Ontological Analysis of Coronavirus Associated Human Genes at the COVID-19 Disease Portal
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. The COVID-19 Disease Portal
3.2. Human COVID-19-Associated Gene Analysis
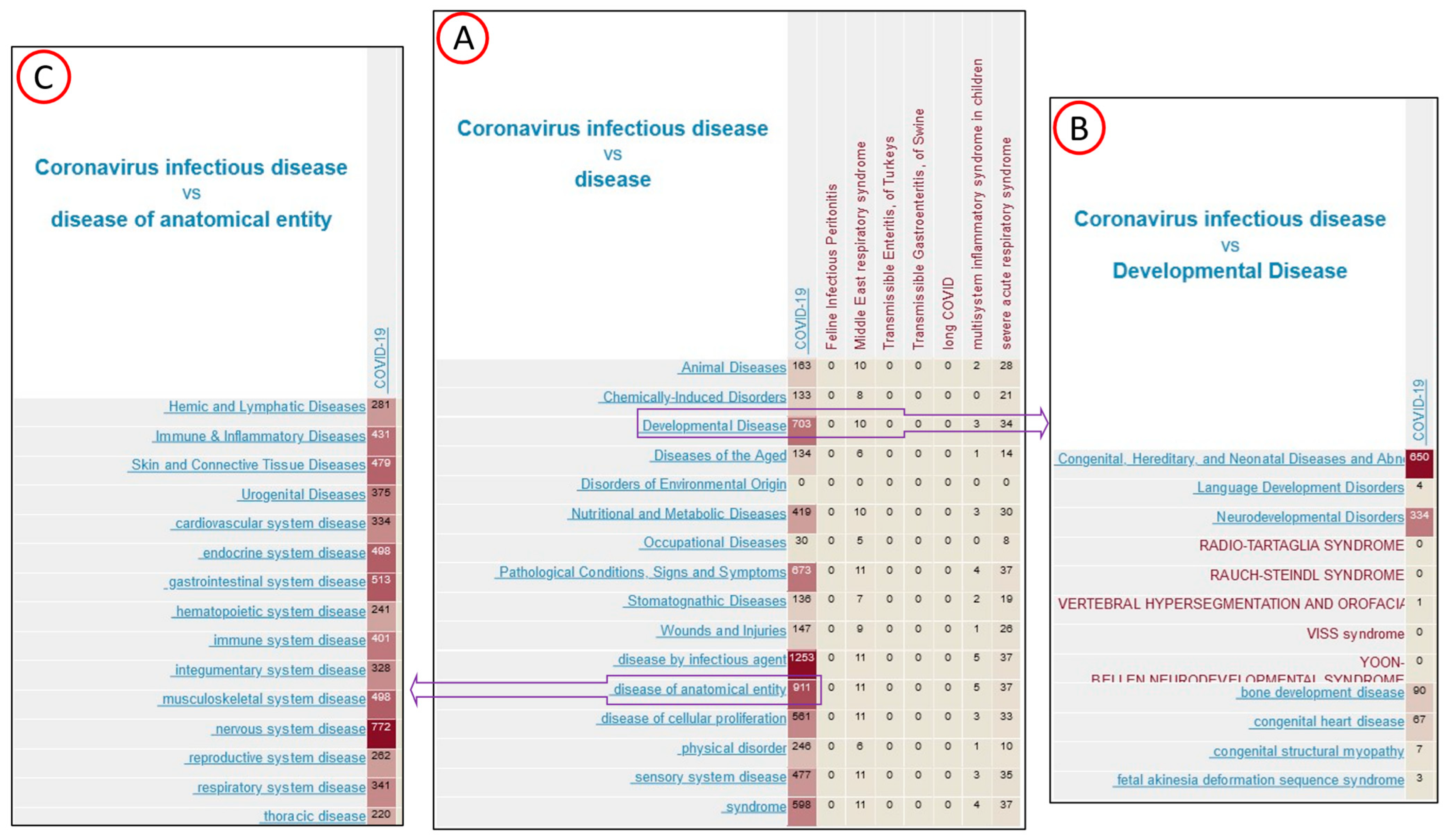
3.3. Gene Disease Association
3.4. Disease Term Enrichment Analysis
3.5. COVID-19 Affected Organ Systems
3.6. Gene Ontology Enrichment of COVID Genes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- da Silva, C.G.L.; Santana, J.R.P.; Pinheiro, L.F.G.; de Sousa, G.O.; Galvao, L.M.A.; Gomes, K.G.S.; Medeiros, K.A.; Diniz, L.A.; de Oliveira, I.G.P.; Felix, E.B.G.; et al. Anatomopathological Aspects and Clinical Correlation of COVID-19: A Systematic Review. Adv. Exp. Med. Biol. 2021, 1353, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Osuchowski, M.F.; Winkler, M.S.; Skirecki, T.; Cajander, S.; Shankar-Hari, M.; Lachmann, G.; Monneret, G.; Venet, F.; Bauer, M.; Brunkhorst, F.M.; et al. The COVID-19 puzzle: Deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir. Med. 2021, 9, 622–642. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Tang, Y.; He, W.; Yang, R.; Lan, Z.; Chen, R.; Zhang, P. Potential Pathophysiological Mechanisms Underlying Multiple Organ Dysfunction in Cytokine Release Syndrome. Mediat. Inflamm. 2022, 2022, 7137900. [Google Scholar] [CrossRef]
- Xu, B.; Li, G.; Guo, J.; Ikezoe, T.; Kasirajan, K.; Zhao, S.; Dalman, R.L. Angiotensin-converting enzyme 2, coronavirus disease 2019, and abdominal aortic aneurysms. J. Vasc. Surg. 2021, 74, 1740–1751. [Google Scholar] [CrossRef]
- Li, M.Y.; Li, L.; Zhang, Y.; Wang, X.S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect. Dis. Poverty 2020, 9, 45. [Google Scholar] [CrossRef]
- Chen, I.Y.; Chang, S.C.; Wu, H.Y.; Yu, T.C.; Wei, W.C.; Lin, S.; Chien, C.L.; Chang, M.F. Upregulation of the chemokine (C-C motif) ligand 2 via a severe acute respiratory syndrome coronavirus spike-ACE2 signaling pathway. J. Virol. 2010, 84, 7703–7712. [Google Scholar] [CrossRef]
- Haidar, M.A.; Shakkour, Z.; Reslan, M.A.; Al-Haj, N.; Chamoun, P.; Habashy, K.; Kaafarani, H.; Shahjouei, S.; Farran, S.H.; Shaito, A.; et al. SARS-CoV-2 involvement in central nervous system tissue damage. Neural Regen. Res. 2022, 17, 1228–1239. [Google Scholar] [CrossRef]
- Srivastava, A.; Rockman-Greenberg, C.; Sareen, N.; Lionetti, V.; Dhingra, S. An insight into the mechanisms of COVID-19, SARS-CoV2 infection severity concerning beta-cell survival and cardiovascular conditions in diabetic patients. Mol. Cell Biochem. 2022, 477, 1681–1695. [Google Scholar] [CrossRef]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef]
- Deer, R.R.; Rock, M.A.; Vasilevsky, N.; Carmody, L.; Rando, H.; Anzalone, A.J.; Basson, M.D.; Bennett, T.D.; Bergquist, T.; Boudreau, E.A.; et al. Characterizing Long COVID: Deep Phenotype of a Complex Condition. EBioMedicine 2021, 74, 103722. [Google Scholar] [CrossRef] [PubMed]
- Phetsouphanh, C.; Darley, D.R.; Wilson, D.B.; Howe, A.; Munier, C.M.L.; Patel, S.K.; Juno, J.A.; Burrell, L.M.; Kent, S.J.; Dore, G.J.; et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat. Immunol. 2022, 23, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Zinatizadeh, M.R.; Zarandi, P.K.; Zinatizadeh, M.; Yousefi, M.H.; Amani, J.; Rezaei, N. Efficacy of mRNA, adenoviral vector, and perfusion protein COVID-19 vaccines. Biomed. Pharmacother. 2022, 146, 112527. [Google Scholar] [CrossRef] [PubMed]
- Lamb, Y.N. Remdesivir: First Approval. Drugs 2020, 80, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Moreno, S.; Alcazar, B.; Duenas, C.; Del Castillo, J.G.; Olalla, J.; Antela, A. Use of Antivirals in SARS-CoV-2 Infection. Critical Review of the Role of Remdesivir. Drug Des. Dev. Ther. 2022, 16, 827–841. [Google Scholar] [CrossRef] [PubMed]
- Kaldunski, M.L.; Smith, J.R.; Hayman, G.T.; Brodie, K.; De Pons, J.L.; Demos, W.M.; Gibson, A.C.; Hill, M.L.; Hoffman, M.J.; Lamers, L.; et al. The Rat Genome Database (RGD) facilitates genomic and phenotypic data integration across multiple species for biomedical research. Mamm. Genome 2022, 33, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.R.; Hayman, G.T.; Wang, S.J.; Laulederkind, S.J.F.; Hoffman, M.J.; Kaldunski, M.L.; Tutaj, M.; Thota, J.; Nalabolu, H.S.; Ellanki, S.L.R.; et al. The Year of the Rat: The Rat Genome Database at 20: A multi-species knowledgebase and analysis platform. Nucleic Acids Res. 2020, 48, D731–D742. [Google Scholar] [CrossRef]
- Laulederkind, S.J.F.; Hayman, G.T.; Wang, S.J.; Hoffman, M.J.; Smith, J.R.; Bolton, E.R.; De Pons, J.; Tutaj, M.A.; Tutaj, M.; Thota, J.; et al. Rat Genome Databases, Repositories, and Tools. Methods Mol. Biol. 2019, 2018, 71–96. [Google Scholar] [CrossRef]
- Vedi, M.; Nalabolu, H.S.; Lin, C.W.; Hoffman, M.J.; Smith, J.R.; Brodie, K.; De Pons, J.L.; Demos, W.M.; Gibson, A.C.; Hayman, G.T.; et al. MOET: A web-based gene set enrichment tool at the Rat Genome Database for multiontology and multispecies analyses. Genetics 2022, 220, iyac005. [Google Scholar] [CrossRef]
- Davis, A.P.; Grondin, C.J.; Johnson, R.J.; Sciaky, D.; Wiegers, J.; Wiegers, T.C.; Mattingly, C.J. Comparative Toxicogenomics Database (CTD): Update 2021. Nucleic Acids Res. 2021, 49, D1138–D1143. [Google Scholar] [CrossRef]
- Liu, W.; Laulederkind, S.J.; Hayman, G.T.; Wang, S.J.; Nigam, R.; Smith, J.R.; De Pons, J.; Dwinell, M.R.; Shimoyama, M. OntoMate: A text-mining tool aiding curation at the Rat Genome Database. Database 2015, 2015, bau129. [Google Scholar] [CrossRef] [PubMed]
- Hayman, G.T.; Laulederkind, S.J.; Smith, J.R.; Wang, S.J.; Petri, V.; Nigam, R.; Tutaj, M.; De Pons, J.; Dwinell, M.R.; Shimoyama, M. The Disease Portals, disease-gene annotation and the RGD disease ontology at the Rat Genome Database. Database 2016, 2016, baw034. [Google Scholar] [CrossRef] [PubMed]
- Nadendla, S.; Jackson, R.; Munro, J.; Quaglia, F.; Meszaros, B.; Olley, D.; Hobbs, E.T.; Goralski, S.M.; Chibucos, M.; Mungall, C.J.; et al. ECO: The Evidence and Conclusion Ontology, an update for 2022. Nucleic Acids Res. 2022, 50, D1515–D1521. [Google Scholar] [CrossRef] [PubMed]
- Binns, D.; Dimmer, E.; Huntley, R.; Barrell, D.; O’Donovan, C.; Apweiler, R. QuickGO: A web-based tool for Gene Ontology searching. Bioinformatics 2009, 25, 3045–3046. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, X.; Chen, Z.; Duan, J.; Hashimoto, K.; Yang, L.; Liu, C.; Yang, C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020, 87, 18–22. [Google Scholar] [CrossRef]
- Moftah, M.B.; Eswayah, A. Intricate relationship between SARS-CoV-2-induced shedding and cytokine storm generation: A signaling inflammatory pathway augmenting COVID-19. Health Sci. Rev. 2022, 2, 100011. [Google Scholar] [CrossRef]
- Montalvan, V.; Lee, J.; Bueso, T.; De Toledo, J.; Rivas, K. Neurological manifestations of COVID-19 and other coronavirus infections: A systematic review. Clin. Neurol. Neurosurg. 2020, 194, 105921. [Google Scholar] [CrossRef]
- Merad, M.; Martin, J.C. Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages. Nat. Rev. Immunol. 2020, 20, 355–362. [Google Scholar] [CrossRef]
- Wang, S.J.; Laulederkind, S.J.; Hayman, G.T.; Smith, J.R.; Petri, V.; Lowry, T.F.; Nigam, R.; Dwinell, M.R.; Worthey, E.A.; Munzenmaier, D.H.; et al. Analysis of disease-associated objects at the Rat Genome Database. Database 2013, 2013, bat046. [Google Scholar] [CrossRef][Green Version]
- Stolp, B.; Stern, M.; Ambiel, I.; Hofmann, K.; Morath, K.; Gallucci, L.; Cortese, M.; Bartenschlager, R.; Ruggieri, A.; Graw, F.; et al. SARS-CoV-2 variants of concern display enhanced intrinsic pathogenic properties and expanded organ tropism in mouse models. Cell Rep. 2022, 38, 110387. [Google Scholar] [CrossRef]
- Deng, H.; Lin, H.; Mai, Y.; Liu, H.; Chen, W. Clinical features and predictive factors related to liver injury in SARS-CoV-2 Delta and Omicron variant-infected patients. Eur. J. Gastroenterol. Hepatol. 2022, 34, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Nardo, A.D.; Schneeweiss-Gleixner, M.; Bakail, M.; Dixon, E.D.; Lax, S.F.; Trauner, M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. 2021, 41, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lei, J.; Li, Z.; Yan, L. Potential Effects of Coronaviruses on the Liver: An Update. Front. Med. 2021, 8, 651658. [Google Scholar] [CrossRef] [PubMed]
- D’Ardes, D.; Boccatonda, A.; Cocco, G.; Fabiani, S.; Rossi, I.; Bucci, M.; Guagnano, M.T.; Schiavone, C.; Cipollone, F. Impaired coagulation, liver dysfunction and COVID-19: Discovering an intriguing relationship. World J. Gastroenterol. 2022, 28, 1102–1112. [Google Scholar] [CrossRef]
- Heinz, S.; Braspenning, J. Measurement of Blood Coagulation Factor Synthesis in Cultures of Human Hepatocytes. Methods Mol. Biol. 2015, 1250, 309–316. [Google Scholar] [CrossRef]


| A. Evidence Code (ECO) Distribution | B. Unique ECO among Genes | |||
|---|---|---|---|---|
| ECO Type | Annotation Count | Gene Count | Gene/Unique ECO | Gene Count |
| IAGP | 21 | 19 | 1 | 221 |
| IDA | 4 | 3 | 2 | 28 |
| IMP | 3 | 3 | 3 | 7 |
| IEP | 145 | 63 | 4 | 1 |
| HEP | 1195 | 1175 | ||
| EXP | 37 | 37 | ||
| ISO | 2 | 2 | ||
| Total | 1407 | 1257 | ||
| Top 40 Enriched Disease Ontology Terms from MOET and Set Analyzer | ||||
|---|---|---|---|---|
| A. MOET | ||||
| Rank | Disease Term (ID) | p * | Count # | Ref Count & |
| 1 | Respiratory Tract Infections (DOID:9008680) | 1.92 × 10−40 | 135 | 553 |
| 2 | liver disease (DOID:409) | 2.16 × 10−35 | 335 | 2638 |
| 3 | hepatocellular carcinoma (DOID:684) | 9.75 × 10−34 | 161 | 853 |
| 4 | liver carcinoma (DOID:686) | 1.13 × 10−33 | 161 | 854 |
| 5 | hepatobiliary disease (DOID:3118) | 1.49 × 10−32 | 337 | 2745 |
| 6 | liver cancer (DOID:3571) | 5.51 × 10−32 | 164 | 908 |
| 7 | hepatobiliary system cancer (DOID:0080355) | 7.52 × 10−32 | 171 | 975 |
| 8 | Adenocarcinoma (DOID:299) | 1.31 × 10−28 | 228 | 1617 |
| 9 | Liver Neoplasms (DOID:9007188) | 1.05 × 10−27 | 176 | 1100 |
| 10 | rheumatic disease (DOID:1575) | 1.18 × 10−27 | 133 | 698 |
| 11 | autoimmune disease of musculoskeletal system (DOID:0060032) | 7.15 × 10−27 | 156 | 922 |
| 12 | allergic disease (DOID:1205) | 3.50 × 10−26 | 122 | 624 |
| 13 | pneumonia (DOID:552) | 1.18 × 10−25 | 79 | 292 |
| 14 | nephritis (DOID:10952) | 7.85 × 10−25 | 91 | 388 |
| 15 | bacterial infectious disease (DOID:104) | 4.20 × 10−24 | 130 | 728 |
| 16 | respiratory allergy (DOID:0060496) | 2.25 × 10−23 | 90 | 397 |
| 17 | Glomerulonephritis (DOID:2921) | 2.83 × 10−23 | 82 | 337 |
| 18 | Wounds and Injuries (DOID:9001600) | 3.76 × 10−23 | 147 | 906 |
| 19 | Immediate Hypersensitivity (DOID:9002850) | 8.83 × 10−23 | 99 | 477 |
| 20 | Bacterial Infections and Mycoses (DOID:9004384) | 1.24 × 10−22 | 149 | 936 |
| 21 | Glomerular Diseases (DOID:9000104) | 1.26 × 10−22 | 82 | 344 |
| 22 | Inflammation (DOID:9005372) | 3.57 × 10−22 | 297 | 2605 |
| 23 | parasitic protozoa infectious disease (DOID:2789) | 3.64 × 10−22 | 56 | 170 |
| 24 | rheumatoid arthritis (DOID:7148) | 3.65 × 10−22 | 94 | 444 |
| 25 | obstructive lung disease (DOID:2320) | 6.72 × 10−22 | 96 | 464 |
| 26 | carcinoma (DOID:305) | 1.02 × 10−21 | 346 | 3235 |
| 27 | arthritis (DOID:848) | 2.80 × 10−21 | 140 | 875 |
| 28 | bone inflammation disease (DOID:3342) | 5.18 × 10−21 | 143 | 910 |
| 29 | lung disease (DOID:850) | 9.56 × 10−21 | 248 | 2066 |
| 30 | parasitic infectious disease (DOID:1398) | 9.89 × 10−21 | 77 | 327 |
| 31 | Orthomyxoviridae Infections (DOID:9001499) | 8.07 × 10−20 | 49 | 144 |
| 32 | asthma (DOID:2841) | 8.18 × 10−20 | 80 | 361 |
| 33 | hepatitis (DOID:2237) | 1.05 × 10−19 | 71 | 293 |
| 34 | Thoracic Injuries(DOID:9001954) | 1.35 × 10−19 | 68 | 272 |
| 35 | bronchial disease (DOID:1176) | 1.57 × 10−19 | 145 | 962 |
| 36 | lower respiratory tract disease (DOID:0050161) | 1.92 × 10−19 | 248 | 2110 |
| 37 | influenza (DOID:8469) | 2.23 × 10−19 | 48 | 141 |
| 38 | autoimmune disease of central nervous system (DOID:0060004) | 4.09 × 10−19 | 72 | 307 |
| 39 | respiratory system disease (DOID:1579) | 4.16 × 10−19 | 343 | 3306 |
| 40 | Lung Injury (DOID:9000310) | 1.17 × 10−18 | 66 | 267 |
| B. Set Analyzer | ||||
| Rank | MESH Term (ID) | p* | Count # | Ref Count & |
| 1 | Neoplasms (MESH:D009369) | 5.22 × 10−126 | 387 | 3777 |
| 2 | Digestive System Diseases (MESH:D004066) | 1.52 × 10−117 | 325 | 2810 |
| 3 | Liver Diseases (MESH:D008107) | 1.22 × 10−114 | 272 | 1974 |
| 4 | Neoplasms by Site (MESH:D009371) | 4.75 × 10−99 | 310 | 2978 |
| 5 | Immune System Diseases (MESH:D007154) | 2.63 × 10−91 | 195 | 1219 |
| 6 | Carcinoma (MESH:D002277) | 1.28 × 10−84 | 196 | 1341 |
| 7 | Digestive System Neoplasms (MESH:D004067) | 8.85 × 10−79 | 197 | 1461 |
| 8 | Respiratory Tract Diseases (MESH:D012140) | 2.39 × 10−77 | 171 | 1099 |
| 9 | Carcinoma, Hepatocellular (MESH:D006528) | 7.71 × 10−76 | 122 | 515 |
| 10 | Adenocarcinoma (MESH:D000230) | 1.00 × 10−74 | 163 | 1028 |
| 11 | Liver Neoplasms(MESH:D008113) | 1.47 × 10−72 | 136 | 707 |
| 12 | Skin and Connective Tissue Diseases (MESH:D017437) | 7.70 × 10−72 | 200 | 1650 |
| 13 | Lung Diseases (MESH:D008171) | 1.13 × 10−71 | 151 | 910 |
| 14 | Urogenital Diseases (MESH:D000091642) | 1.08 × 10−70 | 226 | 2135 |
| 15 | Nervous System Diseases(MESH:D009422) | 3.17 × 10−69 | 253 | 2695 |
| 16 | Female Urogenital Diseases and Pregnancy Complications (MESH:D005261) | 1.42 × 10−65 | 185 | 1535 |
| 17 | Respiratory Tract Infections (MESH:D012141) | 2.29 × 10−65 | 74 | 177 |
| 18 | Infections (MESH:D007239) | 1.62 × 10−64 | 114 | 543 |
| 19 | Skin Diseases (MESH:D012871) | 2.51 × 10−63 | 170 | 1336 |
| 20 | Pneumonia (MESH:D011014) | 1.51 × 10−62 | 57 | 93 |
| 21 | Female Urogenital Diseases (MESH:D052776) | 3.36 × 10−62 | 172 | 1392 |
| 22 | Pneumonia, Viral(MESH:D011024) | 1.02 × 10−57 | 38 | 38 |
| 23 | Fibrosis (MESH:D005355) | 4.37 × 10−57 | 142 | 1020 |
| 24 | Male Urogenital Diseases (MESH:D052801) | 3.56 × 10−56 | 172 | 1528 |
| 25 | COVID-19 (MESH:D000086382) | 3.99 × 10−56 | 37 | 37 |
| 26 | Urologic Diseases (MESH:D014570) | 6.12 × 10−55 | 128 | 852 |
| 27 | Autoimmune Diseases (MESH:D001327) | 1.01 × 10−53 | 104 | 552 |
| 28 | Virus Diseases (MESH:D014777) | 5.58 × 10−48 | 78 | 324 |
| 29 | Kidney Diseases (MESH:D007674) | 2.34 × 10−47 | 108 | 694 |
| 30 | Musculoskeletal Diseases (MESH:D009140) | 5.70 × 10−47 | 159 | 1529 |
| 31 | Hypersensitivity (MESH:D006967) | 4.50 × 10−45 | 76 | 331 |
| 32 | Nephritis (MESH:D009393) | 6.61 × 10−45 | 52 | 126 |
| 33 | Gastrointestinal Diseases (MESH:D005767) | 2.78 × 10−44 | 129 | 1072 |
| 34 | Liver Cirrhosis(MESH:D008103) | 3.25 × 10−44 | 118 | 897 |
| 35 | Vascular Diseases (MESH:D014652) | 5.72 × 10−44 | 122 | 965 |
| 36 | Cardiovascular Diseases (MESH:D002318) | 4.13 × 10−42 | 151 | 1515 |
| 37 | Connective Tissue Diseases (MESH:D003240) | 5.25 × 10−41 | 88 | 521 |
| 38 | Liver Cirrhosis, Experimental (MESH:D008106) | 6.80 × 10−41 | 106 | 777 |
| 39 | Central Nervous System Diseases (MESH:D002493) | 3.99 × 10−40 | 138 | 1330 |
| 40 | Glomerulonephritis (MESH:D005921) | 4.90 × 10−40 | 46 | 109 |
| Nervous System Disease (772) | Gastrointestinal System Disease (513) | Endocrine System Disease (498) | Musculoskeletal System Disease (498) | Skin and Connective Tissue Disease (479) | Immune & Inflammatory Disease (431) |
|---|---|---|---|---|---|
| central nervous system disease (676) | gastrointestinal disease (401) | liver disease (335) | connective tissue disease (376) | bone disease (270) | primary innunodeficiency disease (348) |
| sensory system disease (477) | digestive system neoplasm (339) | endocrine gland neoplasm (301) | bone disease (270) | connective tissue neoplasm (161) | autoimmune disease (246) |
| neurologic Manifestation (356) | liver disease (335) | diabetes Mellitus (134) | neuromusclular disease (174) | breast disase (161) | lymphatic system disease (176) |
| neurodegenerative disease (278) | intestinal disease (248) | pancreas disease (119) | musculoskeletal annormalities (157) | rheumatic disease (123) | rheumatic disease (133) |
| peripheral nervous system disease (232) | mouth disease (120) | gonaldal disease (109) | autoimmune disease of musculoskeletal system (156) | genetic skin disease (119) | allergic disease (121) |
| nervous system neoplasm (148) | stomach disease (99) | parathyoid gland disease (88) | joint disease (156) | interstitial lung disease (85) | immunoproliferative disorders (127) |
| nervous system malformation (122) | gastroenteritis (97) | autoimmune disease of endcrine system (61) | muscular disease (113) | dermatitis (78) | immune ststem cancer (99) |
| nervous system trauma (98) | biliary tract disease (66) | thyroid gland disease (53) | musculoskeletal system cancer (93) | collagen disease (65) | gastroenteritis (97) |
| autoimmune disease of the nervous system (83) | esophageal disease (56) | dwarfism (29) | jaw disease (37) | eczematous skin disease (57) | dermatitis (78) |
| congenital nervous system abnormality (75) | autoimmune disease of gastrointestinal tract (55) | adrenal gland disease (24) | musculoskeketal system benign neoplasm (10) | infectious skin disease (62) | pneumonia (79) |
| A. Biological Process | ||||
| Rank | Term (ID) | p * | Count # | Ref Count & |
| 1 | immune system process (GO:0002376) | 5.93 × 10−76 | 422 | 3246 |
| 2 | immune response (GO:0006955) | 3.97 × 10−74 | 331 | 2154 |
| 3 | adaptive immune response (GO:0002250) | 1.28 × 10−65 | 186 | 805 |
| 4 | defense response (GO:0006952) | 6.89 × 10−52 | 286 | 2067 |
| 5 | response to external stimulus (GO:0009605) | 6.18 × 10−45 | 382 | 3525 |
| 6 | biological process involved in interspecies interaction between organisms (GO:0044419) | 8.29 × 10−42 | 269 | 2093 |
| 7 | response to stimulus (GO:0050896) | 3.90 × 10−39 | 752 | 10120 |
| 8 | response to other organism (GO:0051707) | 1.58 × 10−38 | 249 | 1925 |
| 9 | response to biotic stimulus (GO:0009607) | 1.93 × 10−38 | 253 | 1977 |
| 10 | response to external biotic stimulus (GO:0043207) | 2.09 × 10−38 | 249 | 1928 |
| 11 | response to stress (GO:0006950) | 2.16 × 10−38 | 443 | 4670 |
| 12 | defense response to other organism (GO:0098542) | 3.13 × 10−38 | 198 | 1327 |
| 13 | complement activation classical pathway (GO:0006958) | 3.51 × 10−38 | 59 | 123 |
| 14 | leukocyte activation (GO:0045321) | 1.03 × 10−37 | 183 | 1171 |
| 15 | humoral immune response (GO:0006959) | 1.11 × 10−37 | 101 | 394 |
| 16 | humoral immune response mediated by circulating immunoglobulin (GO:0002455) | 6.29 × 10−37 | 61 | 138 |
| 17 | adaptive immune response based on somatic recombination of immune receptors built from immunoglobulin superfamily domains (GO:0002460) | 8.14 × 10−37 | 103 | 418 |
| 18 | complement activation (GO:0006956) | 5.99 × 10−36 | 63 | 153 |
| 19 | cell activation (GO:0001775) | 1.53 × 10−35 | 194 | 1337 |
| 20 | innate immune response (GO:0045087) | 3.71 × 10−35 | 166 | 1035 |
| 21 | leukocyte mediated immunity (GO:0002443) | 4.46 × 10−35 | 113 | 520 |
| 22 | lymphocyte mediated immunity (GO:0002449) | 6.42 × 10−35 | 99 | 405 |
| 23 | positive regulation of immune system process (GO:0002684) | 1.03 × 10−34 | 172 | 1108 |
| 24 | immune effector process (GO:0002252) | 3.67 × 10−34 | 140 | 784 |
| 25 | immunoglobulin mediated immune response (GO:0016064) | 5.01 × 10−34 | 74 | 230 |
| 26 | B cell mediated immunity (GO:0019724) | 2.63 × 10−33 | 74 | 235 |
| 27 | regulation of immune system process (GO:0002682) | 1.57 × 10−32 | 221 | 1729 |
| 28 | phagocytosis recognition (GO:0006910) | 4.59 × 10−31 | 54 | 128 |
| 29 | positive regulation of B cell activation (GO:0050871) | 5.68 × 10−31 | 63 | 180 |
| 30 | regulation of B cell activation (GO:0050864) | 1.24 × 10−30 | 72 | 241 |
| 31 | regulation of leukocyte activation (GO:0002694) | 5.52 × 10−30 | 132 | 771 |
| 32 | positive regulation of immune response (GO:0050778) | 1.3 × 10−29 | 123 | 687 |
| 33 | phagocytosis engulfment (GO:0006911) | 6.23 × 10−29 | 56 | 150 |
| 34 | cell surface receptor signaling pathway (GO:0007166) | 1.92 × 10−28 | 319 | 3186 |
| 35 | plasma membrane invagination (GO:0099024) | 2.15 × 10−28 | 57 | 159 |
| 36 | response to bacterium (GO:0009617) | 2.31 × 10−28 | 161 | 1114 |
| 37 | inflammatory response (GO:0006954) | 3.21 × 10−28 | 149 | 983 |
| 38 | lymphocyte activation (GO:0046649) | 3.59 × 10−28 | 148 | 973 |
| 39 | membrane invagination (GO:0010324) | 6.48 × 10−28 | 58 | 168 |
| 40 | positive regulation of response to stimulus (GO:0048584) | 1.09 × 10−27 | 272 | 2545 |
| B. Cellular Component | ||||
| Rank | Term (ID) | p * | Count # | Ref Count & |
| 1 | immunoglobulin complex (GO:0019814) | 2.22 × 10−95 | 114 | 186 |
| 2 | extracellular space (GO:0005615) | 2.41 × 10−51 | 409 | 3812 |
| 3 | external side of plasma membrane (GO:0009897) | 6.87 × 10−42 | 124 | 558 |
| 4 | extracellular region (GO:0005576) | 8.80 × 10−42 | 453 | 4820 |
| 5 | immunoglobulin complex circulating (GO:0042571) | 7.25 × 10−39 | 51 | 90 |
| 6 | cell surface (GO:0009986) | 7.98 × 10−35 | 172 | 1147 |
| 7 | side of membrane (GO:0098552) | 9.48 × 10−34 | 140 | 822 |
| 8 | cell periphery (GO:0071944) | 7.43 × 10−26 | 518 | 6662 |
| 9 | blood microparticle (GO:0072562) | 6.74 × 10−22 | 48 | 153 |
| 10 | plasma membrane (GO:0005886) | 6.93 × 10−22 | 476 | 6160 |
| 11 | vesicle (GO:0031982) | 8.59 × 10−20 | 362 | 4363 |
| 12 | extracellular exosome (GO:0070062) | 4.55 × 10−16 | 214 | 2254 |
| 13 | membrane (GO:0016020) | 1.96 × 10−14 | 674 | 10362 |
| 14 | extracellular vesicle (GO:1903561) | 3.25 × 10−14 | 215 | 2354 |
| 15 | extracellular organelle (GO:0043230) | 4.13 × 10−14 | 215 | 2359 |
| 16 | extracellular membran × 10−bounded organelle (GO:0065010) | 4.13 × 10−14 | 215 | 2359 |
| 17 | intracellular vesicle (GO:0097708) | 5.91 × 10−12 | 229 | 2684 |
| 18 | cytoplasmic vesicle (GO:0031410) | 9.94 × 10−12 | 228 | 2681 |
| 19 | secretory granule lumen (GO:0034774) | 3.68 × 10−10 | 52 | 324 |
| 20 | cytoplasmic vesicle lumen (GO:0060205) | 5.33 × 10−10 | 52 | 327 |
| 21 | vesicle lumen (GO:0031983) | 6.80 × 10−10 | 52 | 329 |
| 22 | lytic vacuole (GO:0000323) | 9.38 × 10−9 | 89 | 801 |
| 23 | lysosome (GO:0005764) | 9.38 × 10−9 | 89 | 801 |
| 24 | protein-containing complex (GO:0032991) | 9.66 × 10−9 | 450 | 6667 |
| 25 | vacuole (GO:0005773) | 3.28 × 10−8 | 95 | 900 |
| 26 | secretory vesicle (GO:0099503) | 6.92 × 10−8 | 115 | 1190 |
| 27 | secretory granule (GO:0030141) | 8.02 × 10−8 | 97 | 942 |
| 28 | IgG immunoglobulin complex (GO:0071735) | 7.01 × 10−6 | 8 | 11 |
| 29 | endomembrane system (GO:0012505) | 9.94 × 10−6 | 340 | 5003 |
| 30 | vacuolar lumen (GO:0005775) | 1.18 × 10−4 | 28 | 176 |
| 31 | endocytic vesicle (GO:0030139) | 1.23 × 10−4 | 45 | 372 |
| 32 | collagen-containing extracellular matrix (GO:0062023) | 1.60 × 10−4 | 52 | 464 |
| 33 | membrane raft (GO:0045121) | 1.90 × 10−4 | 49 | 428 |
| 34 | cellular anatomical entity (GO:0110165) | 1.95 × 10−4 | 1072 | 20217 |
| 35 | membrane microdomain (GO:0098857) | 2.04 × 10−4 | 49 | 429 |
| 36 | tertiary granule lumen (GO:1904724) | 5.41 × 10−4 | 14 | 55 |
| 37 | extracellular matrix (GO:0031012) | 1.06 × 10−3 | 60 | 602 |
| 38 | external encapsulating structure (GO:0030312) | 1.18 × 10−3 | 60 | 604 |
| 39 | cytoplasm (GO:0005737) | 1.26 × 10−3 | 729 | 12592 |
| 40 | specific granule lumen (GO:0035580) | 2.57 × 10−3 | 14 | 62 |
| C. Molecular Function | ||||
| Rank | Term (ID) | p * | Count # | Ref Count & |
| 1 | antigen binding (GO:0003823) | 9.64 × 10−65 | 96 | 201 |
| 2 | immunoglobulin receptor binding (GO:0034987) | 1.43 × 10−36 | 51 | 95 |
| 3 | signaling receptor binding (GO:0005102) | 9.52 × 10−21 | 194 | 1748 |
| 4 | cytokine activity (GO:0005125) | 1.75 × 10−14 | 51 | 240 |
| 5 | cytokine receptor binding (GO:0005126) | 3.61 × 10−13 | 55 | 294 |
| 6 | immune receptor activity (GO:0140375) | 3.27 × 10−11 | 37 | 160 |
| 7 | chemokine activity (GO:0008009) | 4.59 × 10−10 | 20 | 50 |
| 8 | signaling receptor activator activity (GO:0030546) | 1.85 × 10−9 | 70 | 525 |
| 9 | receptor ligand activity (GO:0048018) | 2.34 × 10−9 | 69 | 516 |
| 10 | chemokine receptor binding (GO:0042379) | 5.32 × 10−9 | 23 | 75 |
| 11 | signaling receptor regulator activity (GO:0030545) | 7.91 × 10−9 | 73 | 577 |
| 12 | protein binding (GO:0005515) | 2.21 × 10−8 | 895 | 15279 |
| 13 | cytokine binding (GO:0019955) | 2.29 × 10−8 | 32 | 151 |
| 14 | identical protein binding (GO:0042802) | 3.85 × 10−8 | 200 | 2394 |
| 15 | cytokine receptor activity (GO:0004896) | 7.13 × 10−8 | 25 | 99 |
| 16 | chemokine binding (GO:0019956) | 8.63 × 10−6 | 13 | 33 |
| 17 | binding (GO:0005488) | 8.63 × 10−5 | 985 | 17676 |
| 18 | growth factor activity (GO:0008083) | 1.00 × 10−4 | 28 | 167 |
| 19 | C-C chemokine binding (GO:0019957) | 2.76 × 10−4 | 10 | 24 |
| 20 | heparin binding (GO:0008201) | 2.97 × 10−4 | 29 | 186 |
| 21 | growth factor binding (GO:0019838) | 3.31 × 10−4 | 26 | 156 |
| 22 | enzyme binding (GO:0019899) | 1.75 × 10−3 | 177 | 2362 |
| 23 | C-C chemokine receptor activity (GO:0016493) | 2.19 × 10−3 | 9 | 23 |
| 24 | CXCR chemokine receptor binding (GO:0045236) | 2.70 × 10−3 | 8 | 18 |
| 25 | CCR chemokine receptor binding (GO:0048020) | 2.94 × 10−3 | 13 | 51 |
| 26 | G protein-coupled chemoattractant receptor activity (GO:0001637) | 7.23 × 10−3 | 9 | 26 |
| 27 | chemokine receptor activity (GO:0004950) | 7.23 × 10−3 | 9 | 26 |
| 28 | glycosaminoglycan binding (GO:0005539) | 7.48 × 10−3 | 33 | 264 |
| 29 | protein homodimerization activity (GO:0042803) | 1.00 × 10−2 | 69 | 749 |
| 30 | growth factor receptor binding (GO:0070851) | 1.13 × 10−2 | 23 | 154 |
| 31 | G protein-coupled receptor binding (GO:0001664) | 1.31 × 10−2 | 38 | 333 |
| 32 | molecular function regulator (GO:0098772) | 3.08 × 10−2 | 153 | 2082 |
| 33 | exogenous protein binding (GO:0140272) | 3.91 × 10−2 | 15 | 82 |
| 34 | kinase binding (GO:0019900) | 4.78 × 10−2 | 77 | 904 |
| 35 | cell adhesion molecule binding (GO:0050839) | 4.97 × 10−2 | 55 | 585 |
| 36 | protease binding (GO:0002020) | 6.58 × 10−2 | 22 | 160 |
| 37 | sulfur compound binding (GO:1901681) | 7.12 × 10−2 | 34 | 307 |
| 38 | carbohydrate derivative binding (GO:0097367) | 1.10 × 10−1 | 168 | 2383 |
| 39 | virus receptor activity (GO:0001618) | 1.38 × 10−1 | 14 | 81 |
| 40 | protein-containing complex binding (GO:0044877) | 1.49 × 10−1 | 122 | 1642 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.-J.; Brodie, K.C.; De Pons, J.L.; Demos, W.M.; Gibson, A.C.; Hayman, G.T.; Hill, M.L.; Kaldunski, M.L.; Lamers, L.; Laulederkind, S.J.F.; et al. Ontological Analysis of Coronavirus Associated Human Genes at the COVID-19 Disease Portal. Genes 2022, 13, 2304. https://doi.org/10.3390/genes13122304
Wang S-J, Brodie KC, De Pons JL, Demos WM, Gibson AC, Hayman GT, Hill ML, Kaldunski ML, Lamers L, Laulederkind SJF, et al. Ontological Analysis of Coronavirus Associated Human Genes at the COVID-19 Disease Portal. Genes. 2022; 13(12):2304. https://doi.org/10.3390/genes13122304
Chicago/Turabian StyleWang, Shur-Jen, Kent C. Brodie, Jeffrey L. De Pons, Wendy M. Demos, Adam C. Gibson, G. Thomas Hayman, Morgan L. Hill, Mary L. Kaldunski, Logan Lamers, Stanley J. F. Laulederkind, and et al. 2022. "Ontological Analysis of Coronavirus Associated Human Genes at the COVID-19 Disease Portal" Genes 13, no. 12: 2304. https://doi.org/10.3390/genes13122304
APA StyleWang, S.-J., Brodie, K. C., De Pons, J. L., Demos, W. M., Gibson, A. C., Hayman, G. T., Hill, M. L., Kaldunski, M. L., Lamers, L., Laulederkind, S. J. F., Nalabolu, H. S., Thota, J., Thorat, K., Tutaj, M. A., Tutaj, M., Vedi, M., Zacher, S., Smith, J. R., Dwinell, M. R., & Kwitek, A. E. (2022). Ontological Analysis of Coronavirus Associated Human Genes at the COVID-19 Disease Portal. Genes, 13(12), 2304. https://doi.org/10.3390/genes13122304







