Investigating the Impact of a Curse: Diseases, Population Isolation, Evolution and the Mother’s Curse
Abstract
1. Introduction
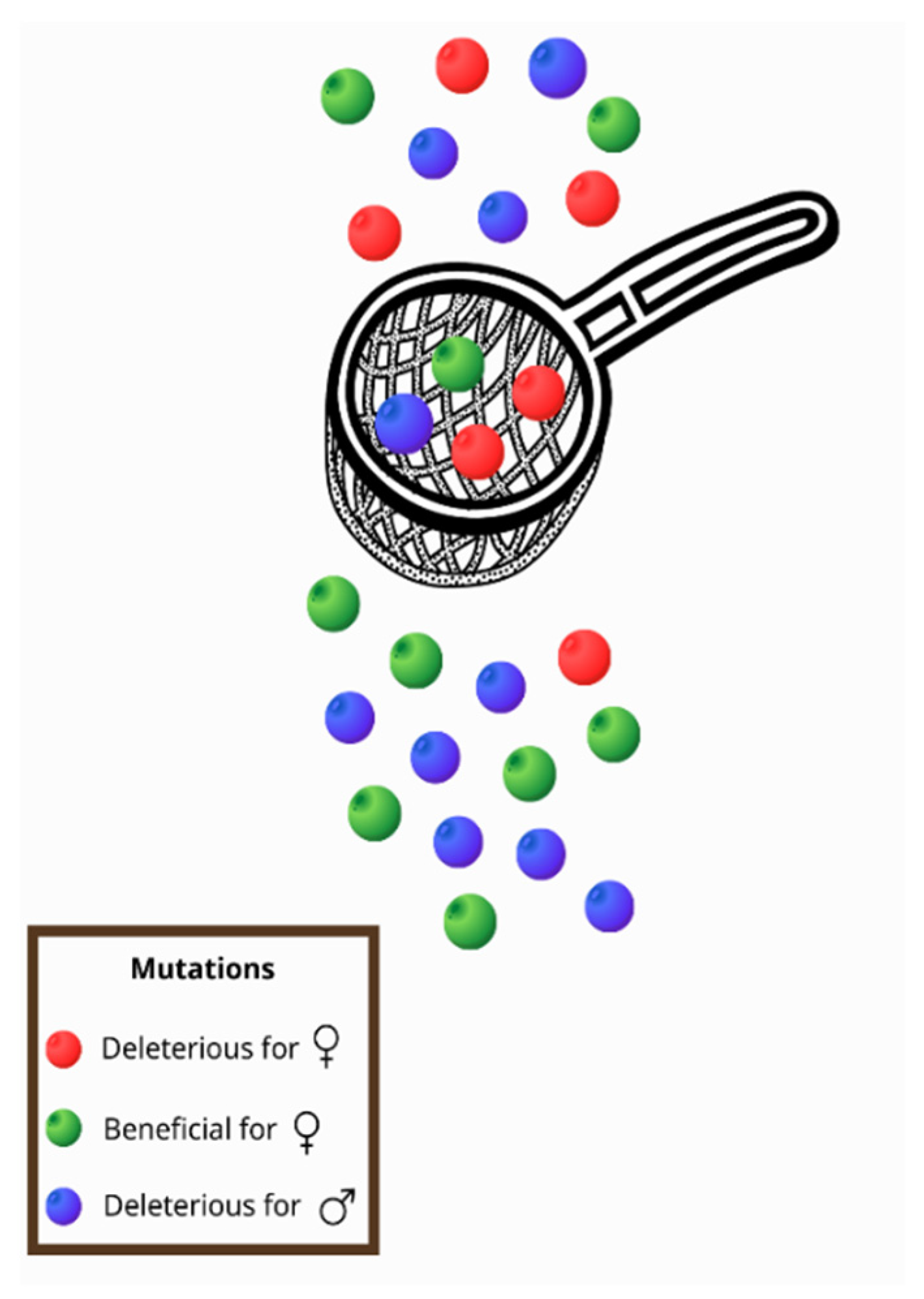
2. The Bad Luck of Being Male—The MC Is Associated with Diseases That Mainly Affect Male Fitness
2.1. Leber’s Hereditary Optic Neuropathy: The First Record of the MC Effect in Humans
2.2. Impact of the MC on the Dimorphic Characters—The Examples of Metabolism and Male Infertility
2.3. MC and the Aging of Males
3. MC Can Lead to the Reproductive Isolation between Populations Affecting Speciation Events
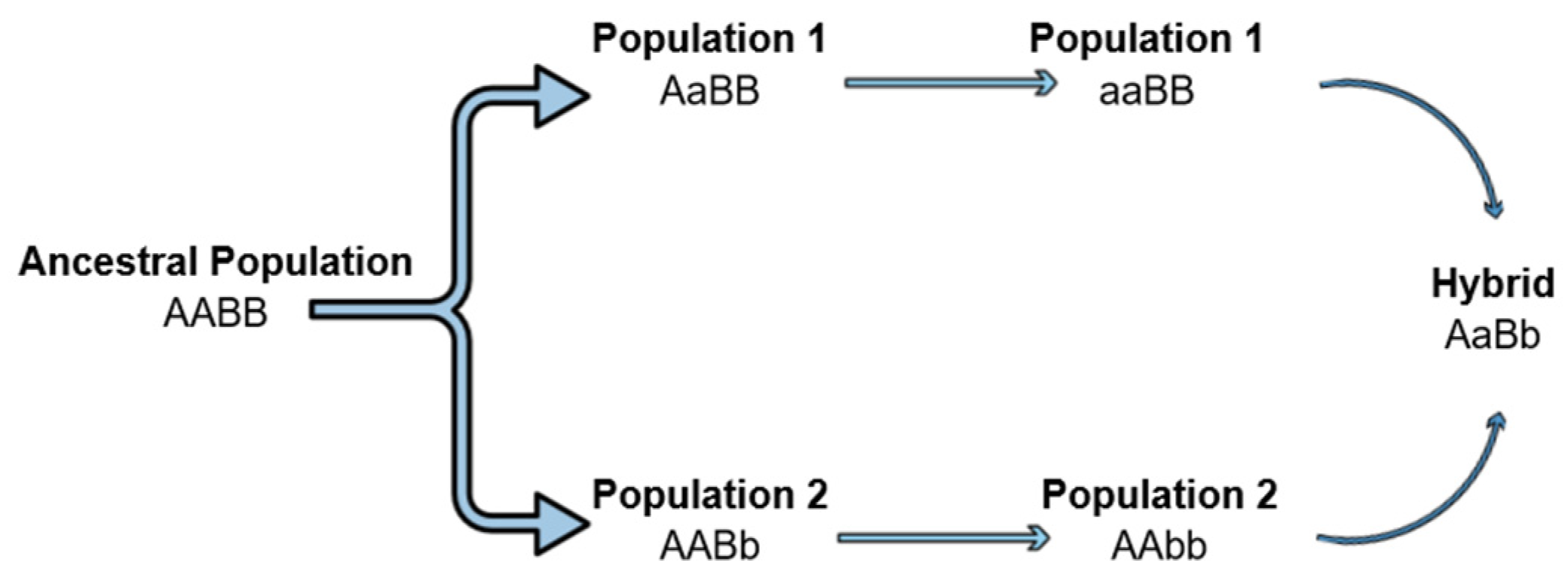
3.1. MC Impact on the Hybrid Breakdown Observed in the Laboratory
3.2. The Fate of the Hybrids and the MC: Examples from Natural Laboratories
3.3. MC as a Step Leading to Speciation, the Driving Forces behind It and the Role of the Environment
4. Future Perspectives and Applications
4.1. MC Application for Pest Management
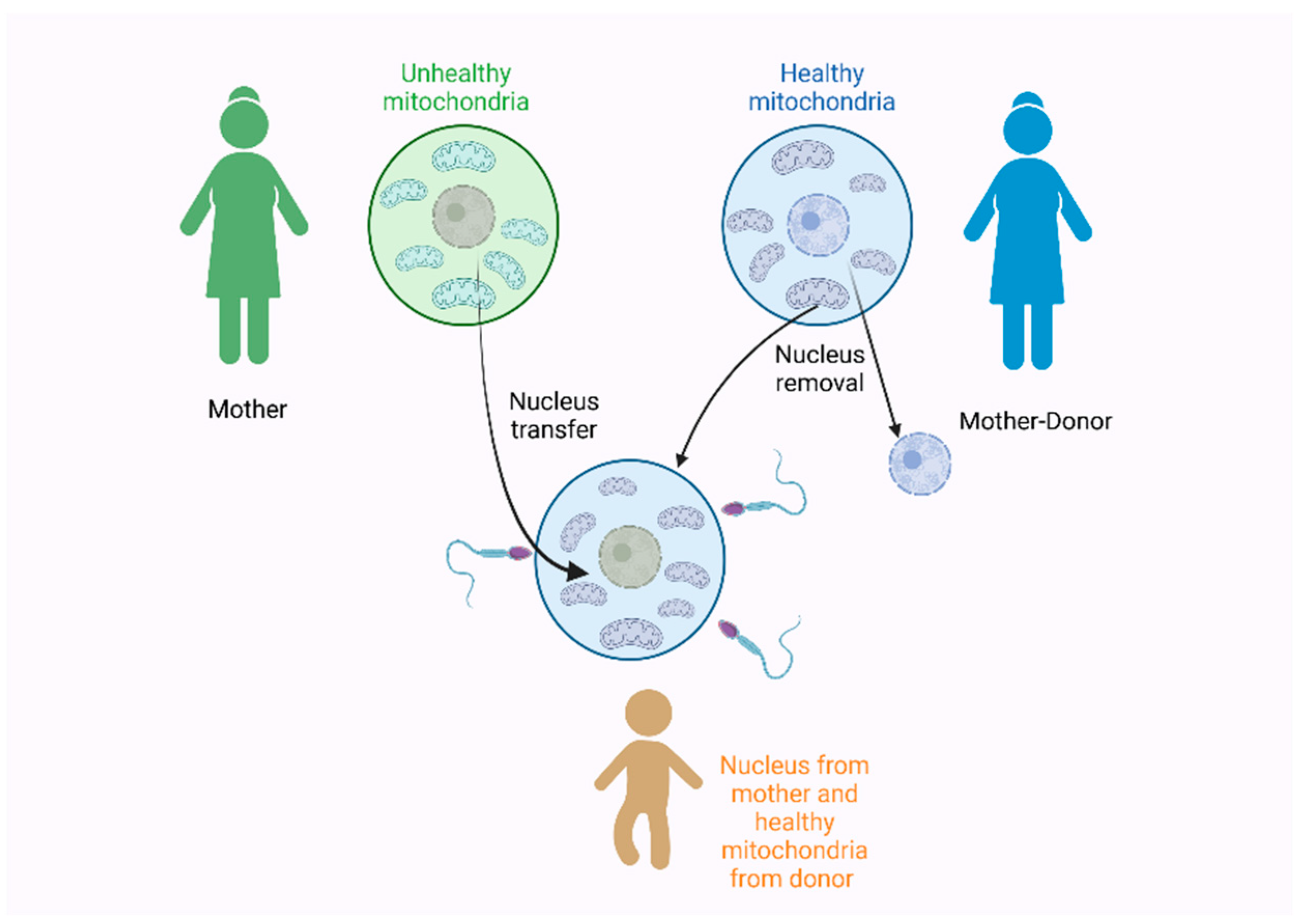
4.2. Implications and Applications of the MC in Genetic Diseases and Medicine—Ethical Considerations
4.3. The Mito-Nuclear Coevolution Challenge in Bivalves
5. Why Is the MC Under-Recorded? The Reverse of a Curse
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Archibald, J.M. Endosymbiosis and Eukaryotic Cell Evolution. Curr. Biol. 2015, 25, R911–R921. [Google Scholar] [CrossRef]
- Martin, W.F.; Garg, S.; Zimorski, V. Endosymbiotic Theories for Eukaryote Origin. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140330. [Google Scholar] [CrossRef]
- Roger, A.J.; Muñoz-Gómez, S.A.; Kamikawa, R. The Origin and Diversification of Mitochondria. Curr. Biol. 2017, 27, R1177–R1192. [Google Scholar] [CrossRef]
- Carvalho, D.S.; Andrade, R.F.S.; Pinho, S.T.R.; Góes-Neto, A.; Lobão, T.C.P.; Bomfim, G.C.; El-Hani, C.N. What Are the Evolutionary Origins of Mitochondria? A Complex Network Approach. PLoS ONE 2015, 10, e0134988. [Google Scholar] [CrossRef]
- Lynch, M.; Koskella, B.; Schaack, S. Mutation Pressure and the Evolution of Organelle Genomic Architecture. Science 2006, 311, 1727–1730. [Google Scholar] [CrossRef]
- Smith, D.R.; Keeling, P.J. Mitochondrial and Plastid Genome Architecture: Reoccurring Themes, but Significant Differences at the Extremes. Proc. Natl. Acad. Sci. USA 2015, 112, 10177–10184. [Google Scholar] [CrossRef]
- Kelly, S. The Economics of Organellar Gene Loss and Endosymbiotic Gene Transfer. Genome Biol. 2021, 22, 345. [Google Scholar] [CrossRef]
- Tang, J.X.; Thompson, K.; Taylor, R.W.; Oláhová, M. Mitochondrial OXPHOS Biogenesis: Co-Regulation of Protein Synthesis, Import, and Assembly Pathways. Int. J. Mol. Sci. 2020, 21, 3820. [Google Scholar] [CrossRef]
- Siekevitz, P. Powerhouse of the Cell. Sci. Am. 1957, 197, 131–144. [Google Scholar] [CrossRef]
- Formosa, L.E.; Ryan, M.T. Mitochondrial OXPHOS Complex Assembly Lines. Nat. Cell Biol. 2018, 20, 511–513. [Google Scholar] [CrossRef]
- Amoutzias, G.D.; Giannoulis, T.; Moutou, K.A.; Psarra, A.M.G.; Stamatis, C.; Tsipourlianos, A.; Mamuris, Z. SNP Identification through Transcriptome Analysis of the European Brown Hare (Lepus europaeus): Cellular Energetics and Mother’s Curse. PLoS ONE 2016, 11, e0159939. [Google Scholar] [CrossRef][Green Version]
- Herst, P.M.; Rowe, M.R.; Carson, G.M.; Berridge, M.V. Functional Mitochondria in Health and Disease. Front. Endocrinol. 2017, 8, 296. [Google Scholar] [CrossRef]
- Abate, M.; Festa, A.; Falco, M.; Lombardi, A.; Luce, A.; Grimaldi, A.; Zappavigna, S.; Sperlongano, P.; Irace, C.; Caraglia, M.; et al. Mitochondria as Playmakers of Apoptosis, Autophagy and Senescence. Semin. Cell Dev. Biol. 2020, 98, 139–153. [Google Scholar] [CrossRef]
- Tait, S.W.G.; Green, D.R. Mitochondria and Cell Signalling. J. Cell Sci. 2012, 125, 807–815. [Google Scholar] [CrossRef]
- Quirós, P.M.; Mottis, A.; Auwerx, J. Mitonuclear Communication in Homeostasis and Stress. Nat. Rev. Mol. Cell Biol. 2016, 17, 213–226. [Google Scholar] [CrossRef]
- Mottis, A.; Herzig, S.; Auwerx, J. Mitocellular Communication: Shaping Health and Disease. Science 2019, 366, 827–832. [Google Scholar] [CrossRef]
- Schirrmacher, V. Mitochondria at Work: New Insights into Regulation and Dysregulation of Cellular Energy Supply and Metabolism. Biomedicines 2020, 8, 526. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, D.; Zhang, D.; Li, P.; Gao, Y. Mitochondrial Protein Translation: Emerging Roles and Clinical Significance in Disease. Front. Cell Dev. Biol. 2021, 9, 675465. [Google Scholar] [CrossRef]
- García-Lepe, U.O.; Bermúdez-Cruz, R.M. Mitochondrial Genome Maintenance: Damage and Repair Pathways. In DNA Repair—An Update; IntechOpen: London, UK, 2019; ISBN 978-1-83880-783-2. [Google Scholar]
- Lawless, C.; Greaves, L.; Reeve, A.K.; Turnbull, D.M.; Vincent, A.E. The Rise and Rise of Mitochondrial DNA Mutations. Open Biol. 2020, 10, 200061. [Google Scholar] [CrossRef]
- Gustafsson, C.M.; Falkenberg, M.; Larsson, N.G. Maintenance and Expression of Mammalian Mitochondrial DNA. Annu. Rev. Biochem. 2016, 85, 133–160. [Google Scholar] [CrossRef]
- Ladoukakis, E.D.; Zouros, E. Evolution and Inheritance of Animal Mitochondrial DNA: Rules and Exceptions. J. Biol. Res. 2017, 24, 2. [Google Scholar] [CrossRef]
- Neiman, M.; Taylor, D.R. The Causes of Mutation Accumulation in Mitochondrial Genomes. Proc. R. Soc. B Biol. Sci. 2009, 276, 1201–1209. [Google Scholar] [CrossRef]
- Muller, H.J. The Relation of Recombination to Mutational Advance. Mutat. Res. Mol. Mech. Mutagen. 1964, 1, 2–9. [Google Scholar] [CrossRef]
- Kauppila, T.E.S.; Bratic, A.; Jensen, M.B.; Baggio, F.; Partridge, L.; Jasper, H.; Grönke, S.; Larsson, N.G. Mutations of Mitochondrial DNA Are Not Major Contributors to Aging of Fruit Flies. Proc. Natl. Acad. Sci. USA 2018, 115, E9620–E9629. [Google Scholar] [CrossRef]
- Sharp, N.P.; Sandell, L.; James, C.G.; Otto, S.P. The Genome-Wide Rate and Spectrum of Spontaneous Mutations Differ between Haploid and Diploid Yeast. Proc. Natl. Acad. Sci. USA 2018, 115, E5046–E5055. [Google Scholar] [CrossRef]
- Sato, M.; Sato, K. Maternal Inheritance of Mitochondrial DNA by Diverse Mechanisms to Eliminate Paternal Mitochondrial DNA. Biochim. Biophys. Acta Mol. Cell Res. 2013, 1833, 1979–1984. [Google Scholar] [CrossRef]
- Gemmell, N.J.; Metcalf, V.J.; Allendorf, F.W. Mother’s Curse: The Effect of MtDNA on Individual Fitness and Population Viability. Trends Ecol. Evol. 2004, 19, 238–244. [Google Scholar] [CrossRef]
- Frank, S.A.; Hurst, L.D. Mitochondria and Male Disease. Nature 1996, 383, 224. [Google Scholar] [CrossRef]
- Beekman, M.; Dowling, D.K.; Aanen, D.K. The Costs of Being Male: Are There Sex-Specific Effects of Uniparental Mitochondrial Inheritance? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130440. [Google Scholar] [CrossRef]
- Dowling, D.K.; Friberg, U.; Lindell, J. Evolutionary Implications of Non-Neutral Mitochondrial Genetic Variation. Trends Ecol. Evol. 2008, 23, 546–554. [Google Scholar] [CrossRef]
- Keaney, T.A.; Wong, H.W.S.; Dowling, D.K.; Jones, T.M.; Holman, L. Mother’s Curse and Indirect Genetic Effects: Do Males Matter to Mitochondrial Genome Evolution? J. Evol. Biol. 2020, 33, 189–201. [Google Scholar] [CrossRef]
- Innocenti, P.; Morrow, E.H.; Dowling, D.K. Experimental Evidence Supports a Sex-Specific Selective Sieve in Mitochondrial Genome Evolution. Science 2011, 332, 845–848. [Google Scholar] [CrossRef]
- Ray, A.; Martinez, B.A.; Berkowitz, L.A.; Caldwell, G.A.; Caldwell, K.A. Mitochondrial Dysfunction, Oxidative Stress, and Neurodegeneration Elicited by a Bacterial Metabolite in a C. Elegans Parkinson’s Model. Cell Death Dis. 2014, 5, e984. [Google Scholar] [CrossRef]
- Fong, S.; Teo, E.; Ng, L.F.; Chen, C.B.; Lakshmanan, L.N.; Tsoi, S.Y.; Moore, P.K.; Inoue, T.; Halliwell, B.; Gruber, J. Energy Crisis Precedes Global Metabolic Failure in a Novel Caenorhabditis Elegans Alzheimer Disease Model. Sci. Rep. 2016, 6, 33781. [Google Scholar] [CrossRef]
- Swerdlow, R.H. Mitochondria and Mitochondrial Cascades in Alzheimer’s Disease. J. Alzheimers. Dis. 2018, 62, 1403–1416. [Google Scholar] [CrossRef]
- Milot, E.; Moreau, C.; Gagnon, A.; Cohen, A.A.; Brais, B.; Labuda, D. Mother’s Curse Neutralizes Natural Selection against a Human Genetic Disease over Three Centuries. Nat. Ecol. Evol. 2017, 1, 1400–1406. [Google Scholar] [CrossRef]
- MacMillan, C.; Johns, T.A.; Fu, K.; Shoubridge, E.A. Predominance of the T14484C Mutation in French-Canadian Families with Leber Hereditary Optic Neuropathy Is Due to a Founder Effect. Am. J. Hum. Genet. 2000, 66, 332–335. [Google Scholar] [CrossRef]
- Macmillan, C.; Kirkham, T.; Fu, K.; Allison, V.; Andermann, E.; Chitayat, D.; Fortier, D.; Gans, M.; Hare, H.; Quercia, N.; et al. Pedigree Analysis of French Canadian Families with T14484C Leber’s Hereditary Optic Neuropathy. Neurology 1998, 50, 417–422. [Google Scholar] [CrossRef]
- Leber, T. Ueber Hereditäre Und Congenital-Angelegte Sehnervenleiden. Albrecht Von Graefes Arch. Für Ophthalmol. 1871, 17, 249–291. [Google Scholar] [CrossRef]
- Hage, R.; Vignal-Clermont, C. Leber Hereditary Optic Neuropathy: Review of Treatment and Management. Front. Neurol. 2021, 12, 651639. [Google Scholar] [CrossRef]
- Laberge, A.M.; Jomphe, M.; Houde, L.; Vézina, H.; Tremblay, M.; Desjardins, B.; Labuda, D.; St-Hilaire, M.; Macmillan, C.; Shoubridge, E.A.; et al. A “Fille Du Roy” Introduced the T14484C Leber Hereditary Optic Neuropathy Mutation in French Canadians. Am. J. Hum. Genet. 2005, 77, 313–317. [Google Scholar] [CrossRef][Green Version]
- Moorad, J.A. Individual Fitness and Phenotypic Selection in Age-Structured Populations with Constant Growth Rates. Ecology 2014, 95, 1087–1095. [Google Scholar] [CrossRef]
- Delaney, D.M.; Hoekstra, L.A.; Janzen, F.J. Age Predicts Risky Investment Better Than Residual Reproductive Value. Am. Nat. 2021, 197, 461–472. [Google Scholar] [CrossRef]
- Moorad, J.A. A Demographic Transition Altered the Strength of Selection for Fitness and Age-Specific Survival and Fertility in a 19th Century American Population. Evolution 2013, 67, 1622–1634. [Google Scholar] [CrossRef]
- Gemmell, N. The Curse of the Filles Du Roy. Nat. Ecol. Evol. 2017, 1, 1228–1229. [Google Scholar] [CrossRef]
- Moore, A.J. The Evolution of Sexual Dimorphism by Sexual Selection: The Separate Effects of Intrasexual Selection and Intersexual Selection. Evolution 1990, 44, 315–331. [Google Scholar] [CrossRef]
- Carnegie, L.; Reuter, M.; Fowler, K.; Lane, N.; Camus, M.F. Mother’s Curse Is Pervasive across a Large Mitonuclear Drosophila Panel. Evol. Lett. 2021, 5, 230–239. [Google Scholar] [CrossRef]
- Aw, W.C.; Garvin, M.R.; Melvin, R.G.; Ballard, J.W.O. Sex-Specific Influences of MtDNA Mitotype and Diet on Mitochondrial Functions and Physiological Traits in Drosophila Melanogaster. PLoS ONE 2017, 12, e0187554. [Google Scholar] [CrossRef]
- Geer, E.B.; Shen, W. Gender Differences in Insulin Resistance, Body Composition, and Energy Balance. Gend. Med. 2009, 6 (Suppl. 1), 60–75. [Google Scholar] [CrossRef]
- Wu, B.N.; O’Sullivan, A.J. Sex Differences in Energy Metabolism Need to Be Considered with Lifestyle Modifications in Humans. J. Nutr. Metab. 2011, 2011, 391809. [Google Scholar] [CrossRef]
- Nagarajan-Radha, V.; Aitkenhead, I.; Clancy, D.J.; Chown, S.L.; Dowling, D.K. Sex-Specific Effects of Mitochondrial Haplotype on Metabolic Rate in Drosophila Melanogaster Support Predictions of the Mother’s Curse Hypothesis. Philos. Trans. R. Soc. B Biol Sci 2020, 375, 20190178. [Google Scholar] [CrossRef]
- Novičić, Z.K.; Immonen, E.; Jelić, M.; Anelković, M.; Stamenković-Radak, M.; Arnqvist, G. Within-Population Genetic Effects of MtDNA on Metabolic Rate in Drosophila Subobscura. J. Evol. Biol. 2015, 28, 338–346. [Google Scholar] [CrossRef]
- Videlier, M.; Careau, V.; Wilson, A.J.; Rundle, H.D. Quantifying Selection on Standard Metabolic Rate and Body Mass in Drosophila Melanogaster. Evolution 2021, 75, 130–140. [Google Scholar] [CrossRef]
- Visconti, P.E. Sperm Bioenergetics in a Nutshell. Biol. Reprod. 2012, 87, 72–73. [Google Scholar] [CrossRef]
- Du Plessis, S.S.; Agarwal, A.; Mohanty, G.; Van Der Linde, M. Oxidative Phosphorylation versus Glycolysis: What Fuel Do Spermatozoa Use? Asian J. Androl. 2015, 17, 230–235. [Google Scholar] [CrossRef]
- Misro, M.M.; Ramya, T. Fuel/Energy Sources of Spermatozoa. In Male Infertility: Contemporary Clinical Approaches, Andrology, ART and Antioxidants; Springer: New York, NY, USA, 2012; pp. 209–223. ISBN 9781461433354. [Google Scholar]
- Smith, S.; Turbill, C.; Suchentrunk, F. Introducing Mother’s Curse: Low Male Fertility Associated with an Imported MtDNA Haplotype in a Captive Colony of Brown Hares. Mol. Ecol. 2010, 19, 36–43. [Google Scholar] [CrossRef]
- Dowling, D.K.; Nowostawski, A.L.; Arnqvist, G. Effects of Cytoplasmic Genes on Sperm Viability and Sperm Morphology in a Seed Beetle: Implications for Sperm Competition Theory? J. Evol. Biol. 2007, 20, 358–368. [Google Scholar] [CrossRef]
- Froman, D.P.; Kirby, J.D. Sperm Mobility: Phenotype in Roosters (Gallus domesticus) Determined by Mitochondrial Function. Biol. Reprod. 2005, 72, 562–567. [Google Scholar] [CrossRef]
- Yee, W.K.W.; Sutton, K.L.; Dowling, D.K. In Vivo Male Fertility Is Affected by Naturally Occurring Mitochondrial Haplotypes. Curr. Biol. 2013, 23, R55–R56. [Google Scholar] [CrossRef]
- Patel, M.R.; Miriyala, G.K.; Littleton, A.J.; Yang, H.; Trinh, K.; Young, J.M.; Kennedy, S.R.; Yamashita, Y.M.; Pallanck, L.J.; Malik, H.S. A Mitochondrial DNA Hypomorph of Cytochrome Oxidase Specifically Impairs Male Fertility in Drosophila Melanogaster. elife 2016, 5, e16923. [Google Scholar] [CrossRef]
- Shamsi, M.B.; Kumar, R.; Bhatt, A.; Bamezai, R.N.K.; Kumar, R.; Gupta, N.P.; Das, T.K.; Dada, R. Mitochondrial DNA Mutations in Etiopathogenesis of Male Infertility. Indian J. Urol. 2008, 24, 150–154. [Google Scholar]
- Holyoake, A.J.; McHugh, P.; Wu, M.; O’Carroll, S.; Benny, P.; Sin, I.L.; Sin, F.Y.T. High Incidence of Single Nucleotide Substitutions in the Mitochondrial Genome Is Associated with Poor Semen Parameters in Men. Int. J. Androl. 2001, 24, 175–182. [Google Scholar] [CrossRef]
- St. John, J.C.; Jokhi, R.P.; Barratt, C.L.R. The Impact of Mitochondrial Genetics on Male Infertility. Int. J. Androl. 2005, 28, 65–73. [Google Scholar] [CrossRef]
- Al Zoubi, M.S.; Al-Talafha, A.M.; Al Sharu, E.; Al-Trad, B.; Alzu’Bi, A.; AbuAlarjah, M.I.; Shehab, Q.; Alsmadi, M.; Al-Batayneh, K.M. Correlation of Sperm Mitochondrial DNA 7345 Bp and 7599 Bp Deletions with Asthenozoospermia in Jordanian Population. J. Reprod. Infertil. 2021, 22, 165–172. [Google Scholar] [CrossRef]
- Karimian, M.; Babaei, F. Large-Scale MtDNA Deletions as Genetic Biomarkers for Susceptibility to Male Infertility: A Systematic Review and Meta-Analysis. Int. J. Biol. Macromol. 2020, 158, 85–93. [Google Scholar] [CrossRef]
- Dzudzor, B.; Bimah, B.; Amarh, V.; Ocloo, A. Sperm Parameters and Mitochondrial DNA Sequence Variants among Patients at a Fertility Clinic in Ghana. PLoS ONE 2021, 16, e0252923. [Google Scholar] [CrossRef]
- Zhu, C.T.; Ingelmo, P.; Rand, D.M. G×G×E for Lifespan in Drosophila: Mitochondrial, Nuclear, and Dietary Interactions That Modify Longevity. PLoS Genet. 2014, 10, e1004354. [Google Scholar] [CrossRef]
- Camus, M.F.; Moore, J.; Reuter, M. Nutritional Geometry of Mitochondrial Genetic Effects on Male Fertility. Biol. Lett. 2020, 16, 20190891. [Google Scholar] [CrossRef]
- Montooth, K.L.; Dhawanjewar, A.S.; Meiklejohn, C.D. Temperature-Sensitive Reproduction and the Physiological and Evolutionary Potential for Mother’s Curse. Integr. Comp. Biol. 2019, 59, 890–899. [Google Scholar] [CrossRef]
- Wolff, J.N.; Tompkins, D.M.; Gemmell, N.J.; Dowling, D.K. Mitonuclear Interactions, MtDNA-Mediated Thermal Plasticity, and Implications for the Trojan Female Technique for Pest Control. Sci. Rep. 2016, 6, 30016. [Google Scholar] [CrossRef]
- McBride, R.S.; Snodgrass, D.J.G.; Adams, D.H.; Rider, S.J.; Colvocoresses, J.A. An Indeterminate Model to Estimate Egg Production of the Highly Iteroparous and Fecund Fish, Dolphinfish (Coryphaena hippurus). Bull. Mar. Sci. 2012, 88, 283–303. [Google Scholar] [CrossRef]
- Lubzens, E.; Young, G.; Bobe, J.; Cerdà, J. Oogenesis in Teleosts: How Eggs Are Formed. Gen. Comp. Endocrinol. 2010, 165, 367–389. [Google Scholar] [CrossRef] [PubMed]
- Hayward, A.; Gillooly, J.F. The Cost of Sex: Quantifying Energetic Investment in Gamete Production by Males and Females. PLoS ONE 2011, 6, e16557. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Hägg, S.; Jylhävä, J. Sex Differences in Biological Aging with a Focus on Human Studies. elife 2021, 10, 936–944. [Google Scholar] [CrossRef]
- Gordon, E.H.; Hubbard, R.E. Do Sex Differences in Chronic Disease Underpin the Sex-Frailty Paradox? Mech. Ageing Dev. 2019, 179, 44–50. [Google Scholar] [CrossRef]
- Gordon, E.H.; Peel, N.M.; Samanta, M.; Theou, O.; Howlett, S.E.; Hubbard, R.E. Sex Differences in Frailty: A Systematic Review and Meta-Analysis. Exp. Gerontol. 2017, 89, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Austad, S.N.; Fischer, K.E. Sex Differences in Lifespan. Cell Metab. 2016, 23, 1022–1033. [Google Scholar] [CrossRef]
- Sun, N.; Youle, R.J.; Finkel, T. The Mitochondrial Basis of Aging. Mol. Cell 2016, 61, 654–666. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Sobenin, I.A.; Revin, V.V.; Orekhov, A.N.; Bobryshev, Y.V. Mitochondrial Aging and Age-Related Dysfunction of Mitochondria. Biomed Res. Int. 2014, 2014, 238463. [Google Scholar] [CrossRef]
- Lionaki, E.; Gkikas, I.; Daskalaki, I.; Ioannidi, M.-K.; Klapa, M.I.; Tavernarakis, N. Mitochondrial Protein Import Determines Lifespan through Metabolic Reprogramming and de Novo Serine Biosynthesis. Nat. Commun. 2022, 13, 651. [Google Scholar] [CrossRef] [PubMed]
- Zapico, S.C.; Ubelaker, D.H. MtDNA Mutations and Their Role in Aging, Diseases and Forensic Sciences. Aging Dis. 2013, 4, 364–380. [Google Scholar] [CrossRef] [PubMed]
- Sebastián, D.; Palacín, M.; Zorzano, A. Mitochondrial Dynamics: Coupling Mitochondrial Fitness with Healthy Aging. Trends Mol. Med. 2017, 23, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Akbari, M.; Kirkwood, T.B.L.; Bohr, V.A. Mitochondria in the Signaling Pathways That Control Longevity and Health Span. Ageing Res. Rev. 2019, 54, 100940. [Google Scholar] [CrossRef]
- Camus, M.F.; Clancy, D.J.; Dowling, D.K. Mitochondria, Maternal Inheritance, and Male Aging. Curr. Biol. 2012, 22, 1717–1721. [Google Scholar] [CrossRef]
- Wolff, J.N.; Gemmell, N.J. Mitochondria, Maternal Inheritance, and Asymmetric Fitness: Why Males Die Younger. BioEssays 2013, 35, 93–99. [Google Scholar] [CrossRef]
- Camus, M.F.; O’Leary, M.; Reuter, M.; Lane, N. Impact of Mitonuclear Interactions on Life-History Responses to Diet. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2020, 375, 20190416. [Google Scholar] [CrossRef]
- Nagarajan-Radha, V.; Rapkin, J.; Hunt, J.; Dowling, D.K. Interactions between Mitochondrial Haplotype and Dietary Macronutrient Ratios Confer Sex-Specific Effects on Longevity in Drosophila Melanogaster. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1573–1581. [Google Scholar] [CrossRef]
- Flanagan, B.A.; Li, N.; Edmands, S. Mitonuclear Interactions Alter Sex-Specific Longevity in a Species without Sex Chromosomes. Proc. R. Soc. B 2021, 288, 20211813. [Google Scholar] [CrossRef]
- Finkel, T. The Metabolic Regulation of Aging. Nat. Med. 2015, 21, 1416–1423. [Google Scholar] [CrossRef]
- Fontana, L.; Partridge, L. Promoting Health and Longevity through Diet: From Model Organisms to Humans. Cell 2015, 161, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Azzu, V.; Valencak, T.G. Energy Metabolism and Ageing in the Mouse: A Mini-Review. Gerontology 2017, 63, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Weir, H.J.; Yao, P.; Huynh, F.K.; Escoubas, C.C.; Goncalves, R.L.; Burkewitz, K.; Laboy, R.; Hirschey, M.D.; Mair, W.B. Dietary Restriction and AMPK Increase Lifespan via Mitochondrial Network and Peroxisome Remodeling. Cell Metab. 2017, 26, 884–896.e5. [Google Scholar] [CrossRef]
- Anton, S.; Leeuwenburgh, C. Fasting or Caloric Restriction for Healthy Aging. Exp. Gerontol. 2013, 48, 1003–1005. [Google Scholar] [CrossRef] [PubMed]
- Lane, N. Power, Sex, Suicide: Mitochondria and the Meaning of Life; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Mishmar, D.; Ruiz-Pesini, E.; Golik, P.; Macaulay, V.; Clark, A.G.; Hosseini, S.; Brandon, M.; Easleyf, K.; Chen, E.; Brown, M.D.; et al. Natural Selection Shaped Regional MtDNA Variation in Humans. Proc. Natl. Acad. Sci. USA 2003, 100, 171–176. [Google Scholar] [CrossRef]
- Silva, G.; Lima, F.P.; Martel, P.; Castilho, R. Thermal Adaptation and Clinal Mitochondrial DNA Variation of European Anchovy. Proc. R. Soc. B Biol. Sci. 2014, 281, 20141093. [Google Scholar] [CrossRef]
- Verma, R.K.; Kalyakulina, A.; Mishra, A.; Ivanchenko, M.; Jalan, S. Role of Mitochondrial Genetic Interactions in Determining Adaptation to High Altitude Human Population. Sci. Rep. 2022, 12, 2046. [Google Scholar] [CrossRef] [PubMed]
- Stelkens, R.B.; Schmid, C.; Seehausen, O. Hybrid Breakdown in Cichlid Fish. PLoS ONE 2015, 10, e0127207. [Google Scholar] [CrossRef]
- Oka, A.; Mita, A.; Sakurai-Yamatani, N.; Yamamoto, H.; Takagi, N.; Takano-Shimizu, T.; Toshimori, K.; Moriwaki, K.; Shiroishi, T. Hybrid Breakdown Caused by Substitution of the X Chromosome Between Two Mouse Subspecies. Genetics 2004, 166, 913–924. [Google Scholar] [CrossRef]
- Turelli, M.; Moyle, L.C. Asymmetric Postmating Isolation: Darwin’s Corollary to Haldane’s Rule. Genetics 2007, 176, 1059–1088. [Google Scholar] [CrossRef]
- Roberts, H.F. Darwin’s Contribution to the Knowledge of Hybridization. Source Am. Nat. 1919, 53, 535–554. [Google Scholar] [CrossRef]
- Brandvain, Y.; Pauly, G.B.; May, M.R.; Turelli, M. Explaining Darwin’s Corollary to Haldane’s Rule: The Role of Mitonuclear Interactions in Asymmetric Postzygotic Isolation among Toads. Genetics 2014, 197, 743–747. [Google Scholar] [CrossRef]
- Bolnick, D.I.; Turelli, M.; Ló Pez-Fernández, H.; Wainwright, P.C.; Near, T.J. Accelerated Mitochondrial Evolution and “Darwin’s Corollary”: Asymmetric Viability of Reciprocal F1 Hybrids in Centrarchid Fishes. Genetics 2008, 178, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Presgraves, D.C. Patterns of Postzygotic Isolation in Lepidoptera. Evolution 2002, 56, 1168–1183. [Google Scholar] [CrossRef] [PubMed]
- Presgraves, D.C. A Fine-Scale Genetic Analysis of Hybrid Incompatibilities in Drosophila. Genetics 2003, 163, 955–972. [Google Scholar] [CrossRef]
- Bolnick, D.I.; Near, T.J. Tempo of Hybrid Inviability in Centrarchid Fishes (Teleostei: Centrarchidae). Evolution 2005, 59, 1754–1767. [Google Scholar] [CrossRef]
- Barreto, F.S.; Burton, R.S. Elevated Oxidative Damage Is Correlated with Reduced Fitness in Interpopulation Hybrids of a Marine Copepod. Proc. R. Soc. B Biol. Sci. 2013, 280, 20131521. [Google Scholar] [CrossRef]
- Lee, H.Y.; Chou, J.Y.; Cheong, L.; Chang, N.H.; Yang, S.Y.; Leu, J.Y. Incompatibility of Nuclear and Mitochondrial Genomes Causes Hybrid Sterility between Two Yeast Species. Cell 2008, 135, 1065–1073. [Google Scholar] [CrossRef]
- Meiklejohn, C.D.; Holmbeck, M.A.; Siddiq, M.A.; Abt, D.N.; Rand, D.M.; Montooth, K.L. An Incompatibility between a Mitochondrial TRNA and Its Nuclear-Encoded TRNA Synthetase Compromises Development and Fitness in Drosophila. PLOS Genet. 2013, 9, e1003238. [Google Scholar] [CrossRef]
- Polačik, M.; Reichard, M. Asymmetric Reproductive Isolation between Two Sympatric Annual Killifish with Extremely Short Lifespans. PLoS ONE 2011, 6, e22684. [Google Scholar] [CrossRef]
- Dobzhansky, T. Studies on Hybrid Sterility. II. Localization of Sterility Factors in Drosophila Pseudoobscura Hybrids. Genetics 1936, 21, 113–135. [Google Scholar] [CrossRef] [PubMed]
- Muller, H.J. Reversibility in Evolution Considered from the Standpoint of Genetics. Biol. Rev. 1939, 14, 185–268. [Google Scholar] [CrossRef]
- Gavrilets, S. Hybrid Zones with Dobzhansky-Type Epistatic Selection. Evolution 1997, 51, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Burton, R.S. The Role of Mitonuclear Incompatibilities in Allopatric Speciation. Cell. Mol. Life Sci. 2022, 79, 103. [Google Scholar] [CrossRef]
- Gershoni, M.; Templeton, A.R.; Mishmar, D. Mitochondrial Bioenergetics as a Major Motive Force of Speciation. Bioessays 2009, 31, 642–650. [Google Scholar] [CrossRef]
- Burton, R.S.; Ellison, C.K.; Harrison, J.S. The Sorry State of F2 Hybrids: Consequences of Rapid Mitochondrial DNA Evolution in Allopatric Populations. Am. Nat. 2006, 168 (Suppl. 6), S14–S24. [Google Scholar] [CrossRef]
- McKenzie, M.; Chiotis, M.; Pinkert, C.A.; Trounce, I.A. Functional Respiratory Chain Analyses in Murid Xenomitochondrial Cybrids Expose Coevolutionary Constraints of Cytochrome b and Nuclear Subunits of Complex III. Mol. Biol. Evol. 2003, 20, 1117–1124. [Google Scholar] [CrossRef]
- McKenzie, M.; Trounce, I.A.; Cassar, C.A.; Pinkert, C.A. Production of Homoplasmic Xenomitochondrial Mice. Proc. Natl. Acad. Sci. USA 2004, 101, 1685–1690. [Google Scholar] [CrossRef]
- Barrientos, A.; Kenyon, L.; Moraes, C.T. Human Xenomitochondrial Cybrids. Cellular Models of Mitochondrial Complex I Deficiency. J. Biol. Chem. 1998, 273, 14210–14217. [Google Scholar] [CrossRef]
- Rand, D.M.; Haney, R.A.; Fry, A.J. Cytonuclear Coevolution: The Genomics of Cooperation. Trends Ecol. Evol. 2004, 19, 645–653. [Google Scholar] [CrossRef]
- Ellison, C.K.; Burton, R.S. Interpopulation Hybrid Breakdown Maps to the Mitochondrial Genome. Evolution 2008, 62, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Ellison, C.K.; Burton, R.S. Disruption of Mitochondrial Function in Interpopulation Hybrids of Tigriopus Californicus. Evolution 2006, 60, 1382–1391. [Google Scholar] [CrossRef] [PubMed]
- Burton, R.S.; Barreto, F.S. A Disproportionate Role for MtDNA in Dobzhansky–Muller Incompatibilities? Mol. Ecol. 2012, 21, 4942–4957. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, G.M. Hybrid Zones-Natural Laboratories for Evolutionary Studies. Trends Ecol. Evol. 1988, 3, 158–167. [Google Scholar] [CrossRef]
- Buggs, R.J.A. Empirical Study of Hybrid Zone Movement. Heredity 2007, 99, 301–312. [Google Scholar] [CrossRef]
- Giannoulis, T.; Plageras, D.; Stamatis, C.; Chatzivagia, E.; Tsipourlianos, A.; Birtsas, P.; Billinis, C.; Suchentrunk, F.; Mamuris, Z. Islands and Hybrid Zones: Combining the Knowledge from “Natural Laboratories” to Explain Phylogeographic Patterns of the European Brown Hare. BMC Evol. Biol. 2019, 19, 17. [Google Scholar] [CrossRef]
- Harrison, R.G.; Larson, E.L. Hybridization, Introgression, and the Nature of Species Boundaries. J. Hered. 2014, 105, 795–809. [Google Scholar] [CrossRef]
- Bilgin, R. Back to the Suture: The Distribution of Intraspecific Genetic Diversity in and Around Anatolia. Int. J. Mol. Sci. 2011, 12, 4080. [Google Scholar] [CrossRef]
- Stamatis, C.; Suchentrunk, F.; Moutou, K.A.; Giacometti, M.; Haerer, G.; Djan, M.; Vapa, L.; Vukovic, M.; Tvrtković, N.; Sert, H.; et al. Phylogeography of the Brown Hare (Lepus europaeus) in Europe: A Legacy of South-Eastern Mediterranean Refugia? J. Biogeogr. 2009, 36, 515–528. [Google Scholar] [CrossRef]
- Kasapidis, P.; Suchentrunk, F.; Magoulas, A.; Kotoulas, G. The Shaping of Mitochondrial DNA Phylogeographic Patterns of the Brown Hare (Lepus europaeus) under the Combined Influence of Late Pleistocene Climatic Fluctuations and Anthropogenic Translocations. Mol. Phylogenet. Evol. 2005, 34, 55–66. [Google Scholar] [CrossRef]
- Pavlova, A.; Amos, J.N.; Joseph, L.; Loynes, K.; Austin, J.J.; Keogh, J.S.; Stone, G.N.; Nicholls, J.A.; Sunnucks, P. Perched at the Mito-Nuclear Crossroads: Divergent Mitochondrial Lineages Correlate with Environment in the Face of Ongoing Nuclear Gene Flow in an Australian Bird. Evolution 2013, 67, 3412–3428. [Google Scholar] [CrossRef]
- Baris, T.Z.; Wagner, D.N.; Dayan, D.I.; Du, X.; Blier, P.U.; Pichaud, N.; Oleksiak, M.F.; Crawford, D.L. Evolved Genetic and Phenotypic Differences Due to Mitochondrial-Nuclear Interactions. PLOS Genet. 2017, 13, e1006517. [Google Scholar] [CrossRef] [PubMed]
- Stacy, E.A.; Johansen, J.B.; Sakishima, T.; Price, D.K. Genetic Analysis of an Ephemeral Intraspecific Hybrid Zone in the Hypervariable Tree, Metrosideros Polymorpha, on Hawai‘i Island. Heredity 2016, 117, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Giannoulis, T.; Stamatis, C.; Tsipourlianos, A.; Mamuris, Z. Mitogenomic Analysis in European Brown Hare (Lepus europaeus) Proposes Genetic and Functional Differentiation between the Distinct Lineages. Mitochondrial DNA Part A 2017, 29, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Morales, H.E.; Pavlova, A.; Joseph, L.; Sunnucks, P. Positive and Purifying Selection in Mitochondrial Genomes of a Bird with Mitonuclear Discordance. Mol. Ecol. 2015, 24, 2820–2837. [Google Scholar] [CrossRef] [PubMed]
- Baumgard, L.; Rhoads, R.P. Effects of Environment on Metabolism. Environ. Physiol. Livest. 2012, 81–100. [Google Scholar] [CrossRef]
- Dingley, S.D.; Polyak, E.; Ostrovsky, J.; Srinivasan, S.; Lee, I.; Rosenfeld, A.B.; Tsukikawa, M.; Xiao, R.; Selak, M.A.; Coon, J.J.; et al. Mitochondrial DNA Variant in COX1 Subunit Significantly Alters Energy Metabolism of Geographically Divergent Wild Isolates in Caenorhabditis Elegans. J. Mol. Biol. 2014, 426, 2199–2216. [Google Scholar] [CrossRef]
- Hill, G.E. Mitonuclear Ecology. Mol. Biol. Evol. 2015, 32, 1917–1927. [Google Scholar] [CrossRef]
- Chen, Y.; Gong, L.; Liu, X.; Chen, X.; Yang, S.; Luo, Y. Mitochondrial DNA Genomes Revealed Different Patterns of High-Altitude Adaptation in High-Altitude Tajiks Compared with Tibetans and Sherpas. Sci. Rep. 2020, 10, 10592. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Dong, X.; Shi, L.; Shi, L.; Lin, K.; Huang, X.; Chu, J. Differences in MtDNA Whole Sequence between Tibetan and Han Populations Suggesting Adaptive Selection to High Altitude. Gene 2012, 496, 37–44. [Google Scholar] [CrossRef]
- Melo-Ferreira, J.; Boursot, P.; Suchentrunk, F.; Ferrand, N.; Alves, P.C. Invasion from the Cold Past: Extensive Introgression of Mountain Hare (Lepus timidus) Mitochondrial DNA into Three Other Hare Species in Northern Iberia. Mol. Ecol. 2005, 14, 2459–2464. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Pesini, E.; Wallace, D.C. Evidence for Adaptive Selection Acting on the TRNA and rRNA Genes of Human Mitochondrial DNA. Hum. Mutat. 2006, 27, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Garvin, M.R.; Thorgaard, G.H.; Narum, S.R. Differential Expression of Genes That Control Respiration Contribute to Thermal Adaptation in Redband Trout (Oncorhynchus mykiss gairdneri). Genome Biol. Evol. 2015, 7, 1404–1414. [Google Scholar] [CrossRef] [PubMed]
- Bar-Yaacov, D.; Hadjivasiliou, Z.; Levin, L.; Barshad, G.; Zarivach, R.; Bouskila, A.; Mishmar, D. Mitochondrial Involvement in Vertebrate Speciation? The Case of Mito-Nuclear Genetic Divergence in Chameleons. Genome Biol. Evol. 2015, 7, 3322–3336. [Google Scholar] [CrossRef] [PubMed]
- Trier, C.N.; Hermansen, J.S.; Sætre, G.P.; Bailey, R.I. Evidence for Mito-Nuclear and Sex-Linked Reproductive Barriers between the Hybrid Italian Sparrow and Its Parent Species. PLoS Genet. 2014, 10, e1004075. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ore, M.J.; Mikkelsen, E.K.; Lee-Yaw, J.; Toews, D.P.L.; Rohwer, S.; Irwin, D. Signatures of Mitonuclear Coevolution in a Warbler Species Complex. Nat. Commun. 2021, 12, 4279. [Google Scholar] [CrossRef]
- Hill, G.E. The Mitonuclear Compatibility Species Concept. Auk 2017, 134, 393–409. [Google Scholar] [CrossRef]
- Kirk, H.; Dorn, S.; Mazzi, D. Molecular Genetics and Genomics Generate New Insights into Invertebrate Pest Invasions. Evol. Appl. 2013, 6, 842–856. [Google Scholar] [CrossRef]
- Bajda, S.; Grigoraki, L. Integrated Pest Management: Novel Tools, Remaining Challenges, and Intriguing Non-Target Effects. Curr. Opin. Insect Sci. 2020, 39, iii–v. [Google Scholar] [CrossRef]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of Pesticides Use in Agriculture: Their Benefits and Hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef]
- Vreysen, M.J.B.; Robinson, A.S.; Hendrichs, J. Area-Wide Control of Insect Pests: From Research to Field Implementation; Springer: Dordrecht, The Netherlands, 2007; pp. 1–789. [Google Scholar] [CrossRef]
- Alphey, L.; Benedict, M.; Bellini, R.; Clark, G.G.; Dame, D.A.; Service, M.W.; Dobson, S.L. Sterile-Insect Methods for Control of Mosquito-Borne Diseases: An Analysis. Vector Borne Zoonotic Dis. 2010, 10, 295–311. [Google Scholar] [CrossRef]
- Gato, R.; Menéndez, Z.; Prieto, E.; Argilés, R.; Rodríguez, M.; Baldoquín, W.; Hernández, Y.; Pérez, D.; Anaya, J.; Fuentes, I.; et al. Sterile Insect Technique: Successful Suppression of an Aedes Aegypti Field Population in Cuba. Insects 2021, 12, 469. [Google Scholar] [CrossRef]
- Ranathunge, T.; Harishchandra, J.; Maiga, H.; Bouyer, J.; Gunawardena, Y.I.N.S.; Hapugoda, M. Development of the Sterile Insect Technique to Control the Dengue Vector Aedes Aegypti (Linnaeus) in Sri Lanka. PLoS ONE 2022, 17, e0265244. [Google Scholar] [CrossRef]
- Chansang, C.; Chansang, U.; Mongkalangoon, P.; Kittayapong, P.; Ninphanomchai, S.; Limohpasmanee, W. Combined Sterile Insect Technique and Incompatible Insect Technique: The First Proof-of-Concept to Suppress Aedes Aegypti Vector Populations in Semi-Rural Settings in Thailand. PLoS Negl. Trop. Dis. 2019, 13, e0007771. [Google Scholar] [CrossRef]
- Gemmell, N.J.; Jalilzadeh, A.; Didham, R.K.; Soboleva, T.; Tompkins, D.M. The Trojan Female Technique: A Novel, Effective and Humane Approach for Pest Population Control. Proc. R. Soc. B Biol. Sci. 2013, 280, 20132549. [Google Scholar] [CrossRef]
- Dowling, D.K.; Tompkins, D.M.; Gemmell, N.J. The Trojan Female Technique for Pest Control: A Candidate Mitochondrial Mutation Confers Low Male Fertility across Diverse Nuclear Backgrounds in Drosophila Melanogaster. Evol. Appl. 2015, 8, 871–880. [Google Scholar] [CrossRef]
- Clancy, D.J. Variation in Mitochondrial Genotype Has Substantial Lifespan Effects Which May Be Modulated by Nuclear Background. Aging Cell 2008, 7, 795–804. [Google Scholar] [CrossRef]
- Wolff, J.N.; Gemmell, N.J.; Tompkins, D.M.; Dowling, D.K. Introduction of a Male-Harming Mitochondrial Haplotype via “Trojan Females” Achieves Population Suppression in Fruit Flies. elife 2017, 6, e23551. [Google Scholar] [CrossRef]
- Jo, A.; Ham, S.; Lee, G.H.; Lee, Y.I.; Kim, S.; Lee, Y.S.; Shin, J.H.; Lee, Y. Efficient Mitochondrial Genome Editing by CRISPR/Cas9. Biomed Res. Int. 2015, 2015, 305716. [Google Scholar] [CrossRef]
- Reinhardt, K.; Dowling, D.K.; Morrow, E.H. Mitochondrial Replacement, Evolution, and the Clinic. Science 2013, 341, 1345–1346. [Google Scholar] [CrossRef]
- Gonzalez, S. The Role of Mitonuclear Incompatibility in Bipolar Disorder Susceptibility and Resilience Against Environmental Stressors. Front. Genet. 2021, 12, 636294. [Google Scholar] [CrossRef]
- Zhang, C.; Montooth, K.L.; Calvi, B.R. Incompatibility between Mitochondrial and Nuclear Genomes during Oogenesis Results in Ovarian Failure and Embryonic Lethality. Development 2017, 144, 2490–2503. [Google Scholar] [CrossRef]
- Dimauro, S. A Brief History of Mitochondrial Pathologies. Int. J. Mol. Sci. 2019, 20, 5643. [Google Scholar] [CrossRef]
- Gorman, G.S.; Chinnery, P.F.; DiMauro, S.; Hirano, M.; Koga, Y.; McFarland, R.; Suomalainen, A.; Thorburn, D.R.; Zeviani, M.; Turnbull, D.M. Mitochondrial Diseases. Nat. Rev. Dis. Prim. 2016, 2, 16080. [Google Scholar] [CrossRef]
- Kato, T. Mitochondrial Dysfunction as the Molecular Basis of Bipolar Disorder: Therapeutic Implications. CNS Drugs 2007, 21, 1–11. [Google Scholar] [CrossRef]
- Kato, T.; Takahashi, Y. Deletion of Leukocyte Mitochondrial DNA in Bipolar Disorder. J. Affect. Disord. 1996, 37, 67–73. [Google Scholar] [CrossRef]
- Kato, T. Mitochondrial Dysfunction in Bipolar Disorder. Biomarkers Bipolar Disord. 2022, 2, 141–156. [Google Scholar] [CrossRef]
- Manji, H.; Kato, T.; Di Prospero, N.A.; Ness, S.; Beal, M.F.; Krams, M.; Chen, G. Impaired Mitochondrial Function in Psychiatric Disorders. Nat. Rev. Neurosci. 2012, 13, 293–307. [Google Scholar] [CrossRef]
- Stahl, E.; Breen, G.; Forstner, A.; McQuillin, A.; Ripke, S.; Trubetskoy, V.; Mattheisen, M.; Wang, Y.; Coleman, J.R.I.; Gaspar, H.A.; et al. Genome-Wide Association Study Identifies 30 Loci Associated with Bipolar Disorder. Nat. Genet. 2019, 51, 793–803. [Google Scholar] [CrossRef]
- Chou, J.Y.; Leu, J.Y. The Red Queen in Mitochondria: Cyto-Nuclear Co-Evolution, Hybrid Breakdown and Human Disease. Front. Genet. 2015, 6, 187. [Google Scholar] [CrossRef]
- Rand, D.M.; Mossman, J.A. Mitonuclear Conflict and Cooperation Govern the Integration of Genotypes, Phenotypes and Environments. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2020, 375, 20190188. [Google Scholar] [CrossRef]
- Asdigian, N.L.; Bear, U.R.; Beals, J.; Manson, S.M.; Kaufman, C.E. Mental Health Burden in a National Sample of American Indian and Alaska Native Adults: Differences between Multiple-Race and Single-Race Subgroups. Soc. Psychiatry Psychiatr. Epidemiol. 2018, 53, 521–530. [Google Scholar] [CrossRef]
- Rose, S.; Bennuri, S.C.; Davis, J.E.; Wynne, R.; Slattery, J.C.; Tippett, M.; Delhey, L.; Melnyk, S.; Kahler, S.G.; MacFabe, D.F.; et al. Butyrate Enhances Mitochondrial Function during Oxidative Stress in Cell Lines from Boys with Autism. Transl. Psychiatry 2018, 8, 42. [Google Scholar] [CrossRef]
- Bennuri, S.C.; Rose, S.; Frye, R.E. Mitochondrial Dysfunction Is Inducible in Lymphoblastoid Cell Lines From Children With Autism and May Involve the TORC1 Pathway. Front. Psychiatry 2019, 10, 269. [Google Scholar] [CrossRef]
- Gonçalves, V.F.; Andreazza, A.C.; Kennedy, J.L. Mitochondrial Dysfunction in Schizophrenia: An Evolutionary Perspective. Hum. Genet. 2015, 134, 13–21. [Google Scholar] [CrossRef]
- Caruso, G.; Benatti, C.; Blom, J.M.C.; Caraci, F.; Tascedda, F. The Many Faces of Mitochondrial Dysfunction in Depression: From Pathology to Treatment. Front. Pharmacol. 2019, 10, 995. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, H.; Luo, S.; Lu, Z.; Chávez-Badiola, A.; Liu, Z.; Yang, M.; Merhi, Z.; Silber, S.J.; Munné, S.; et al. Live Birth Derived from Oocyte Spindle Transfer to Prevent Mitochondrial Disease. Reprod. Biomed. Online 2017, 34, 361–368. [Google Scholar] [CrossRef]
- Schubert, M.B.; Vilarinho, L. Molecular Basis of Leigh Syndrome: A Current Look. Orphanet J. Rare Dis. 2020, 15, 31. [Google Scholar] [CrossRef]
- Swalwell, H.; Kirby, D.M.; Blakely, E.L.; Mitchell, A.; Salemi, R.; Sugiana, C.; Compton, A.G.; Tucker, E.J.; Ke, B.-X.; Lamont, P.J.; et al. Respiratory Chain Complex I Deficiency Caused by Mitochondrial DNA Mutations. Eur. J. Hum. Genet. 2011, 19, 769–775. [Google Scholar] [CrossRef]
- Chinnery, P.F.; Craven, L.; Mitalipov, S.; Stewart, J.B.; Herbert, M.; Turnbull, D.M. The Challenges of Mitochondrial Replacement. PLoS Genet. 2014, 10, e1004315. [Google Scholar] [CrossRef]
- Sendra, L.; García-Mares, A.; Herrero, M.J.; Aliño, S.F. Mitochondrial DNA Replacement Techniques to Prevent Human Mitochondrial Diseases. Int. J. Mol. Sci. 2021, 22, 551. [Google Scholar] [CrossRef]
- Palacios-González, C. Mexico and Mitochondrial Replacement Techniques: What a Mess. Br. Med. Bull. 2018, 128, 97–107. [Google Scholar] [CrossRef]
- Craven, L.; Murphy, J.; Turnbull, D.M.; Taylor, R.W.; Gorman, G.S.; McFarland, R. Scientific and Ethical Issues in Mitochondrial Donation. New Bioeth. 2018, 24, 57–73. [Google Scholar] [CrossRef]
- Zouros, E. Biparental Inheritance Through Uniparental Transmission: The Doubly Uniparental Inheritance (DUI) of Mitochondrial DNA. Evol. Biol. 2012, 40, 1–31. [Google Scholar] [CrossRef]
- Zouros, E.; Ball, A.O.; Saavedra, C.; Freeman, K.R. Mitochondrial DNA Inheritance. Nature 1994, 368, 818. [Google Scholar] [CrossRef]
- Gusman, A.; Lecomte, S.; Stewart, D.T.; Passamonti, M.; Breton, S. Pursuing the Quest for Better Understanding the Taxonomic Distribution of the System of Doubly Uniparental Inheritance of MtDNA. PeerJ 2016, 4, e2760. [Google Scholar] [CrossRef]
- Capt, C.; Bouvet, K.; Guerra, D.; Robicheau, B.M.; Stewart, D.T.; Pante, E.; Breton, S. Unorthodox Features in Two Venerid Bivalves with Doubly Uniparental Inheritance of Mitochondria. Sci. Rep. 2020, 10, 1087. [Google Scholar] [CrossRef]
- Piccinini, G.; Iannello, M.; Puccio, G.; Plazzi, F.; Havird, J.C.; Ghiselli, F. Mitonuclear Coevolution, but Not Nuclear Compensation, Drives Evolution of OXPHOS Complexes in Bivalves. Mol. Biol. Evol. 2021, 38, 2597–2614. [Google Scholar] [CrossRef]
- Breton, S.; Beaupré, H.D.; Stewart, D.T.; Hoeh, W.R.; Blier, P.U. The Unusual System of Doubly Uniparental Inheritance of MtDNA: Isn’t One Enough? Trends Genet. 2007, 23, 465–474. [Google Scholar] [CrossRef]
- Stewart, D.T.; Kenchington, E.R.; Singh, R.K.; Zonros, E. Degree of Selective Constraint as an Explanation of the Different Rates of Evolution of Gender-Specific Mitochondrial DNA Lineages in the Mussel Mytilus. Genetics 1996, 143, 1349–1357. [Google Scholar] [CrossRef]
- Ort, B.S.; Pogson, G.H. Molecular Population Genetics of the Male and Female Mitochondrial DNA Molecules of the California Sea Mussel, Mytilus Californianus. Genetics 2007, 177, 1087–1099. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Soroka, M.; Burzyński, A. Complete Sequences of Maternally Inherited Mitochondrial Genomes in MusselsUnio Pictorum (Bivalvia, Unionidae). J. Appl. Genet. 2010, 51, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Maeda, G.P.; Iannello, M.; McConie, H.J.; Ghiselli, F.; Havird, J.C. Relaxed Selection on Male Mitochondrial Genes in DUI Bivalves Eases the Need for Mitonuclear Coevolution. J. Evol. Biol. 2021, 34, 1722–1736. [Google Scholar] [CrossRef]
- Keaney, T.A.; Wong, H.W.S.; Dowling, D.K.; Jones, T.M.; Holman, L. Sibling Rivalry versus Mother’s Curse: Can Kin Competition Facilitate a Response to Selection on Male Mitochondria? Proc. R. Soc. B Biol. Sci. 2020, 287, 20200575. [Google Scholar] [CrossRef]
- Wade, M.J.; Brandvain, Y. Reversing Mother’s Curse: Selection on Male Mitochondrial Fitness Effects. Evolution 2009, 63, 1084–1089. [Google Scholar] [CrossRef]
- Hill, G.E. Genetic Hitchhiking, Mitonuclear Coadaptation, and the Origins of Mt DNA Barcode Gaps. Ecol. Evol. 2020, 10, 9048–9059. [Google Scholar] [CrossRef]
- Wolff, J.N.; Ladoukakis, E.D.; Enríquez, J.A.; Dowling, D.K. Mitonuclear Interactions: Evolutionary Consequences over Multiple Biological Scales. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2014, 369, 20130443. [Google Scholar] [CrossRef]
- Levin, L.; Blumberg, A.; Barshad, G.; Mishmar, D. Mito-Nuclear Co-Evolution: The Positive and Negative Sides of Functional Ancient Mutations. Front. Genet. 2014, 5, 448. [Google Scholar] [CrossRef]
- Eckardt, N.A. Cytoplasmic Male Sterility and Fertility Restoration. Plant Cell 2006, 18, 515–517. [Google Scholar] [CrossRef]
- Hanson, M.; Bentolila, S. Interactions of Mitochondrial and Nuclear Genes That Affect Male Gametophyte Development. Plant Cell 2004, 16, S154–S169. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y.-G. Male Sterility and Fertility Restoration in Crops. Annu. Rev. Plant Biol. 2014, 65, 579–606. [Google Scholar] [CrossRef] [PubMed]
- Toriyama, K. Molecular Basis of Cytoplasmic Male Sterility and Fertility Restoration in Rice. Plant Biotechnol. 2021, 38, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Hill, G.E. Mitonuclear Compensatory Coevolution. Trends Genet. 2020, 36, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Polovina, E.S.; Parakatselaki, M.E.; Ladoukakis, E.D. Paternal Leakage of Mitochondrial DNA and Maternal Inheritance of Heteroplasmy in Drosophila Hybrids. Sci. Rep. 2020, 10, 2599. [Google Scholar] [CrossRef] [PubMed]
- Dean, M.D.; Ballard, K.J.; Glass, A.; Ballard, J.W.O. Influence of Two Wolbachia Strains on Population Structure of East African Drosophila Simulans. Genetics 2003, 165, 1959–1969. [Google Scholar] [CrossRef] [PubMed]
- Breton, S.; Stewart, D.T. Atypical Mitochondrial Inheritance Patterns in Eukaryotes. Genome 2015, 58, 423–431. [Google Scholar] [CrossRef]
- Gyllensten, U.; Wharton, D.; Josefsson, A.; Wilson, A.C. Paternal Inheritance of Mitochondrial DNA in Mice. Nature 1991, 352, 255–257. [Google Scholar] [CrossRef]
- Payne, B.A.I.; Wilson, I.J.; Yu-Wai-Man, P.; Coxhead, J.; Deehan, D.; Horvath, R.; Taylor, R.W.; Samuels, D.C.; Santibanez-Koref, M.; Chinnery, P.F. Universal Heteroplasmy of Human Mitochondrial DNA. Hum. Mol. Genet. 2013, 22, 384–390. [Google Scholar] [CrossRef]
- Luo, S.; Valencia, C.A.; Zhang, J.; Lee, N.C.; Slone, J.; Gui, B.; Wang, X.; Li, Z.; Dell, S.; Brown, J.; et al. Biparental Inheritance of Mitochondrial DNA in Humans. Proc. Natl. Acad. Sci. USA 2018, 115, 13039–13044. [Google Scholar] [CrossRef]
- Lutz-Bonengel, S.; Parson, W. No Further Evidence for Paternal Leakage of Mitochondrial DNA in Humans Yet. Proc. Natl. Acad. Sci. USA 2019, 116, 1821–1822. [Google Scholar] [CrossRef]
- Hedrick, P.W. Reversing Mother’s Curse Revisited. Evolution 2012, 66, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Kay, T.; Lehmann, L.; Keller, L. Kin Selection and Altruism. Curr. Biol. 2019, 29, R438–R442. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyrgiafini, M.-A.; Giannoulis, T.; Moutou, K.A.; Mamuris, Z. Investigating the Impact of a Curse: Diseases, Population Isolation, Evolution and the Mother’s Curse. Genes 2022, 13, 2151. https://doi.org/10.3390/genes13112151
Kyrgiafini M-A, Giannoulis T, Moutou KA, Mamuris Z. Investigating the Impact of a Curse: Diseases, Population Isolation, Evolution and the Mother’s Curse. Genes. 2022; 13(11):2151. https://doi.org/10.3390/genes13112151
Chicago/Turabian StyleKyrgiafini, Maria-Anna, Themistoklis Giannoulis, Katerina A. Moutou, and Zissis Mamuris. 2022. "Investigating the Impact of a Curse: Diseases, Population Isolation, Evolution and the Mother’s Curse" Genes 13, no. 11: 2151. https://doi.org/10.3390/genes13112151
APA StyleKyrgiafini, M.-A., Giannoulis, T., Moutou, K. A., & Mamuris, Z. (2022). Investigating the Impact of a Curse: Diseases, Population Isolation, Evolution and the Mother’s Curse. Genes, 13(11), 2151. https://doi.org/10.3390/genes13112151





