Transcriptomics Analysis Reveals a More Refined Regulation Mechanism of Methylation in a Drought-Tolerant Variety of Potato
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Cultivation and Treatment
2.2. RNA Extraction, Library Construction, and Transcriptome Sequencing
2.3. Transcriptome Data Analysis
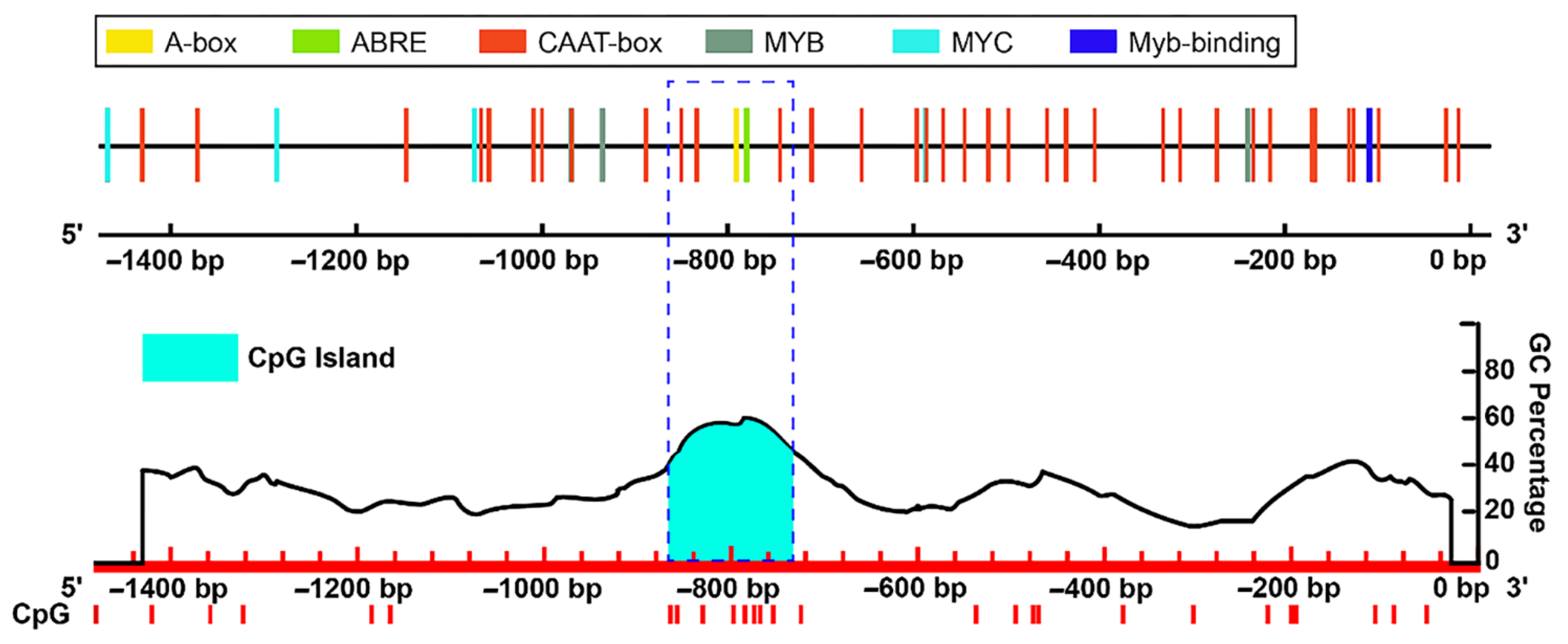
2.4. Promoter Methylation CpG Island Prediction and Cis-Elements Analysis of Important DEGs
2.5. Real-Time qPCR Verification
3. Results
3.1. Transcriptome Sequencing and Functional Annotation and Identification of the DEGs
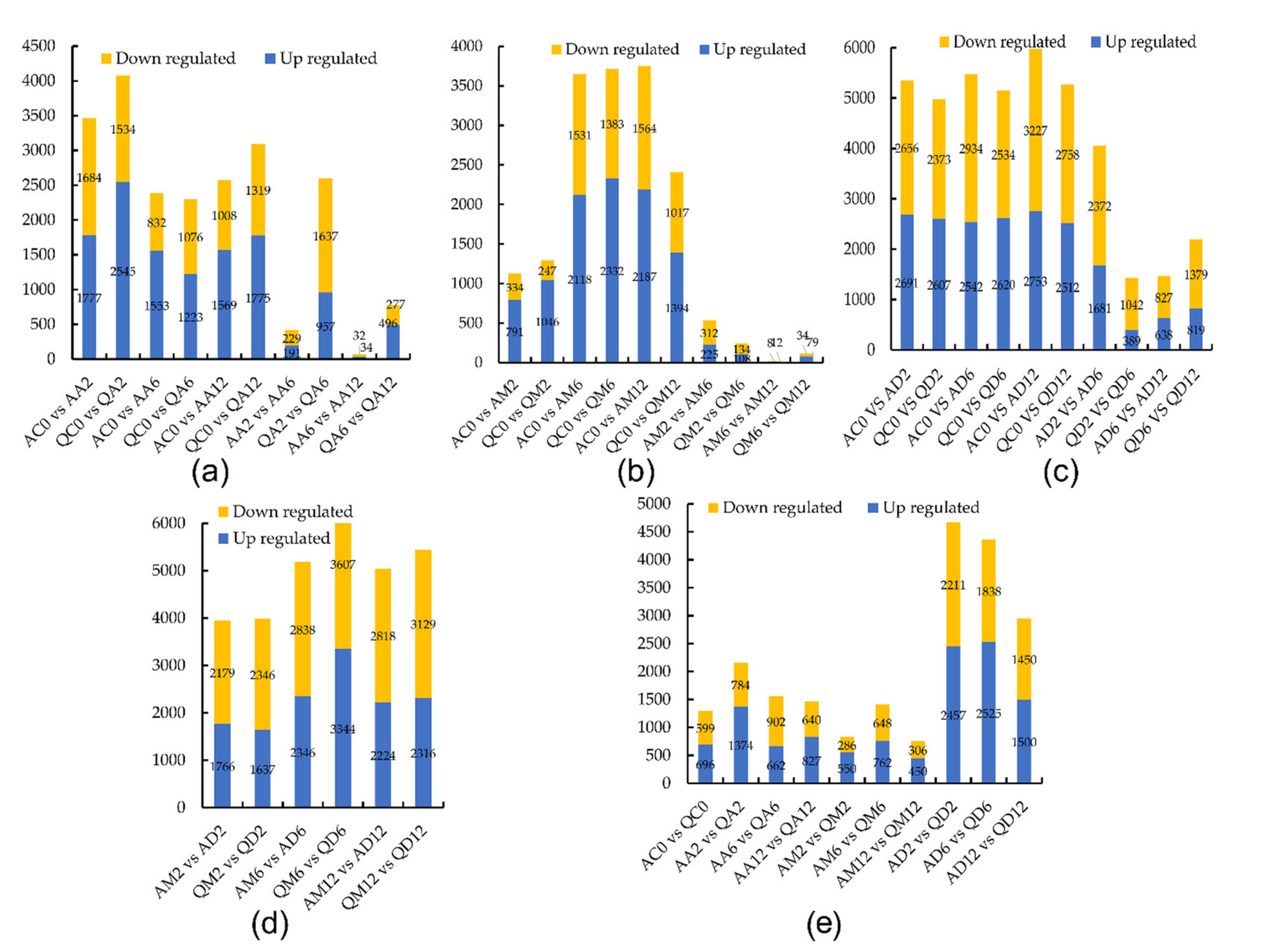
3.1.1. The Number of DEGs in the Drought-Sensitive Variety A
3.1.2. The Number of DEGs in Drought-Tolerant Potato Variety Q
3.1.3. The Number of DEGs of the Two Varieties
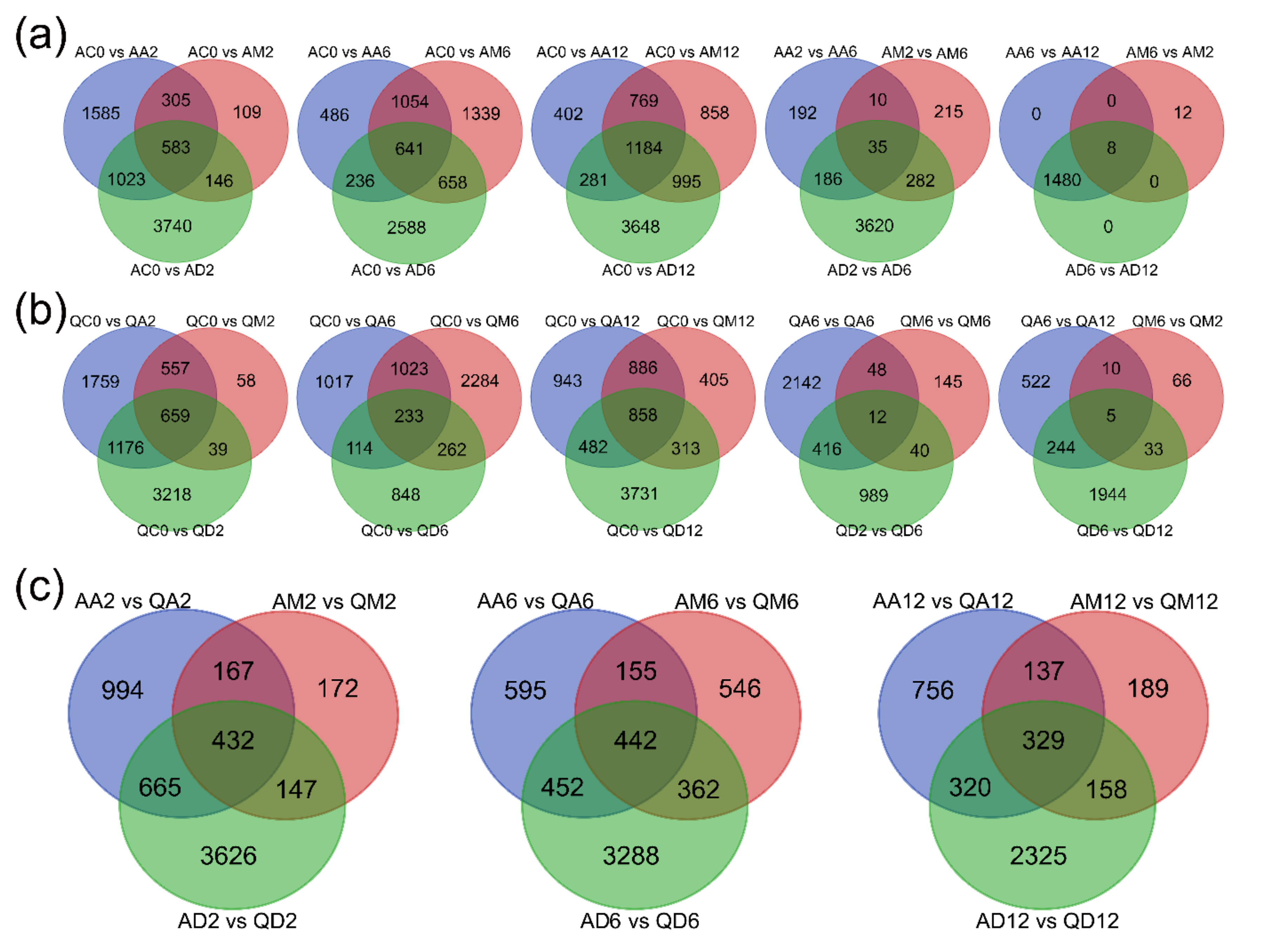
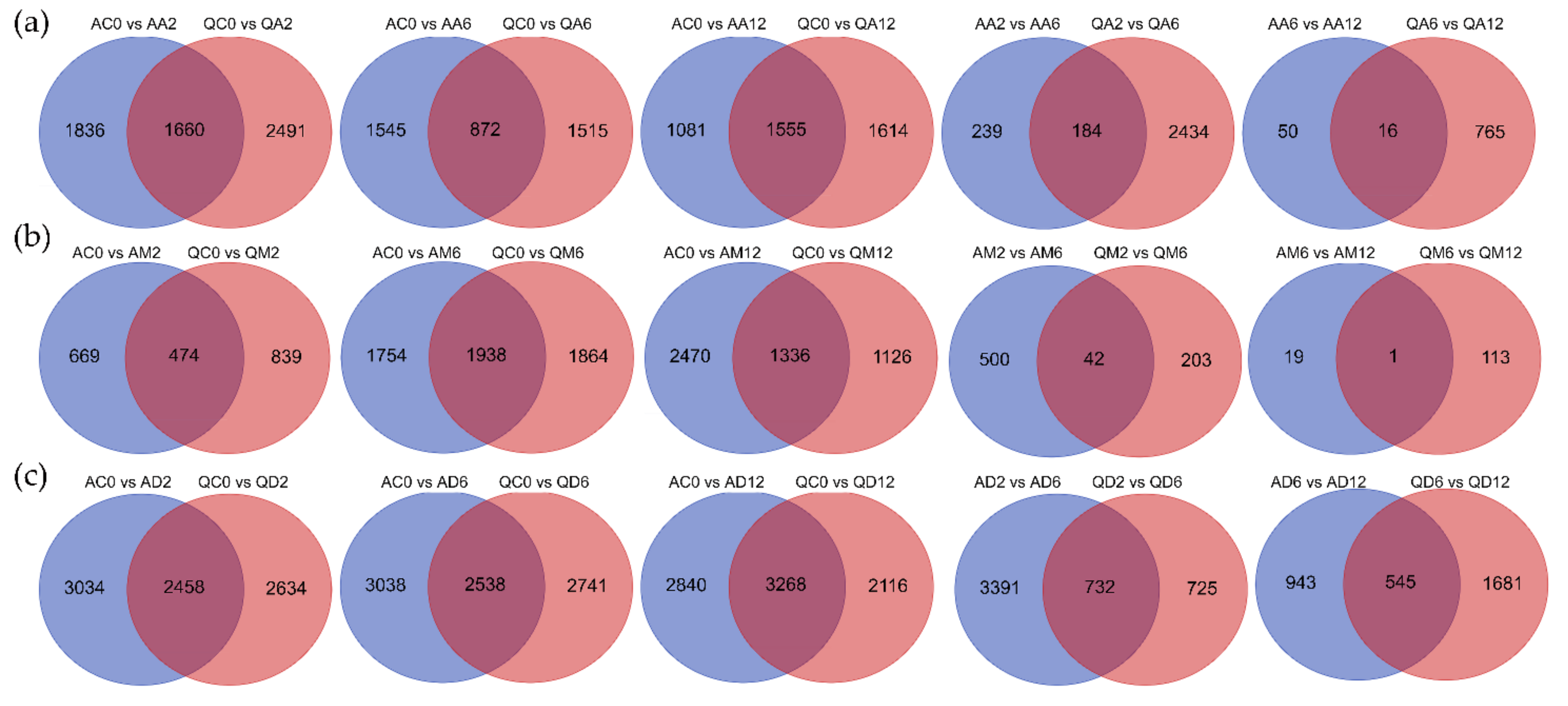
3.1.4. Venn Diagram Statistics of Each Treatment’s DEGs in the Two Varieties
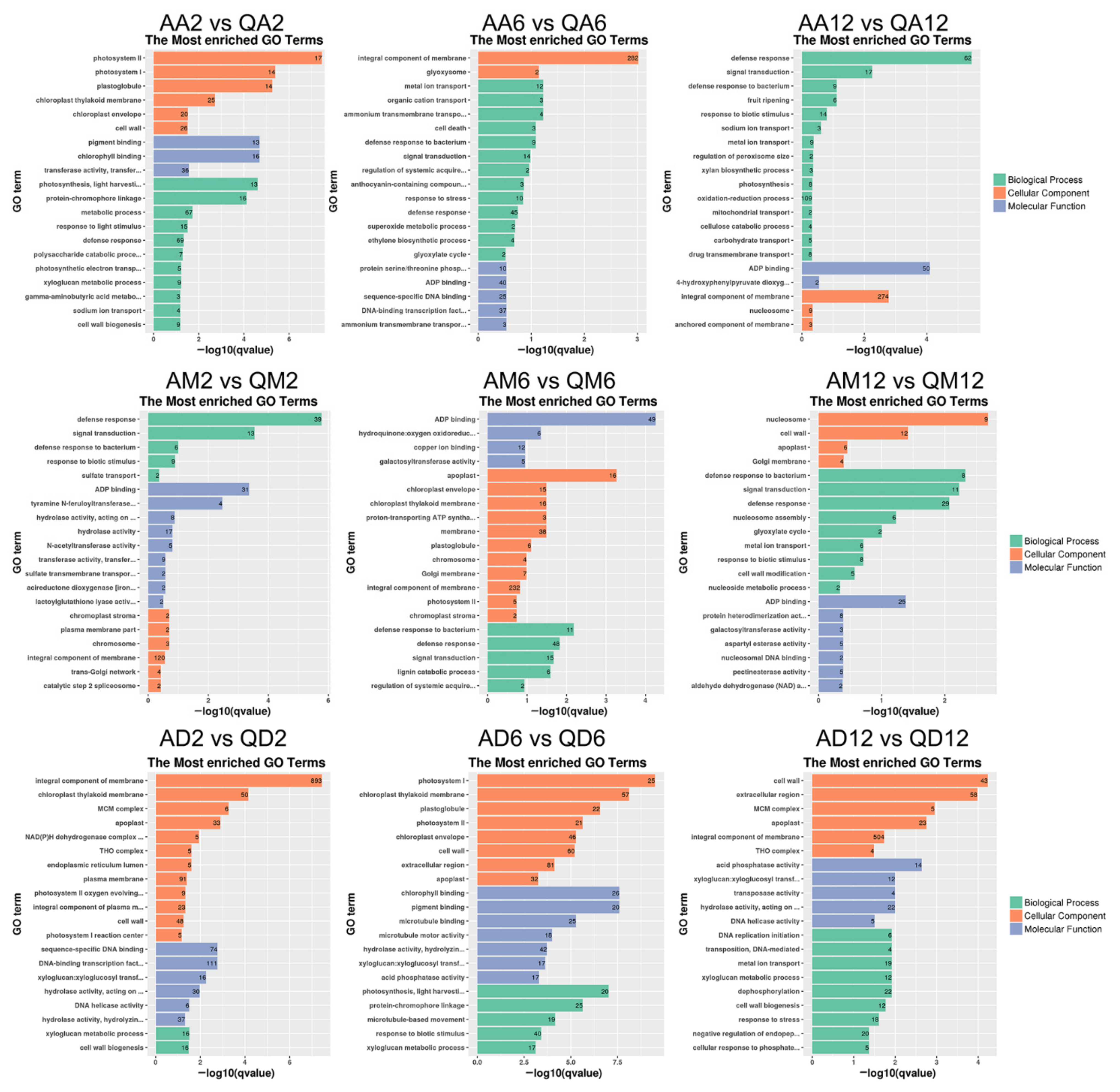
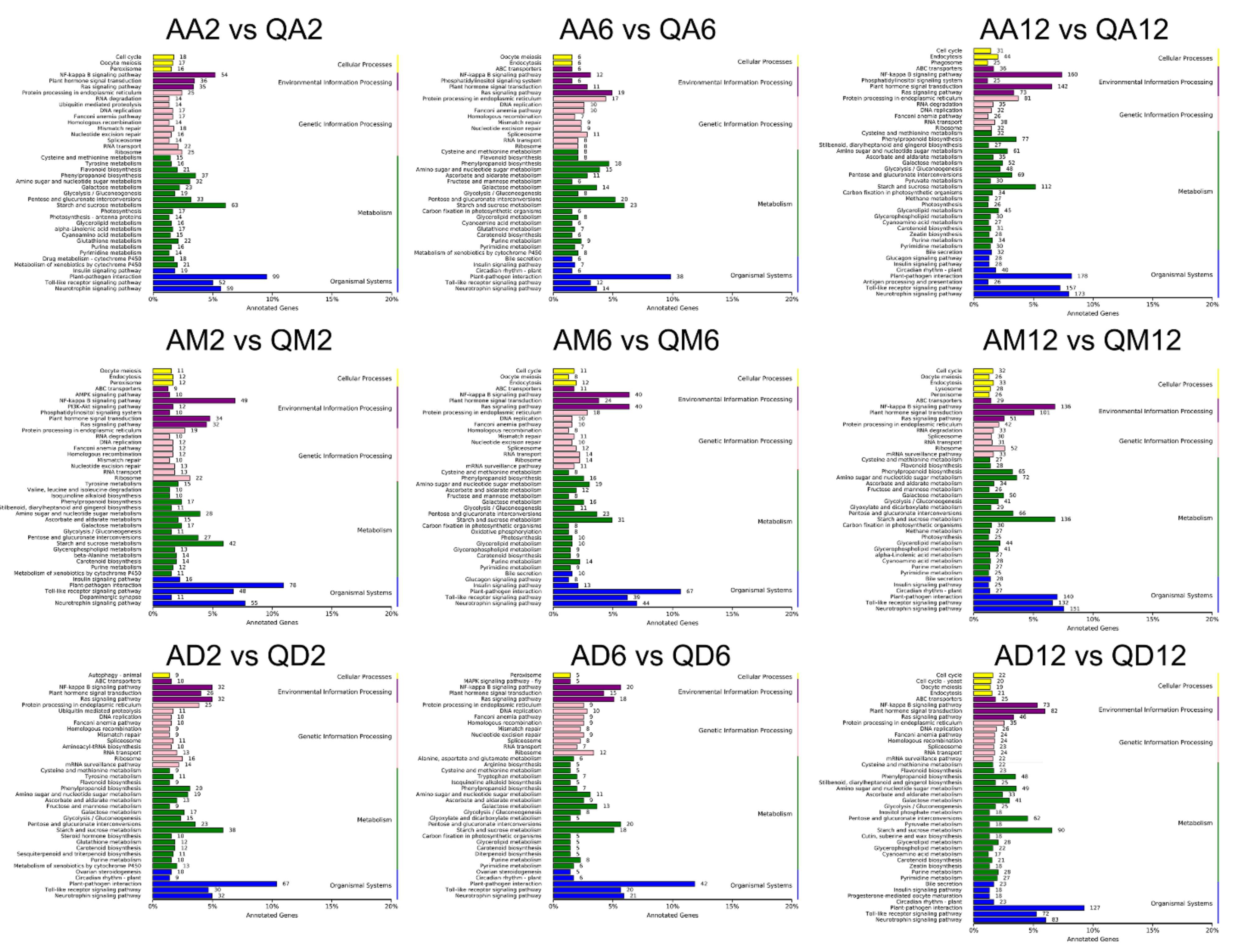
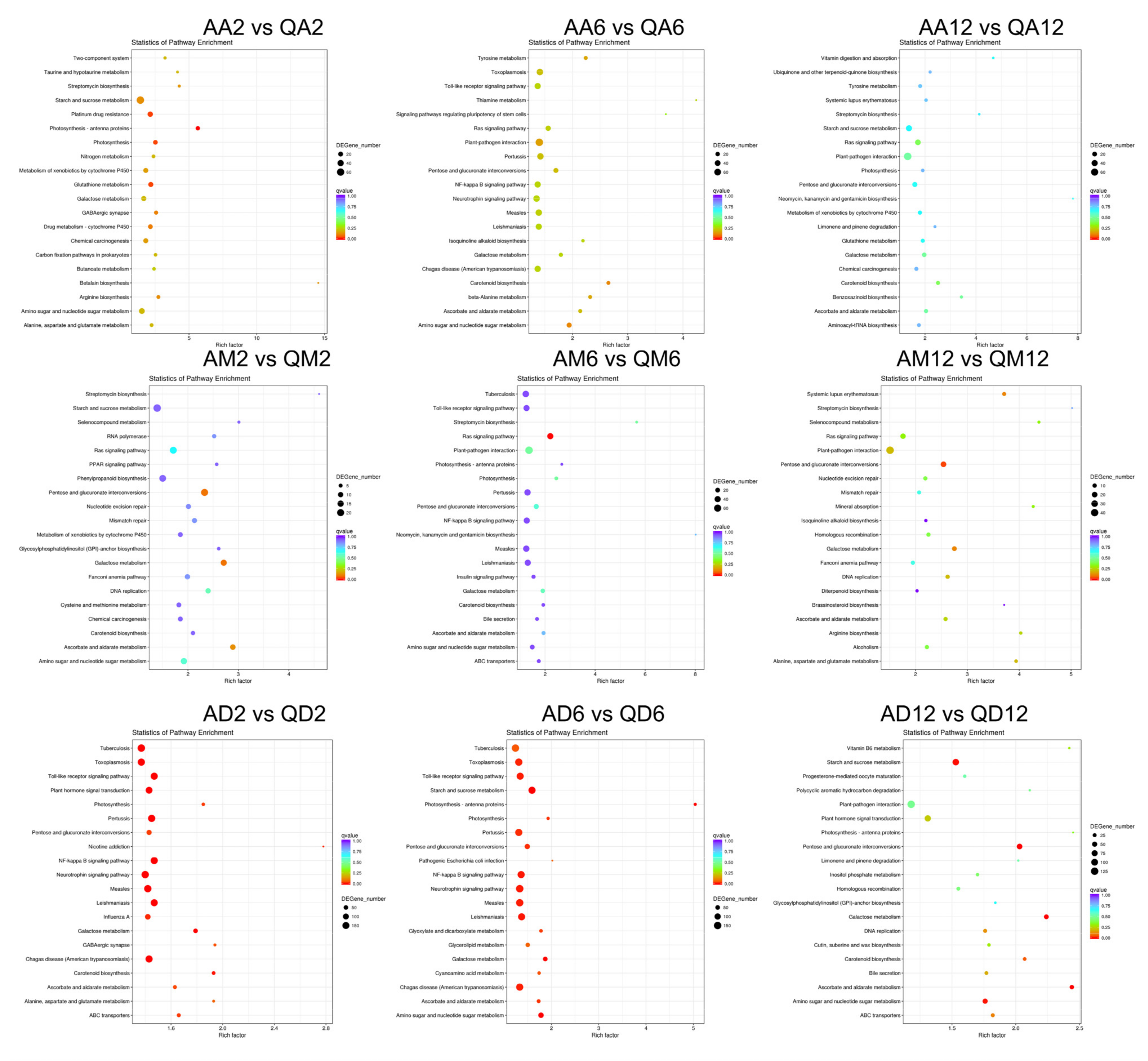
3.2. Functional Annotation Enrichment of DEGs
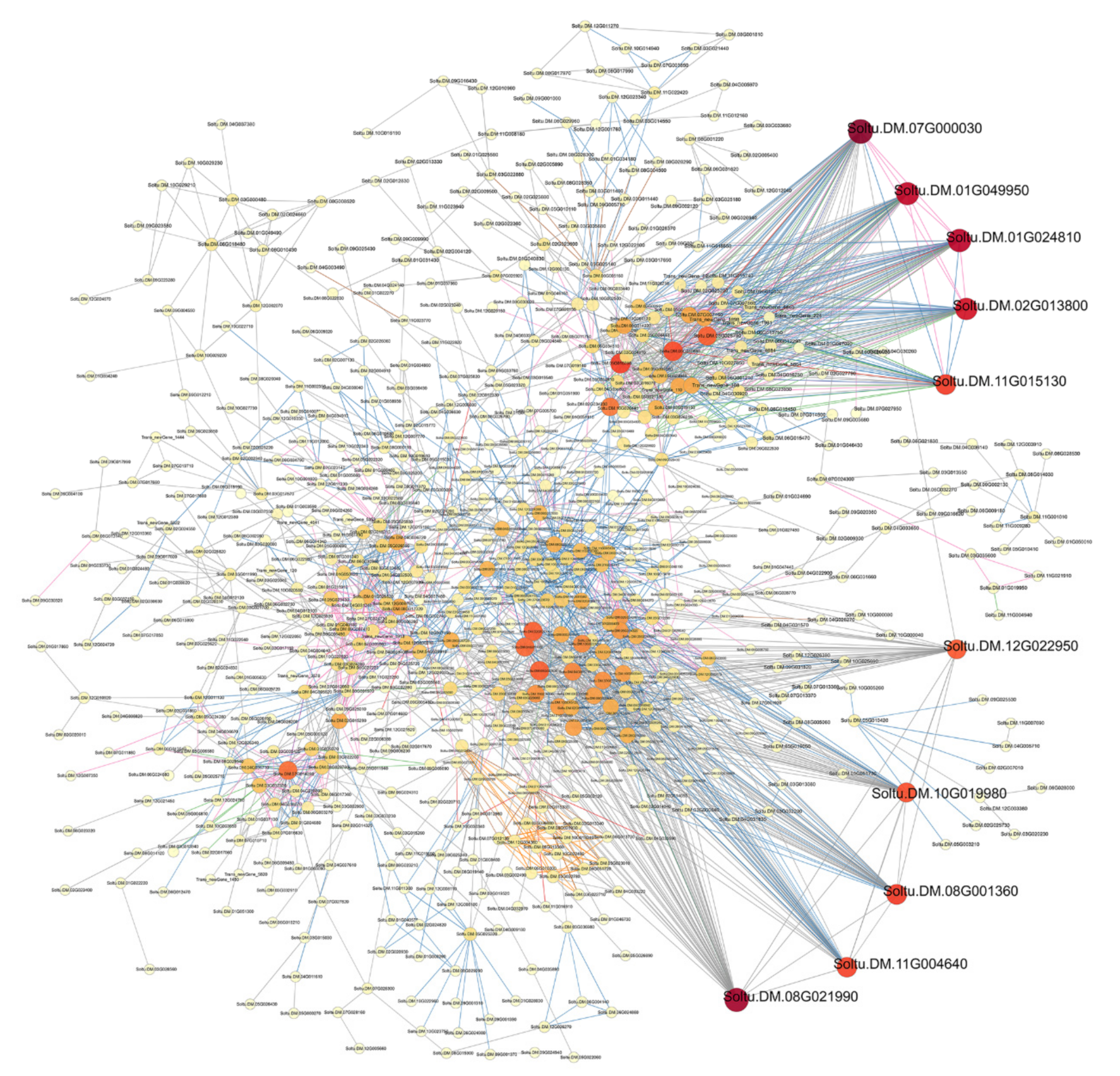
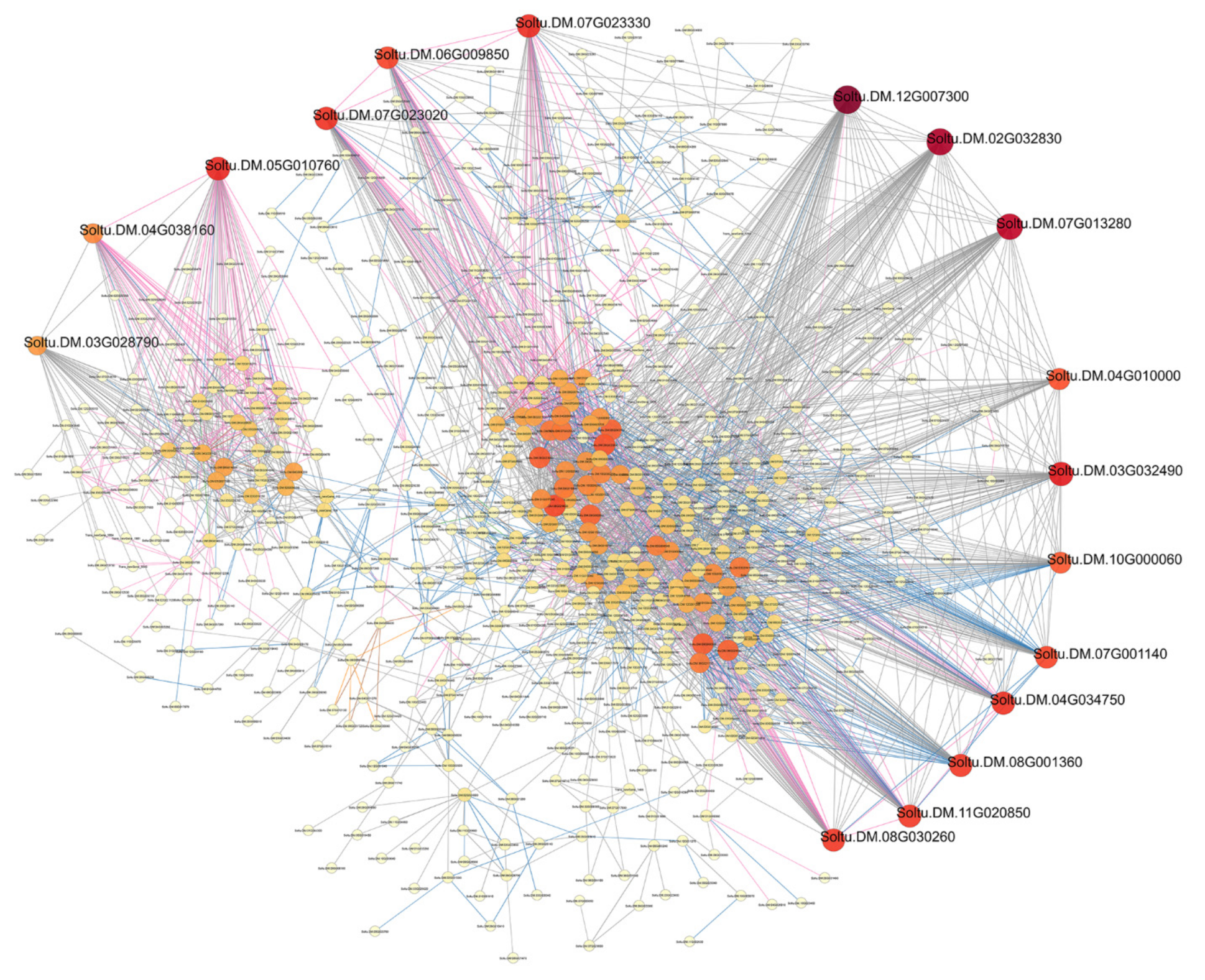
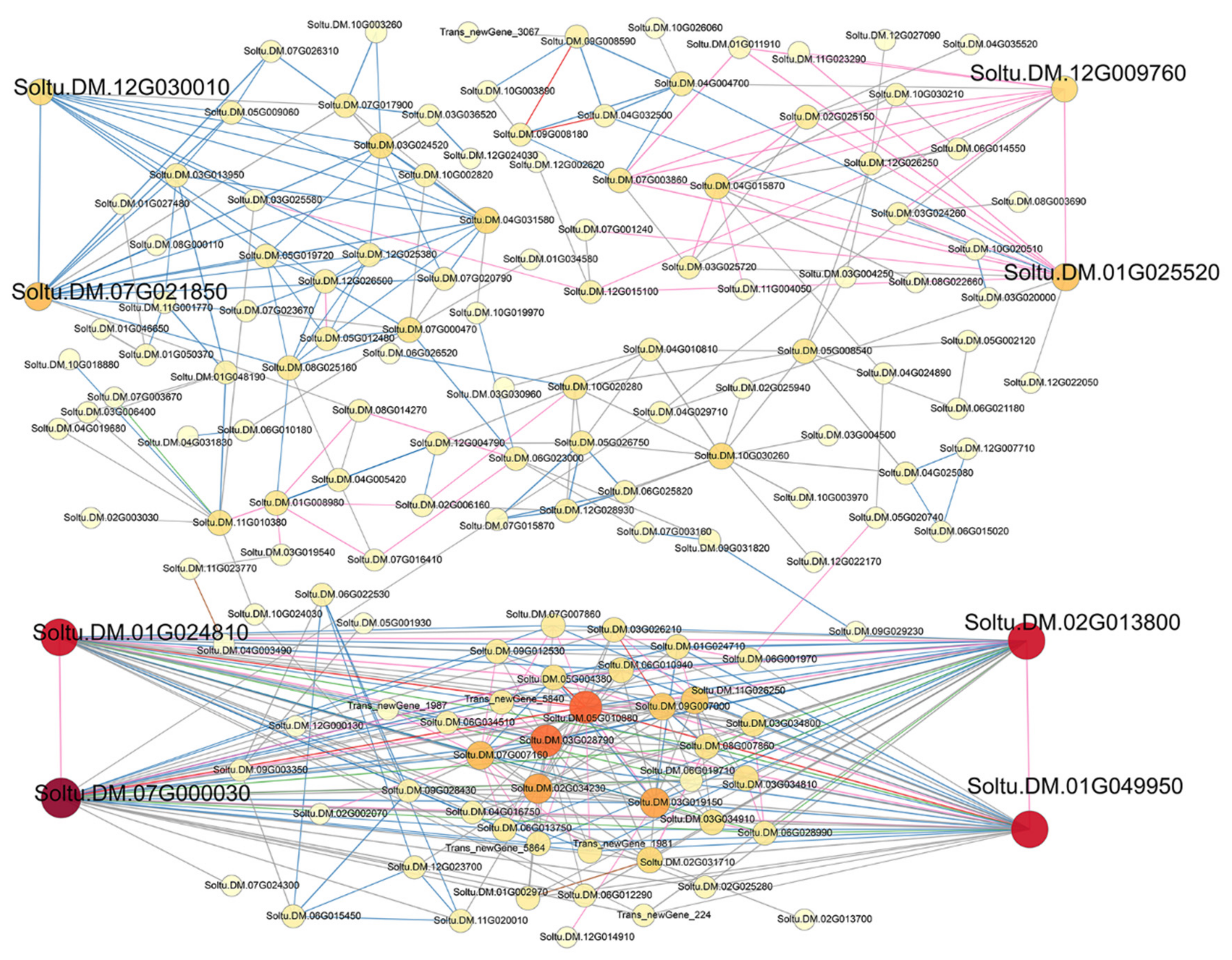
3.3. Protein–Protein Interaction Network Analysis
3.4. Analysis of Methylation Sites in the Promoter of Key DEGs
3.5. Verification of qPCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Golldack, D.; Li, C.; Mohan, H.; Probst, N. Tolerance to drought and salt stress in plants: Unraveling the signaling networks. Front. Plant Sci. 2014, 5, 151. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, K.; Yamaguchi-shinozaki, K.; Seki, M. Regulatory network of gene expression in the drought and cold stress responses. Cur. Opin. Plant Biol. 2003, 6, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, M.A.; Maruyama, K.; Abe, H.; Khan, M.A.; Katsura, K.; Ito, Y.; Yoshiwara, K.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Monitoring expression profiles of rice genes under cold, drought, and high-salinity stresses and abscisic acid application using cDNA microarray and RNA gel-blot analyses. Plant Physiol. 2003, 133, 1755–1767. [Google Scholar] [CrossRef]
- Gallusci, P.; Hodgman, C.; Teyssier, E.; Seymour, G.B. DNA Methylation and Chromatin Regulation during Fleshy Fruit Development and Ripening. Front. Plant Sci. 2016, 7, 807. [Google Scholar] [CrossRef] [PubMed]
- Pikaard, C.S.; Mittelsten, S.O. Epigenetic regulation in plants. Cold Spring Harb. Perspect. Biol. 2014, 6, a019315. [Google Scholar] [CrossRef] [PubMed]
- Vanyushin, B.F.; Ashapkin, V.V. DNA methylation in higher plants: Past, present and future. BBA-Gene Struct. Expr. 2011, 1809, 360–368. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Ye, W.W.; Wang, J.J.; Fan, B.X.; Song, L.Y. Review of DNA Methylation and Plant Stress-Tolerance. Acta Bot. Boreal.-Occident. Sin. 2009, 29, 1479–1489. [Google Scholar]
- Zhang, P.; Wang, J.; Geng, Y.; Dai, J.R.; Zhong, Y.; Chen, Z.Z.; Zhu, K.; Wang, X.Z.; Chen, S.Y. MSAP-based analysis of DNA methylation diversity in tobacco exposed to different environments and at different development phases. Bioch. Syst. Ecol. 2015, 62, 249–260. [Google Scholar] [CrossRef]
- Kaur, A.; Grewal, A.; Sharma, P. Comparative analysis of DNA methylation changes in two contrasting wheat genotypes under water deficit. Biol. Plant. 2018, 62, 471–478. [Google Scholar] [CrossRef]
- Liang, D.; Zhang, Z.; Wu, H.; Huang, C.; Shuai, P.; Ye, C.; Tang, S.; Wang, Y.; Yang, L.; Wang, J.; et al. Single-base-resolution methylomes of Populus trichocarpa reveal the association between DNA methylation and drought stress. BMC Genet. 2014, 15, S9. [Google Scholar] [CrossRef]
- Joel, A.J. Epigenetic responses to drought stress in rice (Oryza sativa L.). Physiol. Mol. Biol. Plants 2013, 19, 379–387. [Google Scholar]
- Garg, R.; Narayana, C.V.; Shankar, R.; Jain, M. Divergent DNA methylation patterns associated with gene expression in rice cultivars with contrasting drought and salinity stress response. Sci. Rep. 2015, 5, 14922. [Google Scholar] [CrossRef] [PubMed]
- Chwialkowska, K.; Nowakowska, U.; Mroziewicz, A.; Szarejko, I.; Kwasniewski, M. Water-deficiency conditions differently modulate the methylome of roots and leaves in barley (Hordeum vulgare L.). J. Exp. Bot. 2016, 67, 1109–1121. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, X.Y.; Deng, X. Analysis of DNA methylation of Boea hygrometrica under dehydration stress based on methylation sensitive amplification polymorphism. J. Fujian Agric. For. Univ. (Nat. Sci. Edit.) 2018, 47, 205–211. [Google Scholar]
- Tang, X.M.; Tao, X.; Wang, Y.; Ma, D.-W.; Li, D.; Yang, H.; Ma, X.-R. Analysis of DNA methylation of perennial ryegrass under drought using the methylation-sensitive amplification polymorphism (MSAP) technique. Mol. Genet. Genom. 2014, 289, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, J.; Mahajan, M.; Yadav, S.K. Comparative analysis of DNA methylation polymorphism in drought sensitive (HPKC2) and tolerant (HPK4) genotypes of horse Gram (Macrotyloma uniflorum). Biochem. Genet. 2013, 51, 493–502. [Google Scholar] [CrossRef]
- Guo, H.X.; Chen, L.; Lou, Q.J.X.; Hui, L.S.; Luo, L.J. Changes in DNA methylation pattern in a water-saving and drought-resistance rice variety at three leaf and four-leaf stages after drought domestication Z. Chin. J. Rice Sci. 2014, 28, 32–40. [Google Scholar]
- Meng, D.W.; Wang, Y.; Li, P.X.; Zhao, Y.W.; Zhou, Y.; Han, Y.; Lang, C.J.; Jin, T.C.; Yang, L.P. Drought-introduced DNA demethylation of AtGSTF14 Gene. Mol. Plant Breed. 2020, 18, 6108–6113. [Google Scholar]
- Feng, S.B. Research on DNA Methylation Variation Induced by Drought Stress in Cassava; Hainan University: Haikou, China, 2017. [Google Scholar]
- Abid, G.; Mingeot, D.; Muhovski, Y.; Mergeai, G.; Aouida, M.; Abdelkarim, S.; Aroua, I.; El Ayed, M.; M’hamdi, M.; Sassi, K.; et al. Analysis of DNA methylation patterns associated with drought stress response in faba bean (Vicia faba L.) using methylation-sensitive amplification polymorphism (MSAP). Environ. Exp. Bot. 2017, 142, 34–44. [Google Scholar] [CrossRef]
- Riddle, N.C.; Richards, E.J. The control of natural variation in cytosine methylation in Arabidopsis. Genetics 2002, 162, 355–363. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhu, S.; Fang, T.T.; Jiang, J.J.; Wang, Y.P. Analysis of drought resistance and DNA methylation level of resynthesized Brassica napus. Acta Agron. Sin. 2019, 45, 693–704. [Google Scholar] [CrossRef]
- Miura, K.; Agetsuma, M.; Kitano, H.; Yoshimura, A.; Matsuoka, M.; Jacobsen, S.E.; Ashikari, M. A metastable DWARF1 epigenetic mutant affecting plant stature in rice. Proc. Natl. Acad. Sci. USA 2009, 106, 11218–11223. [Google Scholar] [CrossRef] [PubMed]
- Kottler, E.J.; Vanwallendael, A.; Franks, S.J. Experimental treatment with a hypomethylating agent alters life history traits and fitness in Brassica rapa. J. bot. 2018, 2018, 7836845. [Google Scholar] [CrossRef]
- Xing, X.C.; Suo, L.G.; Xiao, C.Y.; Liu, M.; Liu, H.; Cui, J. Effect of 5-azacytidine on photosynthesis of cucumber seedlings under low temperature and light intensity. Agric. Res. Arid Areas 2017, 35, 116–123. [Google Scholar]
- Xu, R.; Zhang, W.Z.; Chen, L.L.; Ding, X.; Xie, Z.; Lin, Y.; Xin, J. Research progress on action mechanism of DNA demethylating agents. Drugs Clin. 2017, 32, 152–157. [Google Scholar]
- Mao, R.T. Phylogenetics and Expression of Methyltransferases and Demethylases in Potato; Inner Mongolia University: Hohhot, China, 2014; p. 94. [Google Scholar]
- Zhong, L.; Xu, Y.; Wang, J. The effect of 5-azacytidine on wheat seedlings responses to NaCl stress. Biol. Plant. 2010, 54, 753–756. [Google Scholar] [CrossRef]
- Bai, J. Physiological Response and DNA Methylation of Brassica Napus under Low Temperature Stress; Gansu Agricultural University: Lanzhou, China, 2019. [Google Scholar]
- Ramírez, D.A.; Yactayo, W.; Rens, L.R.; Rolando, J.L.; Palacios, S.; de Mendiburu, F.; Mares, V.; Barreda, C.; Loayza, H.; Monneveux, P.; et al. Defining biological thresholds associated to plant water status for monitoring water restriction effects: Stomatal conductance and photosynthesis recovery as key indicators in potato. Agric. Water Manag. 2016, 177, 369–378. [Google Scholar] [CrossRef]
- Law, R.D.; Suttle, J.C. Transient decreases in methylation at 5′-CCGG-3′ sequences in potato (Solanum tuberosum L.) meristem DNA during progression of tubers through dormancy precede the resumption of sprout growth. Plant Mol. Biol. 2003, 51, 437–447. [Google Scholar] [CrossRef]
- Li, Y.Y.; Cheng, P.; Xiong, X.Y.; Hong, Y.H. DNA methylation in potato under drought stress. Chin. Potato J. 2012, 26, 11–16. [Google Scholar]
- Wang, F.; Shi, R.; Wang, J. Genetic variation of DNA methylation in potato shoot tips after the cryopreservation by vitrification approach. Mol. Plant Breed. 2013, 11, 351–357. [Google Scholar]
- Li, P.C.; Bi, Z.Z.; Liang, W.J.; Sun, C.; Zhang, J.L.; Bai, J.P. DNA methylation involved in regulating drought stress response of potato. Acta Agron. Sin. 2019, 45, 1595–1603. [Google Scholar]
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genom. Biol. 2013, 14, R36. [Google Scholar] [CrossRef] [PubMed]
- Schulze, S.K.; Kanwar, R.; Gölzenleuchter, M.; Therneau, T.M.; Beutler, A.S. SERE: Single-parameter quality control and sample comparison for RNA-Seq. BMC Genom. 2012, 13, 524. [Google Scholar] [CrossRef]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genom. Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qian, W.Q. Mechanisms of DNA methylation and demethylation in plants. Chin. Bull. Life Sci. 2017, 3, 302–309. [Google Scholar]
- Jiao, J.; Wu, J.; Lyu, Z.; Sun, C.; Gao, L.; Yan, X.; Cui, L.; Tang, Z.; Yan, B.; Jia, Y. Methylation-sensitive amplified polymorphism-based genome-wide analysis of cytosine methylation profiles in Nicotiana tabacum cultivars. Genet. Mol. Res. 2015, 14, 15177–15187. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Li, M.; Yan, M.; Qiao, F.; Jiang, X. Integrated Transcriptome Analysis and Single-Base Resolution Methylomes of Watermelon (Citrullus lanatus) Reveal Epigenome Modifications in Response to Osmotic Stress. Front. Plant Sci. 2021, 12, 769712. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Ma, B.; Cai, C.; Xu, J. Transcriptome and methylome changes in two contrasting mungbean genotypes in response to drought stress. BMC Genom. 2022, 25, 80. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, Z.; Wang, Y.; Li, P.; Li, C.; Liu, Y.; Sun, C.; Yao, P.; Liu, Y.; Liu, Z.; Bai, J. Transcriptomics Analysis Reveals a More Refined Regulation Mechanism of Methylation in a Drought-Tolerant Variety of Potato. Genes 2022, 13, 2260. https://doi.org/10.3390/genes13122260
Bi Z, Wang Y, Li P, Li C, Liu Y, Sun C, Yao P, Liu Y, Liu Z, Bai J. Transcriptomics Analysis Reveals a More Refined Regulation Mechanism of Methylation in a Drought-Tolerant Variety of Potato. Genes. 2022; 13(12):2260. https://doi.org/10.3390/genes13122260
Chicago/Turabian StyleBi, Zhenzhen, Yihao Wang, Pengcheng Li, Chengju Li, Yindu Liu, Chao Sun, Panfeng Yao, Yuhui Liu, Zhen Liu, and Jiangping Bai. 2022. "Transcriptomics Analysis Reveals a More Refined Regulation Mechanism of Methylation in a Drought-Tolerant Variety of Potato" Genes 13, no. 12: 2260. https://doi.org/10.3390/genes13122260
APA StyleBi, Z., Wang, Y., Li, P., Li, C., Liu, Y., Sun, C., Yao, P., Liu, Y., Liu, Z., & Bai, J. (2022). Transcriptomics Analysis Reveals a More Refined Regulation Mechanism of Methylation in a Drought-Tolerant Variety of Potato. Genes, 13(12), 2260. https://doi.org/10.3390/genes13122260






