Abstract
Background: Inactivating NSD1 mutations causing Sotos syndrome have been previously associated with a specific genome-wide DNA methylation (DNAm) pattern. Sotos syndrome is characterized by phenotypic overlap with other overgrowth syndromes, and a definite diagnosis might not be easily reached due to the high prevalence of variants of unknown significance (VoUS) that are identified in patients with a suggestive phenotype. Objective: we performed microarray DNAm profiling in a set of 11 individuals with a clinical suspicion of Sotos syndrome and carrying an NSD1 VoUS or previously unreported variants to solve uncertainty in defining pathogenicity of the observed variants. The impact of the training cohort size on sensitivity and prediction confidence of the classifier was assessed. Results: The Sotos syndrome-specific DNAm signature was validated in six individuals with a clinical diagnosis of Sotos syndrome and carrying bona fide pathogenic NSD1 variants. Applying this approach to the remaining 11 individuals with NSD1 variants, we succeeded in confirming pathogenicity in eight subjects and excluding the diagnosis of Sotos syndrome in three. The sensitivity and prediction confidence of the classifier based on the different sizes of the training sets did not show substantial differences, though the overall performance was improved by using a data balancing strategy. Conclusions: The present approach solved uncertainty in cases with NDS1 VoUS, further demonstrating the clinical utility of DNAm profiling.
1. Introduction
Overgrowth syndromes are characterized by a global or regional excess of growth compared to equivalent body parts or age-related peer groups [1]. Sotos syndrome (OMIM #117550) is an autosomal dominant condition characterized by a triad including overgrowth (increased height, macrosomia, and macrocephaly), distinctive facial features, and developmental delay (DD)/intellectual disabilities (ID) [2]. The typical facies include dolichocephaly, broad and prominent forehead, sparse fronto-temporal hair, downslanted palpebral fissures, long and narrow face, and long/pointed chin. Sotos syndrome is caused by heterozygous inactivating variants in the nuclear receptor-binding SET domain protein gene (NSD1, OMIM #606681), which encodes a histone methyltransferase that preferentially methylates lysine 36 of histone H3 and lysine 20 of histone H4, either negatively or positively influencing transcription, depending on the cellular context [3,4]. NSD1 haploinsufficiency is supported by the finding of microdeletions of the 5q35 chromosomal region in a subset of patients [3,5]. The overall prevalence of Sotos syndrome is estimated at 1 in 14,000 live births. More than 95% of the cases arise from de novo mutations; however, few cases of familial segregation have been reported [6,7]. The majority of NSD1 pathogenic variants are point mutations causing an early termination of transcription (nonsense or frameshift variants), though changes causing amino acid substitutions within specific domains of the protein are also observed [4].
NSD1 is characterized by low tolerance to LoF variants (pLI = 1). On the other hand, missense variants seem to be constrained to a lower extent (Z = 3.41), with pathogenic changes showing a clear positional clustering [8,9]. Among the 519 missense/splice site variants reported in gnomAD (https://gnomad.broadinstitute.org; accessed on 5 October 2022), 396 are classified as variant of unknown significance (VoUS) or have a conflicting interpretation in ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/ (accessed on 5 October 2022)). The pathogenic missense variants largely map within functional domains implicated in chromatin regulation at the 3′ of the gene (i.e., PHD, SAC, and SET domains), and predominantly occur at the consensus cysteine and histidine residues that define these domains, with few exceptions [4,9]. The uncertainty of an increasing number of NSD1 variants that are routinely revealed by massive parallel sequencing in the diagnostic setting is generally solved by segregation analysis, or might require dedicated functional validation, which is further more relevant in patients with atypical or incomplete presentation of the disorder.
A large proportion of overgrowth syndromes is caused by pathogenic variants in genes that encode proteins involved in epigenetic regulation, indicating that the functional disruption of the epigenetic machinery is a recurrent mechanism causing these disorders [4,10,11,12]. Epigenetic marks, including DNA methylation (DNAm) and histone modifications, have emerged as crucial genome-wide regulatory events modulating the transcriptome temporally and spatially to drive normal developmental and cellular processes [13]. Recently, dozens of genes involved, directly or indirectly, in chromatin remodeling have been associated, when mutated, with a unique genome-wide DNAm profile (known as “episignature”), which is defined as the cumulative DNAm pattern occurring at multiple CpG dinucleotides across the genome [14,15,16,17]. These disease-specific DNAm signatures are detectable in peripheral blood despite the variable nature and complexity of diseases and the high variance characterizing the DNAm of genomes from different cells and tissues [18]. For this reason, DNAm testing can be effectively used as highly specific and robust tool and has recently been implemented in the diagnostic path of patients with rare disorders [19,20,21]. Sotos syndrome is the first overgrowth condition for which a specific DNAm signature was identified [22].
Genome-wide DNAm profiling is becoming a powerful functional tool in clinical setting [23] as a second-tier diagnostic strategy for the classification of VoUS, allowing to validate or rule out the clinical suspicion, and even confirm a diagnosis in exceptional cases, such as in individuals carrying hypomorphic or mosaic alleles [13]. This approach has successfully been applied to solve the clinical significance of a subset of NSD1 VoUS [22]. Here, we employed microarray DNAm profiling to classify a variegated set of NSD1 VoUS and private variants identified in the routine diagnostic setting. These cases included both subjects with features suggestive of Sotos syndrome who were assessed by targeted sequencing, and individuals with a non-suggestive phenotype who had been investigated by using an agnostic approach (exome sequencing).
2. Methods
2.1. Study Cohort
All subjects were originally enrolled in the context of a routine diagnostic activity. Clinical data, pictures, DNA specimens and other biological material were collected, used, and stored after signed informed consents from the participating subjects/families were collected. Permission to publish clinical pictures was obtained for all subjects reported in Figure 1.
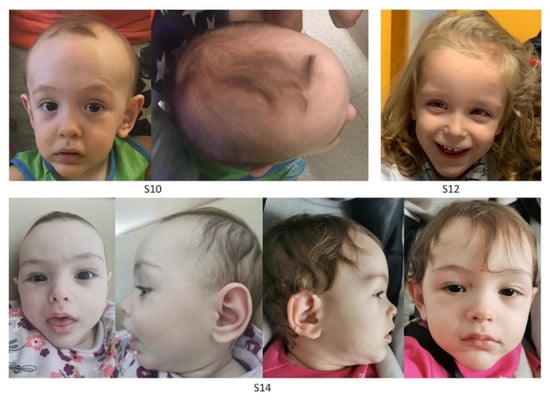
Figure 1.
Facies of three subjects with clinical diagnosis of Sotos syndrome carrying functionally unclassified NSD1 variants. Clinical features of S10 (1 year), S12 (2 years) and S14 (6 months, left panels; 1 year and 3 months, right panels) showing typical Sotos syndrome characteristics. All subjects show macrocephaly, dolichocephaly, broad and prominent forehead, sparse fronto-temporal hair, downslanted palpebral fissures, long and narrow face, and a long/pointed chin.
Six individuals (S1 to S6) presented with both a clinical diagnosis of Sotos syndrome and a molecularly diagnosed pathogenic variant in NSD1, either previously reported or considered bona fide as pathogenic. Their clinical and molecular characterization is summarized in Table 1. A second cohort of 11 individuals (S7 to S17) having a clinical suspicion of Sotos syndrome and an unclassified or a previously unreported variant in NSD1 made up the testing group. Their clinical and molecular characterization is summarized in Table 1, while their detailed description is reported in the supplemental clinical reports.

Table 1.
Clinical and molecular features of the subjects included in the study.
2.2. Molecular Genetics
In subjects S7 to S17, all variants were identified through massive parallel sequencing using different approaches, including target panel resequencing (multigene panel or clinical exome) or trio-based exome sequencing, after the exclusion of any clinically relevant structural variant by high-resolution CMA-array. The identified NSD1 variants with their functional annotation are reported in Table 2. These variants and a general overview of the distribution of the NSD1 variants annotated in ClinVar and gnomAD plotted taking into account the functional domain organization of the protein are shown in Figure 2. Their segregation, when available, has been confirmed by Sanger sequencing.

Table 2.
Functional annotation of the NSD1 variants included in the study.
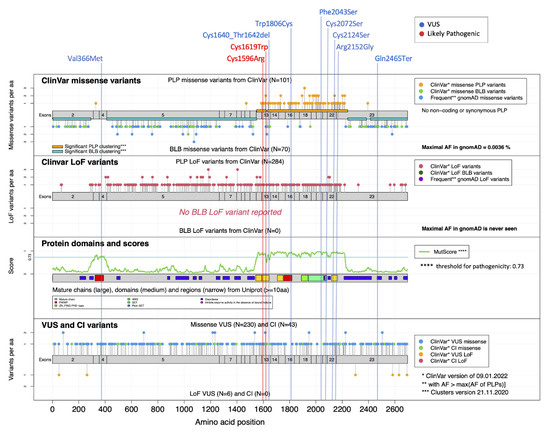
Figure 2.
Mutational landscape for NSD1. MutLand plot for NSD1 (NM_022455.4) [9], showing exons, functional domains and variants reported in ClinVar. Location of the variants included in the present study are also shown, as per different ACMG classification (blue, VoUS; red, likely pathogenic). Positions of the missense and LoF NSD1 variants reported in ClinVar known to be either pathogenic/likely pathogenic (PLP) or benign/likely benign (BLB) are shown (top diagrams), whereas VoUS or variants having conflicting interpretation (CI) reported by ClinVar, either missense or LoF, are shown in the bottom diagram. The cartoons highlight the regions for which MutScore detects significant PLP clustering (orange horizontal bars). A diagram reporting the functional domains of the protein (PHD, yellow; PWWP, red; SET, light green), and plotting the MutScore referred for the entire protein sequence is also shown (third plot from the top).
2.3. Methylation Analysis
Peripheral blood DNA was extracted using standard techniques. Bisulphite conversion was performed, and samples were analyzed using Infinium Methylation EPIC BeadChip (Illumina, San Diego, CA, USA), according to the manufacturer’s protocol. The 17 samples with NSD1 variants were analyzed, alongside 295 controls.
IDAT files containing methylated and unmethylated signal intensities were imported into R V.4.2.1 for subsequent analysis using the ChAMP package (V.2.26.0) [24]. Firstly, intensity values were corrected for background using the Illumina’s method implemented in the minfi package [25] and evaluated for differences in cell type composition [26]. Afterwards, probes located on the X/Y chromosomes or known to cross-react with chromosomal locations other than their target regions, containing SNPs at or near the CpG sites, and having a detection p-value > 0.01 were excluded, resulting in 721,325 high-quality probes used in the subsequent analyses. Normalized methylation levels (β values) for each sample were compared using the established DNAm signature for Sotos syndrome (considering 110 high-quality probes out of 112) [17], and clustered by means of multidimensional scaling (MDS), considering the pair-wise Euclidean distances between samples. The training of the Support Vector Machine (SVM) machine learning (ML)-based classifier was performed with a linear kernel using the e1071 R package V.1.7 and nu-classification option. To determine the best hyperparameter and measure the accuracy of the model, the whole dataset was split into a training set (75% of samples) and a test set (25% of samples), and a 5-fold cross-validation was performed during the training process. This procedure was repeated four times to verify that each sample was used at least once for testing. Finally, a SMOTE (imbalance R package V.1.0.2.1) oversampling technique was carried out to overcome class imbalance between affected and control individuals used in the training process [27]. Scores from the SVM classifier below 0.25 were considered as control samples, scores from 0.25 to 0.50 were considered inconclusive findings, while scores > 0.50 were deemed to behave as NSD1 pathogenic variants.
3. Results
3.1. DNAm profiling allows the classification of rare/private NSD1 variants, confirming or ruling out the diagnosis of Sotos syndrome
Six individuals diagnosed with Sotos syndrome and carrying pathogenic NSD1 mutations (Table 1) were initially tested to confirm the robustness and specificity of the previously identified disease-specific signature [17]. Subsequently, a MDS analysis considering the six previously confirmed Sotos samples, 11 affected individuals with overlapping clinical phenotypes suggestive of Sotos syndrome (Table 1, Figure 1, supplemental clinical reports) and found heterozygous for an unclassified or previously unreported NSD1 variant, and 295 controls (including 213 neurotypical individuals and 82 affected subjects by different genetic diseases) was performed (Figure 3A). The analyses showed a clear clustering of seven subjects carrying missense/small in-frame deletion variants (S7, S8, S10-S14) together with the Sotos syndrome cohort. On the other hand, three individuals, including two subjects with de novo missense changes (S9 and S15) and one individual with a truncating variant inherited by an unaffected parent (S16), mapped far from the Sotos syndrome cluster and more closely to controls. S17, who carried a rare silent change (c.1782T > C), for which segregation was initially not available, clustered within the control group, ruling out pathogenicity.
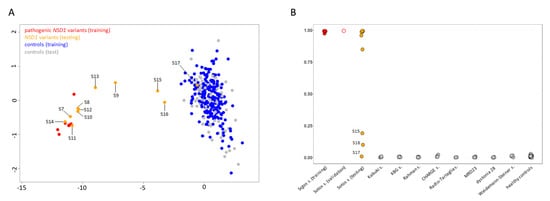
Figure 3.
DNAm array analyses. (A) DNAm profiles in individuals carrying NSD1 variants versus a control cohort. Taking advantage of DNAm levels from the established episignature, MDS plot was used to test/validate sample clustering with respect to the Sotos syndrome training cohort (red, solid circles) and a control group including 295 healthy individuals and subjects with rare neurodevelopmental disorders (blue, solid circles). Tested VoUS are depicted in orange (solid circles), while control subset used for testing are in grey (solid circles). (B) Plot for SVM probability scores from the developed ML-based classifier, showing classification results for the tested samples (VoUS, n = 8, orange solid circles; validation samples, n = 1, red open circles), and the in-house control cohort (grey = testing).
To functionally classify the NSD1 VoUS, an SVM-based ML classifier was applied, taking advantage of the established Sotos syndrome-specific episignature. By training a SVM model on five bona fide pathogenic variants versus our internal control database (~300 samples), the remaining sample with a likely pathogenic variant (S3) was validated, and the 11 tested samples were unambiguously classified (Table 2; Figure 3B). Pathogenic scores (>0.95) were obtained in the seven samples that were identified to cluster together with the Sotos syndrome cohort by MDS analysis. A pathogenic score (0.84) was also obtained for S9, who was definitely reclassified as affected with Sotos syndrome. Again, a control score (<0.10) was reached for S17. The two remaining individuals (S15 and S16) having an ambiguous DNAm profile at the MDS plot were definitively reclassified using the ML-classifier, which ruled out a diagnosis of Sotos syndrome, assigning benign probability scores (<0.25) to their variants.
3.2. Clinical and Molecular Re-Evaluation of Subjects Carrying Reclassified NSD1 Variants
We revised the clinical and molecular characteristics of all the individuals carrying a reclassified NSD1 variant. All subjects with a reclassified pathogenic variant showed a clinical phenotype fulfilling all the elements of the triad requested to suspect a clinical diagnosis of Sotos syndrome, including overgrowth (8/8) and macrocephaly (6/7), suggestive facies (8/8), and DD/ID (8/8). In particular, the cardinal facial features characterized by broad and prominent forehead (8/8), dolichocephaly (7/8), downslanted palpebral fissures (8/8), long and narrow face (8/8), and long/pointed chin (8/8) were invariably observed. On the other hand, the three individuals carrying variants reclassified as non-pathogenic for Sotos syndrome showed a phenotype that only partially fit a clinical diagnosis of the disorder, having less marked suggestive facial features, and not showing hypotonia; among these, S15 and S16 also did not show ID (Table 1). Two of the three subjects (S15 and S17) presented with overgrowth, while the co-occurrence of cystic fibrosis in the third individual (S16) (see supplemental clinical reports) could justify his growth within the normal range.
NSD1 is characterized by an overall PLP clustering score of 0.75, and regions between residues 1,549-1,624 and 1,633-2,241 are identified as significant regions with enriched PLP variants. As shown in Figure 2, all the tested VoUS reclassified as pathogenic by DNAm profiling were predicted as pathogenic by MutScore (>0.73) and map in regions that are classified as significant PLP clusters [9], corresponding to the PHD, PWWP and SET functional domains. Specifically, the majority were located within the PHD and SET domains, mainly at the highly conserved cysteine residues (Figure 2). Among the other missense changes, the variant predicting the Phe-to-Ser change at codon 2,043 within the SET domain (S11) also shows a clear match with the disease-specific DNAm signature, highlighting an important role of this highly conserved residue for the proper stability/function of this domain. Similarly, p.Trp1806Cys (S7) involves a key residue within one of the two PWWP domains (PWWP2). Consistently, subsequent segregation analysis performed in families of S8, S9, S10, S12, S13, and S14 confirmed the de novo origin of the identified variants, further supporting their pathogenic role. When considering the variants that did not fit the DNAm signature, the c.7393C > T (p.Gln2465*) (S16) was observed to map within a functionally uncharacterized region at the C-terminus. Of note, this variant was inherited from an apparently normal father, who only shows macrocrania. Familial inheritance of Sotos syndrome has rarely been observed. Tatton-Brown et al. (2005) [4] screened >300 parents of NSD1 mutation-positive individuals, identifying a mutation in only 11, all of whom had previously been clinically diagnosed with familial Sotos syndrome, and indicating that incomplete penetrance is an extremely rare event for pathogenic NSD1 variants. S17 presented with suggestive features of Sotos syndrome and macrosomia with macrocrania, and had a silent change in NSD1. The lack of signature in this patient allowed us to exclude a role of the observed variant in the pathogenesis of his condition. In line with our conclusions, subsequent segregation analysis documented that the variant was transmitted from a mother with isolated macrocrania and tall stature. Finally, the application of DNAm profiling in S15 allowed to reclassify the de novo c.1096G > A substitution (p.Val366Met), which affects the PWWP1 domain, rarely occurs in the general population (rs1172667661), and has an uncertain interpretation by ClinVar (VCV000451652). Of note, conflicting annotation was also reported by MutScore, which assigned a high score for pathogenicity, though it does not map within a region with a significant PLP clustering (Figure 2).
3.3. Robustness of the SVM Classifier and Sample Size Requirement
To assess how the training cohort size affects the sensitivity and prediction confidence (i.e., probability score) of the classifier, the ML-based classifier training was performed with subsets of the original training cohort. Using different sizes (3, 4 or 5 randomly selected samples with pathogenic NSD1 variants), we performed different rounds of training, testing on the same time if prediction confidence could be further improved using a more balanced dataset by means of oversampling (see Methods). The sensitivity and prediction confidence of different classifiers were compared, showing no substantial differences among probability scores obtained from the models using the full cohort or a minimal cohort of three samples with pathogenic variants as training sets, with class imbalance correction (Table 3). On the other hand, the analysis documented that the performance of the ML-based classifier was relevantly affected by the oversampling procedure, resulting crucial for the classification of three testing individuals (S9, S13, and S15) using all the different training cohorts (Table 3). This is in line with the higher accuracy of the model that was observed following oversampling (0.97 vs. 0.92, with and without oversampling, respectively).

Table 3.
Training sample size requirement for NSD1 variant classification using the SVM classifier.
4. Discussion
DNAm profiling has emerged as a highly informative tool for “functional” validation of putative disease-causing variants. The present findings further confirm the diagnostic utility of this technology in clinical routine diagnostics in solving “real” cases of VoUS for which “traditional” validation using in silico, in vitro and in vivo strategies or segregation analysis are not practicable. The use of DNAm profiling in this case series allowed us to definitively confirm pathogenicity of a new subset of missense and small in-frame deletions that were classified as VoUS and in a small group of previously unreported variants, without recurring to evidence-based of multiple observations eventually classifying them in ClinVar.
The routine use of massive parallel sequencing techniques (targeted sequencing, exome/genome sequencing) has revealed an emerging problem on VoUS interpretation in either highly suggestive or nonsuggestive associated phenotypes. DNAm profiling in a subset of conditions for which a signature has been established is becoming a reflex assay for the assessment of functional significance and reclassification of VoUS. This approach has the advantage of allowing a direct assessment on the primary sample, not requiring a new sampling or a different tissue analysis. If widely applied, this strategy could overcome the “traditional” validation approaches through functional assessment in various model systems, or clinical confirmation in multiple unrelated patients/families, which are usually unavailable for routine screening due to the rarity of these conditions. In the present series, five individuals (S7, S8, S12–S14) carried previously unreported variants, while three subjects (S9-S11) were heterozygous for variants reported as VoUS or having conflicting interpretation in ClinVar. All of them were successfully reclassified as pathogenic by applying this strategy.
Our classifier also managed to exclude pathogenicity of the remaining three assessed cases in the context of Sotos syndrome. One truncating NSD1 variant (S16) was inherited from an apparently unaffected father. S16 showed a co-occurring pathogenic homozygous variant in CFTR (p.Phe508del). She clinically presented with cystic fibrosis and relative macrocrania, which was also observed in her transmitting father. DNAm profiling allowed us to exclude a dual molecular diagnosis expressed with a blended phenotype. The application of the classifier also allowed to directly verify pathogenicity in a variant for which segregation was not available (S11) and exclude pathogenicity in a subject with a highly suggestive presentation (S17). In the latter, thanks to the negative finding, this individual is likely not to have either a deep intronic or an NSD1 intragenic deletion, avoiding further targeted analyses directed to explore other genomic events involving NSD1. Subsequent segregation analysis revealed variant transmission from the mother, who presented with macrocrania, tall stature, and mild learning difficulties, during the scholar period. Finally, S15 presented with many typical facial features of Sotos syndrome (macrocrania, long and triangular face, downslanted palpebral fissure, and deep-set eyes) but lacked two main features (dolicocephaly and pointed chin). Moreover, she did not show hypotonia and ID, thus lacking a main sign to clinically diagnose Sotos syndrome. Her variant was also reported in both ClinVar with uncertain significance and in general population as a rare allele. This variant showed a MutScore value greater than the suggested pathogenicity threshold (0.73) [9]. We cannot exclude a hypomorphic effect for the variants identified in S15–S17 on NSD1 function that might underlie or contribute to the isolated macrocephaly observed in the three patients and their transmitting parents. Our findings, however, definitively exclude a pathogenic role of these variants in Sotos syndrome pathogenesis.
Of note, the present analyses showed that the use of even a small number (down to three) of samples with bona fide pathogenic variants can successfully be used to effectively train a ML-based classifier based on the existing Sotos syndrome-specific DNAm signature, allowing VoUS classification.
In conclusion, the available episignature for Sotos syndrome has demonstrated to be a robust and reliable tool to quickly rule out or confirm a clinical suspicion of this disorder. Episignatures have the great advantage to overcome many technical limitations observed in current sequencing techniques (e.g., the lack of coverage of introns in the search of deep intronic pathogenic variants, promoters, variants involving regulatory regions). If properly used, this assay might be considered as a first-tier analysis in subjects with specific clinical suspicion in whom a condition caused by a gene involved in the epigenetic machinery is suspected. As exemplified in our case (S17), even in the presence of a highly suggestive Sotos syndrome phenotype, this assay could easily rule out the clinical diagnosis without proceeding with more complex targeted analyses directed to explore other genomic events involving NSD1. The application of DNAm analysis in diagnostics, VoUS classification, exploration of disease pathophysiology, as well as its emerging use in biomarker identification applied to precision medicine are becoming progressively more promising and are expected to be routinely applied in the next future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes13112163/s1, File S1: Clinical case reports
Author Contributions
Conceptualization, M.P. and M.T.; patient enrollment and clinical/molecular data collection, M.N., F.C.R., C.M. (Cecilia Mancini), M.I., L.C., T.G., M.A., A.N., A.M., M.M., L.M., F.F., P.P., A.P., M.F.B., R.C., A.B., M.S., C.M. (Corrado Mammì), G.Z., M.C.D., B.D., M.P. and M.T.; DNAm profiling data generation, L.P. and C.C.; DNAm profiling data analyses, M.F., A.C., S.P., E.M. and M.T.; data curation, M.F., A.C., L.P., M.N., F.C.R. and S.P.; writing—original draft preparation, A.C., M.P. and M.T.; writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by funding from the Italian Ministry of Health (Ricerca 5x1000_2019, RCR-2020-23670068_001, and RCR-2022-23682289 to M.T.; Ricerca Corrente 2022 to A.C.; RF-2018-12366931 to B.D. and F.C.R.) and Italian Ministry of Research (FOE 2019 to M.T.).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Ospedale Pediatrico Bambino Gesù (protocol code 1702_OPBG_2018, 11 February 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author (M.T.). The data are not publicly available due to due to privacy/ethical restrictions.
Acknowledgments
The authors wish to thank Claudia Nardini (Ospedale Pediatrico Bambino Gesù, Rome) for her skillful technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
Ethics Approval and Consent to Participate
This study was approved by the Ethical Committee of the Ospedale Pediatrico Bambino Gesù (ref. 1702_OPBG_2018). DNA specimens from the subjects included in this study were collected following procedures in accordance with the ethical standards of the declaration of Helsinki protocols and approved by the Review Boards of all involved institutions, with signed informed consents from the participating subjects/families.
References
- Tatton-Brown, K.; Weksberg, R. Molecular mechanisms of childhood overgrowth. Am. J. Med. Genet. C Semin. Med. Genet. 2013, 163, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Manor, J.; Lalani, S.R. Overgrowth Syndromes-Evaluation, Diagnosis, and Management. Front. Pediatr. 2020, 8, 574857. [Google Scholar] [CrossRef] [PubMed]
- Kurotaki, N.; Imaizumi, K.; Harada, N.; Masuno, M.; Kondoh, T.; Nagai, T.; Ohashi, H.; Naritomi, K.; Tsukahara, M.; Makita, Y.; et al. Haploinsufficiency of NSD1 causes Sotos syndrome. Nat. Genet. 2002, 30, 365–366. [Google Scholar] [CrossRef] [PubMed]
- Tatton-Brown, K.; Douglas, J.; Coleman, K.; Baujat, G.; Cole, T.R.; Das, S.; Horn, D.; Hughes, H.E.; Temple, I.K.; Faravelli, F.; et al. Genotype-phenotype associations in Sotos syndrome: An analysis of 266 individuals with NSD1 aberrations. Am. J. Hum. Genet. 2005, 77, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Douglas, J.; Hanks, S.; Temple, I.K.; Davies, S.; Murray, A.; Upadhyaya, M.; Tomkins, S.; Hughes, H.E.; Cole, T.R.; Rahman, N. NSD1 mutations are the major cause of Sotos syndrome and occur in some cases of Weaver syndrome but are rare in other overgrowth phenotypes. Am. J. Hum. Genet. 2003, 72, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Tatton-Brown, K.; Rahman, N. Sotos syndrome. Eur. J. Hum. Genet. 2007, 15, 264–271. [Google Scholar] [CrossRef]
- Tatton-Brown, K.; Cole, T.R.P.; Rahman, N. Sotos Syndrome. 2004 [Updated 2019 Aug 1]. In GeneReviews® [Internet]; Adam, M.P., Everman, D.B., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993–2022; Available online: https://www.ncbi.nlm.nih.gov/books/ (accessed on 1 August 2019).
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alföldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020, 581, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Quinodoz, M.; Peter, V.G.; Cisarova, K.; Royer-Bertrand, B.; Stenson, P.D.; Cooper, D.N.; Unger, S.; Superti-Furga, A.; Rivolta, C. Analysis of missense variants in the human genome reveals widespread gene-specific clustering and improves prediction of pathogenicity. Am. J. Hum. Genet. 2022, 109, 457–470. [Google Scholar] [CrossRef]
- Bernier, R.; Golzio, C.; Xiong, B.; Stessman, H.A.; Coe, B.P.; Penn, O.; Witherspoon, K.; Gerdts, J.; Baker, C.; Vulto-van Silfhout, A.T.; et al. Disruptive CHD8 mutations define a subtype of autism early in development. Cell 2014, 158, 263–276. [Google Scholar] [CrossRef]
- Tatton-Brown, K.; Loveday, C.; Yost, S.; Clarke, M.; Ramsay, E.; Zachariou, A.; Elliott, A.; Wylie, H.; Ardissone, A.; Rittinger, O.; et al. Mutations in Epigenetic Regulation Genes Are a Major Cause of Overgrowth with Intellectual Disability. Am. J. Hum. Genet. 2017, 100, 725–736. [Google Scholar] [CrossRef]
- Cytrynbaum, C.; Choufani, S.; Weksberg, R. Epigenetic signatures in overgrowth syndromes: Translational opportunities. Am. J. Med. Genet. Part C 2019, 81, 491–501. [Google Scholar] [CrossRef]
- Chater-Diehl, E.; Goodman, S.J.; Cytrynbaum, C.; Turinsky, A.L.; Choufani, S.; Weksberg, R. Anatomy of DNA methylation signatures: Emerging insights and applications. Am. J. Hum. Genet. 2021, 108, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.J.; Goodman, S.J.; Kobor, M.S. DNA methylation and healthy human aging. Aging Cell. 2015, 14, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Aref-Eshghi, E.; Schenkel, L.C.; Ainsworth, P.; Lin, H.; Rodenhiser, D.I.; Cutz, J.C.; Sadikovic, B. Genomic DNA methylation-derived algorithm enables accurate detection of malignant prostate tissues. Front. Oncol. 2018, 8, 100. [Google Scholar] [CrossRef] [PubMed]
- Martin-Herranz, D.E.; Aref-Eshghi, E.; Bonder, M.J.; Stubbs, T.M.; Choufani, S.; Weksberg, R.; Stegle, O.; Sadikovic, B.; Reik, W.; Thornton, J.M. Screening for genes that accelerate the epigenetic aging clock in humans reveals a role for the H3K36 methyltransferase NSD1. Genome Biol. 2019, 20, 146–166. [Google Scholar] [CrossRef] [PubMed]
- Aref-Eshghi, E.; Kerkhof, J.; Pedro, V.P.; France, G.D.; Barat-Houari, M.; Ruiz-Pallares, N.; Andrau, J.C.; Lacombe, D.; Van-Gils, J.; Fergelot, P.; et al. Evaluation of DNA Methylation Episignatures for Diagnosis and Phenotype Correlations in 42 Mendelian Neurodevelopmental Disorders. Am. J. Hum. Genet. 2020, 106, 356–370. [Google Scholar] [CrossRef] [PubMed]
- Sadikovic, B.; Levy, M.A.; Kerkhof, J.; Aref-Eshghi, E.; Schenkel, L.; Stuart, A.; McConkey, H.; Henneman, P.; Venema, A.; Schwartz, C.E.; et al. Clinical epigenomics: Genome-wide DNA methylation analysis for the diagnosis of Mendelian disorders. Genet. Med. 2021, 23, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Ciolfi, A.; Aref-Eshghi, E.; Pizzi, S.; Pedace, L.; Miele, E.; Kerkhof, J.; Flex, E.; Martinelli, S.; Radio, F.C.; Ruivenkamp, C.A.L.; et al. Frameshift mutations at the C-terminus of HIST1H1E result in a specific DNA hypomethylation signature. Clin. Epigenetics 2020, 12, 7. [Google Scholar] [CrossRef]
- Radio, F.C.; Pang, K.; Ciolfi, A.; Levy, M.A.; Hernández-García, A.; Pedace, L.; Pantaleoni, F.; Liu, Z.; de Boer, E.; Jackson, A.; et al. SPEN haploinsufficiency causes a neurodevelopmental disorder overlapping proximal 1p36 deletion syndrome with an episignature of X chromosomes in females. Am. J. Hum. Genet. 2021, 108, 502–516. [Google Scholar] [CrossRef]
- Ciolfi, A.; Foroutan, A.; Capuano, A.; Pedace, L.; Travaglini, L.; Pizzi, S.; Andreani, M.; Miele, E.; Invernizzi, F.; Reale, C.; et al. Childhood-onset dystonia-causing KMT2B variants result in a distinctive genomic hypermethylation profile. Clin. Epigenetics 2021, 13, 157. [Google Scholar] [CrossRef]
- Choufani, S.; Cytrynbaum, C.; Chung, B.H.; Turinsky, A.L.; Grafodatskaya, D.; Chen, Y.A.; Cohen, A.S.; Dupuis, L.; Butcher, D.T.; Siu, M.T.; et al. NSD1 mutations generate a genome-wide DNA methylation signature. Nat. Commun. 2015, 6, 10207. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.A.; Relator, R.; McConkey, H.; Pranckeviciene, E.; Kerkhof, J.; Barat-Houari, M.; Bargiacchi, S.; Biamino, E.; PalomaresBralo, M.; Cappuccio, G.; et al. Functional correlation of genome-wide DNA methylation profiles in genetic neurodevelopmental disorders. Hum. Mutat. 2022, 43, 1609–1628. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Morris, T.J.; Webster, A.P.; Yang, Z.; Beck, S.; Feber, A.; Teschendorff, A.E. ChAMP: Updated methylation analysis pipeline for Illumina BeadChips. Bioinformatics 2017, 33, 3982–3984. [Google Scholar] [CrossRef] [PubMed]
- Fortin, J.P.; Triche, T.J.; Hansen, K.D. Preprocessing, normalization and integration of the Illumina HumanMethylationEPIC array with minfi. Bioinformatics 2017, 33, 558–560. [Google Scholar] [CrossRef] [PubMed]
- Salas, L.A.; Koestler, D.C.; Butler, R.A.; Hansen, H.M.; Wiencke, J.K.; Kelsey, K.T.; Christensen, B.C. An optimized library for reference-based deconvolution of whole-blood biospecimens assayed using the Illumina Human Methylation EPIC Bead Array. Genome Biol. 2018, 19, 64. [Google Scholar] [CrossRef]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic Minority Over-sampling Technique. J. Artif. Intell. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).