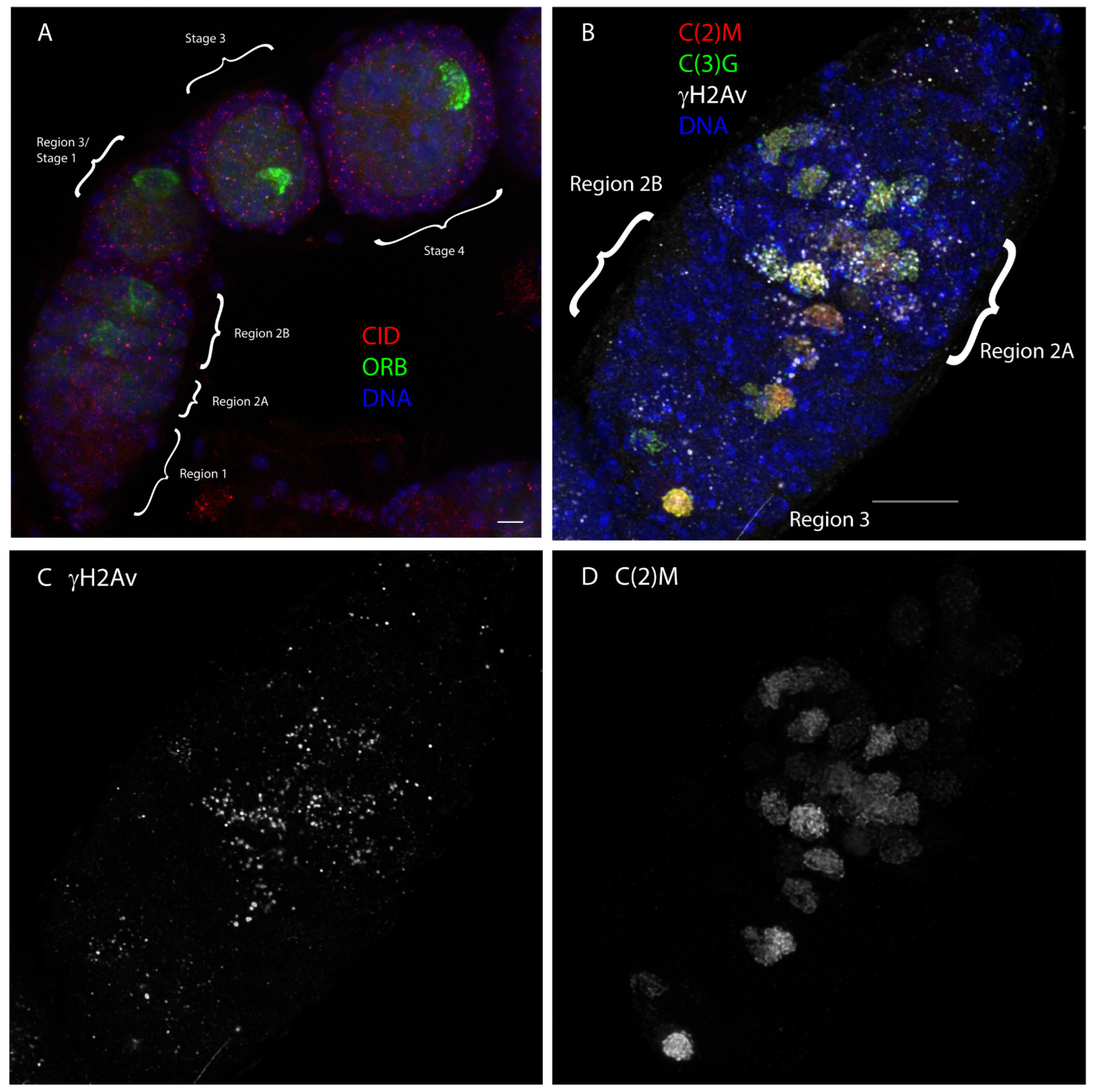
2. The Mitotic Region (Region 1)
3. Meiotic Prophase (Regions 2–3)
4. The Long Pause (Stages 2–12)
5. Entry into the Meiotic Divisions (Stage 13–14)
6. Exit from Meiosis and Fertilization (the Embryo)
7. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Baker, B.S.; Carpenter, A.T.C.; Esposito, M.S.; Esposito, R.E.; Sandler, L. The genetic control of meiosis. Ann. Rev. Genet. 1976, 10, 53–134. [Google Scholar] [CrossRef] [PubMed]
- Hawley, R.S. Exchange and chromosomal segregation in eucaryotes. In Genetic Recombination; Kucherlapati, R., Smith, G., Eds.; American Society of Microbiology: Washington, DC, USA, 1988; pp. 497–527. [Google Scholar]
- Adams, E.E.; He, Q.; McKee, B.D. How noncrossover homologs are conjoined and segregated in Drosophila male meiosis I: Stable but reversible homolog linkers require a novel Separase target protein. PLoS Genet. 2020, 16, e1008997. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.E.; Miller, D.E.; Miller, A.L.; Hawley, R.S. Female Meiosis: Synapsis, Recombination, and Segregation in Drosophila melanogaster. Genetics 2018, 208, 875–908. [Google Scholar] [CrossRef] [PubMed]
- Roth, S.; Lynch, J.A. Symmetry breaking during Drosophila oogenesis. Cold Spring Harb. Perspect. Biol. 2009, 1, a001891. [Google Scholar] [CrossRef]
- Gyuricza, M.R.; Manheimer, K.B.; Apte, V.; Krishnan, B.; Joyce, E.F.; McKee, B.D.; McKim, K.S. Dynamic and Stable Cohesins Regulate Synaptonemal Complex Assembly and Chromosome Segregation. Curr. Biol. 2016, 26, 1688–1698. [Google Scholar] [CrossRef]
- Joyce, E.F.; Apostolopoulos, N.; Beliveau, B.J.; Wu, C.T. Germline Progenitors Escape the Widespread Phenomenon of Homolog Pairing during Drosophila Development. PLoS Genet. 2013, 9, e1004013. [Google Scholar] [CrossRef]
- Christophorou, N.; Rubin, T.; Huynh, J.-R. Synaptonemal Complex Components Promote Centromere Pairing in Pre-meiotic Germ Cells. PLoS Genet. 2013, 9, e1004012. [Google Scholar] [CrossRef]
- Rubin, T.; Christophorou, N.; Huynh, J.-R. How to pre-pair chromosomes for meiosis. Cell Cycle 2016, 15, 609–610. [Google Scholar] [CrossRef][Green Version]
- Sou, I.F.; Pryce, R.M.; Tee, W.-W.; McClurg, U.L. Meiosis initiation: A story of two sexes in all creatures great and small. Biochem. J. 2021, 478, 3791–3805. [Google Scholar] [CrossRef]
- Wei, Y.; Reveal, B.; Reich, J.; Laursen, W.J.; Senger, S.; Akbar, T.; Iida-Jones, T.; Cai, W.; Jarnik, M.; Lilly, M.A. TORC1 regulators Iml1/GATOR1 and GATOR2 control meiotic entry and oocyte development in Drosophila. Proc. Natl. Acad. Sci. USA 2014, 111, E5670–E5677. [Google Scholar] [CrossRef]
- McCarthy, A.; Sarkar, K.; Martin, E.T.; Upadhyay, M.; Jang, S.; Williams, N.D.; Forni, P.E.; Buszczak, M.; Rangan, P. Msl3 promotes germline stem cell differentiation in female Drosophila. Development 2022, 149, dev199625. [Google Scholar] [CrossRef] [PubMed]
- Barr, J.; Gilmutdinov, R.; Wang, L.; Shidlovskii, Y.; Schedl, P. The Drosophila CPEB Protein Orb Specifies Oocyte Fate by a 3’UTR-Dependent Autoregulatory Loop. Genetics 2019, 213, 1431–1446. [Google Scholar] [CrossRef] [PubMed]
- Mach, J.M.; Lehmann, R. An Egalitarian-BicaudalD complex is essential for oocyte specification and axis determination in Drosophila. Genes Dev. 1997, 11, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Lin, H. Asymmetric germ cell division and oocyte determination during Drosophila oogenesis. Int. Rev. Cytol. 2001, 203, 93–138. [Google Scholar] [PubMed]
- Carpenter, A.T.C. Synaptonemal complex and recombination nodules in wild-type Drosophila melanogaster females. Genetics 1979, 92, 511–541. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, A.T.C. Electron microscopy of meiosis in Drosophila melanogaster females. I. Structure, arrangement, and temporal change of the synaptonemal complex in wild-type. Chromosoma 1975, 51, 157–182. [Google Scholar] [CrossRef]
- Manheim, E.; McKim, K.S. The Synaptonemal Complex Component C(2)M Regulates Meiotic Crossing over in Drosophila. Curr. Biol. 2003, 13, 276–285. [Google Scholar] [CrossRef]
- Carpenter, A.T.C. Egalitarian and the choice of cell fates in Drosophila melanogaster oogenesis. Germline Dev. 1994, 182, 223–254. [Google Scholar]
- Carpenter, A.T.C. EM Autoradiographic evidence that DNA synthesis occurs at recombination nodules during meiosis in Drosophila melanogaster females. Chromosoma 1981, 83, 59–80. [Google Scholar] [CrossRef]
- Carpenter, A.T.C. Recombination nodules and synaptonemal complex in recombination-defective females of Drosophila melanogaster. Chromosoma 1979, 75, 259–292. [Google Scholar] [CrossRef]
- Takeo, S.; Hawley, R.S. Rumors of its disassembly have been greatly exaggerated: The secret life of the synaptonemal complex at the centromeres. PLoS Genet. 2012, 8, e1002807. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tanneti, N.S.; Landy, K.; Joyce, E.F.; McKim, K.S. A Pathway for Synapsis Initiation during Zygotene in Drosophila Oocytes. Curr. Biol. 2011, 21, 1852–1857. [Google Scholar] [CrossRef] [PubMed]
- McKim, K.S.; Green-Marroquin, B.L.; Sekelsky, J.J.; Chin, G.; Steinberg, C.; Khodosh, R.; Hawley, R.S. Meiotic synapsis in the absence of recombination. Science 1998, 279, 876–878. [Google Scholar] [CrossRef] [PubMed]
- Dernburg, A.F.; McDonald, K.; Moulder, G.; Barstead, R.; Dresser, M.; Villeneuve, A.M. Meiotic recombination in C. elegans initiates by a conserved mechanism and is dispensable for homologous chromosome synapsis. Cell 1998, 94, 387–398. [Google Scholar] [CrossRef]
- Mehrotra, S.; McKim, K.S. Temporal analysis of meiotic DNA double-strand break formation and repair in Drosophila females. PLoS Genet. 2006, 2, e200. [Google Scholar] [CrossRef]
- Lake, C.M.; Holsclaw, J.K.; Bellendir, S.P.; Sekelsky, J.; Hawley, R.S. The development of a monoclonal antibody recognizing the Drosophila melanogaster phosphorylated histone H2A variant (γ-H2AV). G3 Genes Genomes Genet. 2013, 3, 1539–1543. [Google Scholar]
- Page, S.L.; Hawley, R.S. c(3)G encodes a Drosophila synaptonemal complex protein. Genes Dev. 2001, 15, 3130–3143. [Google Scholar] [CrossRef]
- Collins, K.A.; Unruh, J.R.; Slaughter, B.D.; Yu, Z.; Lake, C.M.; Nielsen, R.J.; Box, K.S.; Miller, D.E.; Blumenstiel, J.P.; Perera, A.G.; et al. Corolla is a novel protein that contributes to the architecture of the synaptonemal complex of Drosophila. Genetics 2014, 198, 219–228. [Google Scholar] [CrossRef]
- Page, S.L.; Khetani, R.S.; Lake, C.M.; Nielsen, R.J.; Jeffress, J.K.; Warren, W.D.; Bickel, S.E.; Hawley, R.S. Corona is required for higher-order assembly of transverse filaments into full-length synaptonemal complex in Drosophila oocytes. PLoS Genet. 2008, 4, e1000194. [Google Scholar] [CrossRef]
- Krishnan, B.; Thomas, S.E.; Yan, R.; Yamada, H.; Zhulin, I.B.; McKee, B.D. Sisters Unbound Is Required for Meiotic Centromeric Cohesion in Drosophila melanogaster. Genetics 2014, 198, 947–965. [Google Scholar] [CrossRef]
- Yan, R.; McKee, B.D. The Cohesion Protein SOLO Associates with SMC1 and Is Required for Synapsis, Recombination, Homolog Bias and Cohesion and Pairing of Centromeres in Drosophila Meiosis. PLoS Genet. 2013, 9, e1003637. [Google Scholar] [CrossRef] [PubMed]
- McKim, K.S.; Hayashi-Hagihara, A. mei-W68 in Drosophila melanogaster encodes a Spo11 homolog: Evidence that the mechanism for initiating meiotic recombination is conserved. Genes Dev. 1998, 12, 2932–2942. [Google Scholar]
- Liu, H.; Jang, J.K.; Kato, N.; McKim, K.S. mei-P22 encodes a chromosome-associated protein required for the initiation of meiotic recombination in Drosophila melanogaster. Genetics 2002, 162, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Robert, T.; Nore, A.; Brun, C.; Maffre, C.; Crimi, B.; Bourbon, H.M.; de Massy, B. The TopoVIB-Like protein family is required for meiotic DNA double-strand break formation. Science 2016, 351, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, A.T.C. Thoughts on Recombination nodules, meiotic recombination, and chiasmata. In Genetic Recombination; Kucherlapati, R., Smith, G., Eds.; American Society of Microbiology: Washington, DC, USA, 1988; pp. 529–548. [Google Scholar]
- Baker, B.S.; Hall, J.C. Meiotic mutants: Genetic control of meiotic recombination and chromosome segregation. In The Genetics and Biology of Drosophila; Ashburner, M., Novitski, E., Eds.; Academic Press: New York, NY, USA, 1976; pp. 351–434. [Google Scholar]
- Carpenter, A.T.C.; Baker, B.S. On the control of the distribution of meiotic exchange in Drosophila melanogaster. Genetics 1982, 101, 81–89. [Google Scholar] [CrossRef]
- Yildiz, O.; Majumder, S.; Kramer, B.; Sekelsky, J.J. Drosophila MUS312 Interacts with the Nucleotide Excision Repair Endonuclease MEI-9 to Generate Meiotic Crossovers. Mol. Cell 2002, 10, 1503–1509. [Google Scholar] [CrossRef][Green Version]
- Sekelsky, J.J.; McKim, K.S.; Chin, G.M.; Hawley, R.S. The Drosophila meiotic recombination gene mei-9 encodes a homologue of the yeast excision repair protein Rad1. Genetics 1995, 141, 619–627. [Google Scholar] [CrossRef]
- Kohl, K.P.; Jones, C.D.; Sekelsky, J. Evolution of an MCM complex in flies that promotes meiotic crossovers by blocking BLM helicase. Science 2012, 338, 1363–1365. [Google Scholar] [CrossRef]
- Hartmann, M.; Kohl, K.P.; Sekelsky, J.; Hatkevich, T. Meiotic MCM Proteins Promote and Inhibit Crossovers during Meiotic Recombination. Genetics 2019, 212, 461–468. [Google Scholar] [CrossRef]
- Andersen, S.L.; Bergstralh, D.T.; Kohl, K.P.; LaRocque, J.R.; Moore, C.B.; Sekelsky, J. Drosophila MUS312 and the vertebrate ortholog BTBD12 interact with DNA structure-specific endonucleases in DNA repair and recombination. Mol. Cell 2009, 35, 128–135. [Google Scholar] [CrossRef]
- Hartmann, M.; Umbanhowar, J.; Sekelsky, J. Centromere-Proximal Meiotic Crossovers in Drosophila melanogaster Are Suppressed by Both Highly Repetitive Heterochromatin and Proximity to the Centromere. Genetics 2019, 213, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Beadle, G. A possible influence of the spindle fiber on crossing over in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 1932, 18, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Lucchesi, J.C.; Suzuki, D.T. The interchromosomal control of recombination. Annu. Rev. Genet. 1968, 2, 53–86. [Google Scholar] [CrossRef]
- Crown, K.N.; Miller, D.E.; Sekelsky, J.; Hawley, R.S. Local Inversion Heterozygosity Alters Recombination throughout the Genome. Curr. Biol. 2018, 28, 2984–2990.e3. [Google Scholar] [CrossRef]
- Joyce, E.F.; McKim, K.S. Chromosome axis defects induce a checkpoint-mediated delay and interchromosomal effect on crossing over during Drosophila meiosis. PLoS Genet. 2010, 6, e1001059. [Google Scholar] [CrossRef]
- Joyce, E.F.; McKim, K.S. Drosophila PCH2 Is Required for a Pachytene Checkpoint That Monitors Double-Strand-Break-Independent Events Leading to Meiotic Crossover Formation. Genetics 2009, 181, 39–51. [Google Scholar] [CrossRef]
- Hawley, R.S. Chromosomal sites necessary for normal levels of meiotic recombination in Drosophila melanogaster. I. Evidence for and mapping of the sites. Genetics 1980, 94, 625–646. [Google Scholar] [CrossRef]
- Sherizen, D.; Jang, J.K.; Bhagat, R.; Kato, N.; McKim, K.S. Meiotic recombination in Drosophila females depends on chromosome continuity between genetically defined boundaries. Genetics 2005, 169, 767–781. [Google Scholar] [CrossRef]
- Gong, W.J.; McKim, K.S.; Hawley, R.S. All Paired Up with No Place to Go: Pairing, Synapsis, and DSB Formation in a Balancer Heterozygote. PLoS Genet. 2005, 1, e67. [Google Scholar] [CrossRef]
- Takeo, S.; Lake, C.M.; Morais-De-Sá, E.; Sunkel, C.E.; Hawley, R.S. Synaptonemal complex-dependent centromeric clustering and the initiation of synapsis in Drosophila oocytes. Curr. Biol. 2011, 21, 1845–1851. [Google Scholar] [CrossRef]
- Theurkauf, W.E.; Hawley, R.S. Meiotic spindle assembly in Drosophila females: Behavior of nonexchange chromosomes and the effects of mutations in the nod kinesin-like protein. J. Cell Biol. 1992, 116, 1167–1180. [Google Scholar] [CrossRef] [PubMed]
- Matthies, H.J.; McDonald, H.B.; Goldstein, L.S.; Theurkauf, W.E. Anastral meiotic spindle morphogenesis: Role of the non-claret disjunctional kinesin-like protein. J. Cell Biol. 1996, 134, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Cullen, C.F.; Ohkura, H. Msps protein is localized to acentrosomal poles to ensure bipolarity of Drosophila meiotic spindles. Nat. Cell Biol. 2001, 3, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.F.; Ohkura, H. The molecular architecture of the meiotic spindle is remodeled during metaphase arrest in oocytes. J. Cell Biol. 2019, 218, 2854–2864. [Google Scholar] [CrossRef]
- Hughes, S.E.; Beeler, J.S.; Seat, A.; Slaughter, B.D.; Unruh, J.R.; Bauerly, E.; Matthies, J.G.; Hawley, R.S. Gamma-Tubulin is required foir bipolar spindle assembly and for proper kinetochore microtubule attachments during prometaphase in Drosophila oocytes. PLoS Genet. 2011, 7, e1002209. [Google Scholar] [CrossRef]
- Theurkauf, W.E. Microtubules and cytoplasmic organization during Drosophila oogenesis. Dev. Biol. 1994, 165, 352–360. [Google Scholar] [CrossRef]
- Hughes, S.E.; Hawley, R.S. Live Imaging of Meiosis I in Late-Stage Drosophila melanogaster Oocytes. Methods Mol. Biol. 2017, 1471, 255–264. [Google Scholar]
- Beaven, R.; Bastos, R.N.; Spanos, C.; Romé, P.; Cullen, C.F.; Rappsilber, J.; Giet, R.; Goshima, G.; Ohkura, H. 14-3-3 regulation of Ncd reveals a new mechanism for targeting proteins to the spindle in oocytes. J. Cell Biol. 2017, 216, 3029–3039. [Google Scholar] [CrossRef]
- Colombié, N.; Cullen, C.F.; Brittle, A.L.; Jang, J.K.; Earnshaw, W.C.; Carmena, M.; McKim, K.; Ohkura, H. Dual roles of Incenp crucial to the assembly of the acentrosomal metaphase spindle in female meiosis. Development 2008, 135, 3239–3246. [Google Scholar] [CrossRef]
- Wang, L.-I.; DeFosse, T.; Jang, J.K.; Battaglia, R.A.; Wagner, V.F.; McKim, K.S. Borealin directs recruitment of the CPC to oocyte chromosomes and movement to the microtubules. J. Cell Biol. 2021, 220, e202006018. [Google Scholar] [CrossRef]
- Radford, S.J.; Jang, J.K.; McKim, K.S. The Chromosomal Passenger Complex is required for Meiotic Acentrosomal Spindle Assembly and Chromosome Bi-orientation. Genetics 2012, 192, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Cesario, J.; McKim, K.S. RanGTP is required for meiotic spindle organization and the initiation of embryonic development in Drosophila. J. Cell Sci. 2011, 124, 3797–3810. [Google Scholar] [CrossRef] [PubMed]
- Romé, P.; Ohkura, H. A novel microtubule nucleation pathway for meiotic spindle assembly in oocytes. J. Cell Biol. 2018, 217, 3431–3445. [Google Scholar] [CrossRef] [PubMed]
- Radford, S.; Hoang, T.L.; Głuszek, A.A.; Ohkura, H.; McKim, K.S. Lateral and End-On Kinetochore Attachments Are Coordinated to Achieve Bi-orientation in Drosophila Oocytes. PLoS Genet. 2015, 11, e1005605. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-I.; Das, A.; McKim, K.S. Sister centromere fusion during meiosis I depends on maintaining cohesins and destabilizing microtubule attachments. PLoS Genet. 2019, 15, e1008072. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.K.; Messina, L.; Erdman, M.B.; Arbel, T.; Hawley, R.S. Induction of metaphase arrest in Drosophila oocytes by chiasma-based kinetochore tension. Science 1995, 268, 1917–1919. [Google Scholar] [CrossRef] [PubMed]
- McKim, K.S.; Jang, J.K.; Theurkauf, W.E.; Hawley, R.S. Mechanical basis of meiotic metaphase arrest. Nature 1993, 362, 364–366. [Google Scholar] [CrossRef]
- Jang, J.K.; Rahman, T.; McKim, K.S. The kinesinlike protein Subito contributes to central spindle assembly and organization of the meiotic spindle in Drosophila oocytes. Mol. Biol. Cell 2005, 16, 4684–4694. [Google Scholar] [CrossRef]
- Giunta, K.L.; Jang, J.K.; Manheim, E.; Subramanian, G.; McKim, K.S. Subito encodes a kinesin-like protein required for meiotic spindle pole formation in Drosophila melanogaster. Genetics 2002, 160, 1489–1501. [Google Scholar] [CrossRef]
- Das, A.; Cesario, J.; Hinman, A.M.; Jang, J.K.; McKim, K.S. Kinesin 6 Regulation in Drosophila Female Meiosis by the Non-conserved N- and C- Terminal Domains. G3 Genes Genomes Genet. 2018, 8, 1555–1569. [Google Scholar] [CrossRef]
- Das, A.; Shah, S.J.; Fan, B.; Paik, D.; Disanto, D.J.; Hinman, A.M.; Cesario, J.M.; Battaglia, R.A.; Demos, N.; McKim, K.S. Spindle Assembly and Chromosome Segregation Requires Central Spindle Proteins in Drosophila Oocytes. Genetics 2016, 202, 61–75. [Google Scholar] [CrossRef]
- McKim, K.S. Highway to hell-thy meiotic divisions: Chromosome passenger complex functions driven by microtubules: CPC interactions with both the chromosomes and microtubules are important for spindle assembly and function: CPC interactions with both the chromosomes and microtubules are important for spindle assembly and function. BioEssays 2022, 44, 2100202. [Google Scholar]
- Fellmeth, J.E.; McKim, K.S. Meiotic CENP-C is a shepherd: Bridging the space between the centromere and the kinetochore in time and space. Essays Biochem. 2020, 64, 251–261. [Google Scholar]
- Weng, K.A.; Jeffreys, C.A.; Bickel, S.E. Rejuvenation of Meiotic Cohesion in Oocytes during Prophase I Is Required for Chiasma Maintenance and Accurate Chromosome Segregation. PLoS Genet. 2014, 10, e1004607. [Google Scholar] [CrossRef]
- Jang, J.K.; Gladstein, A.C.; Das, A.; Shapiro, J.G.; Sisco, Z.L.; McKim, K.S. Multiple pools of PP2A regulate spindle assembly, kinetochore attachments and cohesion in Drosophila oocytes. J. Cell Sci. 2021, 134, jcs254037. [Google Scholar] [CrossRef]
- Bonner, A.M.; Hughes, S.E.; Hawley, R.S. Regulation of Polo Kinase by Matrimony Is Required for Cohesin Maintenance during Drosophila melanogaster Female Meiosis. Curr. Biol. 2020, 30, 715–722.e713. [Google Scholar] [CrossRef]
- Jeffreys, C.A.; Burrage, P.S.; Bickel, S.E. A model system for increased meiotic nondisjunction in older oocytes. Curr. Biol. 2003, 13, 498–503. [Google Scholar] [CrossRef]
- Greenblatt, E.J.; Obniski, R.; Mical, C.; Spradling, A.C. Prolonged ovarian storage of mature Drosophila oocytes dramatically increases meiotic spindle instability. Elife 2019, 8, e49455. [Google Scholar] [CrossRef]
- Perkins, A.T.; Greig, M.M.; Sontakke, A.A.; Peloquin, A.S.; McPeek, M.A.; Bickel, S.E. Increased levels of superoxide dismutase suppress meiotic segregation errors in aging oocytes. Chromosoma 2019, 128, 215–222. [Google Scholar] [CrossRef]
- Perkins, A.T.; Das, T.M.; Panzera, L.C.; Bickel, S.E. Oxidative stress in oocytes during midprophase induces premature loss of cohesion and chromosome segregation errors. Proc. Natl. Acad. Sci. USA 2016, 113, E6823–E6830. [Google Scholar] [CrossRef]
- Hawley, R.S.; Theurkauf, W.E. Requiem for distributive segregation: Achiasmate segregation in Drosophila females. Trends Genet. 1993, 9, 310–317. [Google Scholar] [CrossRef]
- Grell, R.F. Distributive Pairing. In The Genetics and Biology of Drosophila; Ashburner, M., Novitski, E., Eds.; Academic Press: New York, NY, USA, 1976. [Google Scholar]
- Zhang, P.; Knowles, B.A.; Goldstein, L.S.; Hawley, R.S. A kinesin-like protein required for distributive chromosome segregation in Drosophila. Cell 1990, 62, 1053–1062. [Google Scholar] [CrossRef]
- Horner, V.L.; Wolfner, M.F. Mechanical stimulation by osmotic and hydrostatic pressure activates Drosophila oocytes in vitro in a calcium-dependent manner. Dev. Biol. 2008, 316, 100–109. [Google Scholar] [CrossRef]
- Heifetz, Y.; Yu, J.; Wolfner, M.F. Ovulation triggers activation of Drosophila oocytes. Dev. Biol. 2001, 234, 416–424. [Google Scholar] [CrossRef]
- Kaneuchi, T.; Sartain, C.V.; Takeo, S.; Horner, V.L.; Buehner, N.A.; Aigaki, T.; Wolfner, M.F. Calcium waves occur as Drosophila oocytes activate. Proc. Natl. Acad. Sci. USA 2015, 112, 791–796. [Google Scholar] [CrossRef]
- Bourouh, M.; Dhaliwal, R.; Rana, K.; Sinha, S.; Guo, Z.; Swan, A. Distinct and Overlapping Requirements for Cyclins A, B and B3 in Drosophila Female Meiosis. G3 Genes Genomes Genet. 2016, 6, 3711–3724. [Google Scholar] [CrossRef]
- Riparbelli, M.G.; Callaini, G. The meiotic spindle of the Drosophila oocyte: The role of centrosomin and the central aster. J. Cell Sci. 2005, 118, 2827–2836. [Google Scholar] [CrossRef]
- Guo, Z.; Batiha, O.; Bourouh, M.; Fifield, E.; Swan, A. Role of Securin, Separase and Cohesins in female meiosis and polar body formation in Drosophila. J. Cell Sci. 2016, 129, 531–542. [Google Scholar] [CrossRef]
- Kursel, L.E.; Malik, H.S. The cellular mechanisms and consequences of centromere drive. Curr. Opin. Cell Biol. 2018, 52, 58–65. [Google Scholar] [CrossRef]
- Akera, T.; Trimm, E.; Lampson, M.A. Molecular Strategies of Meiotic Cheating by Selfish Centromeres. Cell 2019, 178, 1132–1144.e10. [Google Scholar] [CrossRef]
- Akera, T.; Chmátal, L.; Trimm, E.; Yang, K.; Aonbangkhen, C.; Chenoweth, D.M.; Janke, C.; Schultz, R.M.; Lampson, M.A. Spindle asymmetry drives non-Mendelian chromosome segregation. Science 2017, 358, 668–672. [Google Scholar] [CrossRef]
- Mark, H.F.L.; Zimmering, S. Centromeric effect on the degree of nonrandom disjunction in the female Drosophila melanogaster. Genetics 1977, 86, 121–132. [Google Scholar] [CrossRef]
- Zimmering, S. Genetic and cytogenetic aspects of altered segregation phenomena in Drosophila. In The Genetics and Biology of Drosophila Vol 1b; Ashburner, M., Novitski, E., Eds.; Academic Press: New York, NY, USA, 1976; pp. 569–613. [Google Scholar]
- Novitski, E. Genetic measures of centromere activity in Drosophila melanogaster. J. Cell. Comp. Physiol. 1955, 45, 151–169. [Google Scholar] [CrossRef]
- Novitski, E. Non-random disjunction in Drosophila. Genetics 1951, 36, 267–280. [Google Scholar] [CrossRef]
- Radford, S.J.; Go, A.M.; McKim, K.S. Cooperation between Kinesin Motors Promotes Spindle Symmetry and Chromosome Organization in Oocytes. Genetics 2017, 205, 517–527. [Google Scholar] [CrossRef]
- Wei, K.H.; Reddy, H.M.; Rathnam, C.; Lee, J.; Lin, D.; Ji, S.; Mason, J.M.; Clark, A.G.; Barbash, D.A. A Pooled Sequencing Approach Identifies a Candidate Meiotic Driver in Drosophila. Genetics 2017, 206, 451–465. [Google Scholar] [CrossRef]
- Bridges, C.B. Non-disjunction as proof of the chromosome theory of heredity. Genetics 1916, 1, 1–52. [Google Scholar] [CrossRef]
- Bridges, C.B. Direct proof through non-disjunction that the sex-linked genes of Drosophila are borne by the X-chromosome. Science 1914, 40, 107–109. [Google Scholar] [CrossRef]
- Gowen, M.S.; Gowen, J.W. Complete linkage in Drosophila melanogaster. Am. Nat. 1922, 61, 286–288. [Google Scholar] [CrossRef]
- Baker, B.S.; Carpenter, A.T. Genetic analysis of sex chromosomal meiotic mutants in Drosophilia melanogaster. Genetics 1972, 71, 255–286. [Google Scholar] [CrossRef]
- Sandler, L.; Lindsley, D.L.; Nicoletti, B.; Trippa, G. Mutants affecting meiosis in natural populations of Drosophila melanogaster. Genetics 1968, 60, 525–558. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fellmeth, J.E.; McKim, K.S. A Brief History of Drosophila (Female) Meiosis. Genes 2022, 13, 775. https://doi.org/10.3390/genes13050775
Fellmeth JE, McKim KS. A Brief History of Drosophila (Female) Meiosis. Genes. 2022; 13(5):775. https://doi.org/10.3390/genes13050775
Chicago/Turabian StyleFellmeth, Jessica E., and Kim S. McKim. 2022. "A Brief History of Drosophila (Female) Meiosis" Genes 13, no. 5: 775. https://doi.org/10.3390/genes13050775
APA StyleFellmeth, J. E., & McKim, K. S. (2022). A Brief History of Drosophila (Female) Meiosis. Genes, 13(5), 775. https://doi.org/10.3390/genes13050775





