ON-1 and BA-IX Are the Dominant Sub-Genotypes of Human Orthopneumovirus A&B in Riyadh, Saudi Arabia
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Detection, Typing, and Sequencing of HOPV
2.3. Sequence Data and Phylogenetic Analysis
3. Results
3.1. Prevalence of HOPV
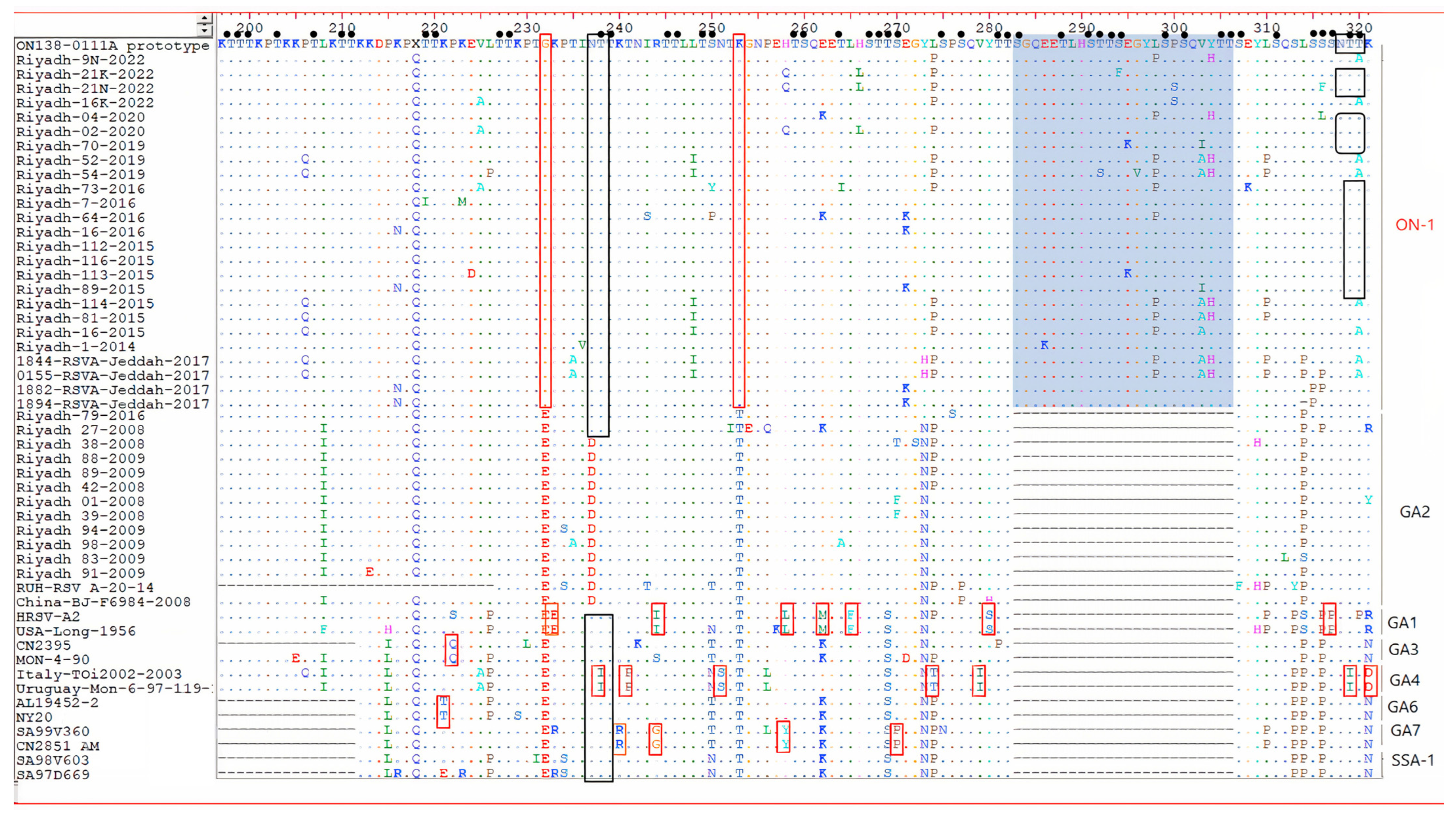
3.2. Genetic Analysis of HOPV Strains
3.3. Amino Acid Sequence Analysis of the G Gene
3.4. N- and O-Glycosylation Site Analysis in Amino Acid Sequence
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Afonso, C.L.; Amarasinghe, G.K.; Bányai, K.; Bào, Y.; Basler, C.F.; Bavari, S.; Bejerman, N.; Blasdell, K.R.; Briand, F.-X.; Briese, T.; et al. Taxonomy of the order Mononegavirales: Update 2016. Arch. Virol. 2016, 161, 2351–2360. [Google Scholar] [CrossRef]
- Mazur, N.I.; Martinón-Torres, F.; Baraldi, E.; Fauroux, B.; Greenough, A.; Heikkinen, T.; Manzoni, P.; Mejias, A.; Nair, H.; Papadopoulos, N.G.; et al. Lower respiratory tract infection caused by respiratory syncytial virus: Current management and new therapeutics. Lancet Respir. Med. 2015, 3, 888–900. [Google Scholar] [CrossRef]
- Mazur, N.I.; Bont, L.; Cohen, A.L.; Cohen, C.; Von Gottberg, A.; Groome, M.J.; Hellferscee, O.; Klipstein-Grobusch, K.; Mekgoe, O.T.; Naby, F.; et al. Severity of Respiratory Syncytial Virus Lower Respiratory Tract Infection with Viral Coinfection in HIV-Uninfected Children. Clin. Infect. Dis. 2017, 64, 443–450. [Google Scholar] [CrossRef]
- Brown, P.M.; Schneeberger, D.L.; Piedimonte, G. Biomarkers of respiratory syncytial virus (RSV) infection: Specific neutrophil and cytokine levels provide increased accuracy in predicting disease severity. Paediatr. Respir. Rev. 2015, 16, 232–240. [Google Scholar] [CrossRef][Green Version]
- Zhong, Q.; Feng, H.; Lü, Q.; Liu, X.; Zhao, Q.; Du, Y.; Zhang, X.-H.; Wang, J.-R. Recurrent wheezing in neonatal pneumonia is associated with combined infection with Respiratory Syncytial Virus and Staphylococcus aureus or Klebsiella pneumoniae. Sci. Rep. 2018, 8, 995. [Google Scholar] [CrossRef]
- Ogunsemowo, O.; Olaleye, D.O.; Odaibo, G.N. Genetic diversity of human respiratory syncytial virus circulating among children in Ibadan, Nigeria. PLoS ONE 2018, 13, e0191494. [Google Scholar] [CrossRef]
- McLellan, J.; Ray, W.C.; Peeples, M.E. Structure and Function of Respiratory Syncytial Virus Surface Glycoproteins. In Challenges and Opportunities for Respiratory Syncytial Virus Vaccines; Anderson, L.J., Graham, B.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 83–104. [Google Scholar]
- Sullender, W.M. Respiratory syncytial virus genetic and antigenic diversity. Clin. Microbiol. Rev. 2000, 13, 1–15. [Google Scholar] [CrossRef]
- Obodai, E.; Odoom, J.K.; Adiku, T.; Goka, B.; Wolff, T.; Biere, B.; Schweiger, B.; Reiche, J. The significance of human respiratory syncytial virus (HRSV) in children from Ghana with acute lower respiratory tract infection: A molecular epidemiological analysis, 2006 and 2013–2014. PLoS ONE 2018, 13, e0203788. [Google Scholar] [CrossRef]
- Esposito, S.; Piralla, A.; Zampiero, A.; Bianchini, S.; Di Pietro, G.; Scala, A.; Pinzani, R.; Fossali, E.; Baldanti, F.; Principi, N. Characteristics and Their Clinical Relevance of Respiratory Syncytial Virus Types and Genotypes Circulating in Northern Italy in Five Consecutive Winter Seasons. PLoS ONE 2015, 10, e0129369. [Google Scholar] [CrossRef]
- Al-Majhdi, F.N.; Al-Jaralla, A.; Elaeed, M.; Latif, A.; Gissmann, L.; Amer, H.M. Prevalence of Respiratory Syncytial Virus Infection in Riyadh During the Winter Season 2007-2008 and Different Risk Factors Impact. Int. J. Virol. 2009, 5, 154–163. [Google Scholar] [CrossRef]
- Almajhdi, F.N.; Farrag, M.A.; Amer, H.M. Genetic diversity in the G protein gene of group A human respiratory syncytial viruses circulating in Riyadh, Saudi Arabia. Arch. Virol. 2014, 159, 73–81. [Google Scholar] [CrossRef]
- Farrag, M.A.; Amer, H.M.; Aziz, I.M.; Alsaleh, A.N.; Almajhdi, F.N. The emergence of subgenotype ON-1 of Human orthopneumovirus type A in Riyadh, Saudi Arabia: A new episode of the virus epidemiological dynamic. J. Med. Virol. 2020, 92, 1133–1140. [Google Scholar] [CrossRef]
- Melero, J.A.; Mas, V.; McLellan, J.S. Structural, antigenic and immunogenic features of respiratory syncytial virus glycoproteins relevant for vaccine development. Vaccine 2017, 35, 461–468. [Google Scholar] [CrossRef]
- Fan, R.; Fan, C.; Zhang, J.; Wen, B.; Lei, Y.; Liu, C.; Chen, L.; Liu, W.; Wang, C.; Qu, X. Respiratory syncytial virus subtype ON1/NA1/BA9 predominates in hospitalized children with lower respiratory tract infections. J. Med. Virol. 2017, 89, 213–221. [Google Scholar] [CrossRef]
- Trento, A.; Galiano, M.; Videla, C.; Carballal, G.; García-Barreno, B.; Melero, J.A.; Palomo, C. Major changes in the G protein of human respiratory syncytial virus isolates introduced by a duplication of 60 nucleotides. J. Gen. Virol. 2003, 84, 3115–3120. [Google Scholar] [CrossRef] [PubMed]
- Eshaghi, A.; Duvvuri, V.R.; Lai, R.; Nadarajah, J.T.; Li, A.; Patel, S.N.; Low, D.E.; Gubbay, J.B. Genetic Variability of Human Respiratory Syncytial Virus A Strains Circulating in Ontario: A Novel Genotype with a 72 Nucleotide G Gene Duplication. PLoS ONE 2012, 7, e32807. [Google Scholar] [CrossRef]
- Choudhary, M.L.; Wadhwa, B.S.; Jadhav, S.M.; Chadha, M.S. Complete Genome Sequences of Two Human Respiratory Syncytial Virus Genotype A Strains from India, RSV-A/NIV1114046/11 and RSV-A/NIV1114073/11. Genome Announc. 2013, 1, e00165-00113. [Google Scholar] [CrossRef]
- Khor, C.-S.; Sam, I.-C.; Hooi, P.-S.; Chan, Y.-F. Displacement of predominant respiratory syncytial virus genotypes in Malaysia between 1989 and 2011. Infect. Genet. Evol. 2013, 14, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-J.; Kim, Y.-J.; Kim, D.-W.; Lee, H.S.; Lee, H.Y.; Kim, K. Complete genome sequence of human respiratory syncytial virus genotype A with a 72-nucleotide duplication in the attachment protein G gene. J. Virol. 2012, 86, 13810–13811. [Google Scholar] [CrossRef]
- Tsukagoshi, H.; Yokoi, H.; Kobayashi, M.; Kushibuchi, I.; Okamoto-Nakagawa, R.; Yoshida, A.; Morita, Y.; Noda, M.; Yamamoto, N.; Sugai, K. Genetic analysis of attachment glycoprotein (G) gene in new genotype ON 1 of human respiratory syncytial virus detected in Japan. Microbiol. Immunol. 2013, 57, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Prifert, C.; Streng, A.; Krempl, C.D.; Liese, J.; Weissbrich, B. Novel Respiratory Syncytial Virus A Genotype, Germany, 2011–2012. Emerg. Infect. Dis. 2013, 19, 1029–1030. [Google Scholar] [CrossRef] [PubMed]
- Hibino, A.; Saito, R.; Taniguchi, K.; Zaraket, H.; Shobugawa, Y.; Matsui, T.; Suzuki, H. Molecular epidemiology of human respiratory syncytial virus among children in Japan during three seasons and hospitalization risk of genotype ON1. PLoS ONE 2018, 13, e0192085. [Google Scholar] [CrossRef] [PubMed]
- Malekshahi, S.S.; Razaghipour, S.; Samieipoor, Y.; Hashemi, F.B.; Manesh, A.A.R.; Izadi, A.; Faghihloo, E.; Ghavami, N.; Mokhtari-Azad, T.; Salimi, V. Molecular characterization of the glycoprotein and fusion protein in human respiratory syncytial virus subgroup A: Emergence of ON-1 genotype in Iran. Infect. Genet. Evol. 2019, 71, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Al-Sharif, H.A.; El-Kafrawy, S.A.; Yousef, J.M.; Kumosani, T.A.; Kamal, M.A.; Khathlan, N.A.; Kaki, R.M.; Alnajjar, A.A.; Azhar, E.I. Dominance of the ON1 Genotype of RSV-A and BA9 Genotype of RSV-B in Respiratory Cases from Jeddah, Saudi Arabia. Genes 2020, 11, 1323. [Google Scholar] [CrossRef] [PubMed]
- Cane, P.A.; Pringle, C.R. Respiratory syncytial virus heterogeneity during an epidemic: Analysis by limited nucleotide sequencing (SH gene) and restriction mapping (N gene). J. Gen. Virol. 1991, 72, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Lam, L.-Y.; Tam, J.S. Typing and subtyping of influenza viruses and respiratory syncytial viruses by multiplex RT-PCR. In International Congress Series; Elsevier: Amsterdam, The Netherlands, 2004; pp. 381–385. [Google Scholar]
- Johnson, P.R.; Spriggs, M.K.; Olmsted, R.A.; Collins, P.L. The G glycoprotein of human respiratory syncytial viruses of subgroups A and B: Extensive sequence divergence between antigenically related proteins. Proc. Natl. Acad. Sci. USA 1987, 84, 5625–5629. [Google Scholar] [CrossRef]
- Ahmed, A.; Parveen, S.; Al-Hassinah, S.M.; Al-Amery, S.F. An overview of respiratory syncytial virus infections in Saudi Arabia. J. Infect. Dev. Ctries. 2018, 12, 929–936. [Google Scholar] [CrossRef]
- Ahmed, A.; Haider, S.H.; Parveen, S.; Arshad, M.; Alsenaidy, H.A.; Baaboud, A.O.; Mobaireek, K.F.; AlSaadi, M.M.; Alsenaidy, A.M.; Sullender, W. Co-Circulation of 72bp Duplication Group A and 60bp Duplication Group B Respiratory Syncytial Virus (RSV) Strains in Riyadh, Saudi Arabia during 2014. PLoS ONE 2016, 11, e0166145. [Google Scholar] [CrossRef]
- Al-Hassinah, S.; Parveen, S.; Somily, A.M.; AlSaadi, M.M.; Alamery, S.F.; Haq, S.H.; Alsenaidy, H.A.; Ahmed, A. Evolutionary analysis of the ON1 genotype of subtype a respiratory syncytial virus in Riyadh during 2008–16. Infect. Genet. Evol. 2020, 79, 104153. [Google Scholar] [CrossRef]
- Tabatabai, J.; Prifert, C.; Pfeil, J.; Grulich-Henn, J.; Schnitzler, P. Novel Respiratory Syncytial Virus (RSV) Genotype ON1 Predominates in Germany during Winter Season 2012–13. PLoS ONE 2014, 9, e109191. [Google Scholar] [CrossRef]
- Calderón, A.; Pozo, F.; Calvo, C.; García-García, M.; González-Esguevillas, M.; Molinero, M.; Casas, I. Genetic variability of respiratory syncytial virus A in hospitalized children in the last five consecutive winter seasons in Central Spain. J. Med. Virol. 2017, 89, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Madi, N.; Chehadeh, W.; Asadzadeh, M.; Al-Turab, M.; Al-Adwani, A. Analysis of genetic variability of respiratory syncytial virus groups A and B in Kuwait. Arch. Virol. 2018, 163, 2405–2413. [Google Scholar] [CrossRef]
- Abou-El-Hassan, H.; Massaad, E.; Soudani, N.; Assaf-Casals, A.; Shaker, R.; Khoury, M.L.; Ghanem, S.; Karam, M.; Andary, R.; Saito, R.; et al. Detection of ON1 and novel genotypes of human respiratory syncytial virus and emergence of palivizumab resistance in Lebanon. PLoS ONE 2019, 14, e0212687. [Google Scholar] [CrossRef]
- Kadji, F.M.N.; Okamoto, M.; Furuse, Y.; Tamaki, R.; Suzuki, A.; Lirio, I.; Dapat, C.; Malasao, R.; Saito, M.; Pedrera-Rico, G.A.G.; et al. Differences in viral load among human respiratory syncytial virus genotypes in hospitalized children with severe acute respiratory infections in the Philippines. Virol. J. 2016, 13, 113. [Google Scholar] [CrossRef][Green Version]
- Oladokun, R.; Muloiwa, R.; Hsiao, N.-Y.; Valley-Omar, Z.; Nuttall, J.; Eley, B. Clinical characterisation and phylogeny of respiratory syncytial virus infection in hospitalised children at Red Cross War Memorial Children’s Hospital, Cape Town. BMC Infect. Dis. 2016, 16, 236. [Google Scholar] [CrossRef] [PubMed]
- Almajhdi, F.N.; Farrag, M.; Amer, H.M. Group B strains of human respiratory syncytial virus in Saudi Arabia: Molecular and phylogenetic analysis. Virus Genes 2014, 48, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.D.; Ochola, R.; Ngama, M.; Okiro, E.A.; Nokes, D.J.; Medley, G.F.; Cane, P.A. Molecular epidemiology of respiratory syncytial virus in Kilifi district, Kenya. J. Med. Virol. 2004, 74, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Kuroiwa, Y.; Nagai, K.; Okita, L.; Yui, I.; Kase, T.; Nakayama, T.; Tsutsumi, H. A phylogenetic study of human respiratory syncytial viruses group A and B strains isolated in two cities in Japan from 1980-2002. J. Med. Virol. 2005, 76, 241–247. [Google Scholar] [CrossRef]
- Zlateva, K.T.; Lemey, P.; Moës, E.; Vandamme, A.-M.; Van Ranst, M. Genetic Variability and Molecular Evolution of the Human Respiratory Syncytial Virus Subgroup B Attachment G Protein. J. Virol. 2005, 79, 9157–9167. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, W.; Shen, K.; Xie, Z.; Sun, L.; Lu, Q.; Liu, C.; Liang, G.; Beeler, J.A.; Anderson, L.J. Genetic variability of group A and B human respiratory syncytial viruses isolated from 3 provinces in China. Arch. Virol. 2007, 152, 1425–1434. [Google Scholar] [CrossRef]
- Botosso, V.F.; Zanotto, P.M.D.A.; Ueda, M.; Arruda, E.; Gilio, A.E.; Vieira, S.; Stewien, K.E.; Peret, T.C.T.; Jamal, L.F.; Pardini, M.I.D.M.C.; et al. Positive Selection Results in Frequent Reversible Amino Acid Replacements in the G Protein Gene of Human Respiratory Syncytial Virus. PLoS Pathog. 2009, 5, e1000254. [Google Scholar] [CrossRef] [PubMed]
- Faghihloo, E.; Salimi, V.; Rezaei, F.; Naseri, M.; Mamishi, S.; Mahmoodi, M.; Mokhtari-Azad, T. Genetic Diversity in the G Protein Gene of Human Respiratory Syncytial Virus among Iranian Children with Acute Respiratory Symptoms. Iran. J. Pediatr. 2011, 21, 58. [Google Scholar] [PubMed]
- Baek, Y.H.; Choi, E.H.; Song, M.-S.; Pascua, P.N.Q.; Kwon, H.-I.; Park, S.-J.; Lee, J.H.; Woo, S.-I.; Ahn, B.-H.; Han, H.-S.; et al. Prevalence and genetic characterization of respiratory syncytial virus (RSV) in hospitalized children in Korea. Arch. Virol. 2012, 157, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Raghuram, S.V.; Khan, W.H.; Deeba, F.; Sullender, W.; Broor, S.; Parveen, S. Retrospective phylogenetic analysis of circulating BA genotype of human respiratory syncytial virus with 60 bp duplication from New Delhi, India during 2007–2010. Virusdisease 2015, 26, 276–281. [Google Scholar] [CrossRef]
- Trento, A.; Casas, I.; Calderón, A.; Garcia-Garcia, M.L.; Calvo, C.; Perez-Breña, P.; Melero, J.A. Ten Years of Global Evolution of the Human Respiratory Syncytial Virus BA Genotype with a 60-Nucleotide Duplication in the G Protein Gene. J. Virol. 2010, 84, 7500–7512. [Google Scholar] [CrossRef]
- Dapat, I.C.; Shobugawa, Y.; Sano, Y.; Saito, R.; Sasaki, A.; Suzuki, Y.; Kumaki, A.; Zaraket, H.; Dapat, C.; Oguma, T.; et al. New Genotypes within Respiratory Syncytial Virus Group B Genotype BA in Niigata, Japan. J. Clin. Microbiol. 2010, 48, 3423–3427. [Google Scholar] [CrossRef]
- Haider, S.H.; Khan, W.H.; Deeba, F.; Ali, S.; Ahmed, A.; Naqvi, I.H.; Dohare, R.; Alsenaidy, H.A.; Alsenaidy, A.M.; Broor, S.; et al. BA9 lineage of respiratory syncytial virus from across the globe and its evolutionary dynamics. PLoS ONE 2018, 13, e0193525. [Google Scholar] [CrossRef]
- Leemans, A.; Boeren, M.; Van der Gucht, W.; Martinet, W.; Caljon, G.; Maes, L.; Cos, P.; Delputte, P. Characterization of the role of N-glycosylation sites in the respiratory syncytial virus fusion protein in virus replication, syncytium formation and antigenicity. Virus Res. 2019, 266, 58–68. [Google Scholar] [CrossRef]


| Virus | Primer Name | Sequence | Amplicon Size (bp) | Ref. | |
|---|---|---|---|---|---|
| For HOPV detection | HOPV | HOPV-U-F | 5′-GGAACAAGTTGTTGAGGTTTATGAATATGC-3′ | 278 | [26] |
| HOPV-U-R | 5′-CTTCTGCTGTCAAGTCTAGTACACTGTAGT-3′ | ||||
| For HOPV typing | HOPV-A | HOPV A-F | 5′-GATGTTACGGTGGGGAGTCT-3′ | 334 | [27] |
| HOPV A-R | 5′-GTACACTGTAGTTAATCACA-3′ | ||||
| HOPV-B | HOPV B-F | 5′-AATGCTAAGATGGGGAGTT-3′ | 183 | ||
| HOPV B-R | 5′-GAAATTG AGTTAATGACAG-3′ | ||||
| For sequencing | HOPV-A | HOPVA-G-F2 | 5′-CAAGATGCAACAAGCCAGATC-3′ | 863 | [12] |
| HOPV-G-R2 | 5′-ACTGCACTGCATGTTGATTG-3′ | ||||
| HOPV-B | HOPVB-G-F2 | 5′-CCTTACTCAAGTCTCACCAGAAAG-3′ | 704 | [28] | |
| HOPV-G-R2 | 5′-CTGTGGATCAGCAACTCCATG-3′ |
| Seasonal and Demographic Details | Number of Samples | Number of Positive Samples (%) | Seasonal HOPV | ||
|---|---|---|---|---|---|
| HOPV-A (%) | HOPV-B (%) | ||||
| Winter season | 2019–2020 | 250 | 40 (16) | 30 (75) | 10(25) |
| 2021–2022 | 50 | 15 (30) | 15 (100) | 0 | |
| Total | 300 | 55 (18.3) | 45 (81.8) | 10(18.2) | |
| Age in years | <1 | 115 | 32 (27.8) | 18 (56.2) | 7(70) |
| 1–2 | 78 | 12 (15.4) | 13 (28.8) | 2(20) | |
| 3–4 | 54 | 9 (16.6) | 7 (15.6) | 1(10) | |
| ≥5 | 53 | 2 (3.8) | 7 (15.6) | - | |
| Gender | Male | 166 | 35 (63.6) | 28 (62.2) | 6(60) |
| Female | 134 | 20 (36.4) | 17 (37.8) | 4(40) | |
| Severity | Mild | 43 | 43 (78.2) | 33 (73.3) | 10(100) |
| Severe | 12 | 12 (21.8) | 12 (26.7) | 0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzayed, R.M.; Aziz, I.M.; Alsaleh, A.N.; Dudin, G.A.; Ahmed, A.A.; Hussain, T.; Alshememry, A.K.; Somily, A.M.; Alsaadi, M.M.; Almajhdi, F.N. ON-1 and BA-IX Are the Dominant Sub-Genotypes of Human Orthopneumovirus A&B in Riyadh, Saudi Arabia. Genes 2022, 13, 2288. https://doi.org/10.3390/genes13122288
Alzayed RM, Aziz IM, Alsaleh AN, Dudin GA, Ahmed AA, Hussain T, Alshememry AK, Somily AM, Alsaadi MM, Almajhdi FN. ON-1 and BA-IX Are the Dominant Sub-Genotypes of Human Orthopneumovirus A&B in Riyadh, Saudi Arabia. Genes. 2022; 13(12):2288. https://doi.org/10.3390/genes13122288
Chicago/Turabian StyleAlzayed, Rasha M., Ibrahim M. Aziz, Asma N. Alsaleh, Gani Asa Dudin, Anwar A. Ahmed, Tajamul Hussain, Abdullah K. Alshememry, Ali M. Somily, Muslim M. Alsaadi, and Fahad N. Almajhdi. 2022. "ON-1 and BA-IX Are the Dominant Sub-Genotypes of Human Orthopneumovirus A&B in Riyadh, Saudi Arabia" Genes 13, no. 12: 2288. https://doi.org/10.3390/genes13122288
APA StyleAlzayed, R. M., Aziz, I. M., Alsaleh, A. N., Dudin, G. A., Ahmed, A. A., Hussain, T., Alshememry, A. K., Somily, A. M., Alsaadi, M. M., & Almajhdi, F. N. (2022). ON-1 and BA-IX Are the Dominant Sub-Genotypes of Human Orthopneumovirus A&B in Riyadh, Saudi Arabia. Genes, 13(12), 2288. https://doi.org/10.3390/genes13122288







