Abstract
Diamond–Blackfan anemia (DBA) is one of the inherited bone marrow failure syndromes marked by erythroid hypoplasia. Underlying variants in ribosomal protein (RP) genes account for 80% of cases, thereby classifying DBA as a ribosomopathy. In addition to RP genes, extremely rare variants in non-RP genes, including GATA1, the master transcription factor in erythropoiesis, have been reported in recent years in patients with a DBA-like phenotype. Subsequently, a pivotal role for GATA-1 in DBA pathophysiology was established by studies showing the impaired translation of GATA1 mRNA downstream of the RP haploinsufficiency. Here, we report on a patient from the Dutch DBA registry, in which we found a novel hemizygous variant in GATA1 (c.220+2T>C), and an Iranian patient with a previously reported variant in the initiation codon of GATA1 (c.2T>C). Although clinical features were concordant with DBA, the bone marrow morphology in both patients was not typical for DBA, showing moderate erythropoietic activity with signs of dyserythropoiesis and dysmegakaryopoiesis. This motivated us to re-evaluate the clinical characteristics of previously reported cases, which resulted in the comprehensive characterization of 18 patients with an inherited GATA-1 defect in exon 2 that is presented in this case-series. In addition, we re-investigated the bone marrow aspirate of one of the previously published cases. Altogether, our observations suggest that DBA caused by GATA1 defects is characterized by distinct phenotypic characteristics, including dyserythropoiesis and dysmegakaryopoiesis, and therefore represents a distinct phenotype within the DBA disease spectrum, which might need specific clinical management.
1. Introduction
Diamond–Blackfan anemia (DBA) is a rare, inherited bone marrow failure syndrome that is characterized by hypoplastic anemia, congenital malformations (in ~50% of patients) and a predisposition to cancer [1,2]. The diagnosis of DBA is mainly based on clinical consensus criteria (Figure 1) [3]. Since the first groundbreaking study identifying RPS19 variants as the underlying cause for DBA in 25% of cases [4], loss of function variants or deletions in more than 20 genes encoding ribosomal proteins (RPs) have been linked to DBA and can be identified in almost 75% of patients [5,6]. These variants cause ribosomal protein haploinsufficiency, leading to impaired ribosome biogenesis and ribosomal stress [7]. How the dysregulation of a basic cellular process such as ribosome biogenesis results in predominantly erythroid lineage-specific defects still remains enigmatic. Possible explanations proposed over the years include the hypersensitivity of erythroblasts to elevated p53 levels and a heme-versus globin-synthesis imbalance [8,9,10,11,12,13,14]. Just recently, it was recognized that p53 activation during ribosome biogenesis itself regulates normal erythroid differentiation [15]. Interestingly, in addition to RP genes, extremely rare variants in non-ribosomal genes such as GATA1 have been linked to DBA-like diseases [16,17,18,19]. This is supported by recent and robust evidence of the impaired translation of GATA1 mRNA downstream of the RP haploinsufficiency, thereby linking these processes and providing an additional explanation for the selective erythroid defect in DBA [19,20,21].
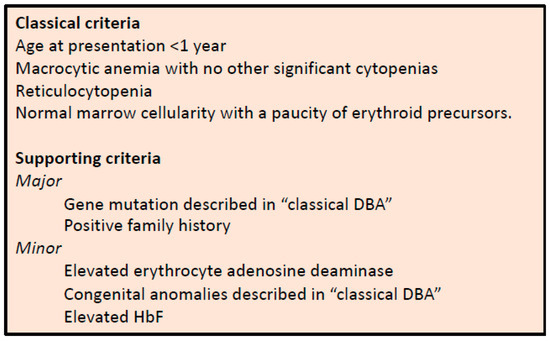
Figure 1.
Diagnostic and supporting criteria for the diagnosis of DBA, as established by Vlachos et al. [3].
GATA-1 is regarded as the “master regulator” of erythropoiesis, regulating processes in both erythroid differentiation and maturation. The protein itself contains two zinc finger domains and an N-terminal transactivation domain. An alternative initiation of translation results in the production of the full-length protein (GATA-1L) and a shorter isoform (GATA-1s) that lacks the N-terminal transactivation domain. GATA-1s is functionally incapable of supporting erythropoiesis adequately as a result of less (efficient) binding of GATA-1s to selective sites within erythroid target genes [22,23,24]. In addition to DBA-like diseases [16,17,18,19], variants in GATA1 have been described in patients with X-linked thrombocytopenia with dyserythropoietic anemia (XLTDA), thrombocytopenia [25,26,27], X-linked (macro)thrombocytopenia with or without severe anemia (XLT) [28,29,30], X-linked thrombocytopenia with ß-thalassemia [31], macrocytic anemia and neutropenia [32], congenital erythropoietic porphyria (CEP) [33], as well as in acquired transient myeloproliferative disorder (TMD) and acute megakaryoblastic leukemia (AMKL) associated with Down syndrome [34,35,36,37]. GATA1 variants associated with a DBA-phenotype all occur within the splice donor site of exon 2, resulting in the impaired production of full-length GATA-1 and the favored production of GATA1s [16,17,18,19].
In this study, we characterized two DBA patients in which GATA1 defects in exon 2 were identified and retrospectively studied the clinical characteristics of previously reported patients with GATA-1 defects and DBA-like phenotypes. Together, our observations suggest that DBA caused by GATA-1 defects constitutes a distinct hematological and clinical phenotype within the DBA syndrome (DBA and DBA-like disease) [38], requiring specific aspects during clinical follow-up.
2. Methods
2.1. Patients and Genetics
The first patient was a patient from the Dutch DBA registry. The coding regions and flanking splice sites of 9 established DBA genes (RPS7, RPS10, RPS17, RPS19, RPS24, RPS26, RPL5, RPL11 and RPL35A), as well as GATA1, were assessed by Sanger sequencing. We found a novel hemizygous variant in GATA1 (c.220+2T>C; p.?)), which was predicted to be pathogenic by in silico analysis (Supplementary File S1). The second patient was an Iranian patient who was identified at the age of five years old with severe anemia and treated at Namazi Hospital, Shiraz, Iran. At the age of thirteen, a next-generation sequencing (NGS) panel for RBC enzyme and membrane genes and targeted NGS panels for bone marrow failure, AML and MDS, were performed in The Netherlands (Sanquin, Amsterdam, The Netherlands), revealing a hemizygous variant in the initiation codon of GATA1 (c.2T>C; p.?).
2.2. Literature Search
A retrospective literature analysis was conducted for all case reports and/or case series regarding inherited GATA1 exon 2 defects in patients with hereditary anemia. The search strategy was therefore broad and comprised the search terms “GATA1” or “GATA-1” and “anemia”. Medline (Pubmed) was searched repeatedly between January 2020 and December 2021. The title and abstract (if provided) were screened, after which, the full text of potentially eligible studies was read. In addition, the reference lists of all the included articles were screened to search for additional records. Subsequently, studies that described patients with variants in exon 2 of GATA1 were included. Data were extracted from the respective papers and their Supplemental Files.
2.3. Bone Marrow Analysis
Bone marrow aspirates and trephine biopsies were performed according to standard procedures and were analyzed by two independent cytologists (aspirates) and pathologists (biopsies), of which the second cytologist/pathologist was blinded from the clinical diagnosis.
2.4. Ethics
Written informed consent was obtained, and clinical and genetic data for our institutional Dutch and Iranian patients were retrieved from their electronic patient records and/or treating physicians.
3. Results
3.1. Hematological Characteristics of Two Novel DBA-Like Patients with GATA1 Defects
In the Dutch DBA registry, a male patient was identified with a novel de novo GATA1 c.220+2T>C splice site variant, which was predicted to cause the skipping of exon 2 and thus produce predominantly GATA-1s protein. The patient was an eleven-year-old boy of Somalian descent that presented in his native country with anemia at the age of seven months old. A bone marrow examination was performed and allegedly reported erythroid hypoplasia. He was treated in both Somalia and Ethiopia with regular blood transfusions before, and after he moved to The Netherlands at the age of four, he was started on glucocorticoid treatment. At that point, his CBC and additional investigations showed mild macrocytic anemia (Hb 9.4 g/dL, MCV 100 fL, references Hb 11.5–14.0 g/dL, MCV 75–85 fL) with a normal reticulocyte count, normal neutrophil and platelet counts, increased fetal hemoglobin (HbF 3.3%) and normal erythrocyte adenosine deaminase activity (eADA 1.0 U/g Hb). No congenital malformations were found, whereas length growth was below the target height range (−2.5 SD). During the follow-up, and while on treatment with glucocorticoids (0.2 mg/kg/day), two bone marrow examinations were performed, demonstrating no significant erythroid hypoplasia (an absence of reticulocytopenia) yet significant dyserythropoiesis and mild dysmegakaryopoiesis (Figure 2). Until the last follow-up, he had a stable disease with glucocorticoid treatment (0.2 mg/kg/day), characterized by moderately severe anemia (Hb 8.7–9.4 g/dL, reference 11.5–15.5 g/dL) with normal reticulocyte counts (70–100 × 109/L), a short stature but a normal growth velocity (at −2.5 SD) and no physical complaints.
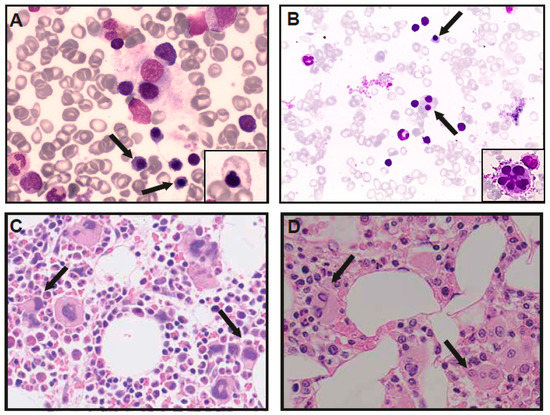
Figure 2.
Bone marrow analysis of GATA-1 DBA patients. Bone marrow aspirates demonstrating dysplastic erythroid precursor cells (arrows), and aberrant shapes (small panel) in patient 1 (A) and dyserytropoiesis (arrows) and dysplastic megakaryocytes (small panel) in patient V-I (B). Trephine biopsies, demonstrating normocellular bone marrow with increased, dysplastic megakaryopoiesis (arrows) in patient 1 (C) and reduced cellularity and erythropoiesis with dysmegakaryopoiesis in patient V-I (D).
The second patient was an Iranian boy who had been treated since the age of five years old for severe macrocytic anemia at Shiraz Medical University hospital (Hb 8.2 g/dL, MCV 97 fL, references Hb 11.5–14.0 g/dL, MCV 75–85 fL). A bone marrow aspirate performed at the age of six years old showed normal cellularity and erythropoiesis (27% erythroblast; reference 8–30%), with megaloblastic changes with no significant dysplastic features (morphology not available). After the first attempt failed, the patient is currently being treated with glucocorticoids (Hb 8.5–10.2 g/dL). Follow-up blood counts often showed increased platelet counts (mean 652 × 109/L), and no congenital malformations were noted. At the age of thirteen, a molecular analysis was performed in The Netherlands, demonstrating the previously reported GATA1 (c.2T>C) variant [18].
3.2. Comparative Analysis with Previously Reported Cases Illustrates Distinct DBA-Like Disease Characteristics
In addition to our two novel patients, sixteen patients from six families were identified in our PubMed literature search [16,17,18,19,32]. All patients were males and had variants in either exon 2 or the start codon, in both cases leading to the predominant/exclusive synthesis of the short isoform of GATA1 (GATA1s) (Table 1). For all patients, data were available on the age of onset, demonstrating that ten patients (10/18, 56%) were diagnosed before the age of one year old. Thirteen patients (13/18, 72%) presented with macrocytic anemia. Regarding other hematopoietic lineages, seven patients (7/18, 39%) had a leukocyte count below the normal range and mild to severe neutropenia. Absolute reticulocytopenia was present in four patients in which data were available (4/14, 29%). However, when a “bone marrow responsiveness index” (BMRI) was calculated, a measure to consider the reticulocyte count relative to the degree of anemia, eight additional patients proved to have relative reticulocytopenia (85.7%), comparable to DBA based on RP-gene defects (Figure 3) [39]. So far, no malignancies have been reported in the GATA1 mutated patients, except for the one with MDS [18]. However, we cannot eliminate an accidental association in this small number of patients.

Table 1.
Clinical and molecular characteristics of reported cases.
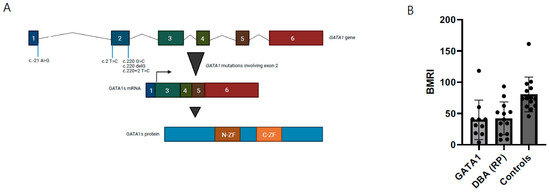
Figure 3.
Functional characteristics of GATA-1 DBA. (A) Overview of genetic defects at exon 2 in GATA1, inducing GATA1s exclusively (figure adapted from Ling & Crispino) [40]. (B) Bone marrow responsiveness index (BMRI), calculated as [(absolute reticulocyte count) × (patient’s Hb/normal Hb)], depicted for DBA patients with GATA1 defects, ribosomal protein defects from the Dutch DBA Registry and healthy controls.
From fifteen patients (including this report), bone marrow characteristics were reported, demonstrating normocellular bone marrow with a paucity of erythroid precursors in six patients (6/15, 40%). One of these patients (Index XIII) was diagnosed with myelodysplastic syndrome (MDS) by the age of four. (Moderate) hypocellularity was noted in six patients (6/15, 40%), and in one patient, moderate hypercellularity was found (index VI-I). Dysplastic features were reported in 9/15 (60%) patients and in 7/15 (47%) patients in the erythroid lineage specifically (dyserythropoiesis). In addition, we re-analyzed the bone marrow aspirate of patient V-I, which had been described as “erythroid hypoplasia with otherwise normal cellularity”. Revision by two blinded cytologists illustrated dysplastic erythropoiesis with dysmorphic cells and dysmegakaryopoiesis (micromegakaryocytes and fragmented megakaryocytes), which had also been reported in patients from family I (Table 1, Figure 1C,D). Patient V-I was not treated with glucocorticoids at the time of the bone marrow analysis. Nine (9/18, 50%) patients treated with glucocorticoids had an initial or partial treatment response, yet four patients (4/9, 44%) became transfusion-dependent after cessation of effect. Three patients were treated with a hematopoietic stem cell transplantation (HSCT), which cured their hematological disease. Indications for HSCT were severe combined anemia and neutropenia (Index I-IV and V) and MDS (Index IV-I). Seven patients are on regular erythrocyte transfusions (7/18, 38%), three patients receive no treatment (17%) and two patients died (2/18, 11%), reportedly from severe pneumonia (Index II) and an unrelated cause (index VIII).
In summary, this suggests that DBA based on specific GATA1 defects is characterized by macrocytic anemia, with neutropenia in a significant number of patients, and specific bone marrow cytomorphological abnormalities, including dyserythropoiesis, dysmegakaryopoiesis with micromegakaryocytes and fragmented megakaryocytes. In addition, other specific characteristics of DBA, including reticulocytopenia, congenital malformations and elevated eADA, are missing. Fetal Hb (HbF) was elevated in all of the analyzed patients. The distribution of patients between treatment modalities is in line with the whole DBA population.
4. Discussion
In this study, we reported on two new DBA-like patients with variants in exon 2 of the erythroid transcription factor GATA-1 and reviewed all published cases. Our subsequent comprehensive review of the cases illustrates that inherited GATA1 defects in exon 2 represent a hematological phenotype that displays distinct characteristics within the DBA spectrum, including (severe) neutropenia and dyserythropoiesis with or without dysmegakaryopoiesis, instead of the typical quantitative intrinsic erythroid defect (hypoplastic anemia). GATA-1 is considered the master transcription factor of erythropoiesis and is known to regulate processes in both early and late erythroid development. In addition to erythroid cells, GATA-1 is expressed in megakaryocytes, eosinophils and basophils, yet is not expressed in non-hematological tissues [41]. In mouse models, the knockdown of Gata1 leads to maturation arrest at the proerythroblast stage, thrombocytopenia and the increased proliferation of megakaryocytes, whereas Gata1-null mice are embryonically lethal due to severe anemia [42,43]. The GATA-1 protein has three important functional domains for erythropoiesis: the N-terminal transactivation domain (N-TAD), an N-terminal zinc finger domain (N-ZF) and a C-terminal zinc finger domain (C-ZF) [40]. The short isoform of GATA-1, GATA-1s, starts at a methionine at position 37 and does not include the N-TAD, and therefore cannot support erythropoiesis adequately. GATA1 variants have been associated with a variety of hematological phenotypes, which can be largely explained by their implication for GATA-1 protein function and GATA-1 binding to FOG-1 [22,40]. GATA-1 also directly or indirectly regulates the development of other hematopoietic lineages, including megakaryopoiesis and granulopoiesis, through interaction with the myeloid master transcription factor PU.1 and by the downregulation of GATA-2 expression [22,40,44], which could explain the abnormalities in megakaryocyte and platelet numbers in patients. Within the spectrum of GATA-1 red cell disorders, the DBA-like phenotype is associated with variants at exon 2 boundaries, resulting in a favored production of the short isoform at the expense of the FL GATA-1 protein. Since it was demonstrated that GATA1 translation is impaired downstream of the RP haploinsufficiency in DBA, this provides an additional explanation for the selective erythroid defect in the case of RP haploinsufficiency, and the clinical characteristics of DBA patients with GATA-1 defects [20,21]. However, it could also point out the distinct phenotypic features, illustrated by an exclusively hematological phenotype in GATA-1 DBA-like patients, compared with a multiorgan disease, encompassing congenital malformations and an increased risk to also develop solid tumors (in addition to hematological malignancies) in the case of DBA with genetic defects in genes encoding RP. While the number of patients studied is too small to make a conclusive statement, solid tumors have not been diagnosed in all GATA-1 DBA-like patients so far. In one patient, MDS evolution has been identified without ruling out an accidental event not related to the GATA1 gene variant. Importantly, some RP genes (e.g., RPL5, RPL11 and RPS20) are known to be tumor-suppressor genes, which is not the case for GATA1. Theoretically, this could play a role in the absence of malignancies, in particular solid tumors in GATA-1 DBA-like patients [45,46,47].
Our retrospective, comprehensive characterization illustrates that the vast majority of patients do not meet the widely used diagnostic criteria for classical DBA, illustrated by the absence of reticulocytopenia in ten analyzed patients, neutropenia in seven (39%) patients and no typical bone marrow morphological findings [3]. Although leukocyte abnormalities are not rare in DBA related to RP defects, these predominantly encompass lymphocytes and lymphocyte subsets (NK-cells, T-, and B-lymphocytes), whereas neutropenia in DBA is associated with RPL35a defects [48,49]. In addition, no congenital malformations were described, and increased HbF is arguably not specific for DBA (minor criteria), yet it largely increased in GATA-1 DBA-like patients. Since eADA was only analyzed in six out of eighteen patients, this cannot be compared with DBA patients with RP defects, in which eADA is elevated in the majority (>80%) of patients [50]. Of special interest in GATA-1 DBA-like patients are their bone marrow cytomorphology findings. Contrary to the classical hypoplastic anemia reported in DBA patients with RP defects, dysplastic features and dyserythropoiesis are reported in 60% and 47% of patients with GATA-1 defects, respectively. Dyserythropoiesis, as well as dysmegakaryopoiesis, was also present in the Dutch patient we reported on. In addition, a blinded revision of previously reported, typical DBA findings in the Swedish GATA-1 patient also showed clear dysplastic erythropoiesis in addition to dysmegakaryopoiesis (including micro- and fragmented megakaryocytes), a finding that was previously reported in the Brazilian family by Hollanda et al [32]. These findings suggest that the bone marrow characteristics in GATA-1 DBA-like patients are distinct from the typical bone marrow findings in DBA with RP gene variants, characterized by a quantitative intrinsic erythroid defect or erythroblastopenia (absence or less than 5% of erythroblasts in otherwise normocellular bone marrow with no signs of dysplasia).
In conclusion, our observations suggest that DBA caused by GATA1 defects is characterized by distinct phenotypic characteristics, including dyserythropoiesis, abnormal megakaryopoiesis and neutropenia, and therefore represents a distinct phenotype within the DBA disease spectrum, which might require specific clinical management.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes13030447/s1, Supplementary Information S1: In silico predictions and GATA1 variant electropherogram.
Author Contributions
B.v.D. performed the study and the wrote manuscript; S.K.K., N.D., R.L., E.N., W.v.S. and R.v.W. reviewed the final version of the manuscript, R.L. and O.F. performed the morphology and reviewed the final version of the manuscript, L.D.C. wrote the manuscript, M.B. performed and supervised the study and the wrote manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
European Union’s Horizon 2020 research and innovation program under the EJP RD COFUND-EJP No 825575 (L.D.C. and M.B.), ANR EJPRD/ANR-19-RAR4-0016 and Laboratory of Excellence for Red Cells [(LABEX GR-Ex)-ANR Avenir-11-LABX-0005-02 (L.D.C.).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki. Ethical review and approval were waived for this study in accordance with institutional standards.
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Acknowledgments
Swedish Research Council (2020-01947 to ND). L.D.C. and M.B. performed this study on behalf of the RiboEurope Research consortium.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Diamond, L.; Blackfan, K. Hypoplastic anemia. Am. J. Dis. Child 1938, 56, 464–467. [Google Scholar]
- Lipton, J.M.; Ellis, S.R. Diamond-Blackfan Anemia: Diagnosis, Treatment, and Molecular Pathogenesis. Hematol. Clin. N. Am. 2009, 23, 261–282. [Google Scholar] [CrossRef] [PubMed]
- Vlachos, A.; Ball, S.; Dahl, N.; Alter, B.P.; Sheth, S.; Ramenghi, U.; Meerpohl, J.; Karlsson, S.; Liu, J.M.; Leblanc, T.; et al. Diagnosing and treating Diamond Blackfan anaemia: Results of an international clinical consensus conference. Br. J. Haematol. 2008, 142, 859–876. [Google Scholar] [CrossRef] [PubMed]
- Draptchinskaia, N.; Gustavsson, P.; Andersson, B.; Pettersson, M.; Willig, T.-N.; Dianzani, I.; Ball, S.; Tchernia, G.; Klar, J.; Matsson, H.; et al. The gene encoding ribosomal protein S19 is mutated in Diamond-Blackfan anaemia. Nat. Genet. 1999, 21, 169–175. [Google Scholar] [CrossRef]
- Ulirsch, J.C.; Verboon, J.M.; Kazerounian, S.; Guo, M.H.; Yuan, D.; Ludwig, L.S.; Handsaker, R.E.; Abdulhay, N.J.; Fiorini, C.; Genovese, G.; et al. The Genetic Landscape of Diamond-Blackfan Anemia. Am. J. Hum. Genet. 2018, 103, 930–947. [Google Scholar] [CrossRef]
- Lezzerini, M.; Penzo, M.; O’Donohue, M.-F.; Vieira, C.M.D.S.; Saby, M.; Elfrink, H.L.; Diets, I.J.; Hesse, A.-M.; Couté, Y.; Gastou, M.; et al. Ribosomal protein gene RPL9 variants can differentially impair ribosome function and cellular metabolism. Nucleic Acids Res. 2019, 48, 770–787. [Google Scholar] [CrossRef]
- Choesmel, V.; Bacqueville, D.; Rouquette, J.; Noaillac-Depeyre, J.; Fribourg, S.; Crétien, A.; Leblanc, T.; Tchernia, G.; Da Costa, L.; Gleizes, P.-E. Impaired ribosome biogenesis in Diamond-Blackfan anemia. Blood 2007, 109, 1275–1283. [Google Scholar] [CrossRef]
- Fumagalli, S.; Thomas, G. The Role of p53 in Ribosomopathies. Semin. Hematol. 2011, 48, 97–105. [Google Scholar] [CrossRef]
- Dutt, S.; Narla, A.; Lin, K.; Mullally, A.; Abayasekara, N.; Megerdichian, C.; Wilson, F.H.; Currie, T.; Khanna-Gupta, A.; Berliner, N.; et al. Haploinsufficiency for ribosomal protein genes causes selective activation of p53 in human erythroid progenitor cells. Blood 2011, 117, 2567–2576. [Google Scholar] [CrossRef]
- Horos, R.; Von Lindern, M. Molecular mechanisms of pathology and treatment in Diamond Blackfan Anaemia. Br. J. Haematol. 2012, 159, 514–527. [Google Scholar] [CrossRef]
- Yang, Z.; Keel, S.B.; Shimamura, A.; Liu, L.; Gerds, A.T.; Li, H.Y.; Wood, B.L.; Scott, B.L.; Abkowitz, J.L. Delayed globin synthesis leads to excess heme and the macrocytic anemia of Diamond Blackfan anemia and del(5q) myelodysplastic syndrome. Sci. Transl. Med. 2016, 8, 338ra67. [Google Scholar] [CrossRef] [PubMed]
- Moniz, H.; DBA Group of Société d’Hématologie et d’Immunologie Pédiatrique (SHIP); Gastou, M.; Leblanc, T.; Hurtaud, C.; Crétien, A.; Lécluse, Y.; Raslova, H.; Larghero, J.; Croisille, L.; et al. Primary hematopoietic cells from DBA patients with mutations in RPL11 and RPS19 genes exhibit distinct erythroid phenotype in vitro. Cell Death Dis. 2012, 3, e356. [Google Scholar] [CrossRef] [PubMed]
- Rio, S.; Gastou, M.; Karboul, N.; Derman, R.; Suriyun, T.; Manceau, H.; Leblanc, T.; El Benna, J.; Schmitt, C.; Azouzi, S.; et al. Regulation of globin-heme balance in Diamond-Blackfan anemia by HSP70/GATA1. Blood 2019, 133, 1358–1370. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.; Yan, X.; Lausted, C.; Munday, A.D.; Yang, Z.; Yi, D.; Jabbari, N.; Liu, L.; Keel, S.B.; Tian, Q.; et al. Single-cell analyses demonstrate that a heme–GATA1 feedback loop regulates red cell differentiation. Blood 2019, 133, 457–469. [Google Scholar] [CrossRef]
- Le Goff, S.; Boussaid, I.; Floquet, C.; Raimbault, A.; Hatin, I.; Andrieu-Soler, C.; Salma, M.; LeDuc, M.; Gautier, E.-F.; Guyot, B.; et al. p53 activation during ribosome biogenesis regulates normal erythroid differentiation. Blood 2020, 137, 89–102. [Google Scholar] [CrossRef]
- Sankaran, V.G.; Ghazvinian, R.; Do, R.; Thiru, P.; Vergilio, J.-A.; Beggs, A.; Sieff, C.A.; Orkin, S.H.; Nathan, D.G.; Lander, E.S.; et al. Exome sequencing identifies GATA1 mutations resulting in Diamond-Blackfan anemia. J. Clin. Investig. 2012, 122, 2439–2443. [Google Scholar] [CrossRef]
- Klar, J.; Khalfallah, A.; Arzoo, P.S.; Gazda, H.T.; Dahl, N. RecurrentGATA1mutations in Diamond-Blackfan anaemia. Br. J. Haematol. 2014, 166, 949–951. [Google Scholar] [CrossRef]
- Parrella, S.; Aspesi, A.; Quarello, P.; Garelli, E.; Pavesi, E.; Carando, A.; Nardi, M.; Ellis, S.R.; Ramenghi, U.; Dianzani, I. Loss of GATA-1 full length as a cause of Diamond-Blackfan anemia phenotype. Pediatr. Blood Cancer 2014, 61, 1319–1321. [Google Scholar] [CrossRef]
- Ludwig, L.S.; Gazda, H.T.; Eng, J.C.; Eichhorn, S.W.; Thiru, P.; Ghazvinian, R.; George, T.; Gotlib, J.R.; Beggs, A.H.; Sieff, C.A.; et al. Altered translation of GATA1 in Diamond-Blackfan anemia. Nat. Med. 2014, 20, 748–753. [Google Scholar] [CrossRef]
- Khajuria, R.K.; Munschauer, M.; Ulirsch, J.; Fiorini, C.; Ludwig, L.S.; McFarland, S.K.; Abdulhay, N.J.; Specht, H.; Keshishian, H.; Mani, D.R.; et al. Ribosome Levels Selectively Regulate Translation and Lineage Commitment in Human Hematopoiesis. Cell 2018, 173, 90–103.e19. [Google Scholar] [CrossRef]
- Boussaid, I.; Le Goff, S.; Floquet, C.; Gautier, E.-F.; Raimbault, A.; Viailly, P.-J.; Al Dulaimi, D.; Burroni, B.; Dusanter-Fourt, I.; Hatin, I.; et al. Integrated analyses of translatome and proteome identify the rules of translation selectivity in RPS14-deficient cells. Haematologica 2021, 106, 746–758. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, L.; Caballero, N.; Fernández-Calleja, L.; Karkoulia, E.; Strouboulis, J. Regulation of GATA1 levels in erythropoiesis. IUBMB Life 2019, 72, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Byrska-Bishop, M.; VanDorn, D.; Campbell, A.E.; Betensky, M.; Arca, P.R.; Yao, Y.; Gadue, P.; Costa, F.F.; Nemiroff, R.L.; Blobel, G.A.; et al. Pluripotent stem cells reveal erythroid-specific activities of the GATA1 N-terminus. J. Clin. Investig. 2015, 125, 993–1005. [Google Scholar] [CrossRef] [PubMed]
- Chlon, T.M.; McNulty, M.; Goldenson, B.; Rosinski, A.; Crispino, J.D. Global transcriptome and chromatin occupancy analysis reveal the short isoform of GATA1 is deficient for erythroid specification and gene expression. Haematologica 2015, 100, 575–584. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nichols, K.E.; Crispino, J.; Poncz, M.; White, J.G.; Orkin, S.H.; Maris, J.M.; Weiss, M. Familial dyserythropoietic anaemia and thrombocytopenia due to an inherited mutation in GATA1. Nat. Genet. 2000, 24, 266–270. [Google Scholar] [CrossRef]
- Russo, R.; Andolfo, I.; Gambale, A.; De Rosa, G.; Manna, F.; Arillo, A.; Wandroo, F.; Bisconte, M.G.; Iolascon, A. GATA1 erythroid-specific regulation of SEC23B expression and its implication in the pathogenesis of congenital dyserythropoietic anemia type II. Haematologica 2017, 102, e371–e374. [Google Scholar] [CrossRef]
- Bouchghoul, H.; Quelin, C.; Loget, P.; Encha-Razavi, F.; Senat, M.-V.; Maheut, L.; Galimand, J.; Collardeau-Frachon, S.; Da Costa, L.; Martinovic, J. Fetal cerebral hemorrhage due to X-linkedGATA1gene mutation. Prenat. Diagn. 2018, 38, 772–778. [Google Scholar] [CrossRef]
- Mehaffey, M.G.; Newton, A.L.; Gandhi, M.J.; Crossley, M.; Drachman, J.G. X-linked thrombocytopenia caused by a novel mutation ofGATA-1. Blood 2001, 98, 2681–2688. [Google Scholar] [CrossRef]
- Freson, K.; Wijgaerts, A.; Van Geet, C. GATA1 gene variants associated with thrombocytopenia and anemia. Platelets 2017, 28, 731–734. [Google Scholar] [CrossRef]
- Freson, K.; Matthijs, G.; Thys, C.; Mariën, P.; Hoylaerts, M.F.; Vermylen, J.; Van Geet, C. Different substitutions at residue D218 of the X-linked transcription factor GATA1 lead to altered clinical severity of macrothrombocytopenia and anemia and are associated with variable skewed X inactivation. Hum. Mol. Genet. 2002, 11, 147–152. [Google Scholar] [CrossRef][Green Version]
- Yu, C.; Niakan, K.; Matsushita, M.; Stamatoyannopoulos, G.; Orkin, S.H.; Raskind, W.H. X-linked thrombocytopenia with thalassemia from a mutation in the amino finger of GATA-1 affecting DNA binding rather than FOG-1 interaction. Blood 2002, 100, 2040–2045. [Google Scholar] [CrossRef]
- Hollanda, L.M.; Lima, C.S.P.; Cunha, A.F.; Albuquerque, D.M.; Vassallo, J.; Ozelo, M.C.; Joazeiro, P.P.; Saad, S.T.O.; Costa, F.F. An inherited mutation leading to production of only the short isoform of GATA-1 is associated with impaired erythropoiesis. Nat. Genet. 2006, 38, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.D.; Steensma, D.P.; Pulsipher, M.A.; Spangrude, G.J.; Kushner, J.P. Congenital erythropoietic porphyria due to a mutation in GATA1: The first trans-acting mutation causative for a human porphyria. Blood 2007, 109, 2618–2621. [Google Scholar] [CrossRef] [PubMed]
- Mundschau, G.; Gurbuxani, S.; Gamis, A.S.; Greene, M.E.; Arceci, R.J.; Crispino, J.D. Mutagenesis of GATA1 is an initiating event in Down syndrome leukemogenesis. Blood 2003, 101, 4298–4300. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, J.; Greene, M.E.; McDevitt, M.A.; Anastasi, J.; Karp, J.E.; Le Beau, M.M.; Crispino, J. Acquired mutations in GATA1 in the megakaryoblastic leukemia of Down syndrome. Nat. Genet. 2002, 32, 148–152. [Google Scholar] [CrossRef]
- Yoshida, K.; Toki, T.; Okuno, Y.; Kanezaki, R.; Shiraishi, Y.; Sato-Otsubo, A.; Sanada, M.; Park, M.-J.; Terui, K.; Suzuki, H.; et al. The landscape of somatic mutations in Down syndrome–related myeloid disorders. Nat. Genet. 2013, 45, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Greene, M.E.; Mundschau, G.; Wechsler, J.; McDevitt, M.; Gamis, A.; Karp, J.; Gurbuxani, S.; Arceci, R.; Crispino, J.D. Mutations in GATA1 in both transient myeloproliferative disorder and acute megakaryoblastic leukemia of Down syndrome. Blood Cells Mol. Dis. 2003, 31, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, L.M.; Leblanc, T.M.; Mohandas, N. Diamond-Blackfan anemia. Blood 2020, 136, 1262–1273. [Google Scholar] [CrossRef]
- Barcellini, W.; Fattizzo, B. Clinical Applications of Hemolytic Markers in the Differential Diagnosis and Management of Hemolytic Anemia. Dis. Mark. 2015, 2015, 635670. [Google Scholar] [CrossRef]
- Ling, T.; Crispino, J.D. GATA1 mutations in red cell disorders. IUBMB Life 2019, 72, 106–118. [Google Scholar] [CrossRef]
- Crispino, J.D.; Horwitz, M.S. GATA factor mutations in hematologic disease. Blood 2017, 129, 2103–2110. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, L.; Tsukamoto, S.; Suzuki, M.; Yamamoto-Mukai, H.; Yamamoto, M.; Philipsen, S.; Ohneda, K. Ablation of Gata in adult mice results in aplastic crisis, revealing its essential role in steady-state and stress erythropoiesis. Blood 2008, 111, 4375–4385. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Onodera, K.; Motohashi, H.; Suwabe, N.; Hayashi, N.; Yanai, N.; Nabesima, Y.; Yamamoto, M. Arrest in primitive erythroid cell development caused by promoter-specific disruption of the GATA-1 gene. J. Biol. Chem. 1997, 272, 12611–12615. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.T.; Khandros, E.; Bailey, L.C.; Nichols, K.E.; Vakoc, C.R.; Yao, Y.; Huang, Z.; Crispino, J.D.; Hardison, R.C.; Blobel, G.A.; et al. Graded repression of PU.1/Sfpi1 gene transcription by GATA factors regulates hematopoietic cell fate. Blood 2009, 114, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, T.T.; O’Donohue, M.-F.; Wu, Y.; Lohi, H.; Scherer, S.; Paterson, A.D.; Ellonen, P.; Abdel-Rahman, W.M.; Valo, S.; Mecklin, J.-P.; et al. Germline Mutation of RPS20, Encoding a Ribosomal Protein, Causes Predisposition to Hereditary Nonpolyposis Colorectal Carcinoma without DNA Mismatch Repair Deficiency. Gastroenterology 2014, 147, 595–598.e5. [Google Scholar] [CrossRef]
- Sulima, S.O.; Hofman, I.J.F.; De Keersmaecker, K.; Dinman, J.D. How Ribosomes Translate Cancer. Cancer Discov. 2017, 7, 1069–1087. [Google Scholar] [CrossRef]
- Fancello, L.; Kampen, K.R.; Hofman, I.J.; Verbeeck, J.; De Keersmaecker, K. The ribosomal protein gene RPL5 is a haploinsufficient tumor suppressor in multiple cancer types. Oncotarget 2017, 8, 14462–14478. [Google Scholar] [CrossRef]
- Iskander, D.; Roberts, I.; Rees, C.; Szydlo, R.; Alikian, M.; Neale, M.; Harrington, Y.; Kelleher, P.; Karadimitris, A.; De La Fuente, J. Impaired cellular and humoral immunity is a feature of Diamond-Blackfan anaemia; experience of 107 unselected cases in the United Kingdom. Br. J. Haematol. 2019, 186, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Gianferante, D.M.; Wlodarski, M.W.; Atsidaftos, E.; Da Costa, L.; Delaporta, P.; Farrar, J.E.; Goldman, F.D.; Hussain, M.; Kattamis, A.; Leblanc, T.; et al. Genotype-phenotype association and variant characterization in Diamond-Blackfan anemia caused by pathogenic variants in RPL35A. Haematologica 2020, 106, 1303–1310. [Google Scholar] [CrossRef]
- Fargo, J.H.; Kratz, C.P.; Giri, N.; Savage, S.A.; Wong, C.; Backer, K.; Alter, B.P.; Glader, B. Erythrocyte adenosine deaminase: Diagnostic value for Diamond-Blackfan anaemia. Br. J. Haematol. 2013, 160, 547–554. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).