Phenotyping Mediterranean Durum Wheat Landraces for Resistance to Zymoseptoria tritici in Tunisia
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Experimental Design and Inoculation Method
2.2. Disease Rating
2.3. Statistical Analysis
3. Results
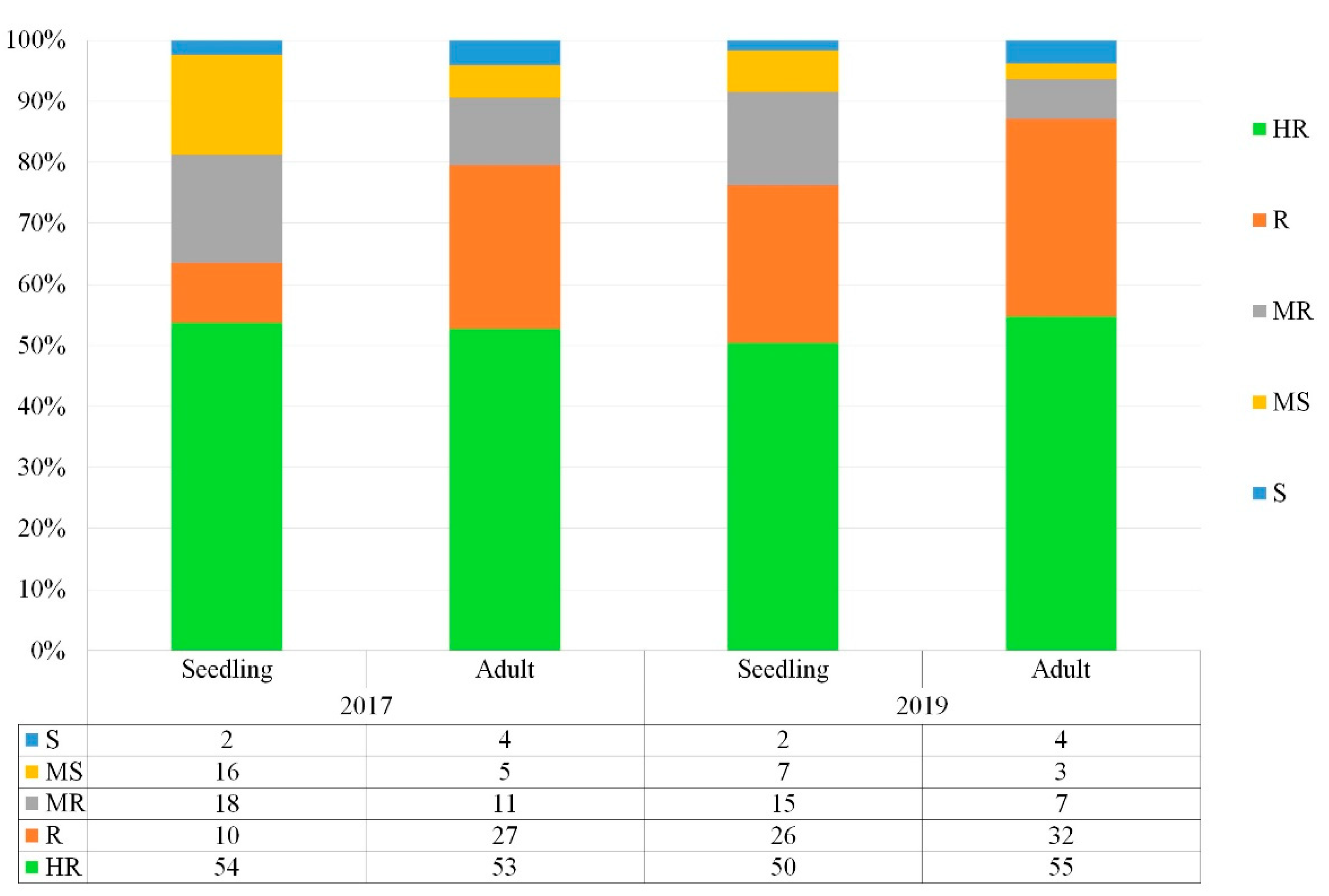
3.1. Disease Response of the USDA Mediterreanean Collection
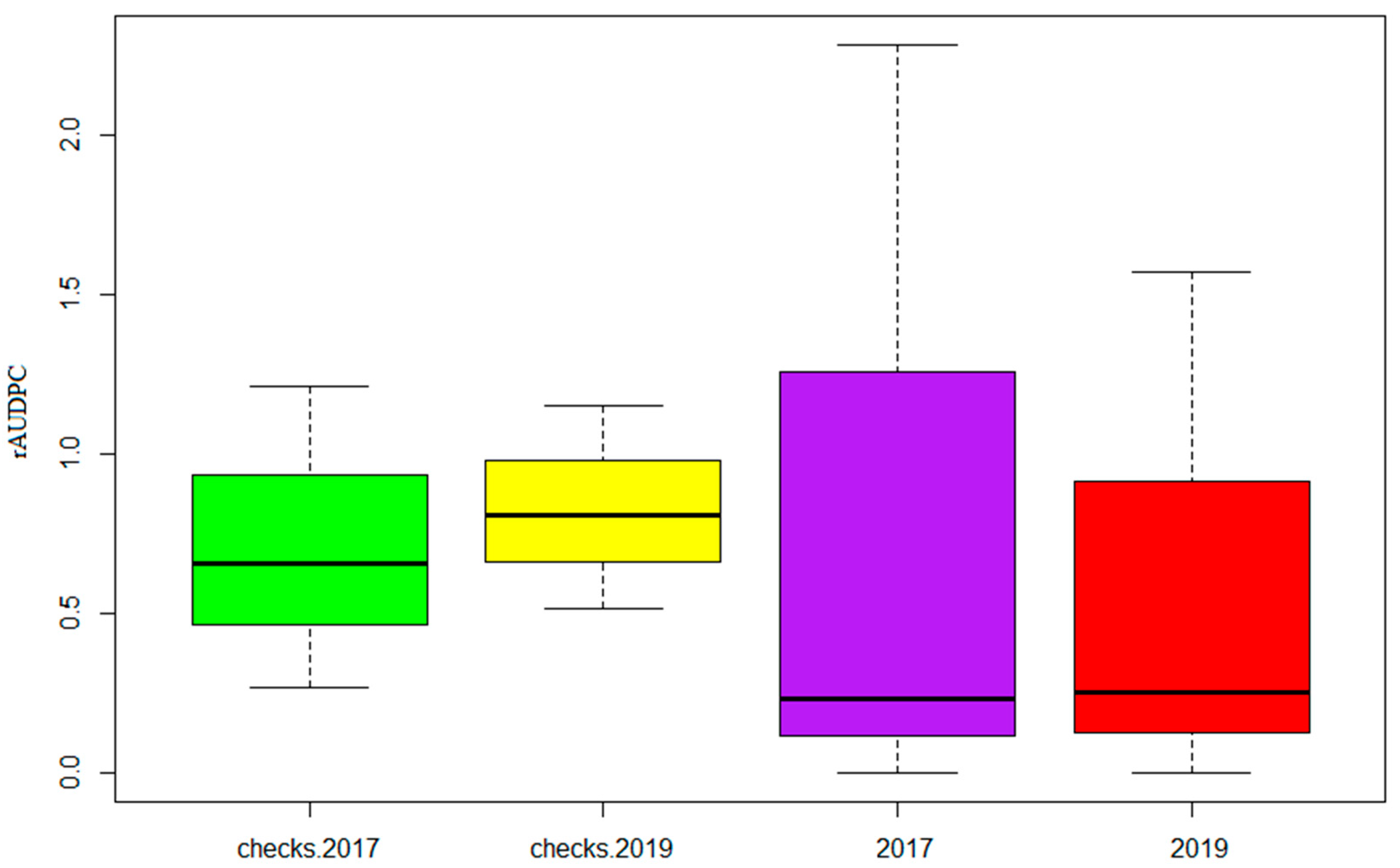
3.2. Reactions of Genotypes across the Two Trials
3.3. Association between Disease Parameters and Plant Height
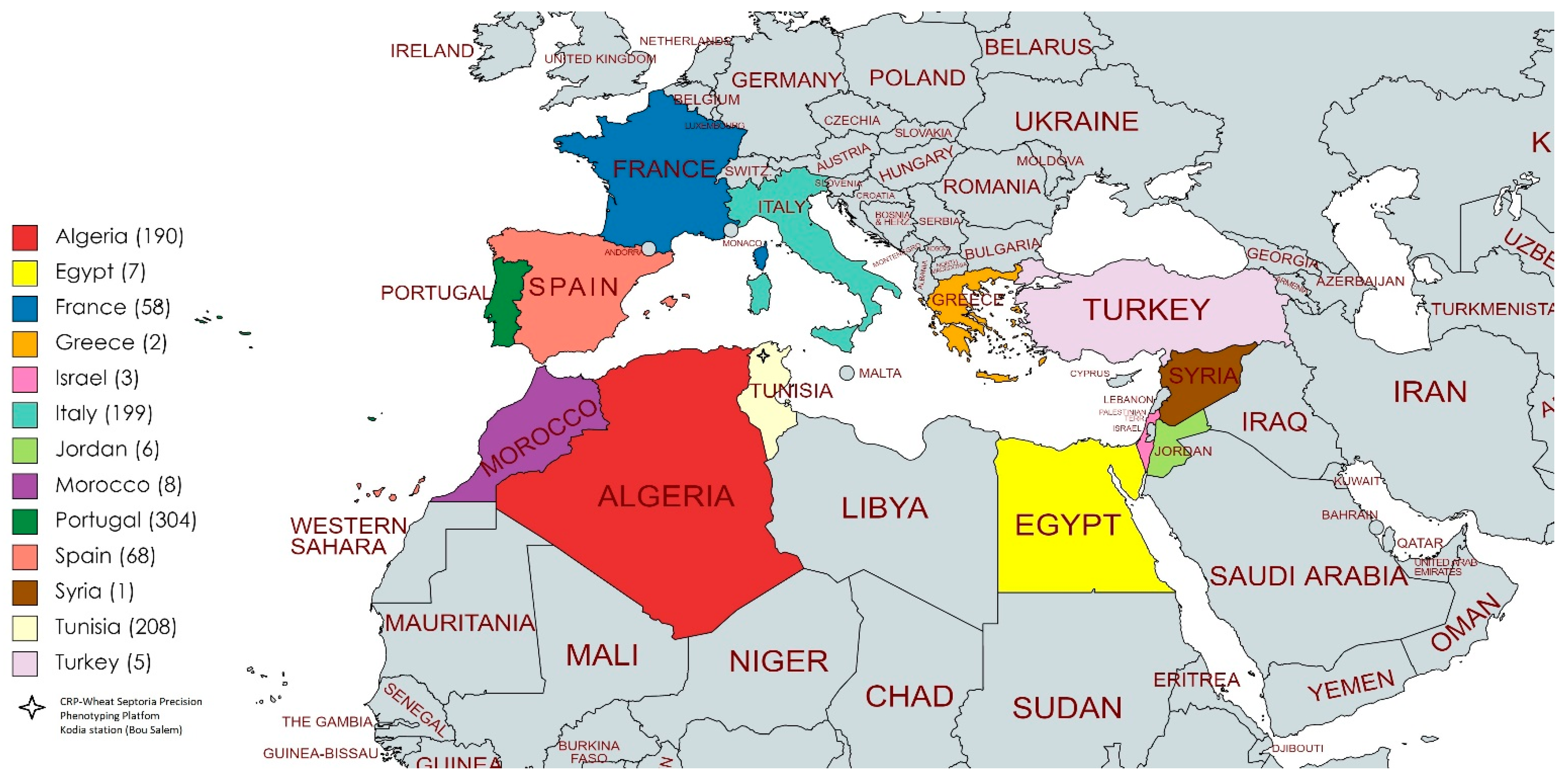
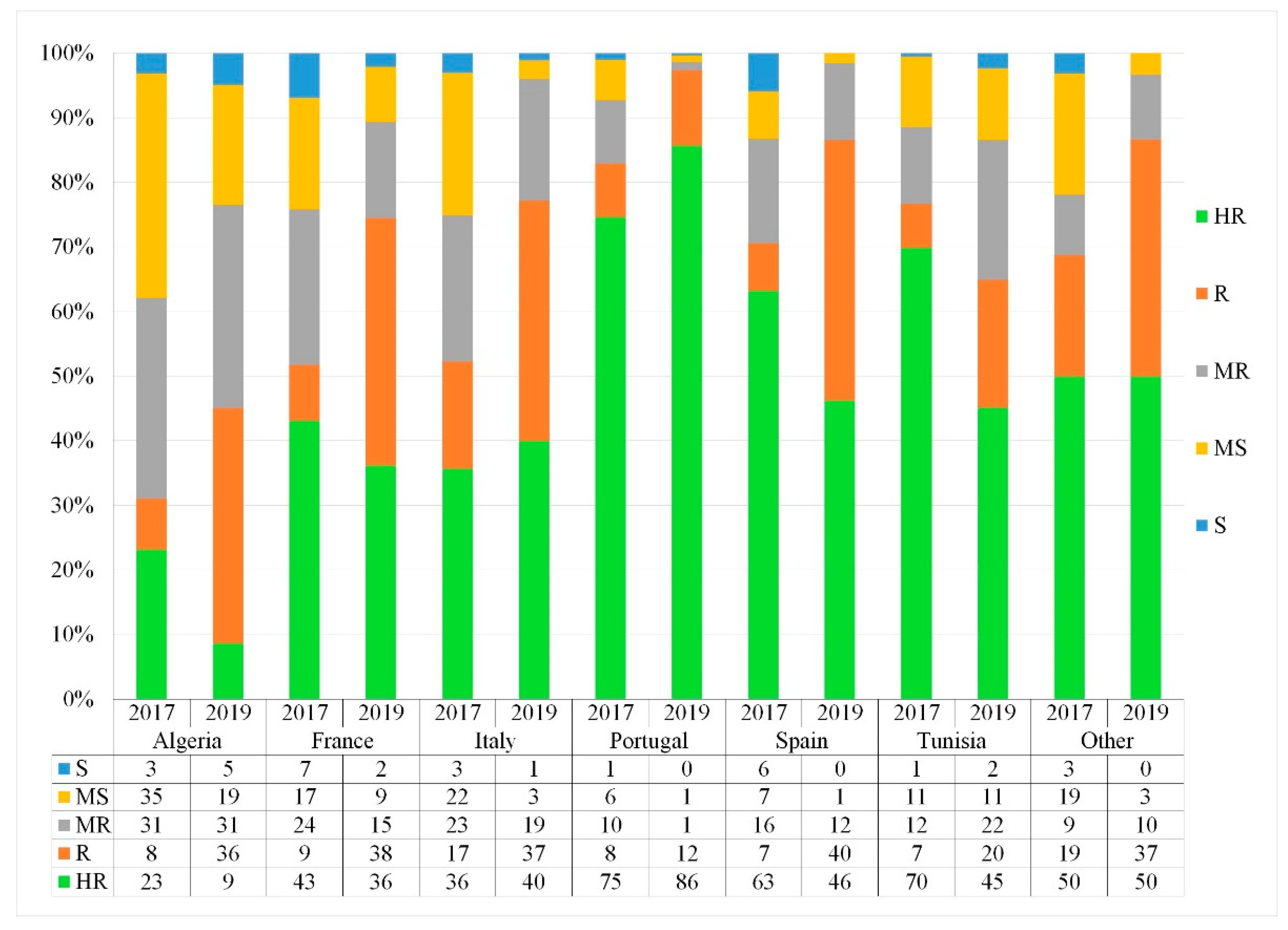
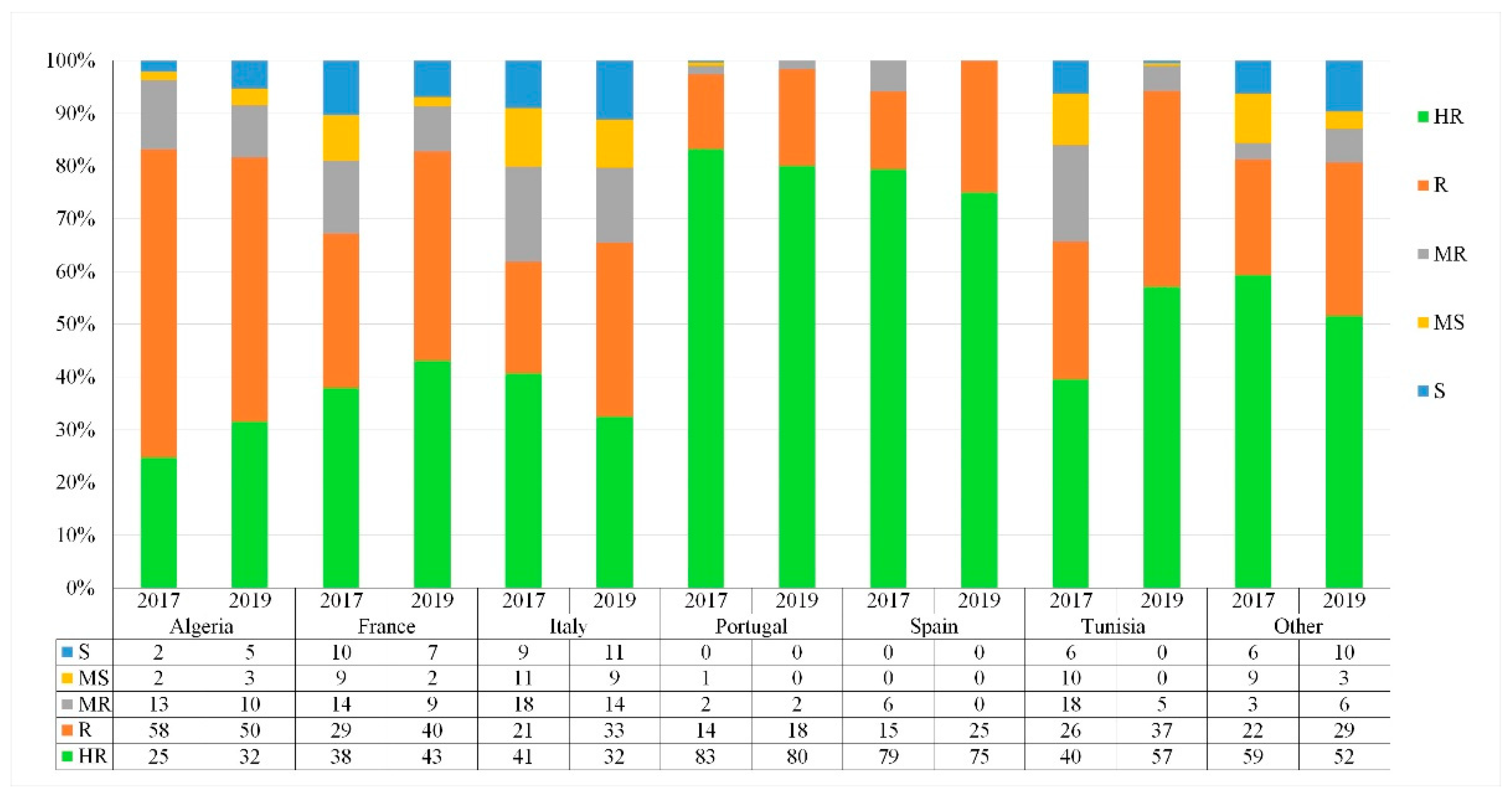
3.4. Geographical Distribution of the Resistant and Susceptible Landraces
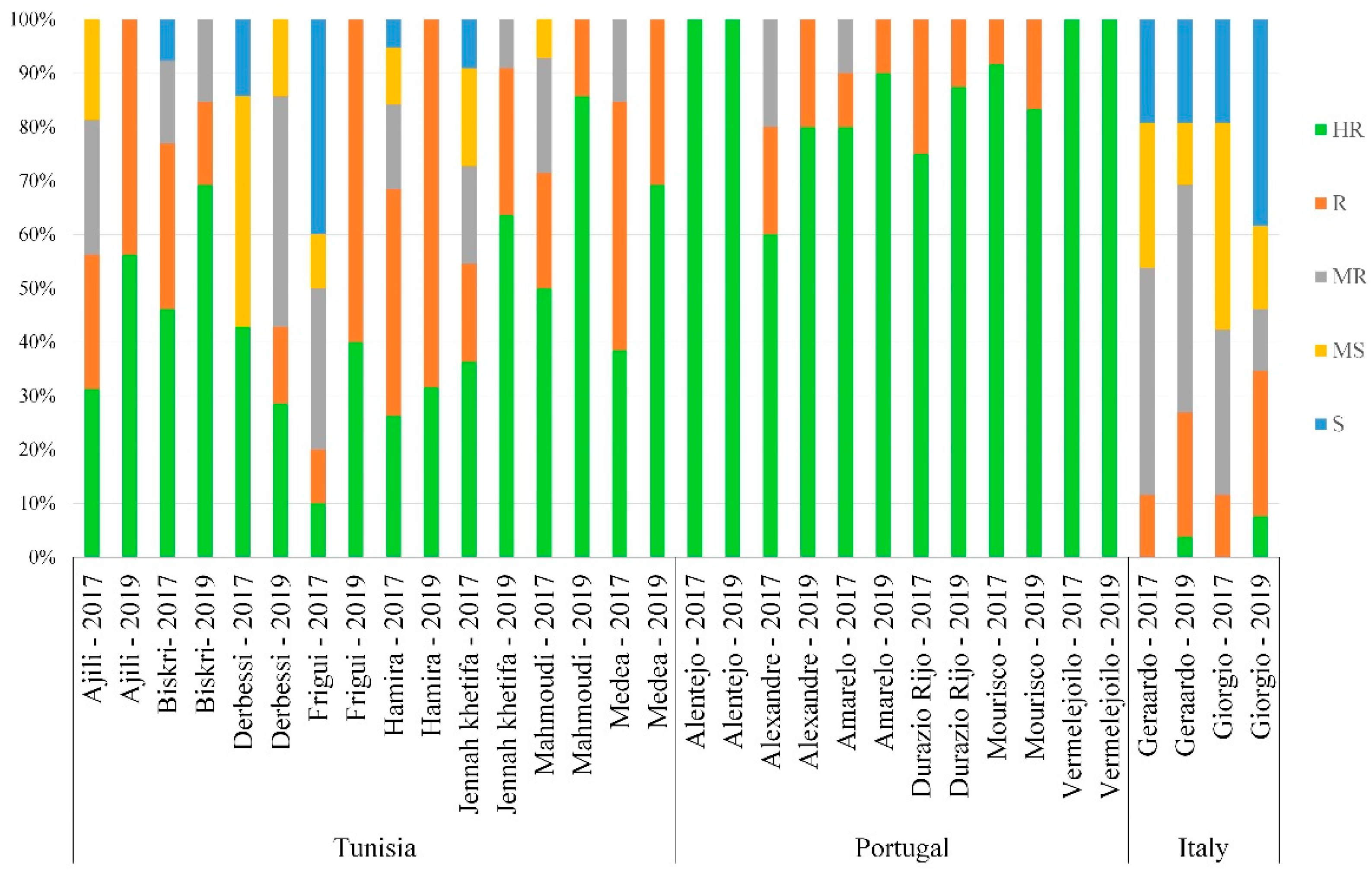
3.5. Distribution of the Reaction Types among and between Populations
4. Discussion
4.1. Mining for Novel Resistance to Septoria Tritici Blotch in the Med-Collection
4.2. Comparison of Seedling and Adult-Plant Resistance
4.3. Diverse Sources of Resistance to STB and the Relationship among Disease-Resistance Traits and Plant Height
4.4. The Effect of Population Origin and Common Name on STB Resistance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mackey, J.M. Wheat: Its Concept, Evolution and Taxonomy. In Durum Wheat Breeding: Current Approaches and Future Strategies; Royo, C., Nachit, M.M., Di Fonzo, N., Araus, J.L., Pfeiffer, W.H., Slafer, G.A., Eds.; Haworth Press: Philadelphia, PA, USA, 2005; pp. 3–61. ISBN 9781482277883. [Google Scholar]
- Feldman, M. Origin of Cultivated Wheat. In The World Wheat Book: A History of Wheat Breeding; Bonjean, A.P., Angus, W.J., Eds.; Lavoisier: Paris, France, 2001; pp. 3–56. [Google Scholar]
- Soriano, J.M.; Villegas, D.; Sorrells, M.E.; Royo, C. Durum Wheat Landraces from East and West Regions of the Mediterranean Basin Are Genetically Distinct for Yield Components and Phenology. Front. Plant Sci. 2018, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Nazco, R.; Villegas, D.; Ammar, K.; Peña, R.J.; Moragues, M.; Royo, C. Can Mediterranean Durum Wheat Landraces Contribute to Improved Grain Quality Attributes in Modern Cultivars? Euphytica 2012, 185, 1–17. [Google Scholar] [CrossRef]
- Faris, J.D. Wheat Domestication: Key to Agricultural Revolutions Past and Future. In Genomics of Plant Genetic Resources; Tuberosa, R., Graner, A., Frison, E., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 439–464. ISBN 978-94-007-7571-8. [Google Scholar]
- Ribeiro-Carvalho, C. High Levels of Genetic Diversity Throughout the Range of the Portuguese Wheat Landrace “Barbela”. Ann. Bot. 2004, 94, 699–705. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aoun, M.; Kolmer, J.A.; Rouse, M.N.; Elias, E.M.; Breiland, M.; Bulbula, W.D.; Chao, S.; Acevedo, M. Mapping of Novel Leaf Rust and Stem Rust Resistance Genes in the Portuguese Durum Wheat Landrace PI 192051. G3 Genes Genomes Genet. 2019, 9, 2535–2547. [Google Scholar] [CrossRef]
- Chacón, E.A.; Vázquez, F.J.; Giraldo, P.; Carrillo, J.M.; Benavente, E.; Rodríguez-Quijano, M. Allelic Variation for Prolamins in Spanish Durum Wheat Landraces and Its Relationship with Quality Traits. Agronomy 2020, 10, 136. [Google Scholar] [CrossRef]
- Brown, A.H.D. Isozymes, Plant Population Genetic Structure and Genetic Conservation. Theor. Appl. Genet. 1978, 52, 145–157. [Google Scholar] [CrossRef]
- Robbana, C.; Kehel, Z.; Ben Naceur, M.; Sansaloni, C.; Bassi, F.; Amri, A. Genome-Wide Genetic Diversity and Population Structure of Tunisian Durum Wheat Landraces Based on DArTseq Technology. Int. J. Mol. Sci. 2019, 20, 1352. [Google Scholar] [CrossRef]
- Soriano, J.M.; Villegas, D.; Aranzana, M.J.; García del Moral, L.F.; Royo, C. Genetic Structure of Modern Durum Wheat Cultivars and Mediterranean Landraces Matches with Their Agronomic Performance. PLoS ONE 2016, 11, e0160983. [Google Scholar] [CrossRef]
- Kabbaj, H.; Sall, A.T.; Al-Abdallat, A.; Geleta, M.; Amri, A.; Filali-Maltouf, A.; Belkadi, B.; Ortiz, R.; Bassi, F.M. Genetic Diversity within a Global Panel of Durum Wheat (Triticum durum) Landraces and Modern Germplasm Reveals the History of Alleles Exchange. Front. Plant Sci. 2017, 8, 1277. [Google Scholar] [CrossRef]
- Kidane, Y.G.; Hailemariam, B.N.; Mengistu, D.K.; Fadda, C.; Pè, M.E.; Dell’Acqua, M. Genome-Wide Association Study of Septoria Tritici Blotch Resistance in Ethiopian Durum Wheat Landraces. Front. Plant Sci. 2017, 8, 1586. [Google Scholar] [CrossRef]
- Marone, D.; Russo, M.A.; Mores, A.; Ficco, D.B.M.; Laidò, G.; Mastrangelo, A.M.; Borrelli, G.M. Importance of Landraces in Cereal Breeding for Stress Tolerance. Plants 2021, 10, 1267. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Newton, A.C. Climate Change, Plant Diseases and Food Security: An Overview: Climate Change and Food Security. Plant Pathol. 2011, 60, 2–14. [Google Scholar] [CrossRef]
- Lopes, M.S.; El-Basyoni, I.; Baenziger, P.S.; Singh, S.; Royo, C.; Ozbek, K.; Aktas, H.; Ozer, E.; Ozdemir, F.; Manickavelu, A.; et al. Exploiting Genetic Diversity from Landraces in Wheat Breeding for Adaptation to Climate Change. J. Exp. Bot. 2015, 66, 3477–3486. [Google Scholar] [CrossRef] [PubMed]
- Nasraoui, B. Maladies Fongiques des Céréales et des Légumineuses; Centre Publication Universitaire: Manouba, Tunisia, 2008; pp. 1–129. [Google Scholar]
- Berraies, S.; Ammar, K.; Salah Gharbi, M.; Yahyaoui, A.; Rezgui, S. Quantitative Inheritance of Resistance to Septoria Tritici Blotch in Durum Wheat in Tunisia. Chil. J. Agric. Res. 2014, 74, 35–40. [Google Scholar] [CrossRef][Green Version]
- Chedli, R.B.H.; M’Barek, S.B.; Yahyaoui, A.; Kehel, Z.; Rezgui, S. Occurrence of Septoria Tritici Blotch (Zymoseptoria tritici) Disease on Durum Wheat, Triticale, and Bread Wheat in Northern Tunisia. Chil. J. Agric. Res. 2018, 78, 559–568. [Google Scholar]
- Saari, E.E.; Prescott, J.M. Scale for Appraising the Foliar Intensity of Wheat Diseases. Plant Dis. Report. 1975, 59, 377–380. [Google Scholar]
- Berraies, S.; Salah Gharbi, M.; Rezgui, S.; Yahyaoui, A. Estimating Grain Yield Losses Caused by Septoria Leaf Blotch on Durum Wheat in Tunisia. Chil. J. Agric. Res. 2014, 74, 432–437. [Google Scholar] [CrossRef]
- McDonald, B.A.; Linde, C. Pathogen Population Genetics, Evolutionary Potential, and Durable Resistance. Annu. Rev. Phytopathol. 2002, 40, 349–379. [Google Scholar] [CrossRef]
- Ben M’Barek, S.; Karisto, P.; Abdedayem, W.; Laribi, M.; Fakhfakh, M.; Kouki, H.; Mikaberidze, A.; Yahyaoui, A. Improved Control of Septoria Tritici Blotch in Durum Wheat Using Cultivar Mixtures. Plant Pathol. 2020, 69, 1655–1665. [Google Scholar] [CrossRef]
- Ammar, K.; Gharbi, M.S.; Deghais, M. Wheat in Tunisia. In The World Wheat Book: A History of Wheat Breeding, 2nd ed.; Bonjean, A.P., Angus, W.J., van Ginkel, M., Eds.; Lavoisier: Paris, France, 2001; pp. 443–463. [Google Scholar]
- Ben Mohamed, L.; Rouaissi, M.; Sebei, A.; Hamza, S.; Harrabi, M. Effet Du Génotype, de La Date de Semis, de La Fertilisation Azotée et Potassique et Des Fongicides Sur Le Développement de Septoria Tritici. Cah. Options Méditerr. 2000, 40, 349–356. [Google Scholar]
- Taher, K.; Graf, S.; Fakhfakh, M.M.; Salah, H.B.H.; Yahyaoui, A.; Rezgui, S.; Nasraoui, B.; Stammler, G. Sensitivity of Zymoseptoria tritici Isolates from Tunisia to Pyraclostrobin, Fluxapyroxad, Epoxiconazole, Metconazole, Prochloraz and Tebuconazole. J. Phytopathol. 2014, 162, 442–448. [Google Scholar] [CrossRef]
- Linde, C.C.; Zhan, J.; McDonald, B.A. Population Structure of Mycosphaerella graminicola: From Lesions to Continents. Phytopathology 2002, 92, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Pettway, R.E.; McDonald, B.A. The Global Genetic Structure of the Wheat Pathogen Mycosphaerella graminicola Is Characterized by High Nuclear Diversity, Low Mitochondrial Diversity, Regular Recombination, and Gene Flow. Fungal Genet. Biol. 2003, 38, 286–297. [Google Scholar] [CrossRef]
- Boukef, S.; McDonald, B.A.; Yahyaoui, A.; Rezgui, S.; Brunner, P.C. Frequency of Mutations Associated with Fungicide Resistance and Population Structure of Mycosphaerella graminicola in Tunisia. Eur. J. Plant Pathol. 2012, 132, 111–122. [Google Scholar] [CrossRef]
- Morais, D.; Duplaix, C.; Sache, I.; Laval, V.; Suffert, F.; Walker, A.-S. Overall Stability in the Genetic Structure of a Zymoseptoria tritici Population from Epidemic to Interepidemic Stages at a Small Spatial Scale. Eur. J. Plant Pathol. 2019, 154, 423–436. [Google Scholar] [CrossRef]
- Hassine, M.; Siah, A.; Hellin, P.; Cadalen, T.; Halama, P.; Hilbert, J.-L.; Hamada, W.; Baraket, M.; Yahyaoui, A.; Legrève, A.; et al. Sexual Reproduction of Zymoseptoria tritici on Durum Wheat in Tunisia Revealed by Presence of Airborne Inoculum, Fruiting Bodies and High Levels of Genetic Diversity. Fungal Biol. 2019, 123, 763–772. [Google Scholar] [CrossRef]
- Tanksley, S.D.; McCouch, S.R. Seed Banks and Molecular Maps: Unlocking Genetic Potential from the Wild. Sci. New Ser. 1997, 277, 1063–1066. [Google Scholar] [CrossRef]
- Feuillet, C.; Langridge, P.; Waugh, R. Cereal Breeding Takes a Walk on the Wild Side. Trends Genet. 2008, 24, 24–32. [Google Scholar] [CrossRef]
- Grandillo, S.; Tanksley, S.D.; Zamir, D. Exploitation of Natural Biodiversity through Genomics. In Genomics-Assisted Crop Improvement; Springer: Dordrecht, The Netherlands, 2007; pp. 121–150. [Google Scholar]
- Ferjaoui, S.; M’Barek, S.B.; Bahri, B.; Slimane, R.B.; Hamza, S. Identification of resistance sources to Septoria tritici blotch in old tunisian durum wheat germplasm applied for the analysis of the Zymoseptoria tritici-durum wheat interaction. J. Plant Pathol. 2015, 1. [Google Scholar] [CrossRef]
- Aouini, L. Durum Wheat and Septoria Tritici Blotch: Genes and Prospects for Breeding; Wageningen University: Wageningen, The Netherlands, 2018. [Google Scholar]
- Ouaja, M.; Aouini, L.; Bahri, B.; Ferjaoui, S.; Medini, M.; Marcel, T.C.; Hamza, S. Identification of Valuable Sources of Resistance to Zymoseptoria Tritici in the Tunisian Durum Wheat Landraces. Eur. J. Plant Pathol. 2020, 156, 647–661. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A Decimal Code for the Growth Stages of Cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Simko, I.; Piepho, H.P. The Area Under the Disease Progress Stairs: Calculation, Advantage, and Application. Phytopathology 2012, 102, 381–389. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 1 November 2021).
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002; ISBN 0-387-95457-0. [Google Scholar]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses; R Package Version 1.0.7; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://CRAN.R-project.org/package=factoextra (accessed on 1 April 2020).
- Maechler, M.; Rousseeuw, P.; Struyf, A.; Hubert, M.; Hornik, K. Cluster: Cluster Analysis Basics and Extensions; R Package Version 2.1.1; Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Kolmer, J.A.; Garvin, D.F.; Hayden, M.; Spielmeyer, W. Adult Plant Leaf Rust Resistance Derived from the Wheat Landrace Cultivar Americano 44d is Conditioned by Interaction of Three QTL. Euphytica 2018, 214, 1–11. [Google Scholar] [CrossRef]
- Gavhane, D.B.; Kulwal, P.L.; Kumbhar, S.D.; Jadhav, A.S.; Sarawate, C.D. Cataloguing of Blast Resistance Genes in Landraces and Breeding Lines of Rice from India. J. Genet. 2019, 98, 106. [Google Scholar] [CrossRef] [PubMed]
- Halder, J.; Zhang, J.; Ali, S.; Sidhu, J.S.; Gill, H.S.; Talukder, S.K.; Kleinjan, J.; Turnipseed, B.; Sehgal, S.K. Mining and Genomic Characterization of Resistance to Tan Spot, Stagonospora Nodorum Blotch (SNB), and Fusarium Head Blight in Watkins Core Collection of Wheat Landraces. BMC Plant Biol. 2019, 19, 480. [Google Scholar] [CrossRef]
- Vikram, P.; Sehgal, D.; Sharma, A.; Bhavani, S.; Gupta, P.; Randhawa, M.; Pardo, N.; Basandra, D.; Srivastava, P.; Singh, S.; et al. Genome-Wide Association Analysis of Mexican Bread Wheat Landraces for Resistance to Yellow and Stem Rust. PLoS ONE 2021, 16, e0246015. [Google Scholar] [CrossRef]
- Royo, C.; Nachit, M.M.; Fonzo, N.D.; Araus, J.L.; Pfeiffer, W.H.; Slafer, G.A. Durum Wheat Breeding: Current Approaches and Future Strategies, 2nd ed.; Royo, C., Miloudi, N., DiFonzo, N., Eds.; Food Products Press; The Haworth Press Inc.: Binghamton, NY, USA, 2005; Volume 1, pp. 1–571. [Google Scholar]
- Kema, G.H.J.; MirzadiGohari, A.; Aouini, L.; Gibriel, H.A.Y.; Ware, S.B.; van den Bosch, F.; Manning-Smith, R.; Alonso-Chavez, V.; Helps, J.; Ben M’Barek, S.; et al. Stress and Sexual Reproduction Affect the Dynamics of the Wheat Pathogen Effector AvrStb6 and Strobilurin Resistance. Nat. Genet. 2018, 50, 375–380. [Google Scholar] [CrossRef]
- Wittenberg, A.H.J.; van der Lee, T.A.J.; Ben M’Barek, S.; Ware, S.B.; Goodwin, S.B.; Kilian, A.; Visser, R.G.F.; Kema, G.H.J.; Schouten, H.J. Meiosis Drives Extraordinary Genome Plasticity in the Haploid Fungal Plant Pathogen Mycosphaerella graminicola. PLoS ONE 2009, 4, e5863. [Google Scholar] [CrossRef]
- Goodwin, S.B.; Ben M’Barek, S.; Dhillon, B.; Wittenberg, A.H.J.; Crane, C.F.; Hane, J.K.; Foster, A.J.; Van der Lee, T.A.J.; Grimwood, J.; Aerts, A.; et al. Finished Genome of the Fungal Wheat Pathogen Mycosphaerella graminicola Reveals Dispensome Structure, Chromosome Plasticity, and Stealth Pathogenesis. PLoS Genet. 2011, 7, e1002070. [Google Scholar] [CrossRef]
- Hartmann, F.E.; McDonald, B.A.; Croll, D. Genome-Wide Evidence for Divergent Selection between Populations of a Major Agricultural Pathogen. Mol. Ecol. 2018, 27, 2725–2741. [Google Scholar] [CrossRef]
- Bari, A.; Street, K.; Mackay, M.; Endresen, D.T.F.; De Pauw, E.; Amri, A. Focused Identification of Germplasm Strategy (FIGS) Detects Wheat Stem Rust Resistance Linked to Environmental Variables. Genet. Resour. Crop Evol. 2012, 59, 1465–1481. [Google Scholar] [CrossRef]
- Maxted, N.; Dulloo, M.E.; Ford-Lloyd, B.V. Enhancing Crop Genepool Use: Capturing Wild Relative and Landrace Diversity for Crop Improvement; CABI: Wallingford, UK, 2016; pp. 1–1049. [Google Scholar]
- Chaabane, R.; Saidi, A.; Bchini, H.; Sassi, M.; Rouissi, M.; Naceur, A.B.; Sayouri, S.; Naceur, M.B.; Masanori, I.; Bari, A.; et al. Identification of Durum Wheat Salt Tolerance Sources in Elite Tunisian Varieties and a Targeted FIGS Subset from ICARDA Gene Bank: Non-Destructive and Easy Way. Am. Acad. Sci. Res. J. Eng. Technol. Sci. 2017, 38, 98–118. [Google Scholar]
- Walters, D.R.; Fountaine, J.M. Practical Application of Induced Resistance to Plant Diseases: An Appraisal of Effectiveness under Field Conditions. J. Agric. Sci. 2009, 147, 523–535. [Google Scholar] [CrossRef]
- Develey-Rivière, M.-P.; Galiana, E. Resistance to Pathogens and Host Developmental Stage: A Multifaceted Relationship within the Plant Kingdom. New Phytol. 2007, 175, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Kuć, J. Induced Immunity to Plant Disease. BioScience 1982, 32, 854–860. [Google Scholar] [CrossRef]
- Hammerschmidt, R. Introduction: Definitions and some history. In Induced Resistance for Plant Defence, 1st ed.; Walters, D., Newton, A., Lyon, G., Eds.; Blackwell Publishing: Oxford, UK, 2007; Volume 3, pp. 154–196. [Google Scholar]
- Goellner, K.; Conrath, U. Priming: It’s All the World to Induced Disease Resistance. Eur. J. Plant Pathol. 2008, 121, 233–242. [Google Scholar] [CrossRef]
- Adhikari, T.B.; Cavaletto, J.R.; Dubcovsky, J.; Gieco, J.O.; Schlatter, A.R.; Goodwin, S.B. Molecular Mapping of the Stb4 Gene for Resistance to Septoria tritici Blotch in Wheat. Phytopathology 2004, 94, 1198–1206. [Google Scholar] [CrossRef]
- Arraiano, L.S.; Worland, A.J.; Ellerbrook, C.; Brown, J.K.M. Chromosomal Location of a Gene for Resistance to Septoria tritici Blotch (Mycosphaerella graminicola) in the Hexaploid Wheat ’Synthetic 6x’. Theor. Appl. Genet. 2001, 103, 758–764. [Google Scholar] [CrossRef]
- Tavella, C.M. Date of Heading and Plant Height of Wheat Varieties, as Related to Septoria Leaf Blotch Damage. Euphytica 1978, 27, 577–580. [Google Scholar] [CrossRef]
- Camacho-Casas, M.A.; Kronstad, W.E.; Scharen, A.L. Septoria tritici Resistance and Associations with Agronomic Traits in a Wheat Cross. Crop Sci. 1995, 35, 971–976. [Google Scholar] [CrossRef]
- Van Beuningen, L.T.; Kohli, M.M. Deviation from the Regression of Infection on Heading and Height as a Measure of Resistance to Septoria tritici Blotch in Wheat. Plant Dis. 1990, 74, 488–493. [Google Scholar] [CrossRef]
- Rosielle, A.A.; Brown, A.G.P. Inheritance, Heritability and Breeding Behaviour of Three Sources of Resistance to Septoria Tritici in Wheat. Euphytica 1979, 28, 385–392. [Google Scholar] [CrossRef]
- Baltazar, B.M.; Scharen, A.L.; Kronstad, W.E. Association between Dwarfing Genes ’Rht ’ and ‘Rht2’ and Resistance to Septoria tritici Blotch in Winter Wheat (Triticum aestivum L. EmThell). Theor. Appl. Genet. 1990, 79, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Arama, P.F.; Parlevliet, J.E.; van Silfhout, C.H. Heading Date and Resistance to Septoria Tritici Blotch in Wheat Not Genetically Associated. Euphytica 1999, 106, 63–68. [Google Scholar] [CrossRef]
- Simón, M.R.; Perelló, A.E.; Cordo, C.A.; Larrán, S.; Putten, P.E.L.; Struik, P.C. Association between Septoria Tritici Blotch, Plant Height, and Heading Date in Wheat. Agron. J. 2005, 97, 1072–1081. [Google Scholar] [CrossRef]
- Arraiano, L.S.; Balaam, N.; Fenwick, P.M.; Chapman, C.; Feuerhelm, D.; Howell, P.; Smith, S.J.; Widdowson, J.P.; Brown, J.K.M. Contributions of Disease Resistance and Escape to the Control of Septoria Tritici Blotch of Wheat. Plant Pathol. 2009, 58, 910–922. [Google Scholar] [CrossRef]
- Chartrain, L.; Berry, S.T.; Brown, J.K.M. Resistance of Wheat Line Kavkaz-K4500 L.6.A.4 to Septoria Tritici Blotch Controlled by Isolate-Specific Resistance Genes. Phytopathology 2005, 95, 664–671. [Google Scholar] [CrossRef]
- Risser, P.; Ebmeyer, E.; Korzun, V.; Hartl, L.; Miedaner, T. Quantitative Trait Loci for Adult-Plant Resistance to Mycosphaerella Graminicola in Two Winter Wheat Populations. Phytopathology 2011, 101, 1209–1216. [Google Scholar] [CrossRef]
- Goudemand, E.; Laurent, V.; Duchalais, L.; Tabib Ghaffary, S.M.; Kema, G.H.J.; Lonnet, P.; Margalé, E.; Robert, O. Association Mapping and Meta-Analysis: Two Complementary Approaches for the Detection of Reliable Septoria Tritici Blotch Quantitative Resistance in Bread Wheat (Triticum aestivum, L.). Mol. Breed. 2013, 32, 563–584. [Google Scholar] [CrossRef]
- McCartney, C.A.; Brûlé-Babel, A.L.; Lamari, L.; Somers, D.J. Chromosomal Location of a Race-Specific Resistance Gene to Mycosphaerella Graminicola in the Spring Wheat ST6. Theor. Appl. Genet. 2003, 107, 1181–1186. [Google Scholar] [CrossRef]
- McDonald, B.A.; Mundt, C.C. How Knowledge of Pathogen Population Biology Informs Management of Septoria tritici Blotch. Phytopathology 2016, 106, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Mundt, C.C. Durable Resistance: A Key to Sustainable Management of Pathogens and Pests. Infect. Genet. Evol. 2014, 27, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Mallick, N.; Chand, S.; Kumari, P.; Sharma, J.B.; Sivasamy, M.; Jayaprakash, P.; Prabhu, K.V.; Jha, S.K. Vinod Marker-Assisted Pyramiding of Thinopyrum-Derived Leaf Rust Resistance Genes Lr19 and Lr24 in Bread Wheat Variety HD2733. J. Genet. 2017, 96, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Herrera-Foessel, S.; Huerta-Espino, J.; Singh, S.; Bhavani, S.; Lan, C.; Basnet, B.R. Progress Towards Genetics and Breeding for Minor Genes Based Resistance to Ug99 and Other Rusts in CIMMYT High-Yielding Spring Wheat. J. Integr. Agric. 2014, 13, 255–261. [Google Scholar] [CrossRef]
- Singh, R.P.; Hodson, D.P.; Huerta-Espino, J.; Jin, Y.; Bhavani, S.; Njau, P.; Herrera-Foessel, S.; Singh, P.K.; Singh, S.; Govindan, V. The Emergence of Ug99 Races of the Stem Rust Fungus Is a Threat to World Wheat Production. Annu. Rev. Phytopathol. 2011, 49, 465–481. [Google Scholar] [CrossRef]
- Singh, R.P.; Hodson, D.P.; Jin, Y.; Lagudah, E.S.; Ayliffe, M.A.; Bhavani, S.; Rouse, M.N.; Pretorius, Z.A.; Szabo, L.J.; Huerta-Espino, J.; et al. Emergence and Spread of New Races of Wheat Stem Rust Fungus: Continued Threat to Food Security and Prospects of Genetic Control. Phytopathology 2015, 105, 872–884. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Singh, P.K.; Rutkoski, J.; Hodson, D.P.; He, X.; Jørgensen, L.N.; Hovmøller, M.S.; Huerta-Espino, J. Disease Impact on Wheat Yield Potential and Prospects of Genetic Control. Annu. Rev. Phytopathol. 2016, 54, 303–322. [Google Scholar] [CrossRef] [PubMed]
- Morgounov, A.; Özdemir, F.; Keser, M.; Akin, B.; Dababat, A.A.; Dreisigacker, S.; Golkari, S.; Koc, E.; Küçükçongar, M.; Muminjanov, H.; et al. Diversity and Adaptation of Currently Grown Wheat Landraces and Modern Germplasm in Afghanistan, Iran, and Turkey. Crops 2021, 1, 54–67. [Google Scholar] [CrossRef]



| Reaction Type at GS (11–20) | Scale 0–5 | Symptom Descriptions |
|---|---|---|
| Seedling HR | 0–1 | Lesion not apparent or very small |
| Seedling R | 2 | Apparent small lesion |
| Seedling MR | 3 | Typical Z. tritici lesions (pycnidia) on leaves 2–3 |
| Seedling MS | 4 | Well-developed lesion up to third and fourth leaves |
| Seedling S | 5 | Clear, susceptible reaction present on all leaves |
| Reaction Types at GS (37–87) | rAUDPC Range | Symptom Descriptions |
|---|---|---|
| Adult HR | <0.2 | No symptoms or small lesions at lower leaves |
| Adult R | 0.2–0.4 | Apparent infection at lower leaves but small lesions |
| Adult MR | 0.4–0.6 | Mid-height infection, and apparent lesions not well developed |
| Adult MS | 0.6–0.8 | Well defined lesions up to flag leaf, and well-developed lesions up to F−1 * |
| Adult S | >0.8 | Well-developed lesions up to flag leaf |
| 2016 | HR | R | MR | MS | S | Missing | TOTAL |
|---|---|---|---|---|---|---|---|
| Subset | 1853 | 800 | 268 | 129 | 97 | 19 | 3166 |
| Algeria | 58 | 119 | 28 | 3 | 6 | 0 | 214 |
| France | 28 | 24 | 10 | 10 | 7 | 0 | 79 |
| Italy | 122 | 62 | 41 | 30 | 20 | 0 | 275 |
| Portugal | 317 | 59 | 10 | 1 | 1 | 1 | 389 |
| Spain | 104 | 18 | 6 | 0 | 0 | 0 | 128 |
| Tunisia | 155 | 111 | 21 | 7 | 5 | 0 | 299 |
| Other | 1069 | 407 | 152 | 78 | 58 | 18 | 1782 |
| Total | 1853 | 800 | 268 | 129 | 97 | 19 | 3166 |
| Physiological Stage | Source of Variation | Sum of Squares | Mean of Squares | F Value | Pr (>F) |
|---|---|---|---|---|---|
| Seedling | Genotype | 3344 | 3.160 | 2.518 | <2 × 10−16 *** |
| Residuals | 1218 | 1.255 | |||
| Year | 1 | 0.635 | 0.282 | 0.595 | |
| Residuals | 4561 | 2.249 | |||
| Genotype × Year | 1236.302 | 0.317 | 0.9238 | 0.9238 | |
| Residuals | 4.000 | 4.00 | |||
| Adult | Year | 0.34 | 0.335 | 6.046 | 0.014 * |
| Residuals | 117.17 | 0.055 | |||
| Genotype | 88.15 | 0.083 | 2.988 | <2 × 10−16 *** | |
| Residuals | 29.36 | 0.027 | |||
| Genotype × Year | 29.164 | 0.027 | 0.524 | 0.832537 | |
| Residuals | 0.529 | 0.529 | |||
| Seedling | 36.1 | 36.17 | 958.6 | <2 × 10−16 *** | |
| Residuals | 76.49 | 0.04 | |||
| PH | 23.45 | 0.868 | 19.48 | <2 × 10−16 *** | |
| Residuals | 89.22 | 0.044 | |||
| Level of improvement | 14.96 | 3.739 | 77.46 | <2 × 10−16 *** | |
| Residuals | 97.71 | 0.048 |
| Seedling 2019 | PH2017 | rAUDPC2019 | |
|---|---|---|---|
| Seedling 2017 | r = 0.431 p < 2.2 × 10−16 *** | - | - |
| PH 2019 | - | r = 0.679 p < 2.2 × 10−16 *** | r = −0.407 p < 2.2 × 10−16 *** |
| rAUDPC 2017 | - | r = −0.264 p < 2.2 × 10−16 *** | r = 0.512 p < 2.2 × 10−16 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben M’Barek, S.; Laribi, M.; Kouki, H.; Castillo, D.; Araar, C.; Nefzaoui, M.; Ammar, K.; Saint-Pierre, C.; Yahyaoui, A.H. Phenotyping Mediterranean Durum Wheat Landraces for Resistance to Zymoseptoria tritici in Tunisia. Genes 2022, 13, 355. https://doi.org/10.3390/genes13020355
Ben M’Barek S, Laribi M, Kouki H, Castillo D, Araar C, Nefzaoui M, Ammar K, Saint-Pierre C, Yahyaoui AH. Phenotyping Mediterranean Durum Wheat Landraces for Resistance to Zymoseptoria tritici in Tunisia. Genes. 2022; 13(2):355. https://doi.org/10.3390/genes13020355
Chicago/Turabian StyleBen M’Barek, Sarrah, Marwa Laribi, Hajer Kouki, Dalma Castillo, Chayma Araar, Meriem Nefzaoui, Karim Ammar, Carolina Saint-Pierre, and Amor Hassine Yahyaoui. 2022. "Phenotyping Mediterranean Durum Wheat Landraces for Resistance to Zymoseptoria tritici in Tunisia" Genes 13, no. 2: 355. https://doi.org/10.3390/genes13020355
APA StyleBen M’Barek, S., Laribi, M., Kouki, H., Castillo, D., Araar, C., Nefzaoui, M., Ammar, K., Saint-Pierre, C., & Yahyaoui, A. H. (2022). Phenotyping Mediterranean Durum Wheat Landraces for Resistance to Zymoseptoria tritici in Tunisia. Genes, 13(2), 355. https://doi.org/10.3390/genes13020355






