Integrated Analysis of the ceRNA Network and M-7474 Function in Testosterone-Mediated Fat Deposition in Pigs
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Sample Collection and RNA Isolation
Tissue Samples
2.2. Library Preparation for Long Noncoding RNA Sequencing and Data Analysis
2.3. Library Preparation for Micro RNA Sequencing and Data Analysis
2.4. Gene Ontology and Kyoto Encyclopedia of Genes and Genomes Enrichment Analysis
2.5. Construction of LncRNA–miRNA–Gene Regulatory Networks
2.6. Quantitative Polymerase Chain Reaction
2.7. Vector Construction
2.8. Dual-Luciferase Reporter Analysis
2.9. Overexpression and Differentiation of Preadipocytes
2.10. Statistical Analysis
3. Results
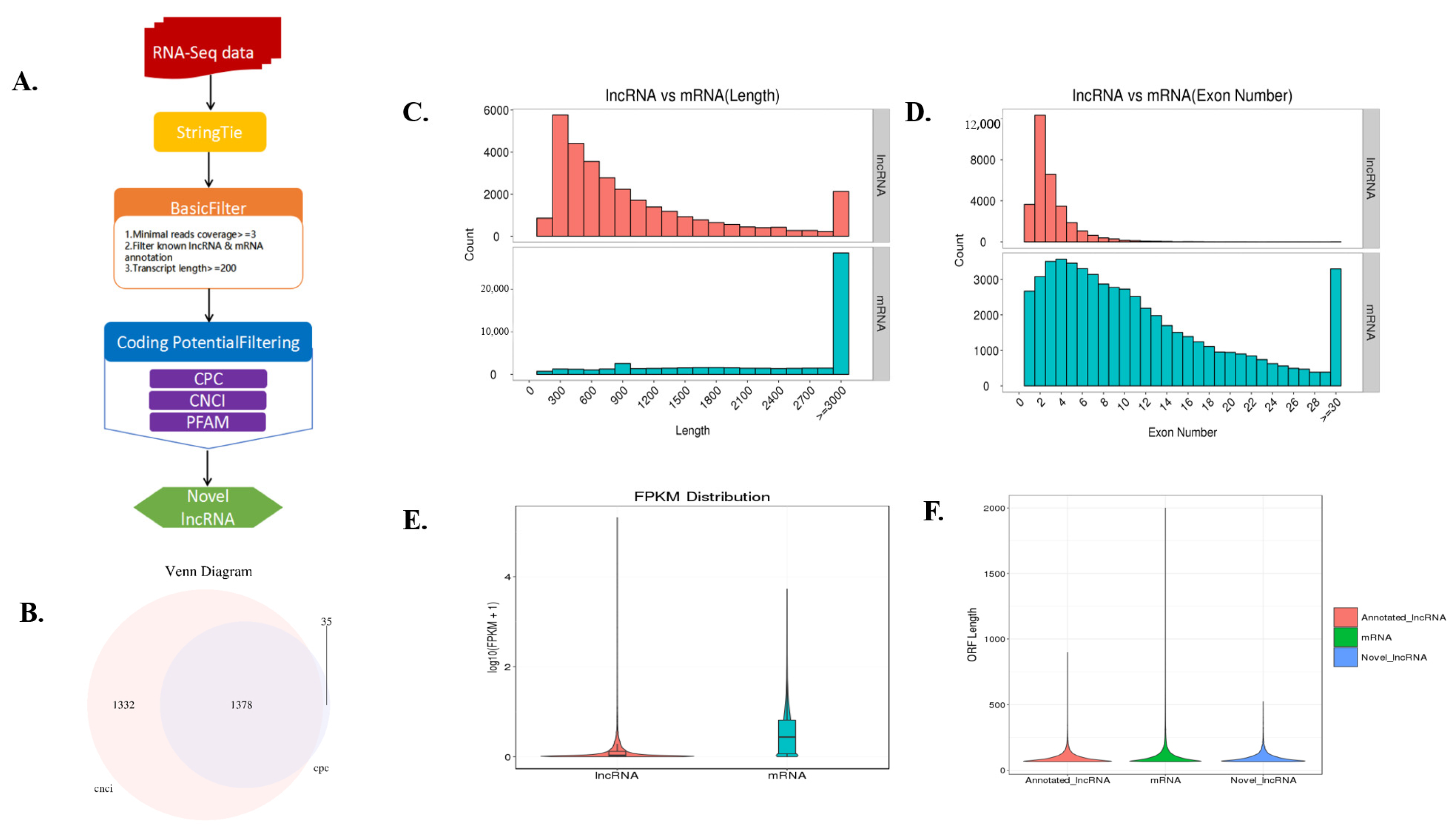
3.1. Overview of the Fat Deposition-Related Long Noncoding RNA, Messenger RNA and microRNA Transcription Profiles
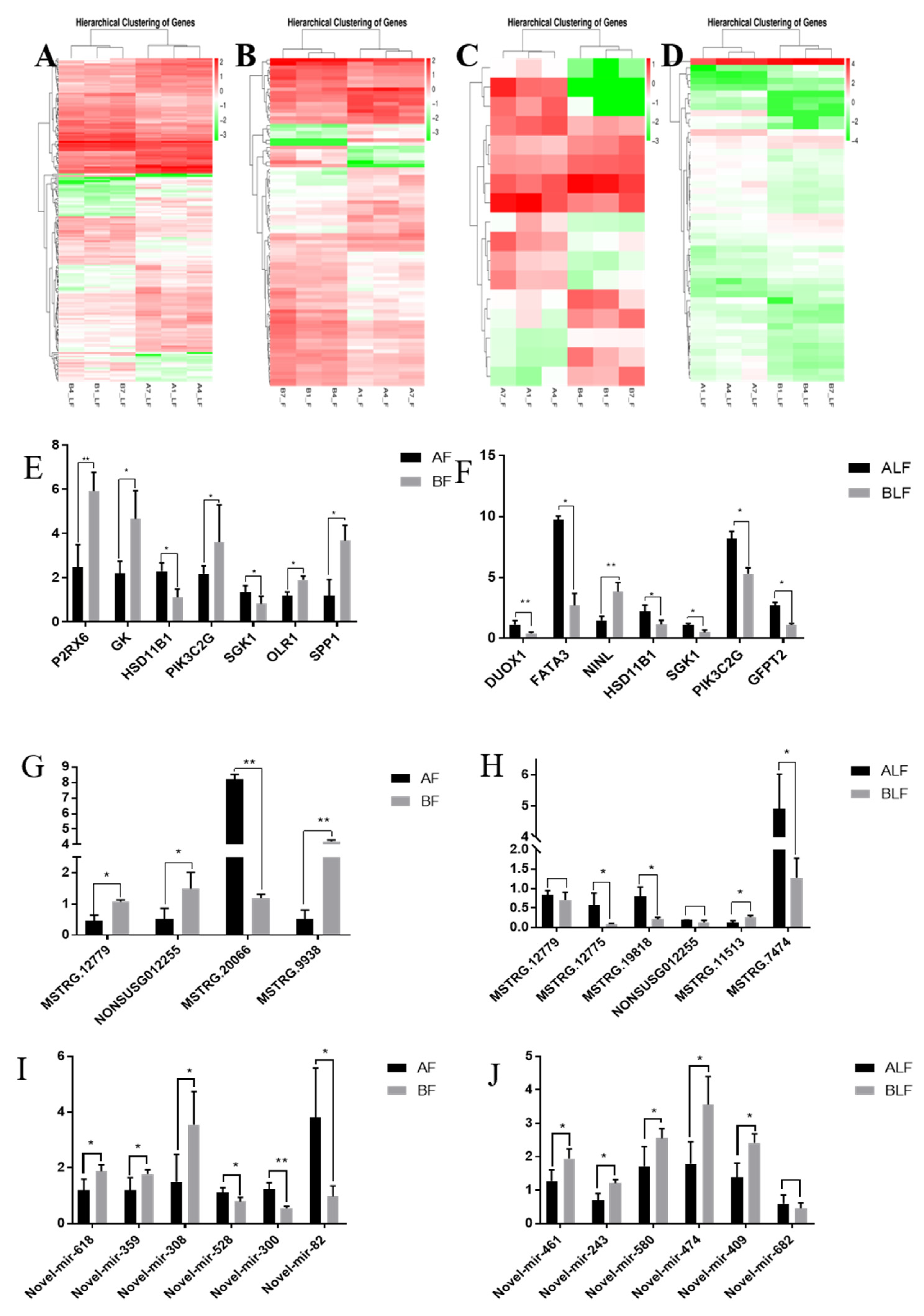
3.2. Differentially Expressed mRNAs and lncRNAs between Castrated and Intact Male Pigs
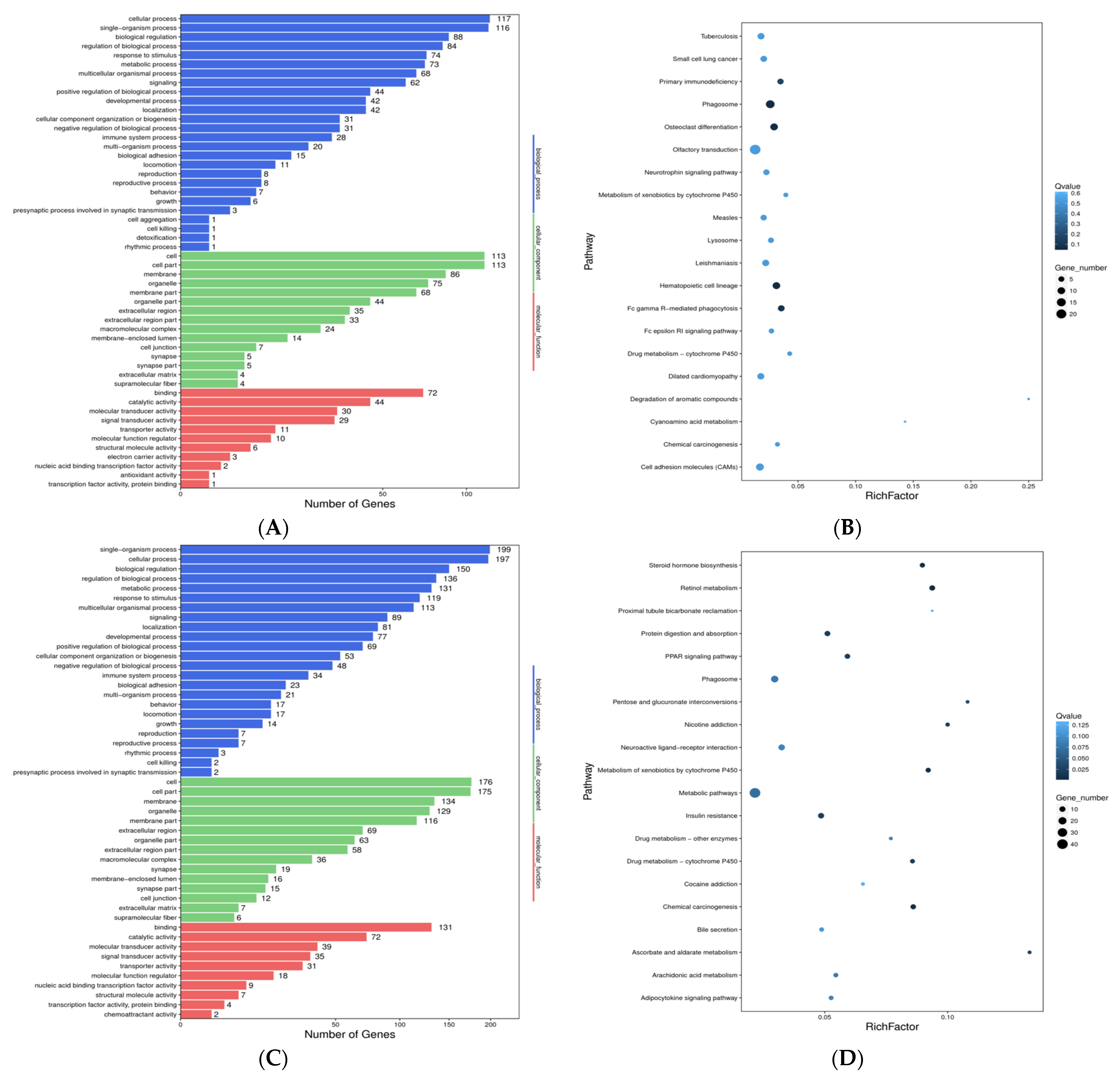
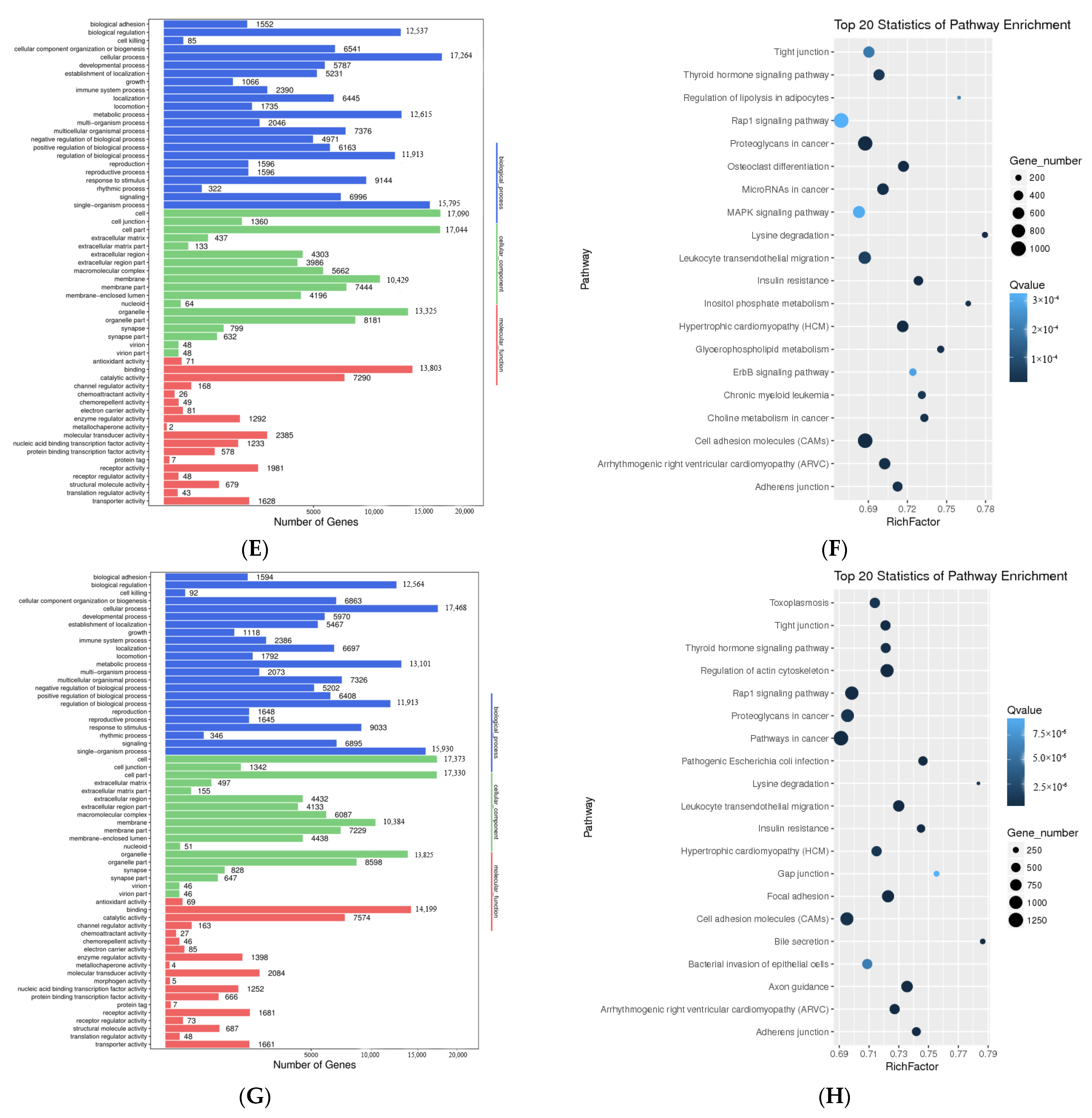
3.3. Functional Analysis of Differentially Expressed Transcripts
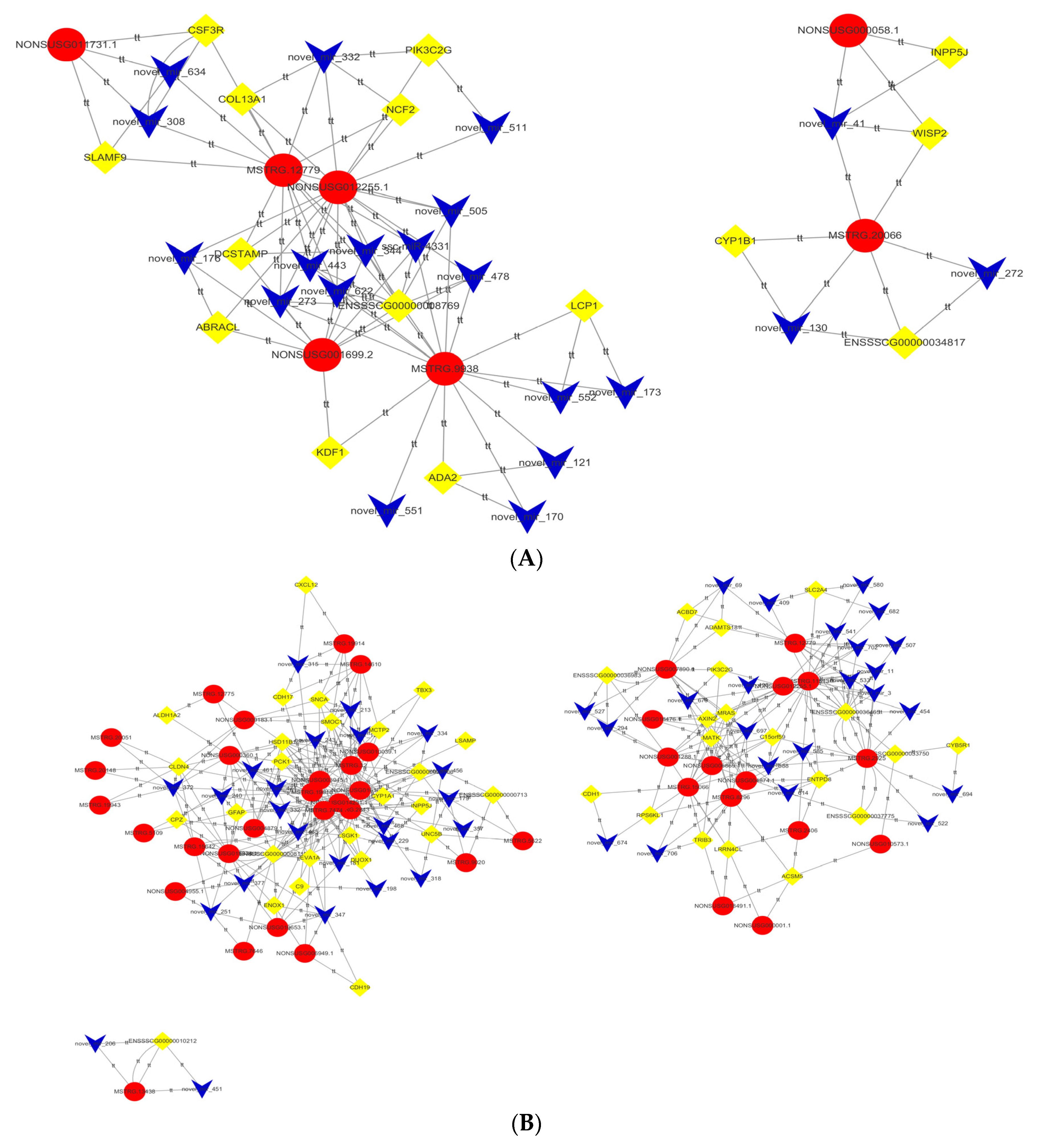
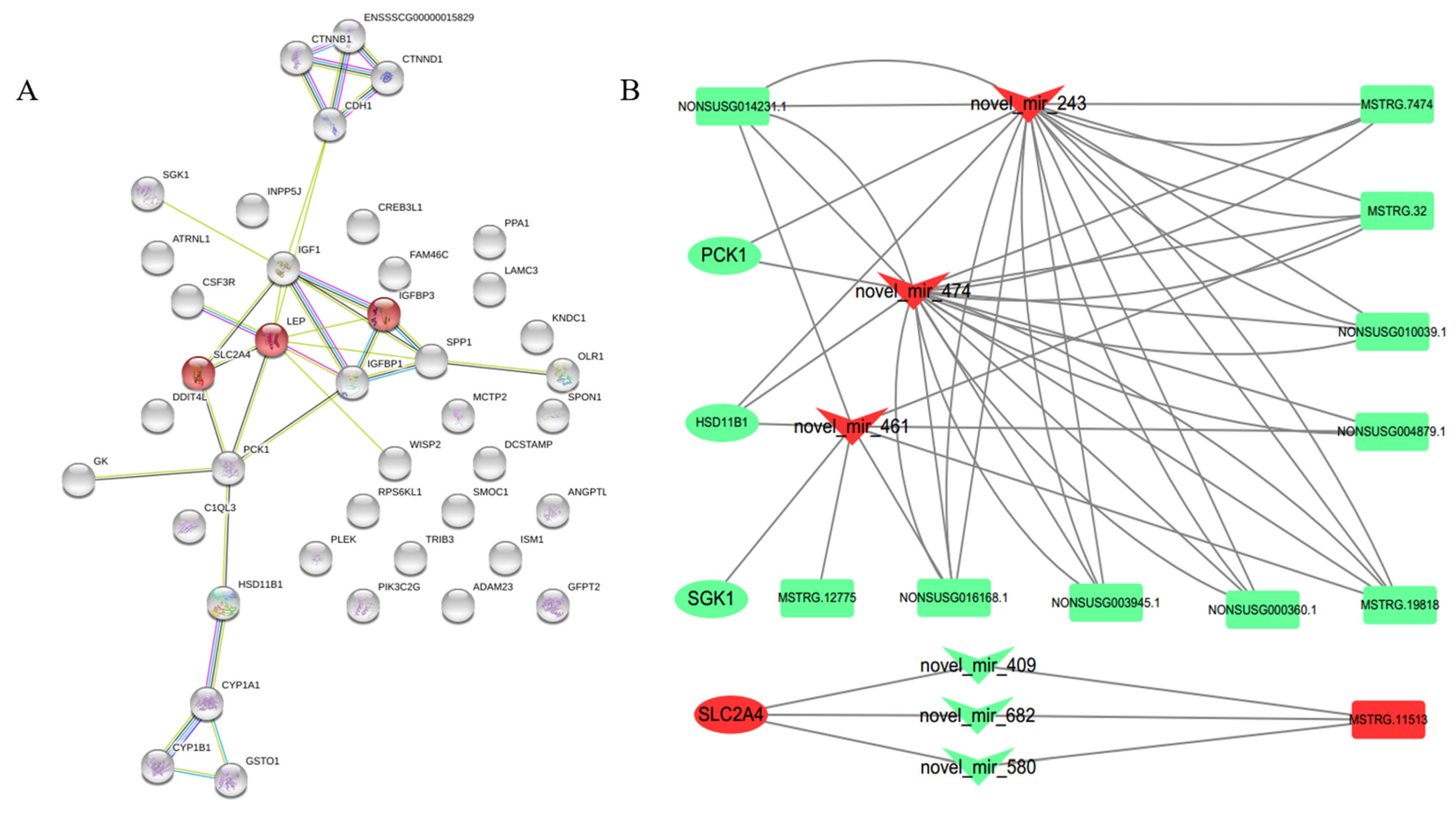
3.4. lncRNA–miRNA–mRNA Network Construction and Visualization
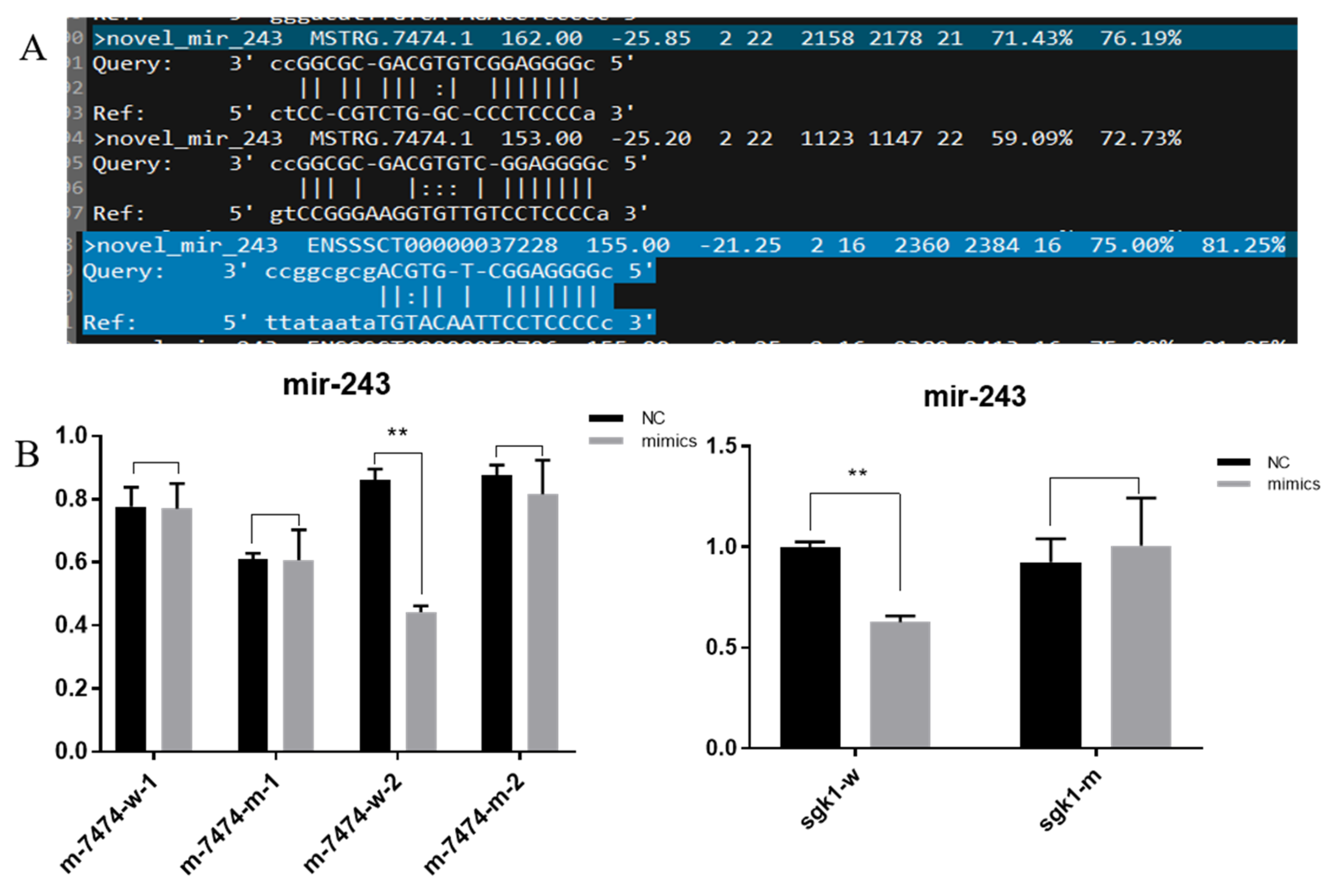
3.5. Double Fluorescence Binding Verification Experiment
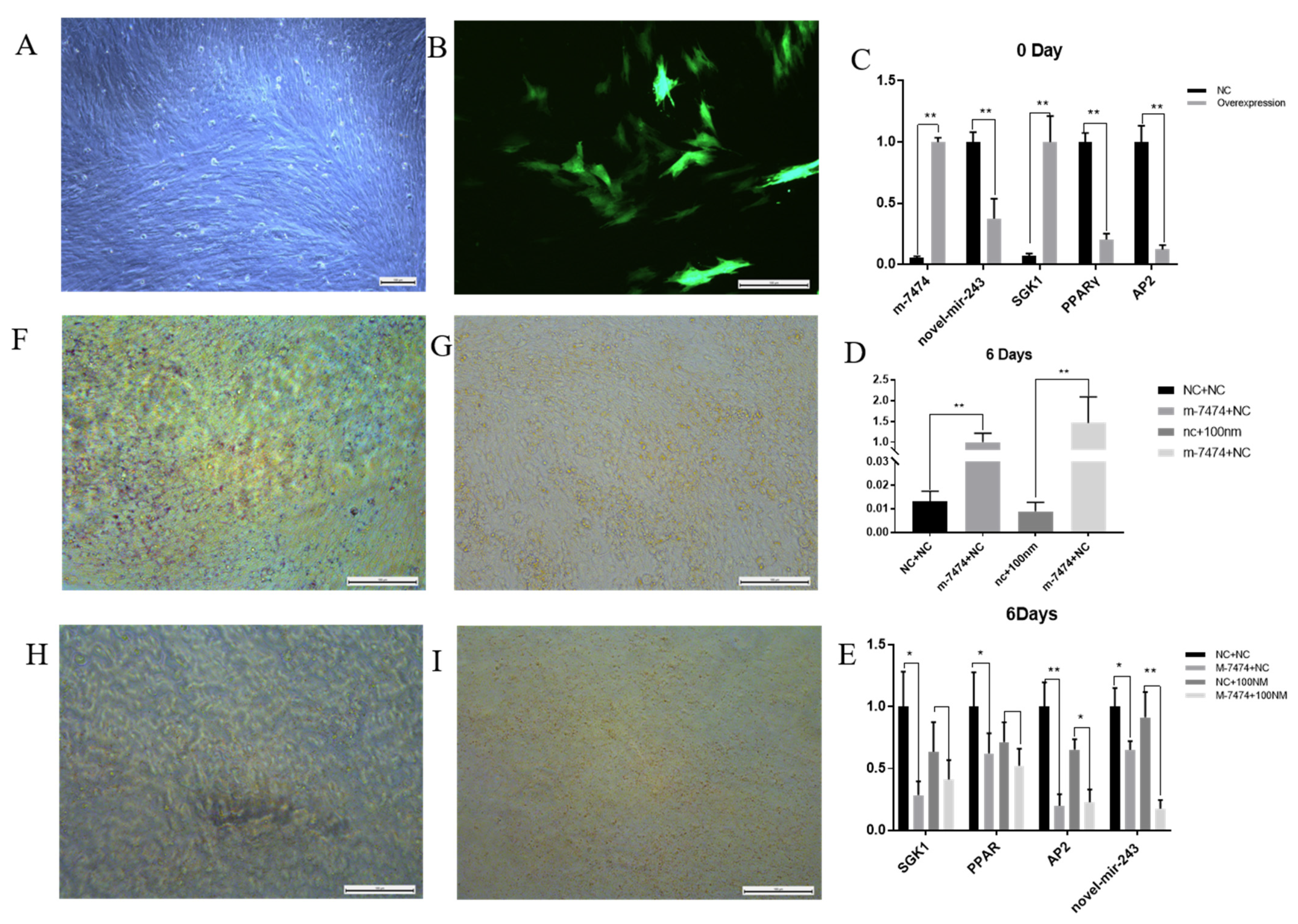
3.6. Functional Verification of Overexpressed M-7474
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsai, E.C.; Boyko, E.J.; Leonetti, D.L.; Fujimoto, W.Y. Low serum testosterone level as a predictor of increased visceral fat in Japanese-American men. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Bender, J.M.; See, M.T.; Hanson, D.J.; Lawrence, T.E.; Cassady, J.P. Correlated responses in growth, carcass, and meat quality traits to divergent selection for testosterone production in pigs. J. Anim. Sci. 2006, 84, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Takeda, H.; Chodak, G.; Mutchnik, S.; Nakamoto, T.; Chang, C. Immunohistochemical localization of androgen receptors with mono- and polyclonal antibodies to androgen receptor. J. Endocrinol. 1990, 126, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Robison, O.W.; Johnson, B.; Lubritz, D. Genetic parameters for testosterone production in boars. J. Anim. Sci. 1991, 69, 3220–3224. [Google Scholar]
- Yeh, S.; Tsai, M.Y.; Xu, Q.; Mu, X.M.; Lardy, H.; Huang, K.E.; Lin, H.; Yeh, S.D.; Altuwaijri, S.; Zhou, X.; et al. Generation and characterization of androgen receptor knockout (ARKO) mice: An in vivo model for the study of androgen functions in selective tissues. Proc. Natl. Acad. Sci. USA 2002, 99, 13498–13503. [Google Scholar] [CrossRef]
- Moverare-Skrtic, S.; Venken, K.; Andersson, N.; Lindberg, M.K.; Svensson, J.; Swanson, C.; Vanderschueren, D.; Oscarsson, J.; Gustafsson, J.A.; Ohlsson, C. Dihydrotestosterone treatment results in obesity and altered lipid metabolism in orchidectomized mice. Obesity 2006, 14, 662–672. [Google Scholar] [CrossRef]
- Clapper, J.A.; Clark, T.M.; Rempel, L.A. Serum concentrations of IGF-I, estradiol-17β, testosterone, and relative amounts of IGF binding proteins (IGFBP) in growing boars, barrows, and gilts. J. Anim. Sci. 2000, 78, 2581–2588. [Google Scholar] [CrossRef]
- Knudson, B.K.; Hogberg, M.G.; Merkel, R.A.; Allen, R.E.; Magee, W.T. Developmental comparisons of boars and barrows: I. Growth rate, carcass and muscle characteristics. J. Anim. Sci. 1985, 61, 789–796. [Google Scholar] [CrossRef]
- Serrano, M.P.; Valencia, D.G.; Fuentetaja, A.; Lázaro, R.; Mateos, G.G. Effect of castration on productive performance, carcass characteristics and meat quality of Iberian pig females reared under intensive management systems. Livest. Sci. 2009, 123, 147–153. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, L.; Chen, M.; Jiang, X.; Xu, N. Castration-induced changes in microRNA expression profiles in subcutaneous adipose tissue of male pigs. J. Appl. Genet. 2014, 55, 259–266. [Google Scholar] [CrossRef]
- Chazenbalk, G.; Singh, P.; Irge, D.; Shah, A.; Abbott, D.H.; Steroids, D.A. Androgens inhibit adipogenesis during human adipose stem cell commitment to preadipocyte formation. Steroids 2013, 78, 920–926. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, M.W.; House, P.J.; Tomlinson, J.W. Understanding androgen action in adipose tissue. J. Steroid Biochem. Mol. Biol. 2014, 143, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.; Greither, T.; Behre, H.M. Androgen-Regulated microRNAs (AndroMiRs) as Novel Players in Adipogenesis. Int. J. Mol. Sci. 2019, 20, 5767. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.D. Role of body fat distribution and the metabolic complications of obesity. J. Clin. Endocrinol. Metab. 2008, 93, S57–S63. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Li, S.; Wang, G.X.; Yu, Q.; Lin, J.D. A Long Noncoding RNA Transcriptional Regulatory Circuit Drives Thermogenic Adipocyte Differentiation. Mol. Cell 2014, 55, 372–382. [Google Scholar] [CrossRef]
- Lanz, R.B.; Mckenna, N.J.; Onate, S.A.; Albrecht, U.; Wong, J.; Tsai, S.Y.; Tsai, M.J.; O’Malley, B.W. A steroid receptor coactivator, SRA, functions as an RNA and is present in an SRC-1 complex. Cell 1999, 97, 17. [Google Scholar] [CrossRef]
- Xu, B.; Isabelle, G.; Miao, H.; Dang, V.P.; Johnson, C.N.; Xu, R.; Chen, X.W.; Cawthorn, W.P.; Macdougald, O.A.; Koenig, R.J. Multiple Roles for the Non-Coding RNA SRA in Regulation of Adipogenesis and Insulin Sensitivity. PLoS ONE 2010, 5, e14199. [Google Scholar] [CrossRef]
- Chen, G.; Yu, D.; Nian, X.; Liu, J.; Koenig, R.J.; Xu, B.; Sheng, L. LncRNA SRA promotes hepatic steatosis through repressing the expression of adipose triglyceride lipase (ATGL). Sci. Rep. 2016, 6, 35531. [Google Scholar] [CrossRef]
- Poklukar, K.; Čandek-Potokar, M.; Vrecl, M.; Batorek-Lukač, N.; Fazarinc, G.; Kress, K.; Weiler, U.; Stefanski, V.; Škrlep, M. The effect of immunocastration on adipose tissue deposition and composition in pigs. Animal 2021, 15, 100118. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Huang, J.M.; Liu, G.; Zhang, J.B.; Wang, J.Y.; Liu, C.K.; Fang, M.Y. A comprehensive microRNA expression profile of the backfat tissue from castrated and intact full-sib pair male pigs. BMC Genom. 2014, 15, 47. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Feinberg, A.P.; Irizarry, R.A.; Fradin, D.; Aryee, M.J.; Murakami, P.; Aspelund, T.; Eiriksdottir, G.; Harris, T.B.; Launer, L.; Gudnason, V.; et al. Personalized epigenomic signatures that are stable over time and covary with body mass index. Sci. Transl. Med. 2010, 2, 49ra67. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Liang, S.; Luo, H.; Bu, D.; Zhao, G.; Yu, K.; Zhang, C.; Liu, Y.; Chen, R.; Yi, Z. Utilizing sequence intrinsic composition to classify protein-coding and long non-coding transcripts. Nucleic Acids Res. 2013, 41, e166. [Google Scholar]
- Lei, K.; Yong, Z.; Ye, Z.Q.; Liu, X.Q.; Zhao, S.Q.; Wei, L.; Ge, G. CPC: Assess the protein-coding potential of transcripts using sequence features and support vector machine. Nucleic Acids Res. 2007, 35, W345–W349. [Google Scholar]
- Punta, M.; Coggill, P.C.; Eberhardt, R.Y.; Mistry, J.; Tate, J.; Boursnell, C.; Pang, N.; Forslund, K.; Ceric, G.; Clements, J. The Pfam protein families database. Nucleic Acids Res. 2004, 28, 263–266. [Google Scholar] [CrossRef]
- Wang, L.; Park, H.J.; Dasari, S.; Wang, S.; Kocher, J.-P.; Li, W. CPAT: Coding-Potential Assessment Tool using an alignment-free logistic regression model. Nucleic Acids Res. 2013, 41, e74. [Google Scholar] [CrossRef]
- Zhou, Y.; Liang, Y.; Lynch, K.H.; Dennis, J.J.; Wishart, D.S. PHAST: A fast phage search tool. Nucleic Acids Res. 2011, 39, W347–W352. [Google Scholar] [CrossRef]
- Siepel, A.; Bejerano, G.; Pedersen, J.S.; Hinrichs, A.S.; Hou, M.; Rosenbloom, K.; Clawson, H.; Spieth, J.; Hillier, L.W.; Richards, S.; et al. Evolutionarily conserved elements in vertebrate, insect, worm, and yeast genomes. Genome Res. 2005, 15, 1034–1050. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Leng, N.; Dawson, J.A.; Thomson, J.A.; Ruotti, V.; Rissman, A.I.; Smits, B.M.G.; Haag, J.D.; Gould, M.N.; Stewart, R.M.; Kendziorski, C. EBSeq: An empirical Bayes hierarchical model for inference in RNA-seq experiments. Bioinformatics 2013, 29, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Tarazona, S.; Garcia-Alcalde, F.; Dopazo, J.; Ferrer, A.; Conesa, A. Differential expression in RNA-seq: A matter of depth. Genome Res. 2011, 21, 2213–2223. [Google Scholar] [CrossRef]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef]
- Friedlnder, M.R.; Mackowiak, S.D.; Na, L.; Wei, C.; Nikolaus, R. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 2012, 40, 37–52. [Google Scholar] [CrossRef]
- Enright, A.J.; John, B.; Gaul, U.; Tuschl, T.; Sander, C.; Marks, D.S. MicroRNA targets in Drosophila. Genome Biol. 2003, 5, R1. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.Y.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef]
- Tay, Y.; Rinn, J.; Pandolfi, P.P. The multilayered complexity of ceRNA crosstalk and competition. Nature 2014, 505, 344–352. [Google Scholar] [CrossRef]
- Agarwal, V.; Bell, G.W.; Nam, J.W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. Elife 2015, 4, e05005. [Google Scholar] [CrossRef]
- Ideker, T. Cytoscape 2.8: New features for data integration and network visualization. Bioinformatics 2011, 27, 431–432. [Google Scholar]
- Dong, X.; Ying, B.; Yi, X.; Xu, Q.; Gang, C.; Fang, M.J.G. Investigation on the transcription factors of porcine 3β-hydroxysteroid dehydrogenase and 17β-hydroxysteroid dehydrogenase genes. Gene 2012, 499, 186–190. [Google Scholar] [CrossRef]
- Gispert, M.; Angels Oliver, M.; Velarde, A.; Suarez, P.; Perez, J.; Font i Furnols, M. Carcass and meat quality characteristics of immunocastrated male, surgically castrated male, entire male and female pigs. Meat Sci. 2010, 85, 664–670. [Google Scholar] [CrossRef]
- Chew, C.L.; Conos, S.A.; Unal, B.; Tergaonkar, V. Noncoding RNAs: Master Regulators of Inflammatory Signaling. Trends Mol. Med. 2018, 24, 66–84. [Google Scholar] [CrossRef]
- Slaby, O.; Laga, R.; Sedlacek, O. Therapeutic targeting of non-coding RNAs in cancer. Biochem. J. 2017, 474, 4219–4251. [Google Scholar] [CrossRef]
- Salmena, L.; Poliseno, L.; Tay, Y.; Kats, L.; Pandolfi, P.P. A ceRNA hypothesis: The Rosetta Stone of a hidden RNA language? Cell 2011, 146, 353–358. [Google Scholar] [CrossRef]
- Ala, U.; Karreth, F.A.; Bosia, C.; Pagnani, A.; Taulli, R.; Leopold, V.; Tay, Y.; Provero, P.; Zecchina, R.; Pandolfi, P.P. Integrated transcriptional and competitive endogenous RNA networks are cross-regulated in permissive molecular environments. Proc. Natl. Acad. Sci. USA 2013, 110, 7154–7159. [Google Scholar] [CrossRef]
- Kornienko, A.E.; Guenzl, P.M.; Barlow, D.P.; Pauler, F.M. Gene regulation by the act of long non-coding RNA transcription. BMC Biol. 2013, 11, 59. [Google Scholar] [CrossRef]
- Esau, C.; Davis, S.; Murray, S.F.; Yu, X.X.; Pandey, S.K.; Pear, M.; Watts, L.; Booten, S.L.; Graham, M.; McKay, R.; et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006, 3, 87–98. [Google Scholar] [CrossRef]
- Zhao, X.; Bai, X.; Guan, L.; Li, J.; Song, X.; Ma, X.; Guo, J.; Zhang, Z.; Du, Q.; Huang, Y.; et al. microRNA-4331 Promotes Transmissible Gastroenteritis Virus (TGEV)-induced Mitochondrial Damage Via Targeting RB1, Upregulating Interleukin-1 Receptor Accessory Protein (IL1RAP), and Activating p38 MAPK Pathway In Vitro. Mol. Cell Proteom. 2018, 17, 190–204. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, Y.; Liu, L.; Yang, W.; Zhang, Q. HNF1A-AS1 Regulates Cell Migration, Invasion and Glycolysis via Modulating miR-124/MYO6 in Colorectal Cancer Cells. Onco Targets Ther. 2020, 13, 1507–1518. [Google Scholar] [CrossRef]
- Wang, X.; Xu, H.; Wang, Y.; Shen, C.; Ma, L.; Zhao, C. MicroRNA-124a contributes to glucocorticoid resistance in acute-on-chronic liver failure by negatively regulating glucocorticoid receptor α. Ann. Hepatol. 2020, 19, 214–221. [Google Scholar] [CrossRef]
- Walden, T.B.; Timmons, J.A.; Keller, P.; Nedergaard, J.; Cannon, B. Distinct expression of muscle-specific microRNAs (myomirs) in brown adipocytes. J. Cell Physiol. 2009, 218, 444–449. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Wang, H.; Ma, X.; Zan, L. MicroRNA-224 impairs adipogenic differentiation of bovine preadipocytes by targeting LPL. Mol. Cell Probes 2019, 44, 29–36. [Google Scholar] [CrossRef]
- Yang, W.M.; Min, K.H.; Park, S.W.; Lee, W. Data on the decreased expression of FOXO1 by miR-1271 in HepG2 hepatocytes. Data Brief. 2017, 15, 800–804. [Google Scholar] [CrossRef]
- Balachandran, A.; Guan, H.; Sellan, M.; van Uum, S.; Yang, K. Insulin and dexamethasone dynamically regulate adipocyte 11beta-hydroxysteroid dehydrogenase type 1. Endocrinology 2008, 149, 4069–4079. [Google Scholar] [CrossRef][Green Version]
- Starr, A.E.; Dufour, A.; Maier, J.; Overall, C.M. Biochemical Analysis of Matrix Metalloproteinase Activation of Chemokines CCL15 and CCL23 and Increased Glycosaminoglycan Binding of CCL16. J. Biol. Chem. 2012, 287, 5848–5860. [Google Scholar] [CrossRef]
- Vianello, E.; Kalousová, M.; Dozio, E.; Tacchini, L.; Zima, T.; Corsi Romanelli, M.M. Osteopontin: The Molecular Bridge between Fat and Cardiac-Renal Disorders. Int. J. Mol. Sci. 2020, 21, 5568. [Google Scholar] [CrossRef]
- Wang, Q.; Shen, B.; Chen, L.; Zheng, P.; Feng, H.; Hao, Q.; Liu, X.; Liu, L.; Xu, S.; Chen, J.; et al. Extracellular calumenin suppresses ERK1/2 signaling and cell migration by protecting fibulin-1 from MMP-13-mediated proteolysis. Oncogene 2015, 34, 1006–1018. [Google Scholar] [CrossRef]
- Ye, J. Mechanisms of Insulin Resistance in Obesity. Front. Med. 2013, 7, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Michael, L.F.; Wu, Z.; Cheatham, R.B.; Puigserver, P.; Adelmant, G.; Lehman, J.J.; Kelly, D.P.; Spiegelman, B.M. Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc. Natl. Acad. Sci. USA 2001, 98, 3820–3825. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hietakangas, V.; Wee, S.; Lim, S.C.; Gunaratne, J.; Cohen, S.M. ER stress potentiates insulin resistance through PERK-mediated FOXO phosphorylation. Genes Dev. 2013, 27, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.V.; Jordan, C.L.; Breedlove, S.M. Testosterone works through androgen receptors to modulate neuronal response to anxiogenic stimuli. Neurosci. Lett. 2021, 753, 135852. [Google Scholar] [CrossRef] [PubMed]
- Zahavi, A.; Perel, M. The information encoded by the sex steroid hormones testosterone and estrogen: A hypothesis. J. Theor. Biol. 2011, 280, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Cooke, P.S.; Walker, W.H. Nonclassical androgen and estrogen signaling is essential for normal spermatogenesis. Semin. Cell Dev. Biol. 2022, 121, 71–81. [Google Scholar] [CrossRef]
- Ren, X.; Fu, X.; Zhang, X.; Chen, S.; Huang, S.; Yao, L.; Liu, G.J.B.P. Testosterone regulates 3T3-L1 pre-adipocyte differentiation and epididymal fat accumulation in mice through modulating macrophage polarization. Biochem. Pharmacol. 2017, 140, 73–88. [Google Scholar] [CrossRef]
- Furman, B.L. Inhibitors of Corticosteroid Synthesis. In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: New York, NY, USA, 2007; pp. 1–2. [Google Scholar] [CrossRef]
- Tchernof, A.; Mansour, M.F.; Pelletier, M.; Boulet, M.-M.; Nadeau, M.; Luu-The, V. Updated survey of the steroid-converting enzymes in human adipose tissues. J. Steroid Biochem. Mol. Biol. 2015, 147, 56–69. [Google Scholar] [CrossRef]
- Lambert, F.; Lammerant, J.; Kolanowski, J. The enhancement of pregnenolone production as the main mechanism of the prolonged stimulatory effect of acth on cortisol production by guinea-pig adrenocortical cells. J. Steroid Biochem. 1984, 21, 299–303. [Google Scholar] [CrossRef]
- Benediktsson, R.; Calder, A.A.; Edwards, C.R.W.; Seckl, J.R.J.C.E. Placental 11 β-hydroxysteroid dehydrogenase: A key regulator of fetal glucocorticoid exposure. Clin. Endocrinol. 2010, 46, 161–166. [Google Scholar] [CrossRef]
- Harno, E.; Cottrell, E.C.; Keevil, B.G.; DeSchoolmeester, J.; Bohlooly, Y.M.; Andersen, H.; Turnbull, A.V.; Leighton, B.; White, A. 11-Dehydrocorticosterone causes metabolic syndrome, which is prevented when 11beta-HSD1 is knocked out in livers of male mice. Endocrinology 2013, 154, 3599–3609. [Google Scholar] [CrossRef] [PubMed][Green Version]
- London, E.; Castonguay, T.W. Diet and the role of 11beta-hydroxysteroid dehydrogenase-1 on obesity. J. Nutr. Biochem. 2009, 20, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.; Huang, Y.; Liang, G.; Li, X. 11beta-hydroxysteroid dehydrogenase type 1 inhibitors as promising therapeutic drugs for diabetes: Status and development. Curr. Med. Chem. 2010, 17, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Vara Prasad, S.S.; Jeya Kumar, S.S.; Kumar, P.U.; Qadri, S.S.; Vajreswari, A. Dietary fatty acid composition alters 11beta-hydroxysteroid dehydrogenase type 1 gene expression in rat retroperitoneal white adipose tissue. Lipids Health Dis. 2010, 9, 111. [Google Scholar] [CrossRef] [PubMed]
- Baudrand, R.; Carvajal, C.A.; Riquelme, A.; Morales, M.; Solis, N.; Pizarro, M.; Escalona, A.; Boza, C.; Perez, G.; Dominguez, A.; et al. Overexpression of 11beta-hydroxysteroid dehydrogenase type 1 in hepatic and visceral adipose tissue is associated with metabolic disorders in morbidly obese patients. Obes. Surg. 2010, 20, 77–83. [Google Scholar] [CrossRef]
- Lang, F.; Stournaras, C.J.H. Serum and glucocorticoid inducible kinase, metabolic syndrome, inflammation, and tumor growth. Hormones 2013, 12, 160–171. [Google Scholar] [CrossRef]
- Dieter, M.; Palmada, M.; Rajamanickam, J.; Aydin, A.; Busjahn, A.; Boehmer, C.; Luft, F.C.; Lang, F. Regulation of glucose transporter SGLT1 by ubiquitin ligase Nedd4-2 and kinases SGK1, SGK3, and PKB. Obes. Res. 2004, 12, 862–870. [Google Scholar] [CrossRef]
- Lang, F.; Bohmer, C.; Palmada, M.; Seebohm, G.; Strutz-Seebohm, N.; Vallon, V. (Patho)physiological Significance of the Serum- and Glucocorticoid-Inducible Kinase Isoforms. Physiol. Rev. 2006, 86, 1151–1178. [Google Scholar] [CrossRef]
- Chakraborty, C.; Bandyopadhyay, S.; Maulik, U.; Agoramoorthy, G. Topology Mapping of Insulin-Regulated Glucose Transporter GLUT4 Using Computational Biology. Cell Biochem. Biophys. 2013, 67, 1261–1274. [Google Scholar] [CrossRef]
- Lacombe, V.A. Expression and Regulation of Facilitative Glucose Transporters in Equine Insulin-Sensitive Tissue: From Physiology to Pathology. Int. Sch. Res. Not. 2014, 2014, 409547. [Google Scholar] [CrossRef]
- Larance, M.; Ramm, G.; James, D.E. The GLUT4 Code. Mol. Endocrinol. 2008, 22, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraj, S.; Boehmer, C.; Lang, F.; Palmada, M. Role of SGK1 kinase in regulating glucose transport via glucose transporter GLUT4. Biochem. Biophys. Res. Commun. 2007, 356, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Hanson, R.W.; Reshef, L. Glyceroneogenesis revisited. Biochimie 2003, 85, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kalhan, S.C.; Hanson, R.W. What is the metabolic role of phosphoenolpyruvate carboxykinase? J. Biol. Chem. 2009, 284, 27025–27029. [Google Scholar] [CrossRef]
- Nye, C.K.; Hanson, R.W.; Kalhan, S.C. Glyceroneogenesis is the dominant pathway for triglyceride glycerol synthesis in vivo in the rat. J. Biol. Chem. 2008, 283, 27565–27574. [Google Scholar] [CrossRef]
- Feingold, K.R.; Moser, A.; Shigenaga, J.K.; Grunfeld, C. Inflammation inhibits the expression of phosphoenolpyruvate carboxykinase in liver and adipose tissue. Innate. Immun. 2012, 18, 231–240. [Google Scholar] [CrossRef]
- Wen, T.Y.; Kang, D.M. Effects of testosterone replacement therapy on glucose and lipid metabolism in middle-aged and elderly high-fat-fed male rats. Biomed. Res. (0970-938x) 2017, 28, 3048–3052. [Google Scholar]
- Arner, P. Effects of testosterone on fat cell lipolysis. Species differences and possible role in polycystic ovarian syndrome. Biochimie 2005, 87, 39–43. [Google Scholar] [CrossRef]
- Segal, D.H.; Raeside, J.I. Androgens in testes and adrenal glands of the fetal pig. J. Steroid Biochem. 1975, 6, 1439–1444. [Google Scholar] [CrossRef]
- Raeside, J.; Sigman, D. Testosterone Levels in Early Fetal Testes of Domestic Pigs. Biol. Reprod. 1975, 13, 318–321. [Google Scholar] [CrossRef][Green Version]
- Raeside, J.I.; Christie, H.L.; Renaud, R.L.; Sinclair, P.A. The boar testis: The most versatile steroid producing organ known. Reprod.-Camb.-Suppl. 2006, 62, 85. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Bai, Y.; Cui, R.; He, S.; Ling, Y.; Wu, C.; Fang, M. Integrated Analysis of the ceRNA Network and M-7474 Function in Testosterone-Mediated Fat Deposition in Pigs. Genes 2022, 13, 668. https://doi.org/10.3390/genes13040668
Liu X, Bai Y, Cui R, He S, Ling Y, Wu C, Fang M. Integrated Analysis of the ceRNA Network and M-7474 Function in Testosterone-Mediated Fat Deposition in Pigs. Genes. 2022; 13(4):668. https://doi.org/10.3390/genes13040668
Chicago/Turabian StyleLiu, Ximing, Ying Bai, Ran Cui, Shuaihan He, Yao Ling, Changxin Wu, and Meiying Fang. 2022. "Integrated Analysis of the ceRNA Network and M-7474 Function in Testosterone-Mediated Fat Deposition in Pigs" Genes 13, no. 4: 668. https://doi.org/10.3390/genes13040668
APA StyleLiu, X., Bai, Y., Cui, R., He, S., Ling, Y., Wu, C., & Fang, M. (2022). Integrated Analysis of the ceRNA Network and M-7474 Function in Testosterone-Mediated Fat Deposition in Pigs. Genes, 13(4), 668. https://doi.org/10.3390/genes13040668




