Genetic Predisposition to Schizophrenia and Depressive Disorder Comorbidity
Abstract
1. Introduction
2. Materials and Methods
- We used keywords “schizophrenia”, “depression”, “comorbidity”, “pathophysiology”, “mechanism”, “genetics”, and their combinations to search for full-text articles in the PubMed, Springer, Wiley Online Library, Taylor & Francis Online, APA PsycInfo, CORE, Science Direct, and eLIBRARY.RU databases.
- We analyzed associative genetic studies, GWAS, cross-sectional studies, case–control studies, case studies, systematic reviews, meta-analyses, and Cochrane reviews. Articles published from January 2011 to November 2021 were analyzed. The final date of the search was 15 November 2021.
- Analyzed data were preselected from identified studies by the titles and abstracts or from the entire publication if titles and abstracts did not provide sufficient information on the type of study.
- English and Russian languages were included.
- Duplicate articles were excluded from the analysis.
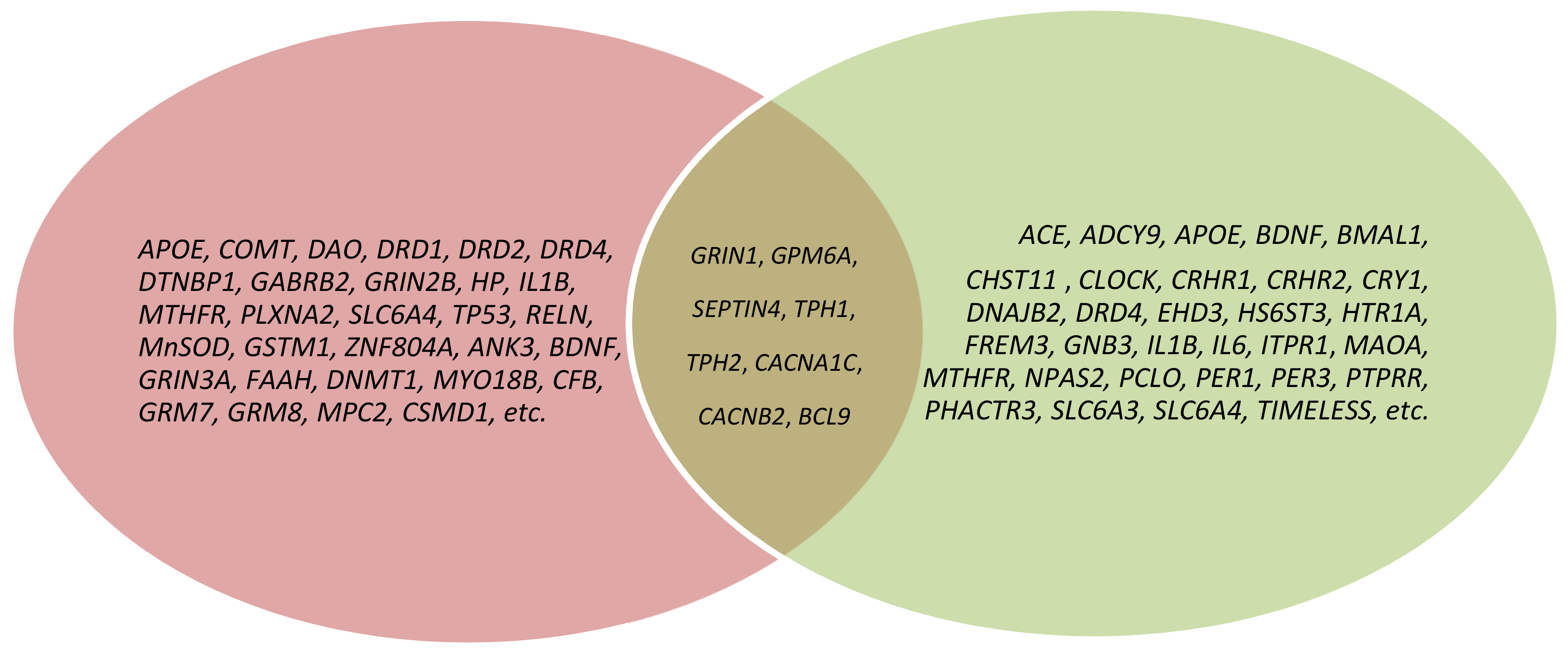
3. Results
3.1. GRIN1
3.2. GPM6A
3.3. SEPTIN4
3.4. TPH1
3.5. TPH2
3.6. CACNA1C
3.7. CACNB2
3.8. BCL9
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ekman, M.; Granström, O.; Omerov, S.; Jacob, J.; Landén, M. The Societal Cost of Schizophrenia in Sweden. J. Ment. Health Policy Econ. 2013, 16, 13–25. [Google Scholar] [CrossRef][Green Version]
- World Health Organization. The Global Burden of Disease: 2004 Update; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Whiteford, H.A.; Degenhardt, L.; Rehm, J.; Baxter, A.J.; Ferrari, A.J.; Erskine, H.E.; Charlson, F.J.; Norman, R.E.; Flaxman, A.D.; Johns, N.; et al. Global burden of disease attributable to mental and substance use disorders: Findings from the Global Burden of Disease Study 2010. Lancet 2013, 382, 1575–1586. [Google Scholar] [CrossRef]
- Buckley, P.F.; Miller, B.J.; Lehrer, D.; Castle, D.J. Psychiatric Comorbidities and Schizophrenia. Schizophr. Bull. 2008, 35, 383–402. [Google Scholar] [CrossRef]
- Häfner, H.; Maurer, K.; der Heiden, W.A. Schizophrenia—A disorder in its own right? Results from 25 years of the ABC study. Nervenarzt 2013, 84, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Häfner, H.; Maurer, K.; Der Heiden, W.A. ABC Schizophrenia study: An overview of results since 1996. Soc. Psychiatry Psychiatr. Epidemiol. 2013, 48, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Sönmez, N.; Romm, K.L.; Andreasssen, O.; Melle, I.; Røssberg, J.I. Depressive symptoms in first episode psychosis: A one-year follow-up study. BMC Psychiatry 2013, 13, 106. [Google Scholar] [CrossRef] [PubMed]
- McGlashan, T.H.; Carpenter, W.T. Postpsychotic Depression in Schizophrenia. Arch. Gen. Psychiatry 1976, 33, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Upthegrove, R.; Birchwood, M.; Ross, K.; Brunett, K.; Mccollum, R.; Jones, L. The evolution of depression and suicidality in first episode psychosis. Acta Psychiatr. Scand. 2009, 122, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Andriopoulos, I.; Ellul, J.; Skokou, M.; Beratis, S. Suicidality in the “prodromal” phase of schizophrenia. Compr. Psychiatry 2011, 52, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Novitskiy, M.; Shnayder, N.; Bugay, V.; Nasyrova, R. Application of Parametric and Questionnaires for the Study of Depressive and Anxiety Disorders in Schizophrenia. Doctor. Ru 2021, 20, 55–61. [Google Scholar] [CrossRef]
- Gozdzik-Zelazny, A.; Borecki, L.; Pokorski, M. Depressive symptoms in schizophrenic patients. Eur. J. Med. Res. 2011, 16, 549–552. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Novitskiy, M.A.; Shnayder, N.A.; Nasyrova, R.F. The incidence of depressive disorders in patients with schizophrenia. V.M. Bekhterev Rev. Psychiatry Med. Psychol. 2021, 56, 45–61. [Google Scholar] [CrossRef]
- Johnson, J.; Horwath, E.; Weissman, M.M. The Validity of Major Depression with Psychotic Features Based on a Community Study. Arch. Gen. Psychiatry 1991, 48, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Ohayon, M.M.; Schatzberg, A.F. Prevalence of Depressive Episodes with Psychotic Features in the General Population. Am. J. Psychiatry 2002, 159, 1855–1861. [Google Scholar] [CrossRef]
- Häfner, H.; Maurer, K.; Trendler, G.; der Heiden, W.A.; Schmidt, M.; Könnecke, R. Schizophrenia and depression: Challenging the paradigm of two separate diseases—A controlled study of schizophrenia, depression and healthy controls. Schizophr. Res. 2005, 77, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Häfner, H.; Löffler, W.; Maurer, K.; Hambrecht, M.; Der Heiden, W.A. Depression, negative symptoms, social stagnation and social decline in the early course of schizophrenia. Acta Psychiatr. Scand. 1999, 100, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Yung, A.; Phillips, L.J.; Yuen, H.P.; Francey, S.M.; McFarlane, C.; Hallgren, M.; McGorry, P.D. Psychosis prediction: 12-month follow up of a high-risk (“prodromal”) group. Schizophr. Res. 2003, 60, 21–32. [Google Scholar] [CrossRef]
- Schothorst, P.F.; Emck, C.; van Engeland, H. Characteristics of early psychosis. Compr. Psychiatry 2006, 47, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-C.; Lee, H.-Y.; Sakong, J.-K.; Jun, T.-Y.; Lee, M.-S.; Kim, J.-M.; Kim, J.-B.; Yim, H.-W.; Park, Y.C. Distinctive Clinical Correlates of Psychotic Major Depression: The CRESCEND Study. Psychiatry Investig. 2014, 11, 281–289. [Google Scholar] [CrossRef]
- Gournellis, R.; Oulis, P.; Howard, R. Psychotic major depression in older people: A systematic review. Int. J. Geriatr. Psychiatry 2014, 29, 784–796. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association DSM-5, Task Force. Diagnostic and Statistical Manual of Mental Disorders: DSM-5; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Lake, C.R. Disorders of Thought Are Severe Mood Disorders: The Selective Attention Defect in Mania Challenges the Kraepelinian Dichotomy A Review. Schizophr. Bull. 2007, 34, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Heckers, S. Diagnostic criteria for schizoaffective disorder. Expert Rev. Neurother. 2012, 12, 1–3. [Google Scholar] [CrossRef][Green Version]
- Möller, H.-J. Occurrence and treatment of depressive comorbidity/cosyndromality in schizophrenic psychoses: Conceptual and treatment issues. World J. Biol. Psychiatry 2005, 6, 247–263. [Google Scholar] [CrossRef] [PubMed]
- Siris, S.G.; Adan, F.; Cohen, M.; Mandeli, J.; Aronson, A.; Casey, E. Postpsychotic depression and negative symptoms: An investigation of syndromal overlap. Am. J. Psychiatry 1988, 145, 1532–1537. [Google Scholar] [CrossRef] [PubMed]
- Birchwood, M.; Iqbal, Z.; Chadwick, P.; Trower, P. Cognitive approach to depression and suicidal thinking in psychosis. Ontogeny of post-psychotic depression. Br. J. Psychiatry 2000, 177, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.A.W. Studies of Depressive Symptoms in Schizophrenia. Br. J. Psychiatry 1981, 139, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Der Heiden, W.A.; Könnecke, R.; Maurer, K.; Ropeter, D.; Hafner, H. Depression in the long-term course of schizophrenia. Eur. Arch. Psychiatry Neurol. Sci. 2005, 255, 174–184. [Google Scholar] [CrossRef]
- Chiappelli, J.; Kochunov, P.; DeRiso, K.; Thangavelu, K.; Sampath, H.; Muellerklein, F.; Nugent, K.L.; Postolache, T.T.; Carpenter, W.T.; Hong, L.E. Testing trait depression as a potential clinical domain in schizophrenia. Schizophr. Res. 2014, 159, 243–248. [Google Scholar] [CrossRef]
- Kirchheiner, J.; Nickchen, K.; Bauer, M.; Wong, M.R.; Licinio, J.; Roots, I.; Brockmöller, J. Pharmacogenetics of antidepres-sants and antipsychotics: The contribution of allelic variations to the phenotype of drug response. Mol. Psychiatry 2004, 9, 442–473. [Google Scholar] [CrossRef]
- Nasyrova, R.F.; Schnaider, N.A.; Mironov, K.; Shipulin, G.; Dribnokhodova, O.P.; Golosov, E.A.; Tolmachev, M.Y.; Andreev, B.V.; Kurylev, A.; Akhmetova, L.S.; et al. Pharmacogenetics of schizophrenia in real clinical practice: A clinical case. Neurol. Neuropsychiatry Psychosom. 2018, 10, 88–93. [Google Scholar] [CrossRef]
- Harrow, M.; Yonan, C.A.; Sands, J.R.; Marengo, J. Depression in Schizophrenia: Are Neuroleptics, Akinesia, or Anhedonia Involved? Schizophr. Bull. 1994, 20, 327–338. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Prosser, E.S.; Csernansky, J.G.; Kaplan, J.; Thiemann, S.; Becker, T.J.; Hollister, L.E. Depression, Parkinsonian Symptoms, and Negative Symptoms in Schizophrenics Treated with Neuroleptics. J. Nerv. Ment. Dis. 1987, 175, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Westermeyer, J. Comorbid Schizophrenia and Substance Abuse: A Review of Epidemiology and Course. Am. J. Addict. 2006, 15, 345–355. [Google Scholar] [CrossRef]
- Turkington, A.; Mulholland, C.C.; Rushe, T.M.; Anderson, R.; McCaul, R.; Barrett, S.L.; Barr, R.S.; Cooper, S.J. Impact of persistent substance misuse on 1-year outcome in first-episode psychosis. Br. J. Psychiatry 2009, 195, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.M.; Kissane, D.W. Demoralization: Its Phenomenology and Importance. Aust. N. Z. J. Psychiatry 2002, 36, 733–742. [Google Scholar] [CrossRef]
- Kudo, J.; Mori, H.; Gomibuchi, T. Loneliness as expressed by schizophrenic patients in the early remission phase. Nagoya J. Med. Sci. 2002, 65, 115–126. [Google Scholar] [PubMed]
- Kalueff, A.V.; Demin, K.A.; Volgin, A.D. Genomic mechanisms of anxiety and depression pathogenesis in experimental models. V.M. Bekhterev Rev. Psychiatry Med. Psychol. 2019, 4, 48–50. [Google Scholar] [CrossRef]
- Rozanov, V.A.; Mazo, G.E.; Kulemin, N.A.; Gorbachev, A.Y.; Kasyanov, E.D. Genome-wide association studies in suicidology—Analysis of main results. V.M. Bekhterev Rev. Psychiatry Med. Psychol. 2019, 4, 58–60. [Google Scholar] [CrossRef]
- Cross-Disorder Group of the Psychiatric Genomics Consortium. Identification of risk loci with shared effects on five major psychiatric disorders: A genome-wide analysis. Lancet 2013, 381, 1371–1379. [Google Scholar] [CrossRef]
- Georgi, A.; Jamra, R.A.; Klein, K.; Villela, A.W.; Schumacher, J.; Becker, T.; Paul, T.; Schmael, C.; Höfels, S.; Klopp, N.; et al. Possible association between genetic variants at the GRIN1 gene and schizophrenia with lifetime history of depressive symptoms in a German sample. Psychiatr. Genet. 2007, 17, 308–310. [Google Scholar] [CrossRef] [PubMed]
- Boks, M.P.; Hoogendoorn, M.; Jungerius, B.J.; Bakker, S.C.; Sommer, I.E.; Sinke, R.J.; Ophoff, R.A.; Kahn, R.S. Do mood symptoms subdivide the schizophrenia phenotype? association of the GMP6A gene with a depression subgroup. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2008, 147B, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Hamshere, M.L.; Williams, N.M.; Norton, N.; Williams, H.; Cardno, A.G.; Zammit, S.; Jones, L.; Murphy, K.; Sanders, R.D.; McCarthy, G.; et al. Genome wide significant linkage in schizophrenia conditioning on occurrence of depressive episodes. J. Med. Genet. 2005, 43, 563–567. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Online Mendelian Inheritance in Man. Available online: https://omim.org/ (accessed on 15 November 2021).
- GeneCards—Human Genes. Gene Database. Gene Search. Available online: https://www.genecards.org/ (accessed on 15 November 2021).
- Fagerberg, L.; Hallström, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the human tis-sue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Masuho, I.; Mototani, Y.; Sahara, Y.; Asami, J.; Nakamura, S.; Kozasa, T.; Inoue, T. Dynamic expression patterns of G protein-regulated inducer of neurite outgrowth 1 (GRIN1) and its colocalization with Gαo implicate significant roles of Gαo-GRIN1 signaling in nervous system. Dev. Dyn. 2008, 237, 2415–2429. [Google Scholar] [CrossRef]
- Nakazawa, K.; Sapkota, K. The origin of NMDA receptor hypofunction in schizophrenia. Pharmacol. Ther. 2020, 205, 107426. [Google Scholar] [CrossRef]
- Nakao, K.; Jeevakumar, V.; Jiang, S.Z.; Fujita, Y.; Diaz, N.B.; Annan, C.A.P.; Jaunarajs, K.L.E.; Hashimoto, K.; Belforte, J.; Nakazawa, K. Schizophrenia-Like Dopamine Release Abnormalities in a Mouse Model of NMDA Receptor Hypofunction. Schizophr. Bull. 2018, 45, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Bygrave, A.M.; Masiulis, S.; Kullmann, D.M.; Bannerman, D.M.; Kätzel, D. Gene-Environment Interaction in a Conditional NMDAR-Knockout Model of Schizophrenia. Front. Behav. Neurosci. 2019, 12, 332. [Google Scholar] [CrossRef]
- Alvarez, R.J.; Pafundo, D.; Zold, C.L.; Belforte, J.E. Interneuron NMDA Receptor Ablation Induces Hippocampus-Prefrontal Cortex Functional Hypoconnectivity after Adolescence in a Mouse Model of Schizophrenia. J. Neurosci. 2020, 40, 3304–3317. [Google Scholar] [CrossRef] [PubMed]
- Mohn, A.R.; Gainetdinov, R.R.; Caron, M.G.; Koller, B.H. Mice with Reduced NMDA Receptor Expression Display Behaviors Related to Schizophrenia. Cell 1999, 98, 427–436. [Google Scholar] [CrossRef]
- Liu, Y.-P.; Ding, M.; Zhang, X.-C.; Liu, Y.; Xuan, J.-F.; Xing, J.-X.; Xia, X.; Yao, J.; Wang, B.-J. Association between polymorphisms in the GRIN1 gene 5′ regulatory region and schizophrenia in a northern Han Chinese population and haplotype effects on protein expression in vitro. BMC Med Genet. 2019, 20, 26. [Google Scholar] [CrossRef] [PubMed]
- Krzystanek, M.; Asman, M.; Witecka, J.; Pałasz, A.; Wiaderkiewicz, R. Selected single-nucleotide variants in GRIN1, GRIN2A, and GRIN2B encoding subunits of the NMDA receptor are not biomarkers of schizophrenia resistant to clozapine: Exploratory study. Pharmacol. Rep. 2021, 73, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Krzystanek, M.; Asman, M.; Witecka, J.; Pałasz, A.; Wiaderkiewicz, R. Exploratory study of selected nucleotide variants in GRIN1, GRIN2A and GRIN2B encoding subunits of the NMDA receptor in a targeted group of schizophrenia patients with chronic cognitive impairment. Pharmacol. Rep. 2021, 73, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Foroughmand, A.M.; Galehdari, H.; Pooryasin, A.; Ajam, T.; Kazemi-Nezhad, S.R. Additive effect of MTHFR and GRIN1 genetic polymorphisms on the risk of schizophrenia. Mol. Biol. Res. Commun. 2015, 4, 33–42. [Google Scholar] [PubMed]
- Formoso, K.; García, M.D.; Frasch, A.C.; Scorticati, C. Filopodia formation driven by membrane glycoprotein M6a depends on the interaction of its transmembrane domains. J. Neurochem. 2015, 134, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, G.I.; Formoso, K.; León, A.; Frasch, A.C.; Scorticati, C. Identification of Potential Interacting Proteins with the Extracellular Loops of the Neuronal Glycoprotein M6a by TMT/MS. Front. Synaptic Neurosci. 2020, 12, 28. [Google Scholar] [CrossRef]
- Fuchsova, B.; Juliá, A.A.; Rizavi, H.; Frasch, A.C.; Pandey, G. Altered expression of neuroplasticity-related genes in the brain of depressed suicides. Neuroscience 2015, 299, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Gu, C.; Huo, Y.; Li, X.; Luo, X.-J. The integrated landscape of causal genes and pathways in schizophrenia. Transl. Psychiatry 2018, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Formoso, K.; Garcia, M.D.; Frasch, A.C.; Scorticati, C. Evidence for a role of glycoprotein M6a in dendritic spine formation and synaptogenesis. Mol. Cell. Neurosci. 2016, 77, 95–104. [Google Scholar] [CrossRef]
- León, A.; Aparicio, G.I.; Scorticati, C. Neuronal Glycoprotein M6a: An Emerging Molecule in Chemical Synapse Formation and Dysfunction. Front. Synaptic Neurosci. 2021, 13, 661681. [Google Scholar] [CrossRef]
- Gottfried, Y.; Rotem, A.; Klein, E.; Larisch, S. The pro-apoptotic ARTS/Sept4 protein is significantly reduced in post-mortem brains from schizophrenic patients. Schizophr. Res. 2007, 96, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.C.; Bagade, S.L.; McQueen, M.B.; Ioannidis, J.P.A.; Kavvoura, F.K.; Khoury, M.J.; Tanzi, R.E.; Bertram, L. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: The SzGene database. Nat. Genet. 2008, 40, 827–834. [Google Scholar] [CrossRef]
- Ioannidis, J.P.; Boffetta, P.; Little, J.; O’Brien, T.R.; Uitterlinden, A.G.; Vineis, P.; Balding, D.; Chokkalingam, A.; Dolan, S.; Flanders, W.D.; et al. Assessment of cumulative evidence on genetic associations: Interim guidelines. Int. J. Epidemiol. 2008, 37, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; He, L. Further clarification of the contribution of the tryptophan hydroxylase (TPH) gene to suicidal behavior using systematic allelic and genotypic meta-analyses. Qual. Life Res. 2006, 119, 233–240. [Google Scholar] [CrossRef]
- Sand, P.G. Comments on the paper by D. Li and L. He: Meta-analysis showed association between the tryptophan hydrox-ylase (TPH) gene and schizophrenia. Hum. Genet. 2007, 122, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Abbar, M.; Courtet, P.; Bellivier, F.; Leboyer, M.; Boulenger, J.P.; Castelhau, D.; Ferreira, M.; Lambercy, C.; Mouthon, D.; Paoloni-Giacobino, A.; et al. Suicide attempts and the tryptophan hydroxylase gene. Mol. Psychiatry 2001, 6, 268–273. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brown, S.M.; Peet, E.; Manuck, S.B.; Williamson, D.; Dahl, R.; Ferrell, R.; Hariri, A.R. A regulatory variant of the human tryptophan hydroxylase-2 gene biases amygdala reactivity. Mol. Psychiatry 2005, 10, 884–888. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cichon, S.; Winge, I.; Mattheisen, M.; Georgi, A.; Karpushova, A.; Freudenberg, J.; Freudenberg-Hua, Y.; Babadjanova, G.; Van Den Bogaert, A.; Abramova, L.I.; et al. Brain-specific tryptophan hydroxylase 2 (TPH2): A functional Pro206Ser substitution and variation in the 5-prime region are associated with bipolar affective disorder. Hum. Mol. Genet. 2008, 17, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Garriock, H.A.; Allen, J.J.B.; Delgado, P.; Nahaz, Z.; Kling, M.A.; Carpenter, L.; Burke, M.; Burke, W.; Schwartz, T.; Marangell, L.B.; et al. Lack of association of TPH2 exon XI polymorphisms with major depression and treatment resistance. Mol. Psychiatry 2005, 10, 976–977. [Google Scholar] [CrossRef][Green Version]
- Zhang, X.; Gainetdinov, R.R.; Beaulieu, J.-M.; Sotnikova, T.D.; Burch, L.H.; Williams, R.B.; Schwartz, D.A.; Krishnan, K.R.R.; Caron, M.G. Loss-of-Function Mutation in Tryptophan Hydroxylase-2 Identified in Unipolar Major Depression. Neuron 2005, 45, 11–16. [Google Scholar] [CrossRef]
- Bellivier, F.; Chaste, P.; Malafosse, A. Association between the TPH gene A218C polymorphism and suicidal behavior: A meta-analysis. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2003, 124B, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Beaulieu, J.-M.; Sotnikova, T.D.; Gainetdinov, R.R.; Caron, M.G. Tryptophan hydroxylase-2 controls brain sero-tonin synthesis. Science 2004, 305, 217. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.E.; Feldman, D.H.; McCue, A.F.; Brenner, R.; Velicelebi, G.; Ellis, S.B.; Harpold, M.M. Structure and function-al expression of alpha-1, alpha-2, and beta subunits of a novel human neuronal calcium channel subtype. Neuron 1992, 8, 71–84. [Google Scholar] [CrossRef]
- Juraeva, D.; Haenisch, B.; Zapatka, M.; Frank, J.; Witt, S.H.; Mühleisen, T.W.; Treutlein, J.; Strohmaier, J.; Meier, S.; Degenhardt, F.; et al. Integrated Pathway-Based Approach Identifies Association between Genomic Regions at CTCF and CACNB2 and Schizophrenia. PLoS Genet. 2014, 10, e1004345. [Google Scholar] [CrossRef]
- Luo, X.; Huang, L.; Han, L.; Luo, Z.; Hu, F.; Tieu, R.; Gan, L. Systematic prioritization and integrative analysis of copy number variations in schizophrenia reveal key schizophrenia susceptibility genes. Schizophr. Bull. 2014, 40, 1285–1299. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, G.; Ji, W.; Feng, G.; Zhao, Q.; Liu, J.; Li, T.; Li, Y.; Chen, P.; Zeng, Z.; et al. Common Variants in the BCL9 Gene Conferring Risk of Schizophrenia. Arch. Gen. Psychiatry 2011, 68, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Aragam, N.; Li, X.; Villla, E.C.; Wang, L.; Briones, D.; Petty, L.; Posada, Y.; Arana, T.B.; Cruz, G.; et al. BCL9 and C9orf5 Are Associated with Negative Symptoms in Schizophrenia: Meta-Analysis of Two Genome-Wide Association Studies. PLoS ONE 2013, 8, e51674. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Tanaka, S.; Kushima, I.; Koide, T.; Banno, M.; Kikuchi, T.; Nakamura, Y.; Shiino, T.; Yoshimi, A.; Oya-Ito, T.; et al. Association study of BCL9 gene polymorphism rs583583 with schizophrenia and negative symptoms in Japanese population. Sci. Rep. 2015, 5, 15705. [Google Scholar] [CrossRef] [PubMed]
- Samsom, J.N.; Wong, A.H.C. Schizophrenia and Depression Co-Morbidity: What We have Learned from Animal Models. Front. Psychiatry 2015, 6, 13. [Google Scholar] [CrossRef]
- Neznanov, N.G. A paradigm shift to treat psychoneurological disorders. Pers. Psychiatry Neurol. 2021, 1, 1–2. [Google Scholar]
- Novitsky, M.A.; De Sousa, A.; Asadullin, A.R.; Gavrilyuk, O.A.; Petrov, A.V.; Nasyrova, R.F. Possibilities and limitations of antidepressant use to correct depressive and negative symptoms in schizophrenia. Pers. Psychiatry Neurol. 2021, 1, 21–45. [Google Scholar] [CrossRef]
- Verbitskaya, E. Meta-analysis: Problems with Russian Publications. Int. J. Risk Saf. Med. 2015, 27, S89–S90. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Kong, D.; Zhu, X.; Wu, W.; Xue, R.; Li, G.; Xu, Y.; Liu, S.; Tian, H.; Zhuo, C. Rethinking Schizophrenia and Depression Comorbidity as One Psychiatric Disorder Entity: Evidence from Mouse Model. Front. Neurosci. 2020, 14, 115. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, M.B.; Orger, M.B.; Robson, D.N.; Li, J.M.; Keller, P.J. Whole-brain functional imaging at cellular resolution using light-sheet microscopy. Nat. Methods 2013, 10, 413–420. [Google Scholar] [CrossRef] [PubMed]

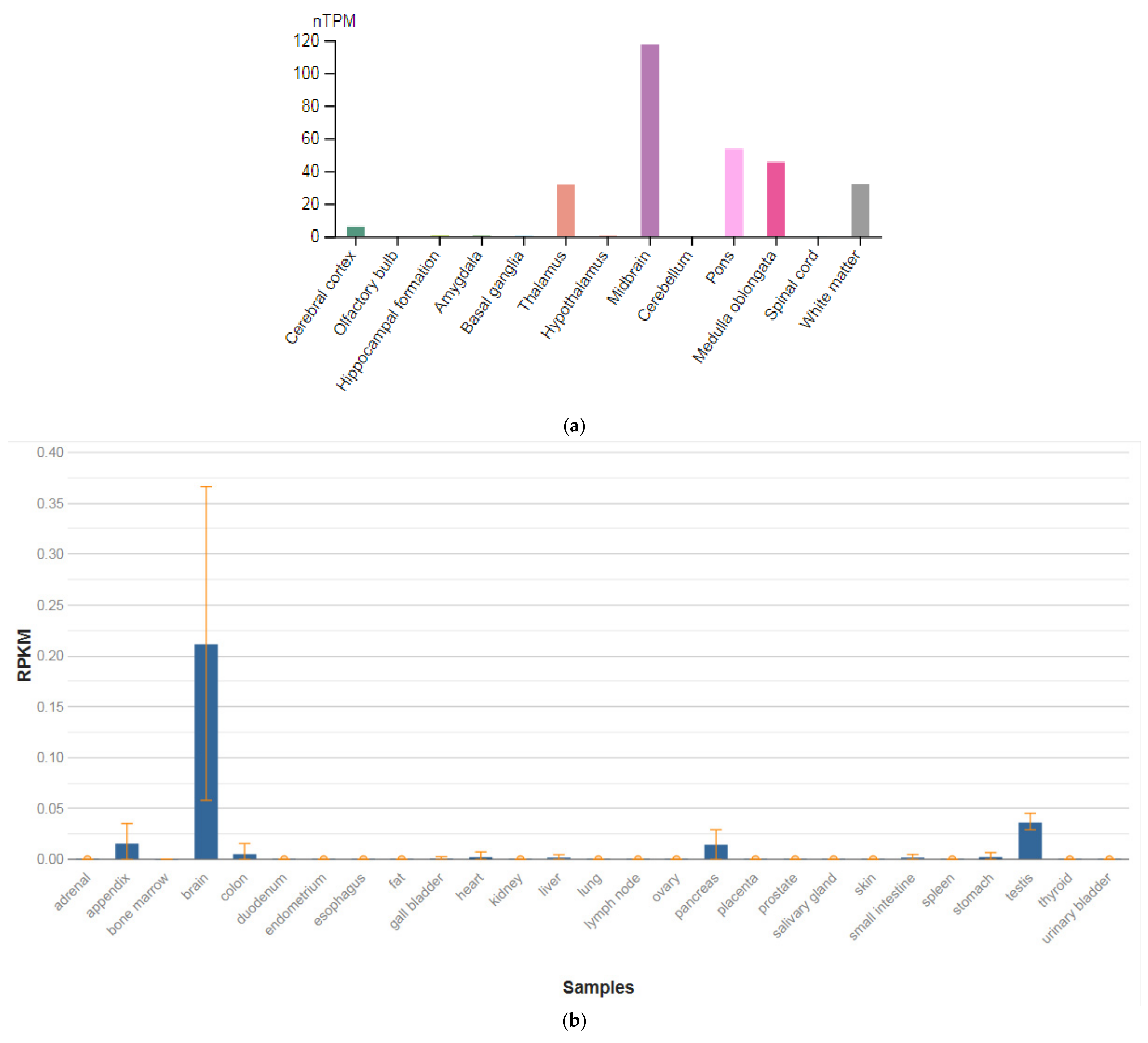
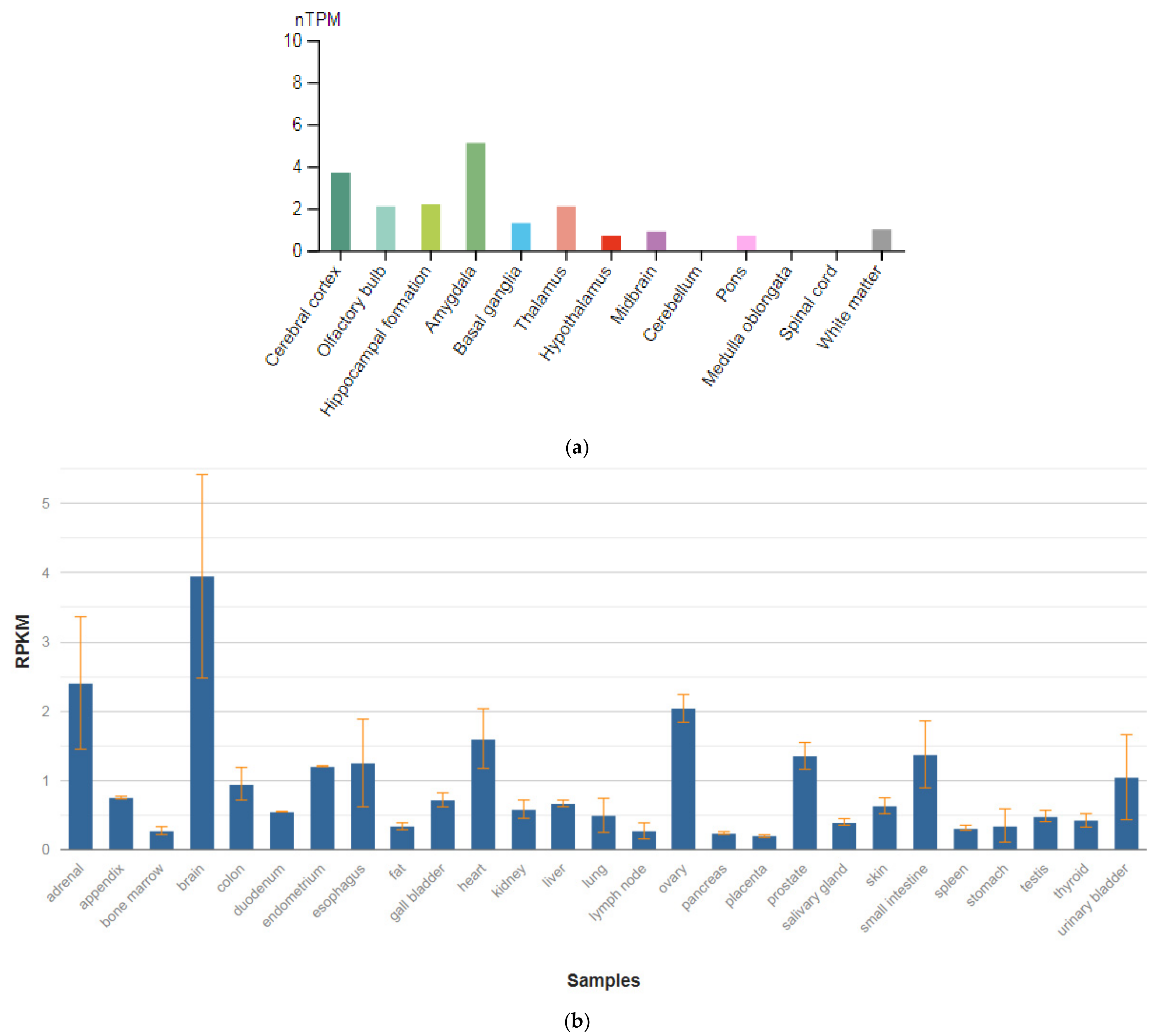
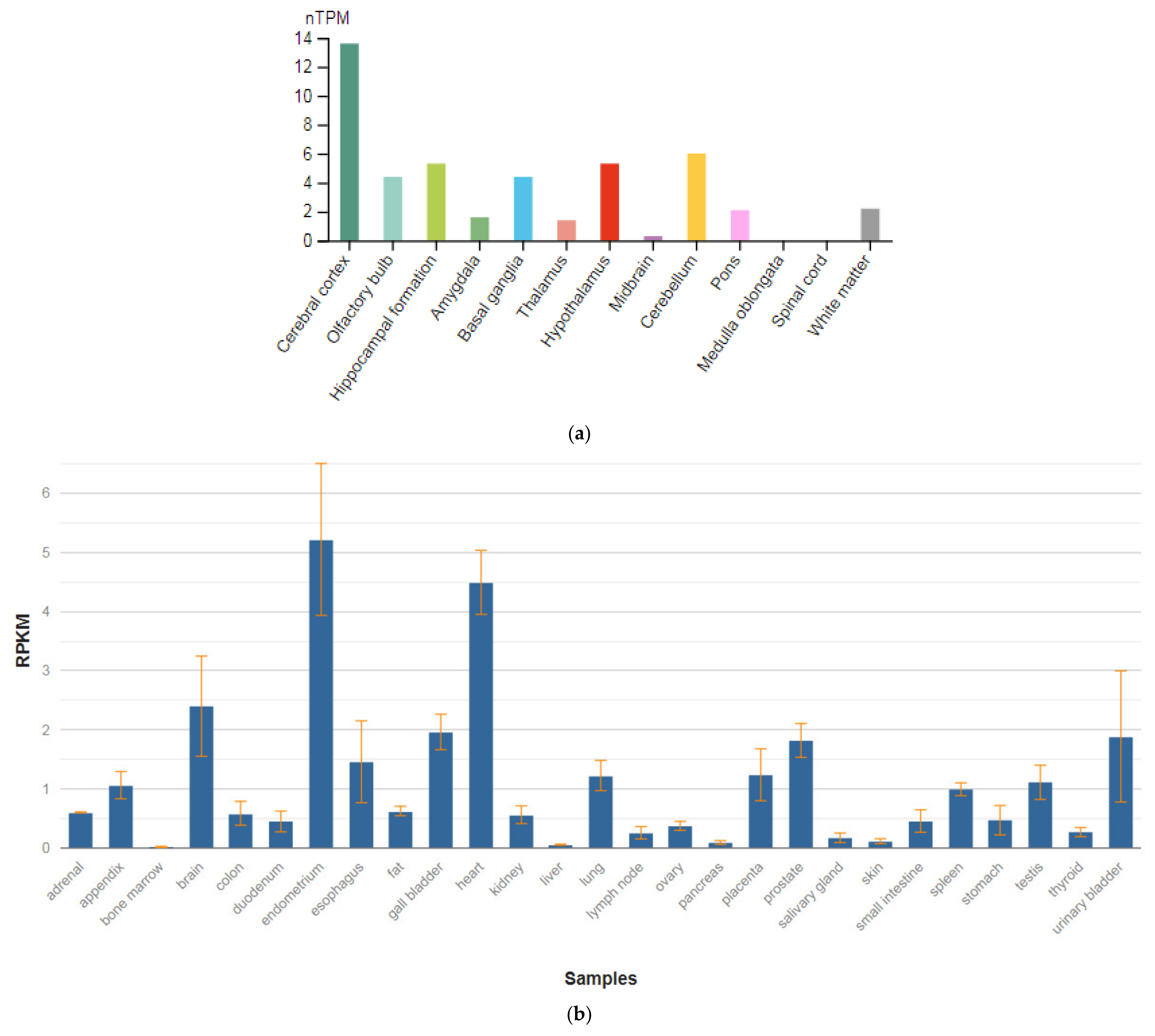
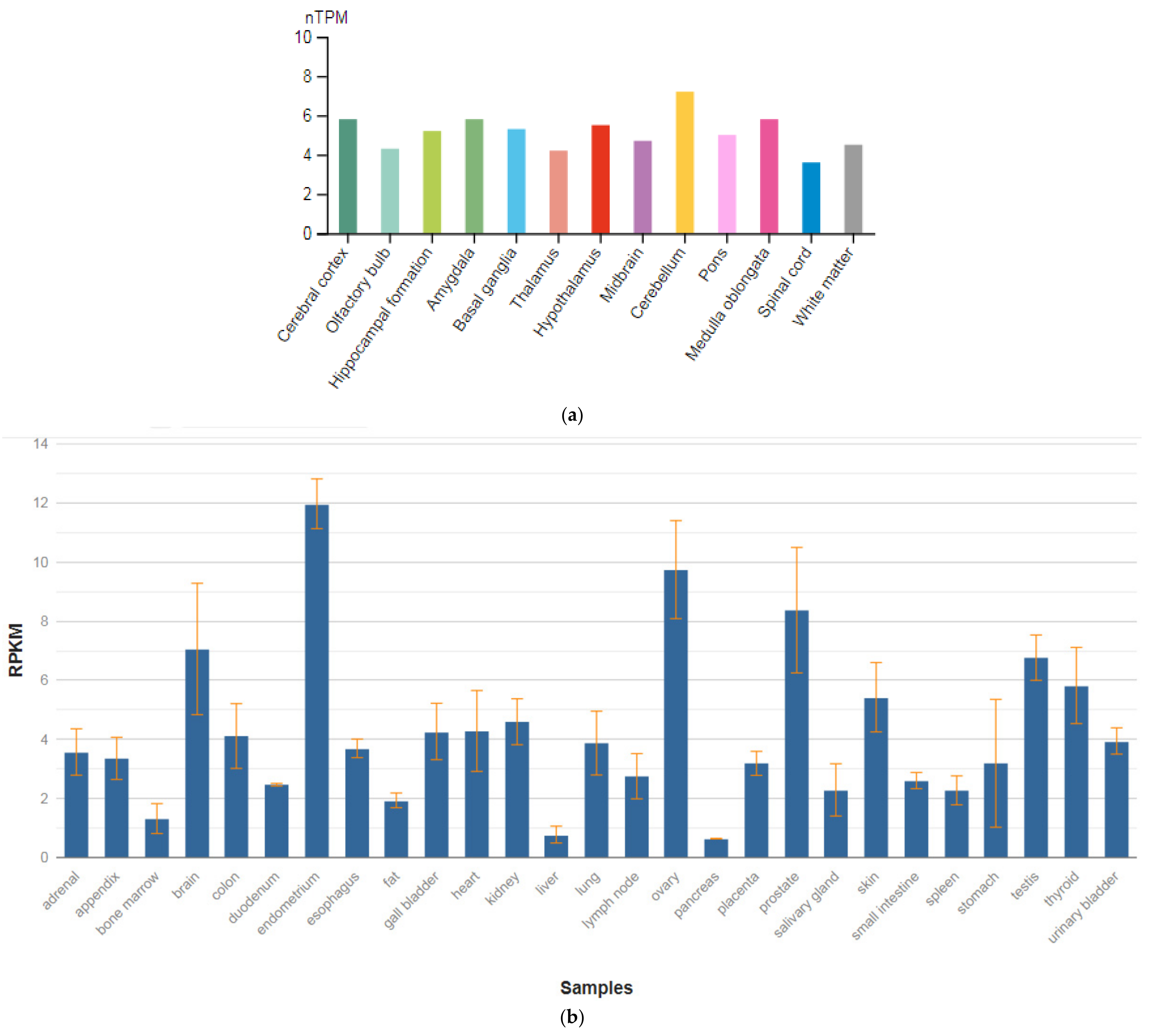
| Gene: MIM (Protein) | Chromosome Location | Clinical Manifestations of Mutation: MIM | Inheritance |
|---|---|---|---|
| GRIN1: 138249 (Glutamate ionotropic receptor NMDA type subunit 1) | 9q34.3 | Neurodevelopmental disorder with or without hyperkinetic movements and seizures, autosomal dominant: 614254 Neurodevelopmental disorder with or without hyperkinetic movements and seizures, autosomal recessive: 617820 Schizophrenia spectrum disorder: 181500 | AD AR Mu |
| GPM6A: 601275 (Glycoprotein M6A) | 4q34.2 | Schizophrenia spectrum disorder: 181500 | Mu |
| SEPTIN4 or SEPT4: 603696 (Septin 4 or Apoptosis-related protein in the TGF-β signaling pathway) | 17q22 | Meckel syndrome, type 1: 249000 Schizophrenia spectrum disorder: 181500 | AR Mu |
| TPH1: 191060 (Tryptophan hydroxylase type 1) | 11p15.1 | Schizophrenia spectrum disorder: 181500 | Mu |
| TPH2: 607478 (Tryptophan hydroxylase type 2 or Neuronal tryptophan hydroxylase) | 12q21.1 | Susceptibility to attention deficit–hyperactivity disorder: 613003 Susceptibility to unipolar depression: 608516 Susceptibility to bipolar affective disorder: 125480 Schizophrenia spectrum disorder: 181500 | Mu Mu Mu Mu |
| CACNA1C: 114205 (α-1C subunit of voltage-dependent calcium channel type L) | 12p13.33 | Brugada syndrome type 3: 611875 Long QT syndrome type 8: 618447 Timothy syndrome: 601005 Schizophrenia spectrum disorder: 181500 | Mu Mu AD Mu |
| CACNB2: 600003 (β2 subunit of voltage-dependent calcium channel) | 10p12.33–p12.31 | Brugada syndrome type 4: 611876 Schizophrenia spectrum disorder: 181500 | Mu Mu |
| BCL9: 602597 (B-cell of lymphoma type 9 transcription coactivator) | 1q21.2 | Schizophrenia spectrum disorder: 181500 | Mu |
| Gene (MIM) | Expression Level in Brain (RPKM) |
|---|---|
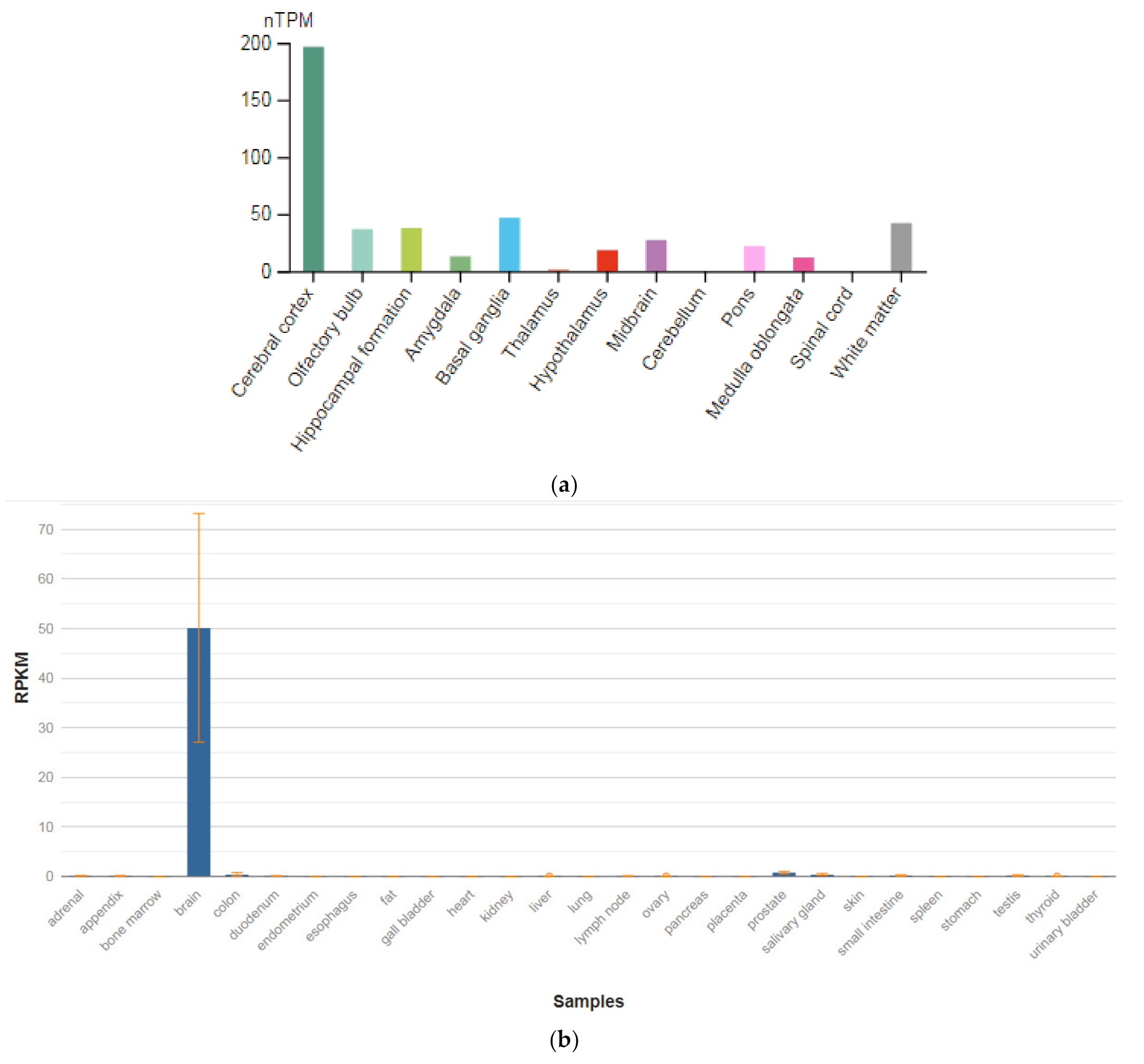
| GRIN1 (138249) | 50.139 ± 23.048 |
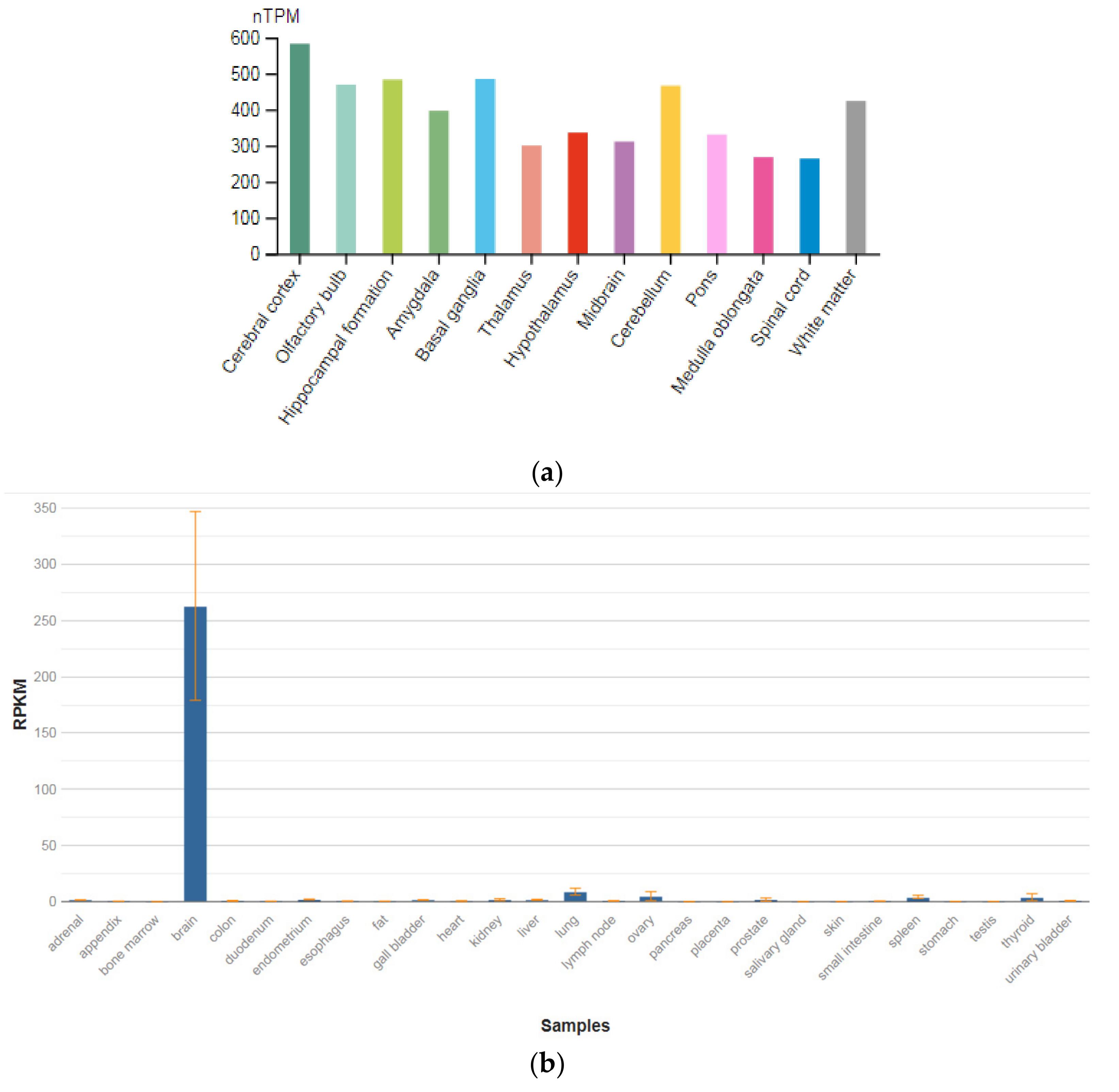
| GPM6A (601275) | 262.992 ± 83.861 |
| SEPTIN4 (603696) | 34.56 ± 5.181 |
| TPH1 (191060) | 0.102 ± 0.025 |
| TPH2 (607478) | 0.212 ± 0.154 |
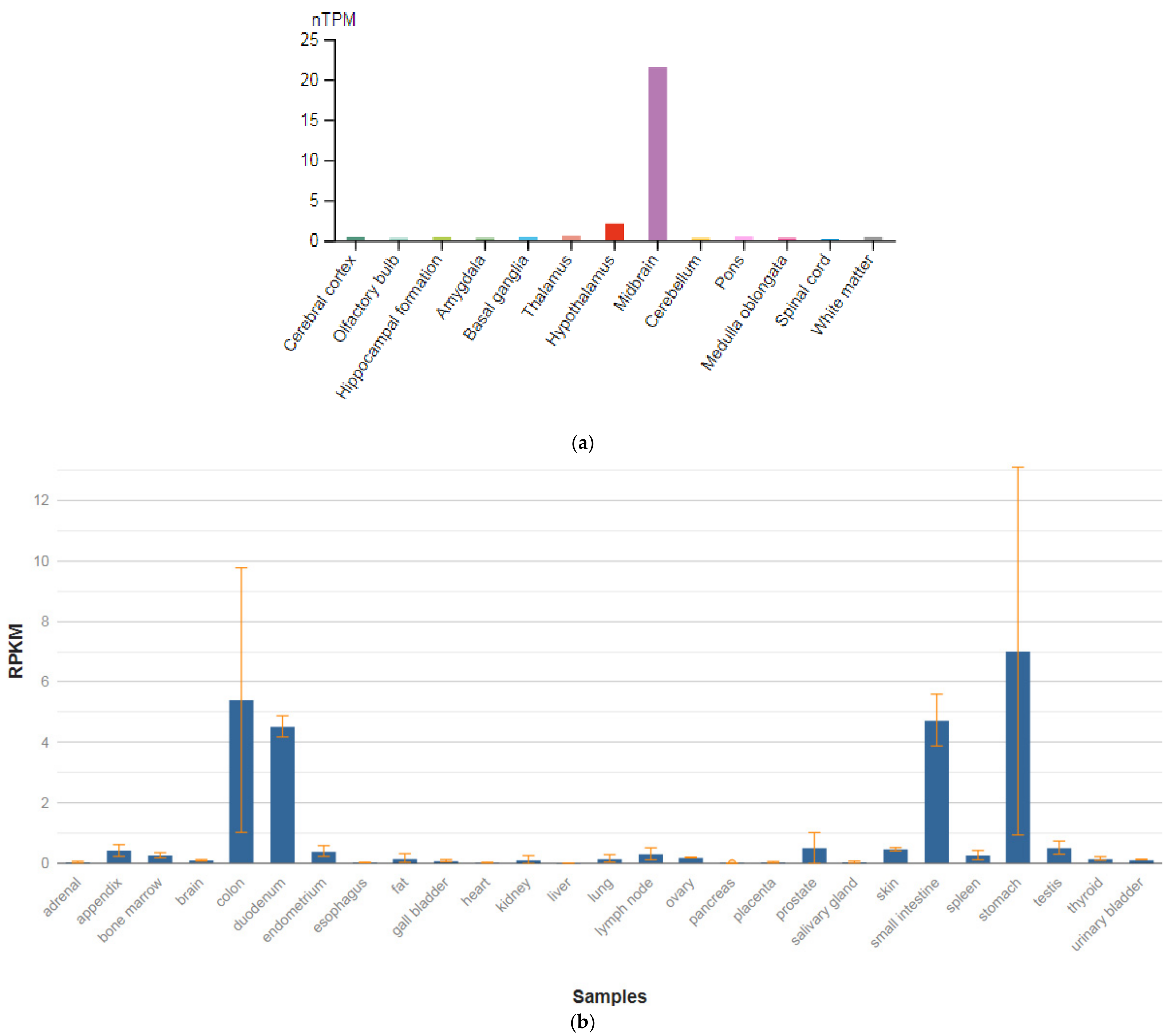
| CACNA1C (114205) | 2.4 ± 0.846 |
| CACNB2 (600003) | 3.947 ± 1.468 |
| BCL9 (602597) | 7.058 ± 2.222 |
| Gene | Chromosome (Locus) | Schizophrenia | Depression | Comorbidity of Schizophrenia and Depression |
|---|---|---|---|---|
| GRIN1 | 9q34.3 | (+) [38] | (+) [38] | (+) [42] |
| GPM6A | 4q34.2 | (+) [39] | (+) [39] | (+) [43] |
| SEPTIN4 | 17q22 | (+) [39] | (−) [39] | (−) [43] |
| TPH1 | 11p15.3-p14 | (+) [43] | (+) [43] | (+) [73] |
| TPH2 | 12q21.1 | (+) [42] | (+) [42] | (−) [69] |
| CACNA1C | 12p13.33 | (+) [45] | (+) [45] | (+) [41] |
| CACNB2 | 10p12.31 | (+) [45] | (+) [45] | (+) [41] |
| Gene | Chromosome Location | Single Nucleotide Variant | Patients Nationality (Race) | References |
|---|---|---|---|---|
| GRIN1 | 9q34.3 | rs4880213 rs11146020 rs6293 rs10747050 | European (German) | [42] |
| GPM6A | 4q34.2 | rs9247 rs3925 rs2286435 rs8135641 rs7748777 rs2240432 rs4917450 rs6434387 rs2293759 | Caucasians and Africans (Americans) | [73] |
| TPH1 | 11p15.3-p14 | T1606C T3792A A218C T465C C160T distal region of exon 11 39 (repeat expansion 5657 base pairs (CT) m (CA) n (CT) p) | Caucasians and African Americans | [73] |
| CACNA1C | 12p13.33 | rs1024582 | Europeans (Norwegians) | [41] |
| CACNB2 | 10p12.31 | rs2799573 | Europeans (Norwegians) | [41] |
| BCL9 | 1q21.1 | rs672607 | Asians (Chinese) | [79] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shnayder, N.A.; Novitsky, M.A.; Neznanov, N.G.; Limankin, O.V.; Asadullin, A.R.; Petrov, A.V.; Dmitrenko, D.V.; Narodova, E.A.; Popenko, N.V.; Nasyrova, R.F. Genetic Predisposition to Schizophrenia and Depressive Disorder Comorbidity. Genes 2022, 13, 457. https://doi.org/10.3390/genes13030457
Shnayder NA, Novitsky MA, Neznanov NG, Limankin OV, Asadullin AR, Petrov AV, Dmitrenko DV, Narodova EA, Popenko NV, Nasyrova RF. Genetic Predisposition to Schizophrenia and Depressive Disorder Comorbidity. Genes. 2022; 13(3):457. https://doi.org/10.3390/genes13030457
Chicago/Turabian StyleShnayder, Natalia A., Maxim A. Novitsky, Nikolay G. Neznanov, Oleg V. Limankin, Azat R. Asadullin, Artem V. Petrov, Diana V. Dmitrenko, Ekaterina A. Narodova, Natalia V. Popenko, and Regina F. Nasyrova. 2022. "Genetic Predisposition to Schizophrenia and Depressive Disorder Comorbidity" Genes 13, no. 3: 457. https://doi.org/10.3390/genes13030457
APA StyleShnayder, N. A., Novitsky, M. A., Neznanov, N. G., Limankin, O. V., Asadullin, A. R., Petrov, A. V., Dmitrenko, D. V., Narodova, E. A., Popenko, N. V., & Nasyrova, R. F. (2022). Genetic Predisposition to Schizophrenia and Depressive Disorder Comorbidity. Genes, 13(3), 457. https://doi.org/10.3390/genes13030457







