Analysis of the PRA1 Genes in Cotton Identifies the Role of GhPRA1.B1-1A in Verticillium dahliae Resistance
Abstract
1. Introduction
2. Materials and Methods
2.1. Phylogenetic Analysis
2.2. Analysis of Gene Structures, Domains, and Motifs
2.3. Analysis of Cis-Acting Elements in the GhPRA1 Promoter Region
2.4. Collinearity Analysis of the GhPRA1 Gene
2.5. Plant Materials and Growth Conditions
2.6. Gene Cloning
2.7. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR) Analysis
2.8. Generating Transgenic Arabidopsis Plants
2.9. VIGS and V. dahliae Inoculation
2.10. Plant Disease Evaluation
2.11. ROS and Callose Deposition Detection
2.12. Measuring Jasmonic Acid, Salicylic Acid, and H2O2 Content
2.13. Statistical Analysis
3. Results
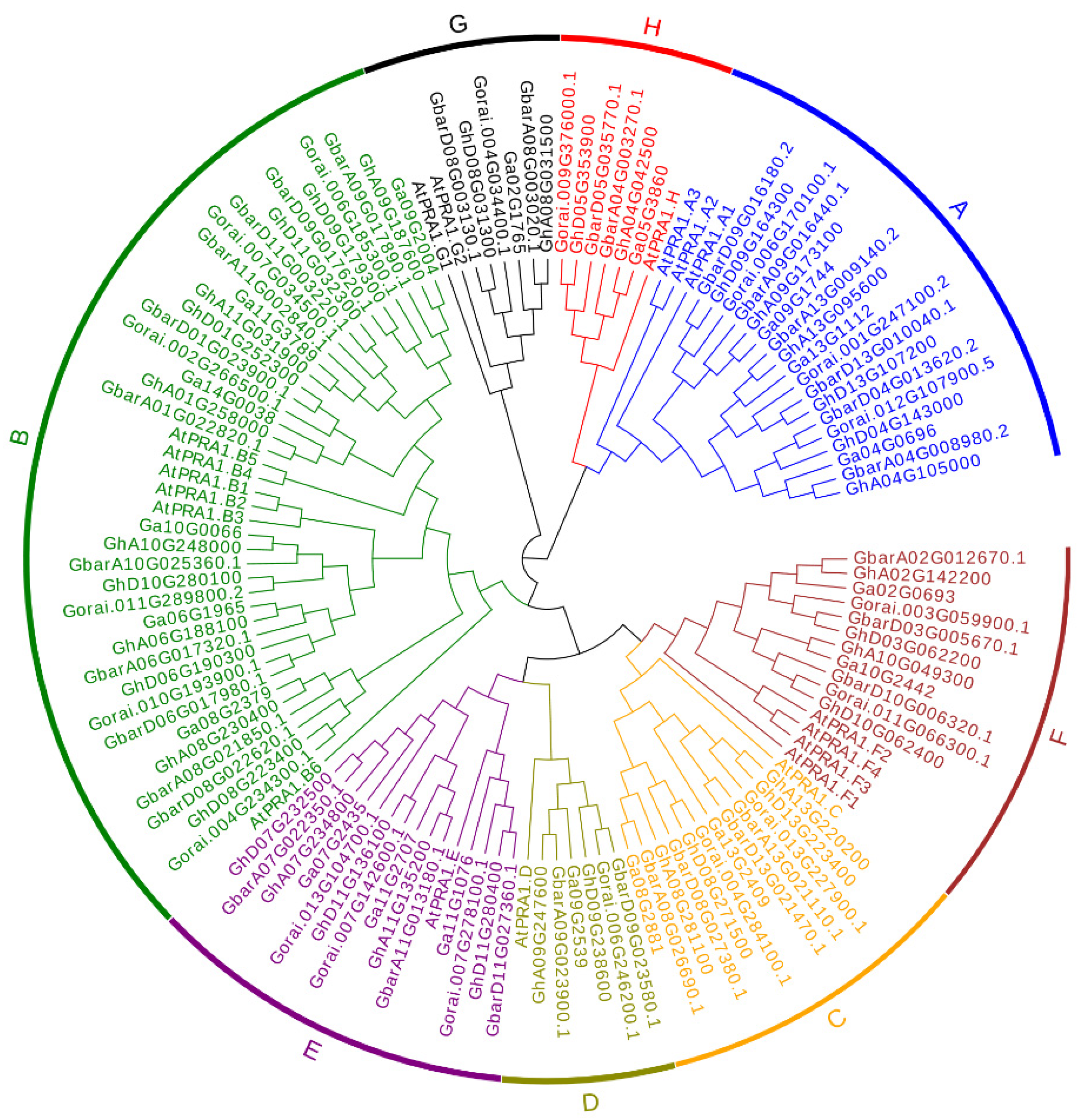
3.1. Identification and Phylogenetic Analysis of PRA1 Family Members
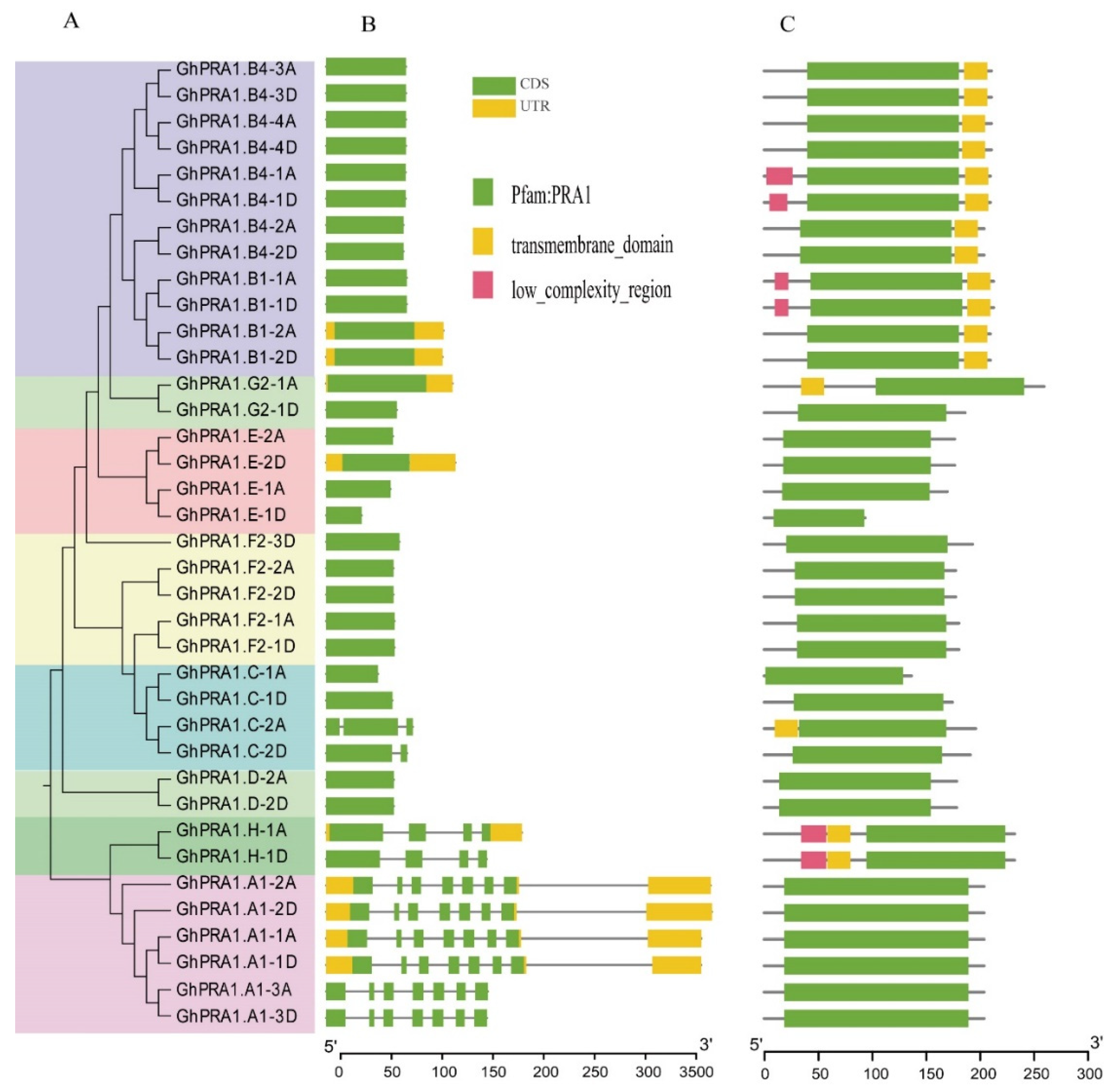
3.2. Analysis of the Gene Organization and Conserved Domains of GhPRA1s
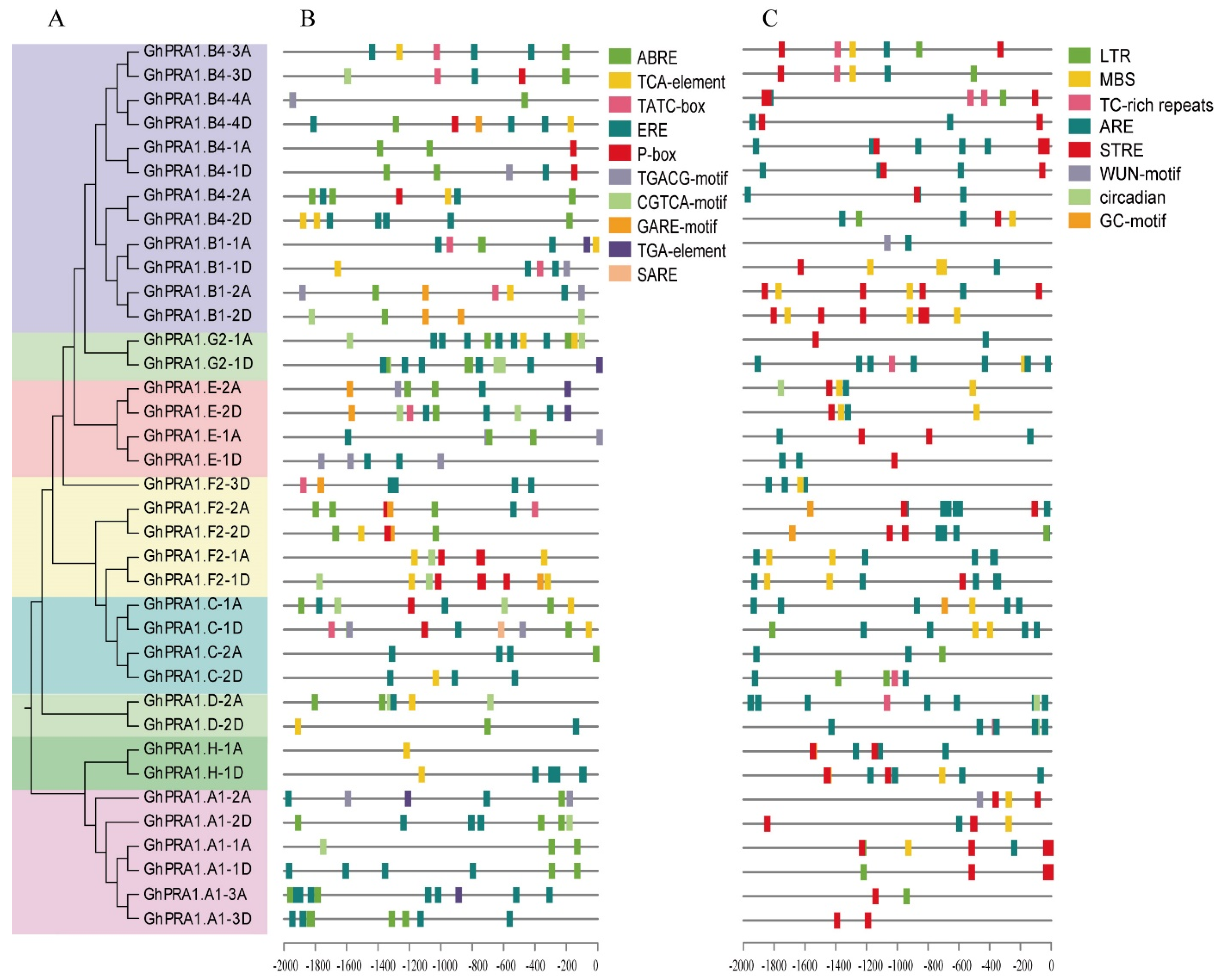
3.3. Analysis of Cis-Acting Elements of PRA1 Promoter
3.4. Chromosomal Location and Gene Synteny Analyses of GhPRA1 Genes
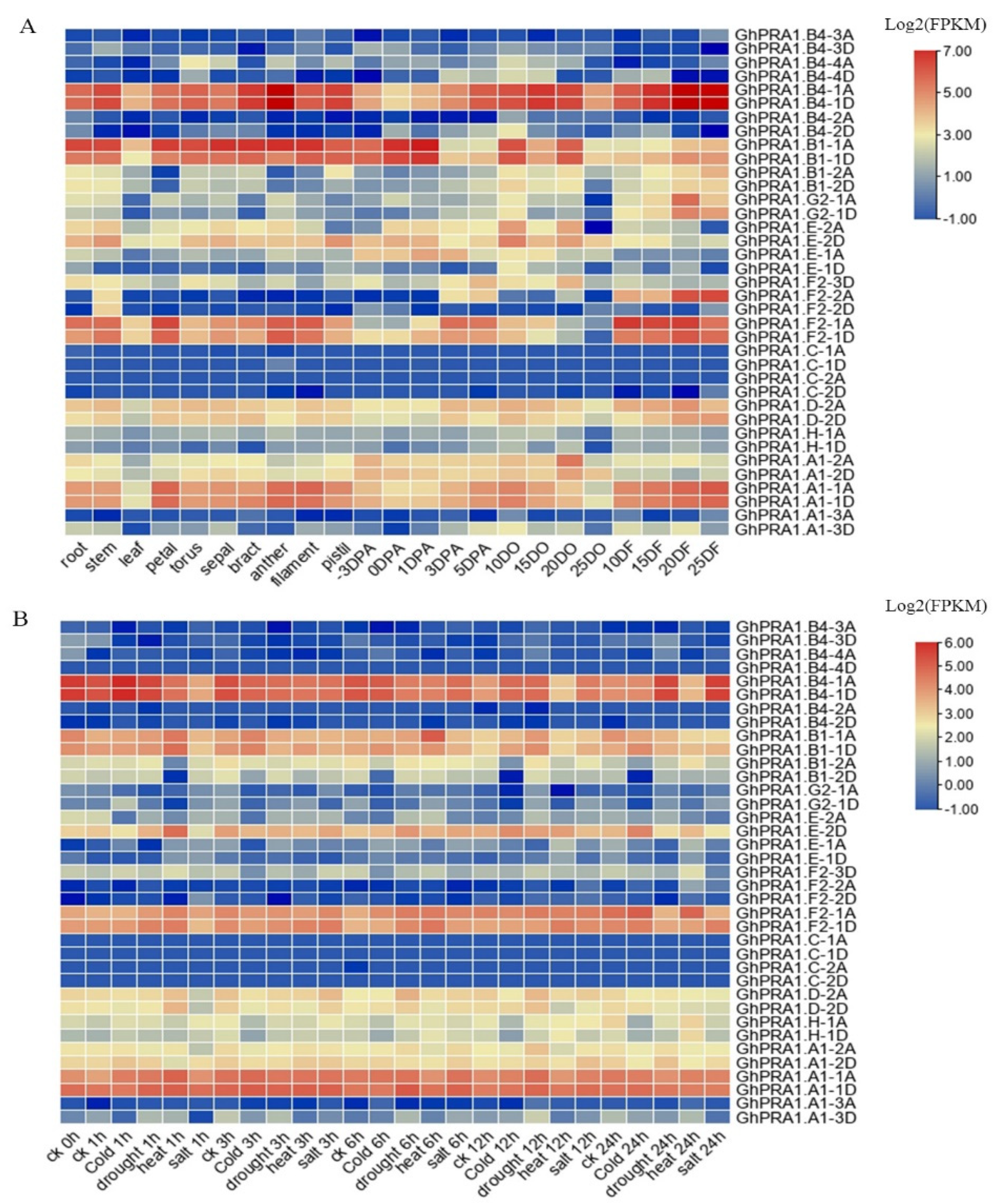
3.5. Expression Profiles of GhPRA1 in Upland Cotton
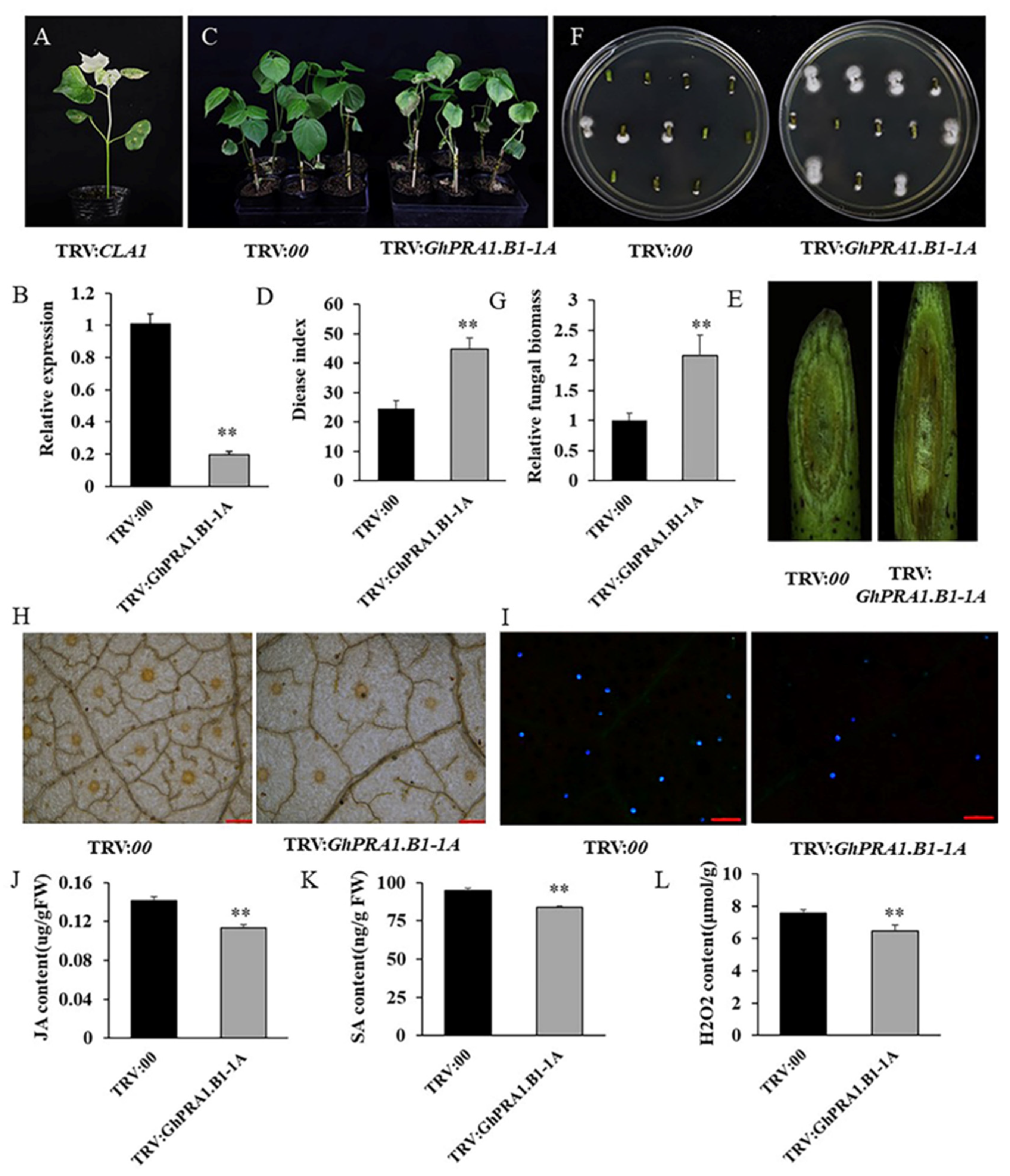
3.6. Overexpression of GhPRA1.B1-1A Enhanced V. dahliae Resistance in Arabidopsis
3.7. Silencing GhPRA1.B1-1A Reduced V. dahliae Resistance in Upland Cotton
3.8. GhPRA1.B1-1A Affected PR Genes’ Expression and Regulated the SA and JA Signaling Pathways
4. Discussion
4.1. Evolution of GhPRA1 Genes
4.2. Expression of GhPRA1 in Tissue and under Abiotic Stress
4.3. GhPRA1.B1-1A Enhanced Cotton’s Resistance to V. dahliae through ROS Accumulation and the SA and JA Signaling Pathways
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ali, F.; Qanmber, G.; Li, Y.; Ma, S.; Lu, L.; Yang, Z.; Wang, Z.; Li, F. Genome-wide identification of Gossypium INDETERMINATE DOMAIN genes and their expression profiles in ovule development and abiotic stress responses. J. Cotton Res. 2019, 2, 1–16. [Google Scholar] [CrossRef]
- Wendel, J.F. New world tetraploid cottons contain old-world cytoplasm. Proc. Natl. Acad. Sci. USA 1989, 86, 4132–4136. [Google Scholar] [CrossRef] [PubMed]
- Shaban, M.; Miao, Y.; Ullah, A.; Khan, A.Q.; Menghwar, H.; Khan, A.H.; Ahmed, M.M.; Tabassum, M.A.; Zhu, L. Physiological and molecular mechanism of defense in cotton against Verticillium dahliae. Plant Physiol. Biochem. 2018, 125, 193–204. [Google Scholar] [CrossRef]
- Daayf, F. Verticillium wilts in crop plants: Pathogen invasion and host defence responses. Can. J. Plant Pathol. 2015, 37, 8–20. [Google Scholar] [CrossRef]
- Zhao, P.; Zhao, Y.L.; Jin, Y.; Zhang, T.; Guo, H.S. Colonization process of Arabidopsis thaliana roots by a green fluorescent protein-tagged isolate of Verticillium dahliae. Protein Cell 2014, 5, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Talboys, P.W. Association of tylosis and hyperplasia of the xylem with vascular invasion of the hop by Verticillium alboatrum. Trans. Br. Mycol. Soc. 1958, 41, 249–260. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, Y.; Yang, J.; Zhang, M.; Ma, Q.; Wang, X.; Ma, Z. The G-protein α subunit GhGPA positively regulates Gossypium hirsutum resistance to Verticillium dahliae via induction of SA and JA signaling pathways and ROS accumulation. Crop J. 2021, 9, 823–833. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, L.; Wang, X.; Chen, B.; Zhao, J.; Cui, J.; Li, Z.; Yang, J.; Wu, L.; Wu, J. The cotton laccase gene GhLAC15 enhances Verticillium wilt resistance via an increase in defence-induced lignification and lignin components in the cell walls of plants. Mol. Plant Pathol. 2019, 20, 309–322. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.; Wang, X.; Wang, W.; Li, Z.; Wu, J.; Wang, G.; Wu, L.; Zhang, G.; Ma, Z. HyPRP1 performs a role in negatively regulating cotton resistance to V. dahliae via the thickening of cell walls and ROS accumulation. BMC Plant Biol. 2018, 18, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhou, L.; Jamieson, P.; Zhang, L.; Zhao, Z.; Babilonia, K.; Shao, W.; Wu, L.; Mustafa, R.; Amin, I. The cotton wall-associated kinase GhWAK7A mediates responses to fungal wilt pathogens by complexing with the chitin sensory receptors. Plant Cell 2020, 32, 3978–4001. [Google Scholar] [CrossRef]
- Feng, H.; Li, C.; Zhou, J.; Yuan, Y.; Feng, Z.; Shi, Y.; Zhao, L.; Zhang, Y.; Wei, F.; Zhu, H. A cotton WAKL protein interacted with a DnaJ protein and was involved in defense against Verticillium dahliae. Int. J. Biol. Macromol. 2021, 167, 633–643. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, X.; Sun, Y.; Wang, P.; Li, X.; Pei, Y.; Li, F.; Hou, Y. Molecular evidence for the involvement of a polygalacturonase-inhibiting protein, GhPGIP1, in enhanced resistance to Verticillium and Fusarium wilts in cotton. Sci. Rep. 2017, 7, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Liu, S.; Zhang, Z.; Sun, L.; He, X.; Lindsey, K.; Zhu, L.; Zhang, X. GhCyP3 improves the resistance of cotton to Verticillium dahliae by inhibiting the E3 ubiquitin ligase activity of GhPUB17. Plant Mol. Biol. 2019, 99, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Stenmark, H. Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 2009, 10, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Stenmark, H.; Olkkonen, V.M. The rab gtpase family. Genome Biol. 2001, 2, 1–7. [Google Scholar] [CrossRef][Green Version]
- Agarwal, P.; Reddy, M.; Sopory, S.; Agarwal, P.K. Plant rabs: Characterization, functional diversity, and role in stress tolerance. Plant Mol. Biol. Rep. 2009, 27, 417–430. [Google Scholar] [CrossRef]
- Gao, W.; Tian, Z.; Liu, J.; Lin, Y.; Xie, C. Isolation, characterization and functional analysis of StRab, a cDNA clone from potato encoding a small GTP-binding protein. Sci. Hortic. 2012, 135, 80–86. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, H.; Zhang, G.; Zhao, R.; Bie, T.; Zhang, R.; Gao, D.; Xing, L.; Cao, A. Characterization of a small GTP-binding protein gene TaRab18 from wheat involved in the stripe rust resistance. Plant Physiol. Biochem. 2017, 113, 40–50. [Google Scholar] [CrossRef]
- Kwon, S.I.; Cho, H.J.; Kim, S.R.; Park, O.K. The Rab GTPase RabG3b positively regulates autophagy and immunity-associated hypersensitive cell death in Arabidopsis. Plant Pphysiol. 2013, 161, 1722–1736. [Google Scholar] [CrossRef]
- Nielsen, M.E.; Jürgens, G. Thordal-Christensen H: VPS9a activates the Rab5 GTPase ARA7 to confer distinct pre-and postinvasive plant innate immunity. Plant Cell 2017, 29, 1927–1937. [Google Scholar] [CrossRef]
- Taefehshokr, N.; Yin, C.; Heit, B. Rab GTPases in the differential processing of phagocytosed pathogens versus efferocytosed apoptotic cells. Histol. Histopathol. 2021, 36, 123–135. [Google Scholar] [PubMed]
- Alvim Kamei, C.L.; Boruc, J.; Vandepoele, K.; Van den Daele, H.; Maes, S.; Russinova, E.; Inzé, D.; De Veylder, L. The PRA1 gene family in Arabidopsis. Plant Physiol. 2008, 147, 1735–1749. [Google Scholar] [CrossRef]
- Yang, X.; Matern, H.T.; Gallwitz, D. Specific binding to a novel and essential Golgi membrane protein (Yip1p) functionally links the transport GTPases Ypt1p and Ypt31p. EMBO J. 1998, 17, 4954–4963. [Google Scholar] [CrossRef]
- Calero, M.; Winand, N.J.; Collins, R.N. Identification of the novel proteins Yip4p and Yip5p as Rab GTPase interacting factors. FEBS Lett. 2002, 515, 89–98. [Google Scholar] [CrossRef]
- Hutt, D.M.; Da-Silva, L.F.; Chang, L.H.; Prosser, D.C.; Ngsee, J.K. PRA1 inhibits the extraction of membrane-bound rab GTPase by GDI1. J. Biol. Chem. 2000, 275, 18511–18519. [Google Scholar] [CrossRef]
- Figueroa, C.; Taylor, J.; Vojtek, A.B. Prenylated Rab acceptor protein is a receptor for prenylated small GTPases. J. Biol. Chem. 2001, 276, 28219–28225. [Google Scholar] [CrossRef]
- Jung, C.J.; Hui Lee, M.; Ki Min, M.; Hwang, I. Localization and trafficking of an isoform of the AtPRA1 family to the golgi apparatus depend on both N- and C- terminal sequence motifs. Traffic 2011, 12, 185–200. [Google Scholar] [CrossRef]
- Rho, H.S.; Heo, J.B.; Bang, W.Y.; Hwang, S.M.; Nahm, M.Y.; Kwon, H.J.; Kim, S.W.; Lee, B.H.; Bahk, J.D. The role of OsPRA1 in vacuolar trafficking by OsRab GTPases in plant system. Plant Sci. 2009, 177, 411–417. [Google Scholar] [CrossRef]
- Pizarro, L.; Leibman-Markus, M.; Schuster, S.; Bar, M.; Avni, A. SlPRA1A/RAB attenuate EIX immune responses via degradation of LeEIX2 pattern recognition receptor. Plant Signal Behav. 2018, 13, e1467689. [Google Scholar] [CrossRef]
- Paterson, A.H.; Wendel, J.F.; Gundlach, H.; Guo, H.; Jenkins, J.; Jin, D.; Llewellyn, D.; Showmaker, K.C.; Shu, S.; Udall, J.; et al. Repeated polyploidization of Gossypium genomes and the evolution of spinnable cotton fibres. Nature 2012, 492, 423–427. [Google Scholar] [CrossRef]
- Du, X.; Huang, G.; He, S.; Yang, Z.; Sun, G.; Ma, X.; Li, N.; Zhang, X.; Sun, J.; Liu, M.; et al. Resequencing of 243 diploid cotton accessions based on an updated A genome identifies the genetic basis of key agronomic traits. Nat. Genet. 2018, 50, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tu, L.; Yuan, D.; Zhu, D.; Shen, C.; Li, J.; Liu, F.; Pei, L.; Wang, P.; Zhao, G.; et al. Reference genome sequences of two cultivated allotetraploid cottons, Gossypium hirsutum and Gossypium barbadense. Nat. Genet. 2019, 51, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ge, X.; Yang, Z.; Qin, W.; Sun, G.; Wang, Z.; Li, Z.; Liu, J.; Wu, J.; Wang, Y.; et al. Extensive intraspecific gene order and gene structural variations in upland cotton cultivars. Nat. Commun. 2019, 10, 2989. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Jung, S.; Cheng, C.H.; Ficklin, S.P.; Lee, T.; Zheng, P.; Jones, D.; Percy, R.G.; Main, D. CottonGen: A genomics, genetics and breeding database for cotton research. Nucleic Acids Res. 2014, 42, D1229–D1236. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. 20 years of the SMART protein domain annotation resource. Nucleic Acids Res. 2018, 46, D493–D496. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.h.; Jin, H.; Marler, B.; Guo, H. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR. Methods 2002, 25, 402–408. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Gao, X.; Wheeler, T.; Li, Z.; Kenerley, C.M.; He, P.; Shan, L. Silencing GhNDR1 and GhMKK2 compromises cotton resistance to Verticillium wilt. Plant J. 2011, 66, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.P.; Shen, J.L.; Li, W.J.; Wu, N.; Chen, C.; Hou, Y.X. Evolutionary and characteristic analysis of RING-DUF1117 E3 ubiquitin ligase genes in Gossypium discerning the role of GhRDUF4D in Verticillium dahliae resistance. Biomolecules 2021, 11, 1145. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ma, Q.; Zhang, Y.; Wang, X.; Zhang, G.; Ma, Z. Molecular cloning and functional analysis of GbRVd, a gene in Gossypium barbadense that plays an important role in conferring resistance to Verticillium wilt. Gene 2016, 575, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Rong, W.; Yang, J.; Ma, Z. Island cotton enhanced disease susceptibility 1 gene encoding a lipase-like protein plays a crucial role in response to Verticillium dahliae by regulating the SA level and H2O2 accumulation. Front. Plant Sci. 2016, 7, 1830. [Google Scholar]
- Liu, S.; Sun, R.; Zhang, X.; Feng, Z.; Wei, F.; Zhao, L.; Zhang, Y.; Zhu, L.; Feng, H.; Zhu, H. Genome-wide analysis of OPR family genes in cotton identified a role for GhOPR9 in Verticillium dahliae resistance. Genes 2020, 11, 1134. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, J.; Fang, L.; Zhang, Z.; Ma, W.; Niu, Y.; Ju, L.; Deng, J.; Zhao, T.; Lian, J.; et al. Gossypium barbadense and Gossypium hirsutum genomes provide insights into the origin and evolution of allotetraploid cotton. Nat. Genet. 2019, 51, 739–748. [Google Scholar] [CrossRef]
- Jwa, N.S.; Hwang, B.K. Convergent evolution of pathogen effectors toward reactive oxygen species signaling networks in plants. Front. Plant Sci. 2017, 8, 1687. [Google Scholar] [CrossRef]
- Noman, A.; Aqeel, M.; Lou, Y. PRRs and NB-LRRs: From signal perception to activation of plant innate immunity. Int. J. Mol. Sci. 2019, 20, 1882. [Google Scholar] [CrossRef]
- Ben Khaled, S.; Postma, J.; Robatzek, S. A moving view: Subcellular trafficking processes in pattern recognition receptor–triggered plant immunity. Annu Rev. Phytopathol. 2015, 53, 379–402. [Google Scholar] [CrossRef]
- Li, F.; Fan, G.; Lu, C.; Xiao, G.; Zou, C.; Kohel, R.J.; Ma, Z.; Shang, H.; Ma, X.; Wu, J. Genome sequence of cultivated Upland cotton (Gossypium hirsutum TM-1) provides insights into genome evolution. Nat. Biotechnol. 2015, 33, 524–530. [Google Scholar] [CrossRef]
- Lee, M.H.; Yoo, Y.J.; Kim, D.H.; Hanh, N.H.; Kwon, Y.; Hwang, I. The prenylated rab GTPase receptor PRA1. F4 contributes to protein exit from the Golgi apparatus. Plant Physiol. 2017, 174, 1576–1594. [Google Scholar] [CrossRef]
- Spoel, S.H.; Johnson, J.S.; Dong, X. Regulation of tradeoffs between plant defenses against pathogens with different lifestyles. Proc. Nati. Acad. Sci. USA 2007, 104, 18842–18847. [Google Scholar] [CrossRef]
- Bari, R.; Jones, J.D. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef]
- Betsuyaku, S.; Katou, S.; Takebayashi, Y.; Sakakibara, H.; Nomura, N.; Fukuda, H. Salicylic acid and jasmonic acid pathways are activated in spatially different domains around the infection site during effector-triggered immunity in Arabidopsis thaliana. Plant Cell Physiol. 2018, 59, 8–16. [Google Scholar] [CrossRef]
- Long, L.; Xu, F.C.; Zhao, J.R.; Li, B.; Xu, L.; Gao, W. GbMPK3 overexpression increases cotton sensitivity to Verticillium dahliae by regulating salicylic acid signaling. Plant Sci. 2020, 292, 110374. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, T.; Ling, X.; Ma, Z. Gbvdr6, a gene encoding a receptor-like protein of cotton (Gossypium barbadense), confers resistance to Verticillium wilt in Arabidopsis and upland cotton. Front. Plant Sci. 2018, 8, 2272. [Google Scholar] [CrossRef]
- Guo, W.; Jin, L.; Miao, Y.; He, X.; Hu, Q.; Guo, K.; Zhu, L.; Zhang, X. An ethylene response-related factor, GbERF1-like, from Gossypium barbadense improves resistance to Verticillium dahliae via activating lignin synthesis. Plant Mol. Biol. 2016, 91, 305–318. [Google Scholar] [CrossRef]
- Gong, Q.; Yang, Z.; Wang, X.; Butt, H.I.; Chen, E.; He, S.; Zhang, C.; Zhang, X.; Li, F. Salicylic acid-related cotton (Gossypium arboreum) ribosomal protein GaRPL18 contributes to resistance to Verticillium dahliae. BMC Plant Biol. 2017, 17, 1–15. [Google Scholar] [CrossRef]
- Ali, M.; Cheng, Z.; Ahmad, H. Hayat S: Reactive oxygen species (ROS) as defenses against a broad range of plant fungal infections and case study on ROS employed by crops against Verticillium dahliae wilts. J. Plant Interact. 2018, 13, 353–363. [Google Scholar] [CrossRef]
- Xie, C.; Wang, C.; Wang, X.; Yang, X. Proteomics-based analysis reveals that Verticillium dahliae toxin induces cell death by modifying the synthesis of host proteins. J. Gen. Plant Pathol. 2013, 79, 335–345. [Google Scholar] [CrossRef][Green Version]




| Gene Name | Gene Locus ID | Chr Location | CDS | Size | MW (kDa) | pI | Subcellular Localization |
|---|---|---|---|---|---|---|---|
| GhPRA1.A1-1A | Gh_A04G105000.1 | A04: 70948155–70951205 | 630 | 209 | 24.19 | 10.58 | vacu: 5, plas: 4, extr: 2 |
| GhPRA1.A1-1D | Gh_D04G143000.1 | D04: 44330508–44333557 | 630 | 209 | 24.20 | 10.44 | vacu: 7, plas: 4, golg: 2, |
| GhPRA1.A1-2A | Gh_A09G173100.1 | A09: 74156483–74159609 | 630 | 209 | 24.17 | 10.58 | vacu: 12, plas: 1, golg: 1 |
| GhPRA1.A1-2D | Gh_D09G164300.1 | D09: 43855015–43858151 | 630 | 209 | 24.23 | 10.48 | vacu: 11, plas: 2, golg: 1 |
| GhPRA1.A1-3A | Gh_A13G095600.1 | A13: 40978102–40979417 | 630 | 209 | 23.98 | 10.41 | vacu: 6, plas: 4, golg: 2 |
| GhPRA1.A1-3D | Gh_D13G107200.1 | D13: 24914233–24915541 | 630 | 209 | 24.11 | 10.45 | plas: 5, vacu: 5, golg: 2 |
| GhPRA1.B1-1A | Gh_A06G188100.1 | A06: 117938223–117938879 | 657 | 218 | 23.31 | 9.44 | chlo: 5, ER: 4, plas: 3 |
| GhPRA1.B1-1D | Gh_D06G190300.1 | D06: 58492262–58492918 | 657 | 218 | 23.40 | 9.64 | chlo: 7, ER: 3, plas: 2 |
| GhPRA1.B1-2A | Gh_A10G248000.1 | A10: 114198294–114199248 | 648 | 215 | 23.49 | 9.64 | chlo: 6, ER: 3, plas: 2 |
| GhPRA1.B1-2D | Gh_D10G280100.1 | D10: 65324234–65325180 | 648 | 215 | 23.54 | 9.83 | chlo: 6, ER: 3, plas: 2 |
| GhPRA1.B4-1A | Gh_A01G258000.1 | A01: 115880792–115881439 | 648 | 215 | 23.32 | 7.82 | E.R.: 5, plas: 3, chlo: 2 |
| GhPRA1.B4-1D | Gh_D01G252300.1 | D01: 64514473–64515120 | 648 | 215 | 23.33 | 7.82 | E.R.: 4, plas: 3, chlo: 2, |
| GhPRA1.B4-2A | Gh_A08G230400.1 | A08: 119818691–119819320 | 630 | 209 | 22.74 | 8.68 | vacu: 7, ER: 3, plas: 2 |
| GhPRA1.B4-2D | Gh_D08G223400.1 | D08: 63336820–63337449 | 630 | 209 | 22.81 | 8.68 | vacu: 7, ER: 3, plas: 2 |
| GhPRA1.B4-3A | Gh_A09G187600.1 | A09: 75447349–75447999 | 651 | 216 | 23.70 | 9.03 | chlo: 5, plas: 5, golg: 2 |
| GhPRA1.B4-3D | Gh_D09G179300.1 | D09: 45181994–45182644 | 651 | 216 | 23.64 | 8.97 | chlo: 5, plas: 4, vacu: 2 |
| GhPRA1.B4-4A | Gh_A11G031900.1 | A11: 2693360–2694010 | 651 | 216 | 23.69 | 6.73 | vacu: 10, plas: 3, extr: 1 |
| GhPRA1.B4-4D | Gh_D11G032300.1 | D11: 2630603–2631253 | 651 | 216 | 23.69 | 6.10 | vacu: 12, plas: 1, extr: 1 |
| GhPRA1.C-1A | Gh_A08G281100.1 | A08: 124823067–124823489 | 423 | 140 | 16.10 | 6.71 | plas: 4.5, vacu: 3 |
| GhPRA1.C-1D | Gh_D08G271500.1 | D08: 67997394–67997933 | 540 | 179 | 20.29 | 6.06 | vacu: 9, golg: 3, plas: 1 |
| GhPRA1.C-2A | Gh_A13G220200.1 | A13: 104371813–104372518 | 606 | 201 | 22.70 | 5.11 | vacu: 11, plas: 1, extr: 1 |
| GhPRA1.C-2D | Gh_D13G223400.1 | D13: 59483095–59483754 | 591 | 196 | 22.08 | 8.05 | vacu: 7, plas: 2.5, golg: 2 |
| GhPRA1.D-2A | Gh_A09G247600.1 | A09: 81208181–81208732 | 552 | 183 | 20.15 | 4.71 | plas: 8, ER: 2, chlo: 1 |
| GhPRA1.D-2D | Gh_D09G238600.1 | D09: 50472807–50473358 | 552 | 183 | 20.20 | 4.59 | plas: 8, ER: 3, chlo: 1 |
| GhPRA1.E-1A | Gh_A11G135200.1 | A11: 14400779–14401303 | 525 | 174 | 19.28 | 7.95 | plas: 8, chlo: 3, ER: 3 |
| GhPRA1.E-1D | Gh_D11G136100.1 | D11: 12800664–12800954 | 291 | 96 | 11.19 | 10.84 | chlo: 7, plas: 6, ER: 1 |
| GhPRA1.E-2A | Gh_A07G234800.1 | A07: 93082823–93083368 | 546 | 181 | 20.04 | 9.68 | chlo: 7, plas: 6, ER: 1 |
| GhPRA1.E-2D | Gh_D07G232500.1 | D07: 55224484–55225535 | 546 | 181 | 20.17 | 9.68 | chlo: 7, plas: 6, ER: 1 |
| GhPRA1.F2-1A | Gh_A02G142200.1 | A02: 80712703–80713260 | 558 | 185 | 20.72 | 9.09 | plas: 10, golg: 2, vacu: 1 |
| GhPRA1.F2-1D | Gh_D03G062200.1 | D03: 10655558–10656115 | 558 | 185 | 20.76 | 8.07 | plas: 4, vacu: 3, ER: 3 |
| GhPRA1.F2-2A | Gh_A10G049300.1 | A10: 6090256–6090804 | 549 | 182 | 20.53 | 9.09 | plas: 5, golg: 3, chlo: 2 |
| GhPRA1.F2-2D | Gh_D10G062400.1 | D10: 6067187–6067735 | 549 | 182 | 20.47 | 7.93 | plas: 7, golg: 3, vacu: 2 |
| GhPRA1.F2-3D | Gh_D11G280400.1 | D11: 57861036–57861632 | 597 | 198 | 21.96 | 8.82 | plas: 7, ER: 3, chlo: 1 |
| GhPRA1.G2-1A | Gh_A08G031500.1 | A08: 3124048–3125076 | 801 | 266 | 28.99 | 8.76 | plas: 7, nucl: 2, vacu: 2 |
| GhPRA1.G2-1D | Gh_D08G031300.1 | D08: 2941239–2941814 | 576 | 191 | 20.78 | 7.09 | plas: 4.5, vacu: 3, ER: 3 |
| GhPRA1.H-1A | Gh_A04G042500.1 | A04: 7308990–7310582 | 717 | 238 | 26.79 | 9.14 | vacu: 8, plas: 2, extr: 2 |
| GhPRA1.H-1D | Gh_D05G353900.1 | D05: 56125424–56126730 | 717 | 238 | 26.84 | 9.30 | vacu: 9, plas: 2, extr: 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, N.; Li, W.-J.; Chen, C.; Zhao, Y.-P.; Hou, Y.-X. Analysis of the PRA1 Genes in Cotton Identifies the Role of GhPRA1.B1-1A in Verticillium dahliae Resistance. Genes 2022, 13, 765. https://doi.org/10.3390/genes13050765
Wu N, Li W-J, Chen C, Zhao Y-P, Hou Y-X. Analysis of the PRA1 Genes in Cotton Identifies the Role of GhPRA1.B1-1A in Verticillium dahliae Resistance. Genes. 2022; 13(5):765. https://doi.org/10.3390/genes13050765
Chicago/Turabian StyleWu, Na, Wen-Jie Li, Chen Chen, Yan-Peng Zhao, and Yu-Xia Hou. 2022. "Analysis of the PRA1 Genes in Cotton Identifies the Role of GhPRA1.B1-1A in Verticillium dahliae Resistance" Genes 13, no. 5: 765. https://doi.org/10.3390/genes13050765
APA StyleWu, N., Li, W.-J., Chen, C., Zhao, Y.-P., & Hou, Y.-X. (2022). Analysis of the PRA1 Genes in Cotton Identifies the Role of GhPRA1.B1-1A in Verticillium dahliae Resistance. Genes, 13(5), 765. https://doi.org/10.3390/genes13050765






