Transcriptome Analysis Reveals the Differentially Expressed Genes Associated with Growth in Guangxi Partridge Chickens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Tissue Samples
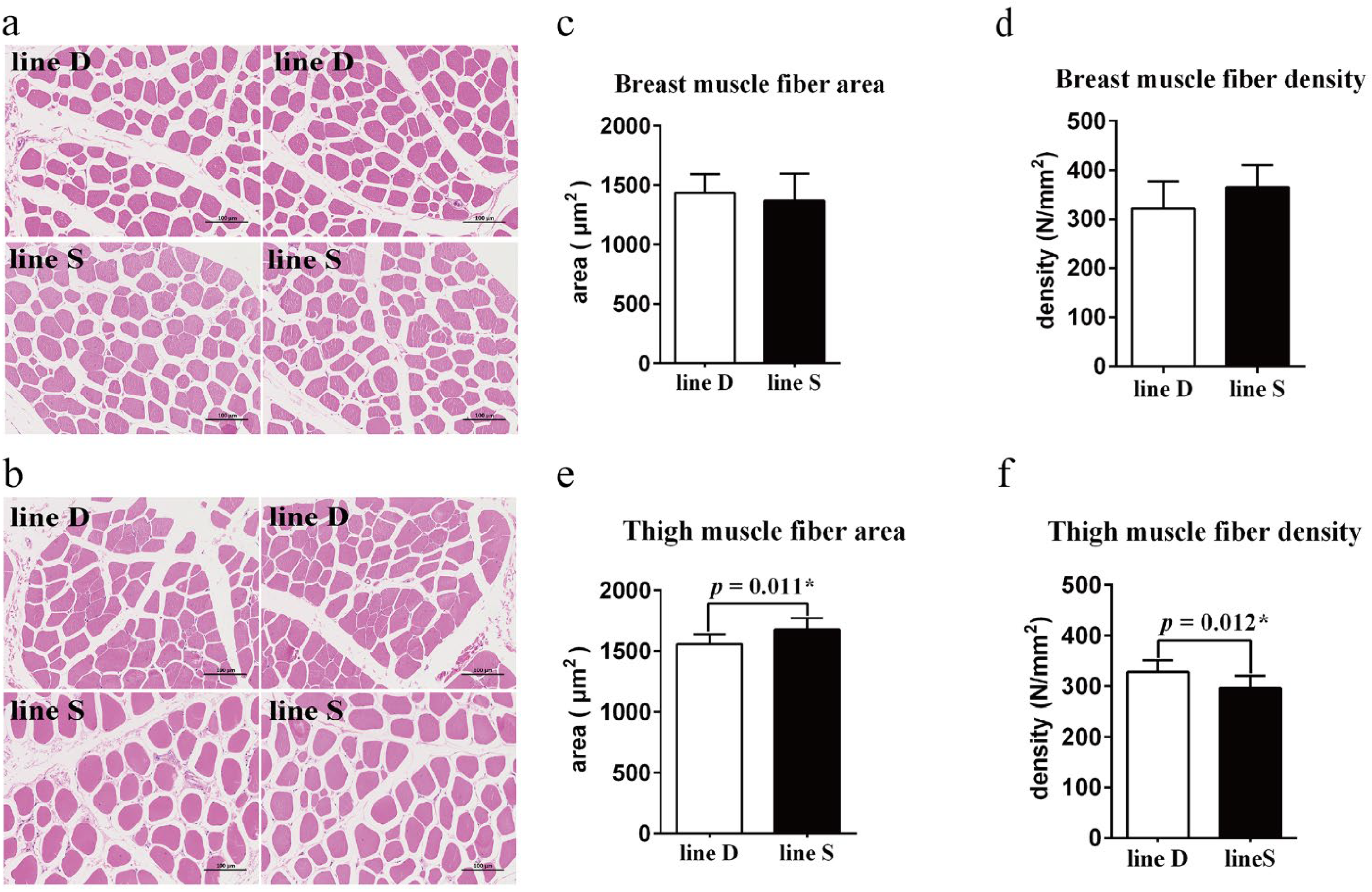
2.2. Examination of Muscle Fibers
2.3. RNA Extraction and Sequencing
2.4. Transcriptome Mapping and Assembly
2.5. RNA-seq Data Analysis
2.6. Validation of RNA Expression by Quantitative-PCR
2.7. Statistical Analysis
3. Results
3.1. Growth Performance and Differences in Muscle Fiber between the Two Lines
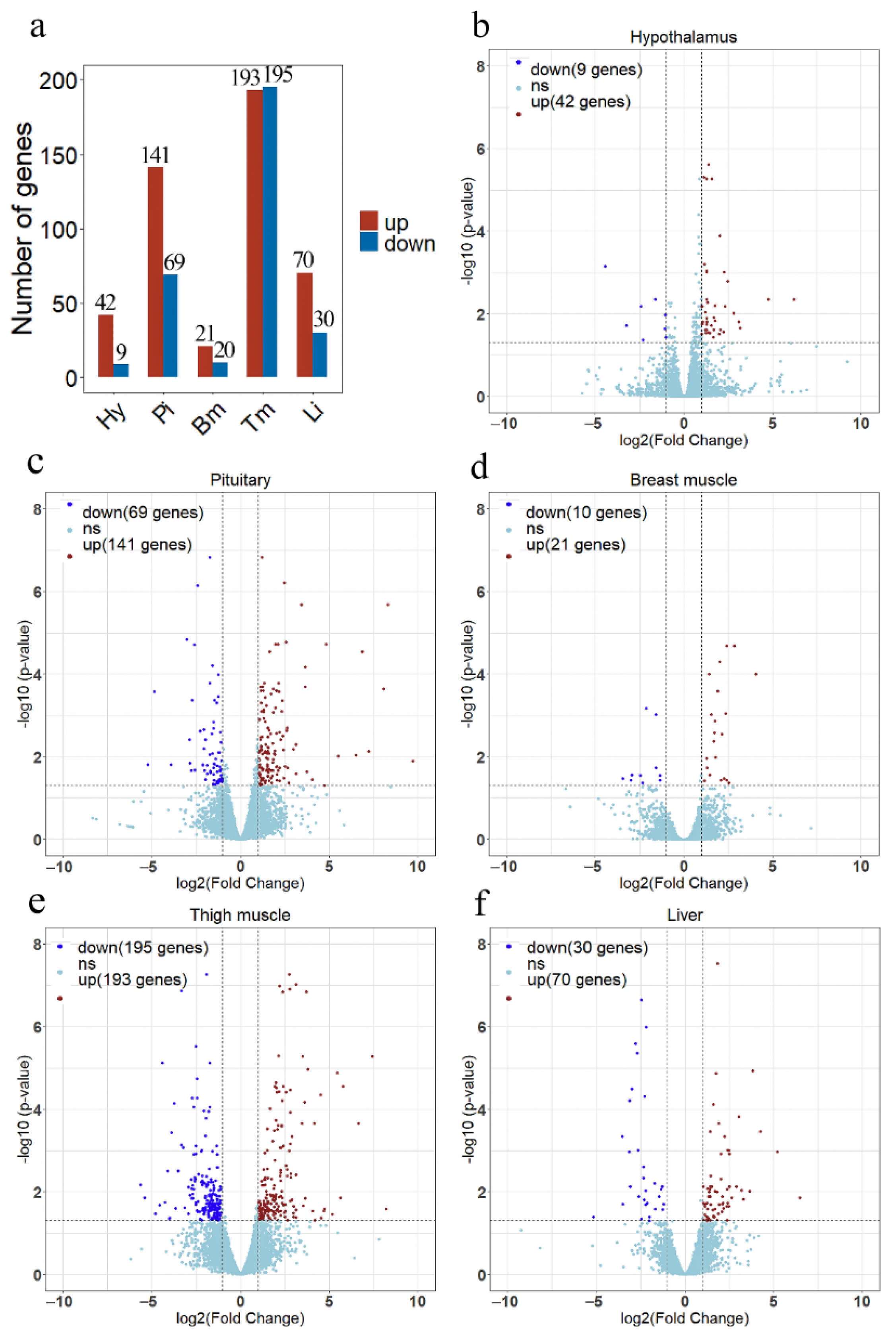
3.2. Analysis of Differentially Expressed Genes
3.2.1. Hypothalamus and Pituitary Tissues
3.2.2. Muscle Tissues
3.2.3. Liver Tissue
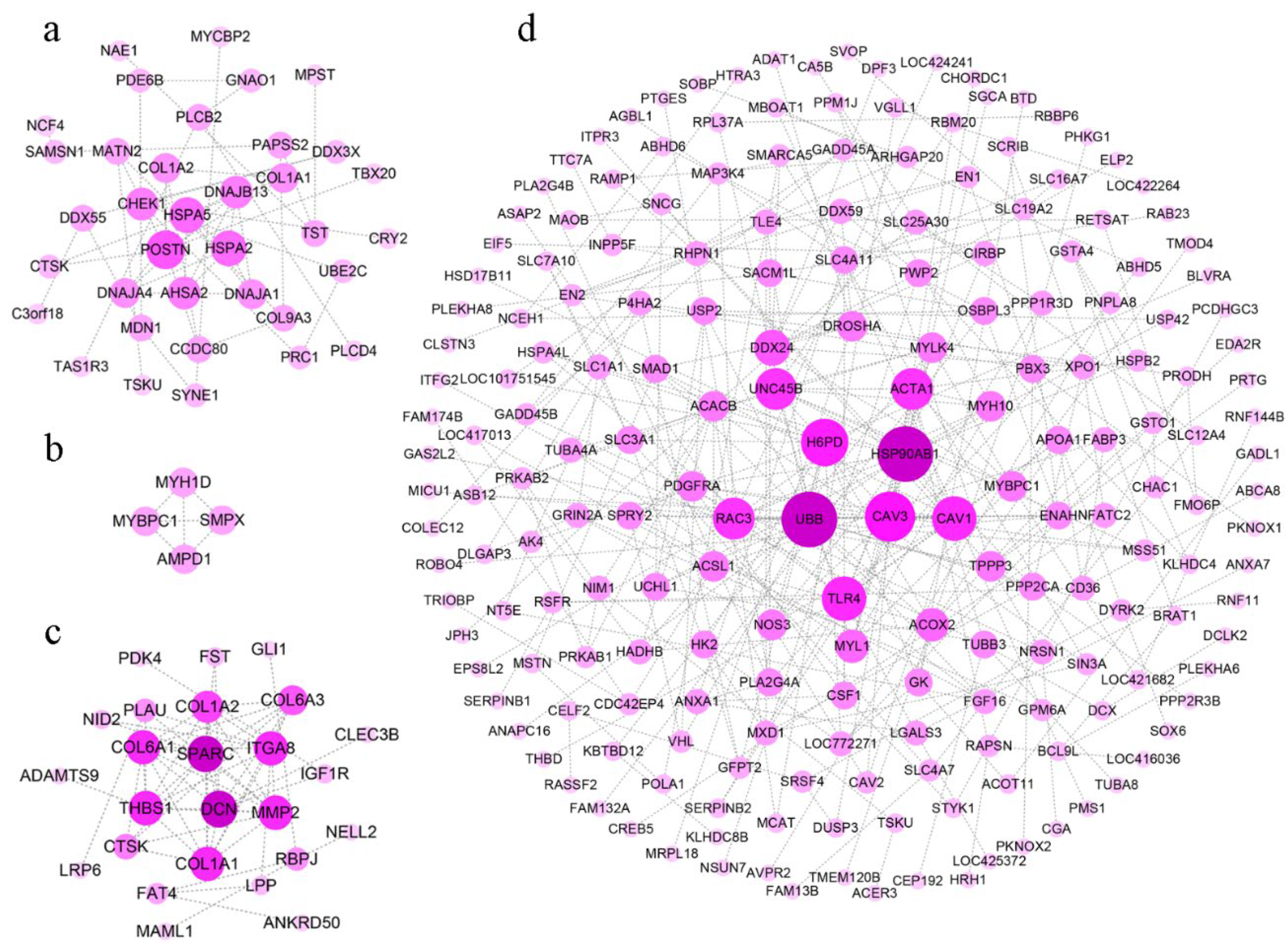
3.3. Interaction Network between DEGs
3.4. Validation of RNA-seq
4. Discussion
4.1. Hypothalamus and Pituitary
4.2. Skeletal Muscle
4.3. Liver
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, P.B. Evolution of the modern broiler and feed efficiency. Annu. Rev. Anim. Biosci. 2014, 2, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, P.; Martinez, D.A.; Weil, J.; Suesuttajit, N.; Umberson, C.; Mullenix, G.; Hilton, K.M.; Beitia, A.; Coon, C.N. Review: Physiological growth trend of current meat broilers and dietary protein and energy management approaches for sustainable broiler production. Anim. Int. J. Anim. Biosci. 2021, 15, 100284. [Google Scholar] [CrossRef] [PubMed]
- Tomas, E.; Kelly, M.; Xiang, X.; Tsao, T.S.; Keller, C.; Keller, P.; Luo, Z.; Lodish, H.; Saha, A.K.; Unger, R.; et al. Metabolic and hormonal interactions between muscle and adipose tissue. Proc. Nutr. Soc. 2004, 63, 381–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Du, H.; Yang, C.; Li, Q.; Qiu, M.; Song, X.; Yu, C.; Jiang, X.; Liu, L.; Hu, C.; et al. Comparative transcriptome analysis reveals regulators mediating breast muscle growth and development in three chicken breeds. Anim. Biotechnol. 2019, 30, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Li, Z.; Zhou, Y.; Liu, X.; Han, R.; Wang, Y.; Yan, F.; Sun, G.; Li, H.; Kang, X. Sequencing and characterization of lncRNAs in the breast muscle of Gushi and Arbor Acres chickens. Genome 2018, 61, 337–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, X.K.; Cheng, J.; Huang, Y.Z.; Wang, X.G.; Ma, Y.L.; Peng, S.J.; Chaogetu, B.; Zhuoma, Z.; Chen, H. Growth Performance and Meat Quality Evaluations in Three-Way Cross Cattle Developed for the Tibetan Plateau and their Molecular Understanding by Integrative Omics Analysis. J. Agric. Food Chem. 2019, 67, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Li, Y.; Wu, J.; Wang, X.; Bian, C.; Tian, Y.; Sun, G.; Han, R.; Liu, X.; et al. Genome-wide association study reveals the genetic determinism of growth traits in a Gushi-Anka F(2) chicken population. Heredity 2021, 126, 293–307. [Google Scholar] [CrossRef]
- Martín, A.I.; Priego, T.; López-Calderón, A. Hormones and Muscle Atrophy. Adv. Exp. Med. Biol. 2018, 1088, 207–233. [Google Scholar] [CrossRef]
- Kühn, E.R.; Geelissen, S.M.; Van der Geyten, S.; Darras, V.M. The release of growth hormone (GH): Relation to the thyrotropic- and corticotropic axis in the chicken. Domest. Anim. Endocrinol. 2005, 29, 43–51. [Google Scholar] [CrossRef]
- Cone, R.D. Studies on the physiological functions of the melanocortin system. Endocr. Rev. 2006, 27, 736–749. [Google Scholar] [CrossRef]
- Piórkowska, K.; Żukowski, K.; Połtowicz, K.; Nowak, J.; Ropka-Molik, K.; Derebecka, N.; Wesoły, J.; Wojtysiak, D. Identification of candidate genes and regulatory factors related to growth rate through hypothalamus transcriptome analyses in broiler chickens. BMC Genom. 2020, 21, 509. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ma, W. Hypothalamic and pituitary transcriptome profiling using RNA-sequencing in high-yielding and low-yielding laying hens. Sci. Rep. 2019, 9, 10285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Julian, R.J. Production and growth related disorders and other metabolic diseases of poultry—A review. Vet. J. 2005, 169, 350–369. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, T.; Xu, C.; Wang, D.; Ren, J.; Li, Y.; Tian, Y.; Wang, Y.; Jiao, Y.; Kang, X.; et al. Transcriptome profile of liver at different physiological stages reveals potential mode for lipid metabolism in laying hens. BMC Genom. 2015, 16, 763. [Google Scholar] [CrossRef] [Green Version]
- Willson, N.L.; Forder, R.E.A.; Tearle, R.; Williams, J.L.; Hughes, R.J.; Nattrass, G.S.; Hynd, P.I. Transcriptional analysis of liver from chickens with fast (meat bird), moderate (F1 layer x meat bird cross) and low (layer bird) growth potential. BMC Genom. 2018, 19, 309. [Google Scholar] [CrossRef] [Green Version]
- van Iterson, M.; van de Wiel, M.A.; Boer, J.M.; de Menezes, R.X. General power and sample size calculations for high-dimensional genomic data. Stat. Appl. Genet. Mol. Biol. 2013, 12, 449–467. [Google Scholar] [CrossRef]
- Koomkrong, N.; Theerawatanasirikul, S.; Boonkaewwan, C.; Jaturasitha, S.; Kayan, A. Breed-Related Number and Size of Muscle Fibres and Their Response to Carcass Quality in Chickens. Ital. J. Anim. Sci. 2015, 14, 4145. [Google Scholar] [CrossRef] [Green Version]
- Huo, W.; Weng, K.; Li, Y.; Zhang, Y.; Zhang, Y.; Xu, Q.; Chen, G. Comparison of muscle fiber characteristics and glycolytic potential between slow- and fast-growing broilers. Poult. Sci. 2021, 101, 101649. [Google Scholar] [CrossRef]
- Chodová, D.; Tůmová, E.; Ketta, M.; Skřivanová, V. Breast meat quality in males and females of fast-, medium- and slow-growing chickens fed diets of 2 protein levels. Poult. Sci. 2021, 100, 100997. [Google Scholar] [CrossRef]
- Shi, K.; Zhao, Q.; Shao, M.; Duan, Y.; Li, D.; Lu, Y.; Tang, Y.; Feng, C. Untargeted Metabolomics Reveals the Effect of Selective Breeding on the Quality of Chicken Meat. Metabolites 2022, 12, 367. [Google Scholar] [CrossRef]
- Dostert, C.; Grusdat, M.; Letellier, E.; Brenner, D. The TNF Family of Ligands and Receptors: Communication Modules in the Immune System and Beyond. Physiol. Rev. 2019, 99, 115–160. [Google Scholar] [CrossRef] [PubMed]
- Te Pas, M.F.W.; Borg, R.; Buddiger, N.J.H.; Wood, B.J.; Rebel, J.M.J.; van Krimpen, M.M.; Calus, M.P.L.; Park, J.E.; Schokker, D. Regulating appetite in broilers for improving body and muscle development-A review. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1819–1834. [Google Scholar] [CrossRef] [PubMed]
- Bastard, J.P.; Maachi, M.; Lagathu, C.; Kim, M.J.; Caron, M.; Vidal, H.; Capeau, J.; Feve, B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur. Cytokine Netw. 2006, 17, 4–12. [Google Scholar] [PubMed]
- Liu, L.; Cui, H.; Fu, R.; Zheng, M.; Liu, R.; Zhao, G.; Wen, J. The regulation of IMF deposition in pectoralis major of fast-and slow-growing chickens at hatching. J. Anim. Sci. Biotechnol. 2017, 8, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manickam, R.; Duszka, K.; Wahli, W. PPARs and Microbiota in Skeletal Muscle Health and Wasting. Int. J. Mol. Sci. 2020, 21, 8056. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Zhang, C.L.; Yu, R.T.; Cho, H.K.; Nelson, M.C.; Bayuga-Ocampo, C.R.; Ham, J.; Kang, H.; Evans, R.M. Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biol. 2004, 2, e294. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.; Zheng, M.; Zhao, G.; Liu, R.; Wen, J. Identification of differentially expressed genes and pathways for intramuscular fat metabolism between breast and thigh tissues of chickens. BMC Genom. 2018, 19, 55. [Google Scholar] [CrossRef]
- Mamoshina, P.; Volosnikova, M.; Ozerov, I.V.; Putin, E.; Skibina, E.; Cortese, F.; Zhavoronkov, A. Machine Learning on Human Muscle Transcriptomic Data for Biomarker Discovery and Tissue-Specific Drug Target Identification. Front. Genet. 2018, 9, 242. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, Y.S.; Zimmers, T.A.; Soleimani, A.; Matzuk, M.M.; Tsuchida, K.; Cohn, R.D.; Barton, E.R. Regulation of muscle mass by follistatin and activins. Mol. Endocrinol. (Baltim. Md.) 2010, 24, 1998–2008. [Google Scholar] [CrossRef] [Green Version]
- Amthor, H.; Nicholas, G.; McKinnell, I.; Kemp, C.F.; Sharma, M.; Kambadur, R.; Patel, K. Follistatin complexes Myostatin and antagonises Myostatin-mediated inhibition of myogenesis. Dev. Biol. 2004, 270, 19–30. [Google Scholar] [CrossRef]
- Dushyanth, K.; Shukla, R.; Chatterjee, R.N.; Bhattacharya, T.K. Expression and polymorphism of Follistatin (FST) gene and its association with growth traits in native and exotic chicken. Anim. Biotechnol. 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fallahshahroudi, A.; Løtvedt, P.; Bélteky, J.; Altimiras, J.; Jensen, P. Changes in pituitary gene expression may underlie multiple domesticated traits in chickens. Heredity 2019, 122, 195–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, W.W.; Myers, M.G., Jr. Leptin and the maintenance of elevated body weight. Nat. Rev. Neurosci. 2018, 19, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Flak, J.N.; Myers, M.G., Jr. Minireview: CNS Mechanisms of Leptin Action. Mol. Endocrinol. (Baltim. Md.) 2016, 30, 3–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bates, S.H.; Stearns, W.H.; Dundon, T.A.; Schubert, M.; Tso, A.W.; Wang, Y.; Banks, A.S.; Lavery, H.J.; Haq, A.K.; Maratos-Flier, E.; et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature 2003, 421, 856–859. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Shimomura, I.; Matsukawa, Y.; Kumada, M.; Takahashi, M.; Matsuda, M.; Ouchi, N.; Kihara, S.; Kawamoto, T.; Sumitsuji, S.; et al. Association of adiponectin mutation with type 2 diabetes: A candidate gene for the insulin resistance syndrome. Diabetes 2002, 51, 2325–2328. [Google Scholar] [CrossRef] [Green Version]
- Cristina, Ó.; Oliver, A.; Noguera, J.L.; Clop, A.; Barragán, C.; Varona, L.; Rodríguez, C.; Toro, M.; Sánchez, A.; Pérez-Enciso, M.; et al. Test for positional candidate genes for body composition on pig chromosome 6. Genet. Sel. Evol. GSE 2002, 34, 465–479. [Google Scholar] [CrossRef] [Green Version]
- El Moujahid El, M.; Chen, S.; Jin, S.; Lu, Y.; Zhang, D.; Ji, C.; Yang, N. Association of leptin receptor gene polymorphisms with growth and feed efficiency in meat-type chickens. Poult. Sci. 2014, 93, 1910–1915. [Google Scholar] [CrossRef]
- Bonen, A.; Dyck, D.J.; Ibrahimi, A.; Abumrad, N.A. Muscle contractile activity increases fatty acid metabolism and transport and FAT/CD36. Am. J. Physiol. 1999, 276, E642–E649. [Google Scholar] [CrossRef]
- Brooke, M.H.; Kaiser, K.K. Muscle fiber types: How many and what kind? Arch. Neurol. 1970, 23, 369–379. [Google Scholar] [CrossRef]
- Jin, S.; Moujahid, E.M.E.; Duan, Z.; Zheng, J.; Qu, L.; Xu, G.; Yang, N.; Chen, S. Association of AMPK subunit gene polymorphisms with growth, feed intake, and feed efficiency in meat-type chickens. Poult. Sci. 2016, 95, 1492–1497. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Iwamoto, H.; Takahara, H. The relationship between muscle growth and the growth of different fiber types in the chicken. Poult. Sci. 1993, 72, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Lim, K.S.; Ko, K.B.; Ryu, Y.C. Estimation of pork quality in live pigs using biopsied muscle fibre number composition. Meat Sci. 2018, 137, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Burguière, A.C.; Nord, H.; von Hofsten, J. Alkali-like myosin light chain-1 (myl1) is an early marker for differentiating fast muscle cells in zebrafish. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2011, 240, 1856–1863. [Google Scholar] [CrossRef] [PubMed]
- Aberle, E.D.; Stewart, T.S. Growth of fiber types and apparent fiber number in skeletal muscle of broiler- and layer-type chickens. Growth 1983, 47, 135–144. [Google Scholar] [PubMed]
- Vallejo, D.; Hernández-Torres, F.; Lozano-Velasco, E.; Rodriguez-Outeiriño, L.; Carvajal, A.; Creus, C.; Franco, D.; Aránega, A.E. PITX2 Enhances the Regenerative Potential of Dystrophic Skeletal Muscle Stem Cells. Stem Cell Rep. 2018, 10, 1398–1411. [Google Scholar] [CrossRef] [Green Version]
- Buckingham, M.; Rigby, P.W. Gene regulatory networks and transcriptional mechanisms that control myogenesis. Dev. Cell 2014, 28, 225–238. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; He, S.; Wang, S.; Zhu, Y.; Xu, H.; Luo, R.; Lan, X.; Cai, Y.; Sun, X. Two New Insertion/Deletion Variants of the PITX2 Gene and their Effects on Growth Traits in Sheep. Anim. Biotechnol. 2018, 29, 276–282. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, H.; Lei, C.; Pan, C.; Chen, H.; Lin, Q.; Lan, X. Effects of genetic variations within goat PITX2 gene on growth traits and mRNA expression. Anim. Biotechnol. 2020, 31, 107–114. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, H.; Kang, Z.; Cai, H.; Dang, R.; Lei, C.; Chen, H.; Guo, X.; Lan, X. Bovine pituitary homeobox 2 (PITX2): mRNA expression profiles of different alternatively spliced variants and association analyses with growth traits. Gene 2018, 669, 1–7. [Google Scholar] [CrossRef]
- Jiang, Y.; Tang, S.; Wang, C.; Wang, Y.; Qin, Y.; Wang, Y.; Zhang, J.; Song, H.; Mi, S.; Yu, F.; et al. A genome-wide association study of growth and fatness traits in two pig populations with different genetic backgrounds. J. Anim. Sci. 2018, 96, 806–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, H.; Dong, X.; Mao, H.; Xu, N.; Yin, Z. Expression Analysis of the PITX2 Gene and Associations between Its Polymorphisms and Body Size and Carcass Traits in Chickens. Animals 2019, 9, 1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, H.; Zhou, W.; Tan, Y.; Xu, X.; Mao, H.; Dong, X.; Xu, N.; Yin, Z. Chronological Expression of PITX2 and SIX1 Genes and the Association between Their Polymorphisms and Chicken Meat Quality Traits. Animals 2021, 11, 445. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zuo, B.; Ren, Z.; Hapsari, A.A.; Lei, M.; Xu, D.; Li, F.; Xiong, Y. Identification of four SNPs and association analysis with meat quality traits in the porcine Pitx2c gene. Sci. China. Life Sci. 2011, 54, 426–433. [Google Scholar] [CrossRef] [Green Version]
- Pandey, S.N.; Cabotage, J.; Shi, R.; Dixit, M.; Sutherland, M.; Liu, J.; Muger, S.; Harper, S.Q.; Nagaraju, K.; Chen, Y.W. Conditional over-expression of PITX1 causes skeletal muscle dystrophy in mice. Biol. Open 2012, 1, 629–639. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Lee, M.; Davis, B.W.; Lamichhaney, S.; Dorshorst, B.J.; Siegel, P.B.; Andersson, L. Mutations Upstream of the TBX5 and PITX1 Transcription Factor Genes Are Associated with Feathered Legs in the Domestic Chicken. Mol. Biol. Evol. 2020, 37, 2477–2486. [Google Scholar] [CrossRef]
- Domyan, E.T.; Kronenberg, Z.; Infante, C.R.; Vickrey, A.I.; Stringham, S.A.; Bruders, R.; Guernsey, M.W.; Park, S.; Payne, J.; Beckstead, R.B.; et al. Molecular shifts in limb identity underlie development of feathered feet in two domestic avian species. eLife 2016, 5, e12115. [Google Scholar] [CrossRef]
- Sabina, R.L.; Ogasawara, N.; Holmes, E.W. Expression of three stage-specific transcripts of AMP deaminase during myogenesis. Mol. Cell. Biol. 1989, 9, 2244–2246. [Google Scholar] [CrossRef]
- Iuliano, R.; Trapasso, F.; Le Pera, I.; Schepis, F.; Samà, I.; Clodomiro, A.; Dumon, K.R.; Santoro, M.; Chiariotti, L.; Viglietto, G.; et al. An adenovirus carrying the rat protein tyrosine phosphatase eta suppresses the growth of human thyroid carcinoma cell lines in vitro and in vivo. Cancer Res. 2003, 63, 882–886. [Google Scholar]
- Wei, C.B.; Wang, J.Q.; Chen, F.Y.; Niu, H.; Li, K. DNA sequence polymorphism within the bovine adenosine monophosphate deaminase 1 (AMPD1) is associated with production traits in Chinese cattle. Genet. Mol. Res. GMR 2015, 14, 1025–1033. [Google Scholar] [CrossRef]
- Wang, L.; Mo, X.; Xu, Y.; Zuo, B.; Lei, M.; Li, F.; Jiang, S.; Deng, C.; Xiong, Y. Molecular characterization and expression patterns of AMP deaminase1 (AMPD1) in porcine skeletal muscle. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2008, 151, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Yam, J.W.; Tse, E.Y.; Ng, I.O. Role and significance of focal adhesion proteins in hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2009, 24, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, R.J.; Foley, A.R.; Dastgir, J.; Asman, S.; Dunn, D.M.; Zou, Y.; Hu, Y.; Donkervoort, S.; Flanigan, K.M.; Swoboda, K.J.; et al. Position of glycine substitutions in the triple helix of COL6A1, COL6A2, and COL6A3 is correlated with severity and mode of inheritance in collagen VI myopathies. Hum. Mutat. 2013, 34, 1558–1567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho Duy, B.; Zhytnik, L.; Maasalu, K.; Kändla, I.; Prans, E.; Reimann, E.; Märtson, A.; Kõks, S. Mutation analysis of the COL1A1 and COL1A2 genes in Vietnamese patients with osteogenesis imperfecta. Hum. Genom. 2016, 10, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kos, K.; Wilding, J.P. SPARC: A key player in the pathologies associated with obesity and diabetes. Nat. Rev. Endocrinol. 2010, 6, 225–235. [Google Scholar] [CrossRef]
- Matsuo, Y.; Tanaka, M.; Yamakage, H.; Sasaki, Y.; Muranaka, K.; Hata, H.; Ikai, I.; Shimatsu, A.; Inoue, M.; Chun, T.H.; et al. Thrombospondin 1 as a novel biological marker of obesity and metabolic syndrome. Metab. Clin. Exp. 2015, 64, 1490–1499. [Google Scholar] [CrossRef] [Green Version]
- Lawler, J. The functions of thrombospondin-1 and-2. Curr. Opin. Cell Biol. 2000, 12, 634–640. [Google Scholar] [CrossRef]
- Järvinen, T.A.; Prince, S. Decorin: A Growth Factor Antagonist for Tumor Growth Inhibition. BioMed Res. Int. 2015, 2015, 654765. [Google Scholar] [CrossRef] [Green Version]


| Line D (n = 10) | Line S (n = 10) | |
|---|---|---|
| Body weight (kg) | 1.509 ± 0.084 *** | 1.724 ± 0.128 *** |
| Heart (g) | 7.61 ± 0.96 ** | 8.99 ± 0.83 ** |
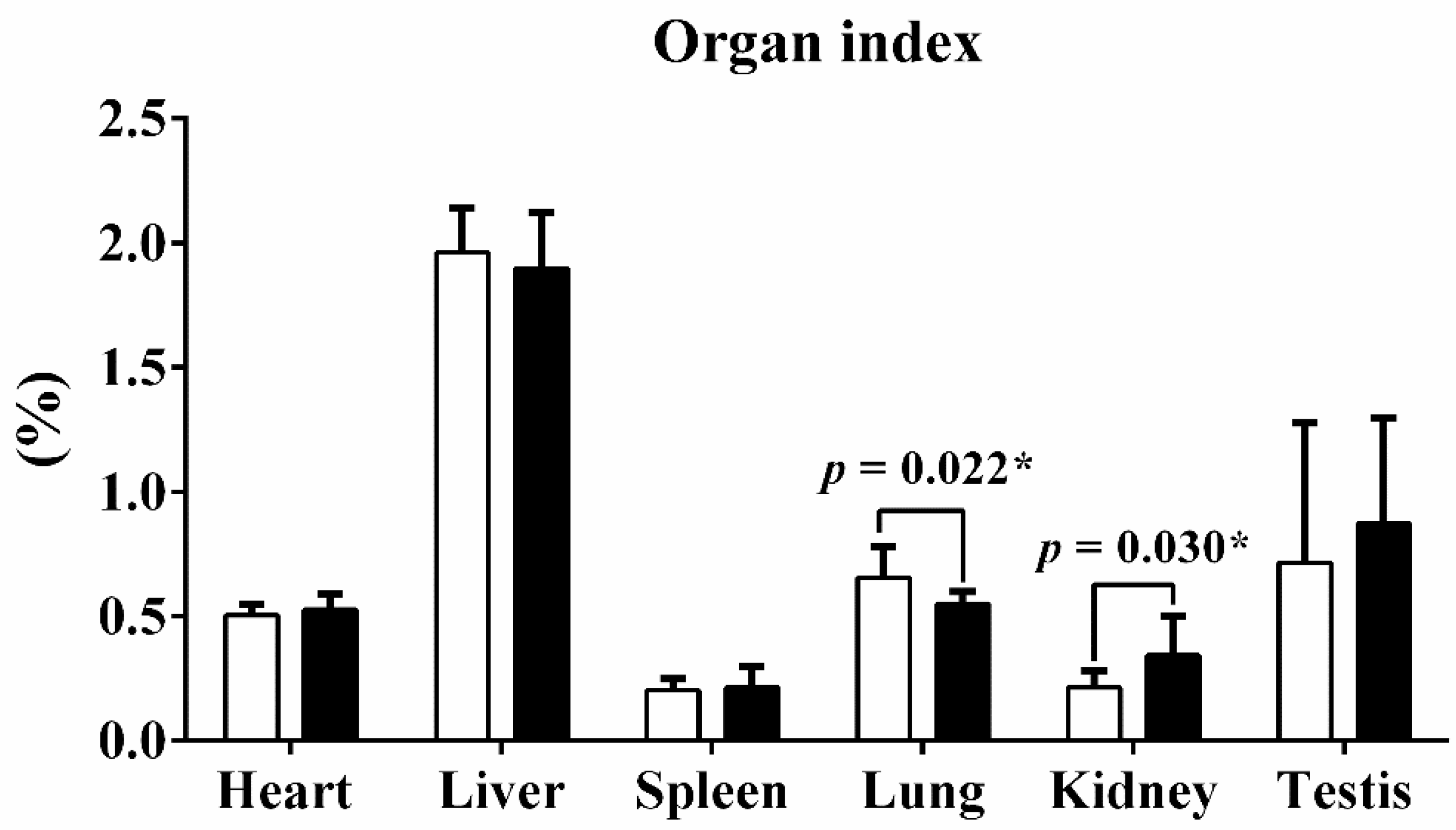
| Liver (g) Lung (g) Kidney (g) Spleen (g) Testis (g) | 29.56 ± 2.81 * 9.88 ± 1.97 3.20 ± 0.96 * 3.03 ± 0.77 10.90 ± 8.80 | 32.55 ± 2.73 * 9.42 ± 0.60 5.88 ± 2.78 * 3.65 ± 1.44 14.89 ± 7.18 |
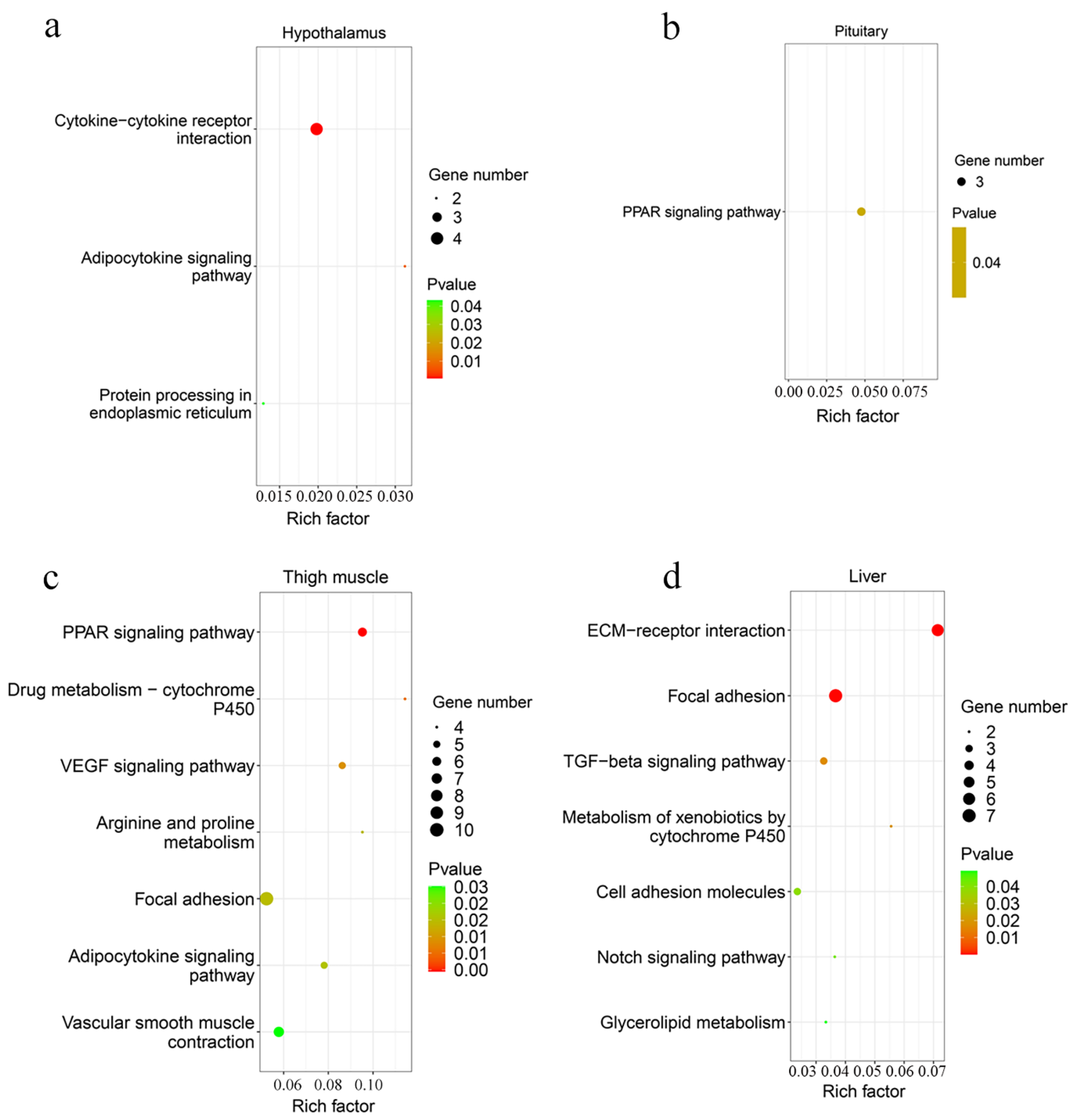
| Tissue | Term and Pathways | p-Value | DEGs No. | Genes | |
|---|---|---|---|---|---|
| Up-Regulated | Down-Regulated | ||||
| Hypothalamus | Cytokine–cytokine receptor interaction | 6.7 × 10−4 | 4 | TNFRSF18/TNFRSF8 /TNFRSF1B | LEPR |
| Adipocytokine signaling pathway | 0.008 | 2 | TNFRSF1B | LEPR | |
| Protein processing in endoplasmic reticulum | 0.043 | 2 | DNAJA1/DNAJB1 | ||
| Pituitary | PPAR signaling pathway | 0.040 | 3 | HMGCS1/ACSL6/PLTP | |
| Thigh muscle | PPAR signaling pathway | 0.004 | 6 | GK2/ACOX2 | APOA1/ACSL1/FABP3/CD36 |
| Drug metabolism—cytochrome P450 | 0.010 | 4 | GSTA4L | GSTO1/MAOB/FMO3 | |
| VEGF signaling pathway | 0.014 | 5 | PLA2G4B/RAC3 | LOC107057170 /NFATC2/PLA2G4A | |
| Arginine and proline metabolism | 0.020 | 4 | P4HA2 | LOC107057170 /PRODH/MAOB | |
| Focal adhesion | 0.020 | 10 | MYLK4/RAC3/MYLPF /CAV3 | TNX/CAPN2 /PDFRA/CAV1 /COL4A6/CAV2 | |
| Adipocytokine signaling pathway | 0.021 | 5 | PRKAB1/PRKAB2 | ACSL1/ACACB/CD36 | |
| Vascular smooth muscle contraction | 0.030 | 7 | PLA2G4B/RAMP1/MYLK4 | PLA2G4A/ITPR3 /KCNMB2/MYH10 | |
| Liver | ECM-receptor interaction | 7.8 × 10−6 | 6 | COL1A1/COL1A2/COL6A3 /ITGA8/COL6A1/THBS1 | |
| Focal adhesion | 9.87 × 10−5 | 7 | COL1A1/COL1A2/COL6A3 /ITGA8/IGF1R/COL6A1 /THBS1 | ||
| TGF-β signaling pathway | 0.017 | 3 | FST/THBS1/DCN | ||
| Metabolism of xenobiotics by cytochrome P450 | 0.019 | 2 | LOC100859645/CYP1B1 | ||
| Cell adhesion molecules | 0.039 | 3 | NECTIN3/SIGLEC1/ITGA8 | ||
| Notch signaling pathway | 0.042 | 2 | MAML1/RBPJ | ||
| Glycerolipid metabolism | 0.049 | 2 | LPIN1 | GPAM | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, M.; Shi, K.; Zhao, Q.; Duan, Y.; Shen, Y.; Tian, J.; He, K.; Li, D.; Yu, M.; Lu, Y.; et al. Transcriptome Analysis Reveals the Differentially Expressed Genes Associated with Growth in Guangxi Partridge Chickens. Genes 2022, 13, 798. https://doi.org/10.3390/genes13050798
Shao M, Shi K, Zhao Q, Duan Y, Shen Y, Tian J, He K, Li D, Yu M, Lu Y, et al. Transcriptome Analysis Reveals the Differentially Expressed Genes Associated with Growth in Guangxi Partridge Chickens. Genes. 2022; 13(5):798. https://doi.org/10.3390/genes13050798
Chicago/Turabian StyleShao, Minghui, Kai Shi, Qian Zhao, Ying Duan, Yangyang Shen, Jinjie Tian, Kun He, Dongfeng Li, Minli Yu, Yangqing Lu, and et al. 2022. "Transcriptome Analysis Reveals the Differentially Expressed Genes Associated with Growth in Guangxi Partridge Chickens" Genes 13, no. 5: 798. https://doi.org/10.3390/genes13050798
APA StyleShao, M., Shi, K., Zhao, Q., Duan, Y., Shen, Y., Tian, J., He, K., Li, D., Yu, M., Lu, Y., Tang, Y., & Feng, C. (2022). Transcriptome Analysis Reveals the Differentially Expressed Genes Associated with Growth in Guangxi Partridge Chickens. Genes, 13(5), 798. https://doi.org/10.3390/genes13050798









