Noninvasive Prenatal Screening for Trisomy 21 in Patients with a Vanishing Twin
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient and Sample Details
3.2. NIPT Results
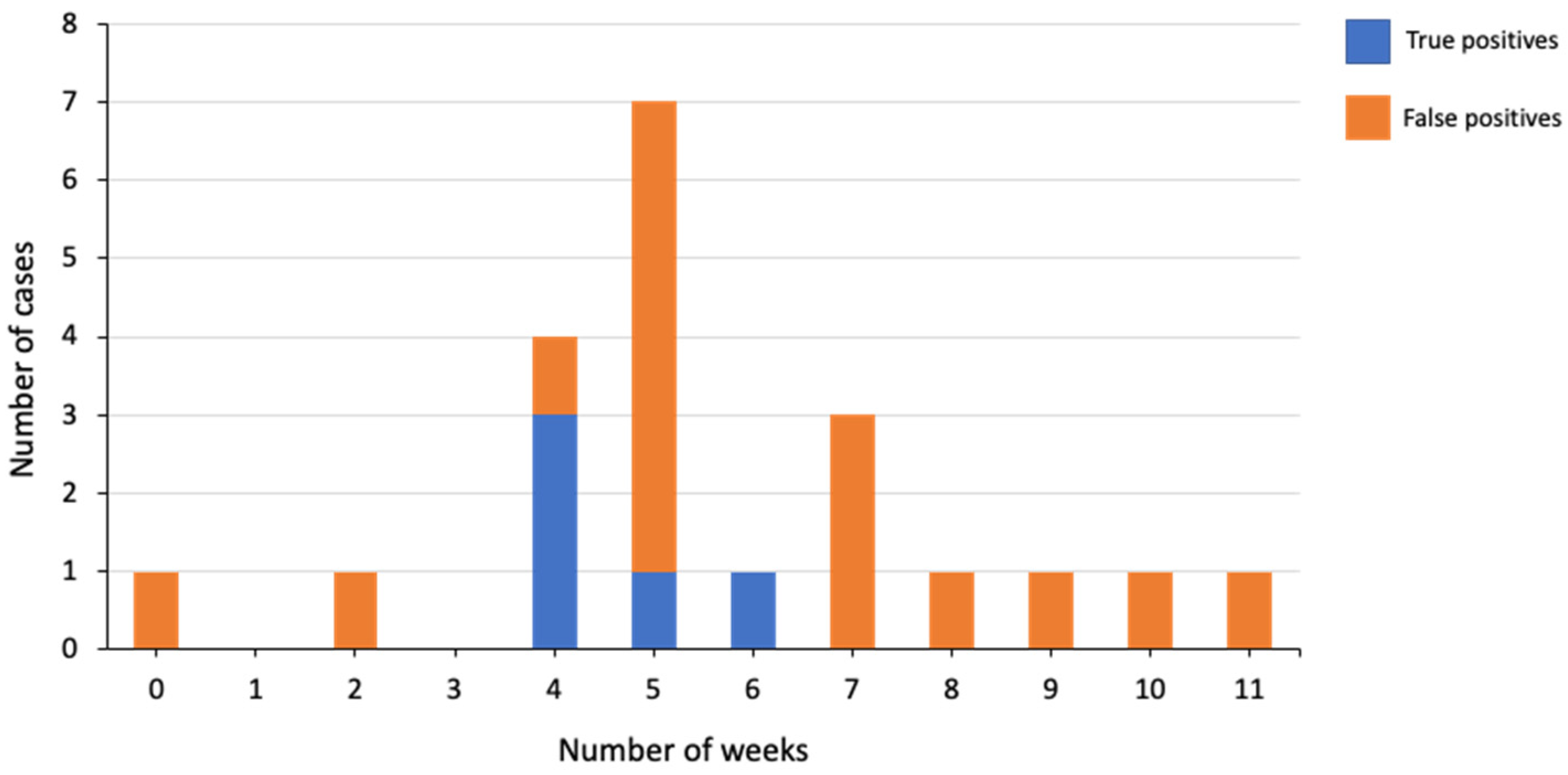
3.3. Outcome Information
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levi, S. Ultrasonic assessment of the high rate of human multiple pregnancy in the first trimester. J. Clin. Ultrasound 1976, 4, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Balaguer, N.; Mateu-Brull, E.; Serra, V.; Simón, C.; Milán, M. Should vanishing twin pregnancies be systematically excluded from cell-free fetal DNA testing? Prenat. Diagn. 2020, 41, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Pinborg, A.; Lidegaard, O.; la Cour Freiesleben, N.; Andersen, A.N. Consequences of vanishing twins in IVF/ICSI pregnancies. Hum. Reprod. 2005, 20, 2821–2829. [Google Scholar] [CrossRef] [PubMed]
- Almog, B.; Levin, I.; Wagman, I.; Kapustiansky, R.; Lessing, J.B.; Amit, A.; Azem, F. Adverse obstetric outcome for the vanishing twin syndrome. Reprod. Biomed. Online 2010, 20, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Dickey, R.P.; Taylor, S.N.; Lu, P.Y.; Sartor, B.M.; Storment, J.M.; Rye, P.H.; Pelletier, W.D.; Zender, J.L.; Matulich, E.M. Spontaneous reduction of multiple pregnancy: Incidence and effect on outcome. Am. J. Obstet. Gynecol. 2002, 186, 77–83. [Google Scholar] [CrossRef]
- Pinborg, A.; Lidegaard, O.; Freiesleben, N.; Andersen, A.N. Vanishing twins: A predictor of small-for-gestational age in IVF singletons. Hum. Reprod. 2007, 22, 2707–2714. [Google Scholar] [CrossRef]
- La Sala, G.B.; Villani, M.T.; Nicoli, A.; Gallinelli, A.; Nucera, G.; Blickstein, I. Effect of the mode of assisted reproductive technology conception on obstetric outcomes for survivors of the vanishing twin syndrome. Fertil. Steril. 2006, 86, 247–249. [Google Scholar] [CrossRef]
- Chasen, S.T.; Luo, G.; Perni, S.C.; Kalish, R.B. Are in vitro fertilization pregnancies with early spontaneous reduction high risk? Am. J. Obstet. Gynecol. 2006, 195, 814–817. [Google Scholar] [CrossRef]
- La Sala, G.B.; Nucera, G.; Gallinelli, A.; Nicoli, A.; Villani, M.T.; Blickstein, I. Spontaneous embryonic loss after in vitro fertilization with and without intracytoplasmic sperm injection. Fertil. Steril. 2004, 82, 1536–1539. [Google Scholar] [CrossRef]
- Tummers, P.; De Sutter, P.; Dhont, M. Risk of spontaneous abortion in singleton and twin pregnancies after IVF/ICSI. Hum. Reprod. 2003, 18, 1720–1723. [Google Scholar] [CrossRef]
- Landy, H.J.; Keith, L.G. The vanishing twin: A review. Hum. Reprod. Update 1998, 4, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Sampson, A.; de Crespigny, L.C. Vanishing twins: The frequency of spontaneous fetal reduction of a twin pregnancy. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 1992, 2, 107–109. [Google Scholar] [CrossRef]
- Márton, V.; Zádori, J.; Kozinszky, Z.; Keresztúri, A. Prevalences and pregnancy outcome of vanishing twin pregnancies achieved by in vitro fertilization versus natural conception. Fertil. Steril. 2016, 106, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Curnow, K.J.; Wilkins-Haug, L.; Ryan, A.; Kırkızlar, E.; Stosic, M.; Hall, M.P.; Sigurjonsson, S.; Demko, Z.; Rabinowitz, M.; Gross, S.J. Detection of triploid, molar, and vanishing twin pregnancies by a single-nucleotide polymorphism-based noninvasive prenatal test. Am. J. Obstet. Gynecol. 2015, 212, e71–e79. [Google Scholar] [CrossRef] [PubMed]
- Spencer, K.; Staboulidou, I.; Nicolaides, K.H. First trimester aneuploidy screening in the presence of a vanishing twin: Implications for maternal serum markers. Prenat. Diagn. 2010, 30, 235–240. [Google Scholar] [CrossRef]
- Niles, K.M.; Murji, A.; Chitayat, D. Prolonged duration of persistent cell-free fetal DNA from vanishing twin. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2018, 52, 547–548. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists (ACOG); Society for Maternal-Fetal Medicine (SMFM). Screening for Fetal Chromosomal Abnormalities: ACOG Practice Bulletin Summary, Number 226. Obstet. Gynecol. 2020, 136, 859–867. [Google Scholar] [CrossRef]
- ACLF. Recommandations pour le de Pistage des Anomalies Chromosomiques Fœtales par l’ETude de l’ADN Libre Circulant (ADNlc). Available online: http://www.eaclf.org/docs/GBP_ADNlc-V4.pdf (accessed on 16 August 2022).
- Pertile, M.D.; Flowers, N.; Vavrek, D.; Andrews, D.; Kalista, T.; Craig, A.; Deciu, C.; Duenwald, S.; Meier, K.; Bhatt, S. Performance of a Paired-End Sequencing-Based Noninvasive Prenatal Screening Test in the Detection of Genome-Wide Fetal Chromosomal Anomalies. Clin. Chem. 2021, 67, 1210–1219. [Google Scholar] [CrossRef]
- Kleinfinger, P.; Lohmann, L.; Luscan, A.; Trost, D.; Bidat, L.; Debarge, V.; Castaigne, V.; Senat, M.V.; Brechard, M.P.; Guilbaud, L.; et al. Strategy for Use of Genome-Wide Non-Invasive Prenatal Testing for Rare Autosomal Aneuploidies and Unbalanced Structural Chromosomal Anomalies. J. Clin. Med. 2020, 9, 2466. [Google Scholar] [CrossRef]
- Gregg, A.R.; Skotko, B.G.; Benkendorf, J.L.; Monaghan, K.G.; Bajaj, K.; Best, R.G.; Klugman, S.; Watson, M.S. Noninvasive prenatal screening for fetal aneuploidy, 2016 update: A position statement of the American College of Medical Genetics and Genomics. Genet. Med. Off. J. Am. Coll. Med. Genet. 2016, 18, 1056–1065. [Google Scholar] [CrossRef]
- Zou, Y.; Cui, L.; Xue, M.; Yan, J.; Huang, M.; Gao, M.; Gao, X.; Gao, Y.; Chen, Z.J. Applications of noninvasive prenatal testing in vanishing twin syndrome pregnancies after treatment of assisted reproductive technology in a single center. Prenat. Diagn. 2021, 41, 226–233. [Google Scholar] [CrossRef] [PubMed]
| Referral Indication | VT + 1 Surviving Fetus | VT + 2 Surviving Fetuses | VT + 3 Surviving Fetuses | Total |
|---|---|---|---|---|
| Parental Robertsonian translocation | 2 | 0 | 0 | 2 |
| First-trimester MSS ≥ 1/1000 | 183 | 1 | 0 | 184 |
| Second-trimester MSS ≥ 1/1000 | 99 | 1 | 0 | 100 |
| Prior pregnancy with aneuploidy | 9 | 0 | 0 | 9 |
| First-tier screening | 386 | 147 | 4 | 537 |
| First-trimester MSS < 1/1000 | 12 | 0 | 0 | 12 |
| Second-trimester MSS < 1/1000 | 3 | 0 | 0 | 3 |
| Total | 694 (81.9%) | 149 (17.6%) | 4 (0.5%) | 847 |
| Cohort | Mean FF, % | Median FF, % | Mean GA, wk | Median GA, wk |
|---|---|---|---|---|
| Study cohort | ||||
| VT + 1 surviving fetus | 10.8 | 10 | 15.6 | 14.4 |
| VT + 2 surviving fetuses | 13.6 | 13 | 15.8 | 15 |
| VT + 3 surviving fetuses | 18 | 17.5 | 13.4 | 13.3 |
| Total | 11.3 | 10 | 15.6 | 14.5 |
| Multiple pregnancies with no VT * | ||||
| 2 fetuses | 12.5 | 12 | 14.8 | 13.5 |
| 3 or 4 fetuses | 14.4 | 14 | 14 | 13 |
| Total | 12.5 | 12 | 14.8 | 13.5 |
| Singleton pregnancies ‡ | 10.3 | 10 | 16.7 | 15.4 |
| NIPT Result | VT + 1 Surviving Fetus | VT + 2 Surviving Fetuses | VT + 3Surviving Fetuses | Total, n (%) | Multiple Pregnancies with No VT, n (%) |
|---|---|---|---|---|---|
| Trisomy 21 | 13 * | 1 | 0 | 14 (1.65) | 46 (0.47) |
| Trisomy 18 | 9 | 0 | 0 | 9 (1.06) | 11 (0.11) |
| Trisomy 13 | 6 | 0 | 0 | 6 (0.71) | 10 (0.10) |
| Low risk | 666 | 148 | 4 | 818 (96.58) | 9610 (99.16) |
| No call | 0 | 0 | 0 | 0 | 14 (0.14) |
| Trisomy (n) | True Positive | False Positive | PPV | PPV for Multiple Pregnancies with No VT |
|---|---|---|---|---|
| Trisomy 21 (14 *) | 6 | 6 | 50% | 79.5% (35/44) |
| Trisomy 18 (9) | 1 | 8 ‡ | 11.1% | 50% (5/10) |
| Trisomy 13 (6) | 0 | 6 | 0% | 0% (0/7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kleinfinger, P.; Luscan, A.; Descourvieres, L.; Buzas, D.; Boughalem, A.; Serero, S.; Valduga, M.; Trost, D.; Costa, J.-M.; Vivanti, A.J.; et al. Noninvasive Prenatal Screening for Trisomy 21 in Patients with a Vanishing Twin. Genes 2022, 13, 2027. https://doi.org/10.3390/genes13112027
Kleinfinger P, Luscan A, Descourvieres L, Buzas D, Boughalem A, Serero S, Valduga M, Trost D, Costa J-M, Vivanti AJ, et al. Noninvasive Prenatal Screening for Trisomy 21 in Patients with a Vanishing Twin. Genes. 2022; 13(11):2027. https://doi.org/10.3390/genes13112027
Chicago/Turabian StyleKleinfinger, Pascale, Armelle Luscan, Léa Descourvieres, Daniela Buzas, Aicha Boughalem, Stéphane Serero, Mylène Valduga, Detlef Trost, Jean-Marc Costa, Alexandre J. Vivanti, and et al. 2022. "Noninvasive Prenatal Screening for Trisomy 21 in Patients with a Vanishing Twin" Genes 13, no. 11: 2027. https://doi.org/10.3390/genes13112027
APA StyleKleinfinger, P., Luscan, A., Descourvieres, L., Buzas, D., Boughalem, A., Serero, S., Valduga, M., Trost, D., Costa, J.-M., Vivanti, A. J., & Lohmann, L. (2022). Noninvasive Prenatal Screening for Trisomy 21 in Patients with a Vanishing Twin. Genes, 13(11), 2027. https://doi.org/10.3390/genes13112027







