Abstract
Clinically relevant glycopeptide antibiotics remain among the most successful classes of natural antibacterials. This success, however, is endangered by the spread of glycopeptide resistance genes, also known as van genes. Thus, it is important to trace and comprehend possible routes of van gene dissemination. In the current work, we present a comprehensive bioinformatic analysis aimed at mapping the occurrence of van genes beyond the Actinobacteria phylum—the most likely natural reservoir of van genes. We show that two additional classes of Gram-positive bacteria, Erysipelotrichia and Ktedonobacteria, as well as one class of Gram-negative bacteria, Anaerolineae, carry van genes. Additionally, we demonstrate that various new genera belonging to the classes Clostridia and Bacilli also carry van genes. The majority of discovered van loci are co-localized with MGE-related genes of various types. Finally, we propose a phylogeny-based scenario for the spread of van genes, unraveling a network of consequential horizontal gene transfer events linking the phylum Actinobacteria with the five other bacterial classes carrying van genes.
1. Introduction
Glycopeptide antibiotics—more precisely, dalbaheptides—represent a large group of natural products produced by soil-dwelling, high-G-C-content, and Gram-positive actinomycetes [1,2]. Dalbaheptides are products of sophisticated biosynthetic machinery involving the non-ribosomal synthesis of the heptapeptide aglycone, followed by numerous and variable modifications. Genes encoding these pathways are co-localized, co-expressed, and organized in large biosynthetic gene clusters (BGCs). Dalbaheptide biosynthesis has been extensively studied and reviewed in recent years [3,4,5,6,7,8,9].
Although biosynthetic and regulatory mechanisms behind the production of dalbaheptides are interesting per se and represent a valuable model for studying other natural products, the real importance of dalbaheptides comes from their clinical relevance [10,11,12,13]. Today, two natural (vancomycin and teicoplanin) and three semi-synthetic (dalbavancin, telavancin, and oritavancin) dalbaheptides are used to treat infections caused by multidrug-resistant (MDR) pathogens [8]. As potent inhibitors of Gram-positive cell-wall biosynthesis, dalbaheptides strongly and selectively bind lipid II [14], essential for the biosynthesis of the bacterial cell wall [15]. They specifically interact with the d-alanyl-d-alanine (d-Ala-d-Ala) termini of the lipid II pentapeptide side stems by forming five defined hydrogen bonds, thus impeding the following transpeptidation and transglycosylation steps [9,16,17].
As Gram-positive bacteria, actinobacterial dalbaheptide producers require the expression of self-resistance mechanisms to avoid suicide during antibiotic biosynthesis and excretion. Resistance mechanisms in model dalbaheptide producers have been investigated in detail in recent years [18,19,20,21]. The most relevant dalbaheptide resistance mechanism involves at least five genes—known as van genes (i.e., vancomycin resistance genes)—commonly organized in two operons [22,23]. The first operon consists of three genes—vanHAX—coding for structural enzymes required for cell-wall remodeling. Here, VanH is an α-ketoacid dehydrogenase, transforming pyruvate to d-lactate (d-Lac); VanA is a d-Ala-d-Lac ligase, while VanX is a d,d-dipeptidase [24]. The second operon—vanRS—encodes a two-component regulatory system tuning vanHAX expression in response to the extracellular presence of a glycopeptide antibiotic. Dalbaheptide-producing actinobacteria carry vanHAXRS genes adjacent to or within the corresponding glycopeptide BGCs.
Based on extensive genomic analysis, we recently reported [25] that vanHAXRS genes are also frequently present in dalbaheptide non-producing actinobacteria, indicating that there is no unique correlation between the presence of glycopeptide BGCs and van genes. However, it remains unclear why dalbaheptide non-producing actinobacteria carry vanHAXRS genes so frequently. Actinobacteria—either dalbaheptide-producing or not—are the most likely primary environmental reservoir of van genes, further spreading to other bacterial taxa [25,26]. The spread of van genes among opportunistic pathogens such as enterococci or staphylococci represents a hot topic of investigation due to their clinical relevance [27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47]. On the other hand, the migration of vanHAXRS genes to non-harmful soil-dwelling bacteria other than Actinobacteria has not attracted much immediate attention, although they likely serve as a bridge to distribute van genes from Actinobacteria spp. to dangerous pathogens [26]. Previous reports highlighted that van genes are also present in two additional classes of Gram-positive soil bacteria, namely, the class Bacilli and the class Clostridia [48,49,50,51,52]. As reported in ESM Table S1, we analyzed in detail the precedent literature and the genomic data available on the bacterial species carrying vanHAXRS genes other than those belonging to the Actinobacteria phylum. It was observed that vanHAXRS genes were mainly found in enterococci (Enterococcus (Ecc.) fecalis and Enterococcus faecium) and Staphylococcus (Scc.) aureus strains, followed by Paenibacillus spp. and a few other species belonging to the Bacilli and Clostridia classes (for example, in Clostridioides (Cld.) difficile AI0499). In many cases, genes were found to be associated with mobile genetic elements (MGEs): transposons and plasmids (ESM Table S1), which are probably involved in their interspecies migration.
Considering the current progress in bacterial genome sequencing, in this work, we screened the vast amount of current publicly available genomic records to investigate vanHAXRS gene distribution in species other than those belonging to the Actinobacteria phylum. Our findings demonstrate for the first time that vanHAXRS and related genes might be found in Anaerolineae [53], Erysipelotrichia [54], and Ktedonobacteria [55] classes. Additional information on van loci spread within Bacilli and Clostridia classes is also reported. Further phylogenetic analysis of Van proteins following the one published earlier on actinobacterial Van proteins [25] adds more details to the overall picture of how vanHAXRS genes spread among bacterial taxa.
2. Methods
2.1. van Genes Discovery
Amino acid (aa) sequences of SCO3594, SCO3595, and SCO3596 (VanH, VanA, and VanX) from Streptomyces (S.) coelicolor A3(2) were used as queries for BLASTP [56] against non-redundant protein sequence databases for each bacterial class listed in the NCBI Taxonomy database [57]. The BLASTP search used default algorithm parameters, but the maximum number of aligned sequences to display was set to 5000. The analysis was performed in May 2022. All obtained hits (with no regard to the corresponding e-values) were further investigated manually and mapped to the corresponding genomic records retrieved from either GenBank [58] or RefSeq [59] databases. Every bacterial taxon in which genes coding for SCO3594-95-96 homologs were found to be co-localized was further investigated. In total, we obtained 1 set of van genes for Anaerolineae, 48 for Bacilli, 23 for Clostridia, 2 for Erysipelotrichia, and 7 for Ktedonobacteria; the accession numbers for the discovered nucleic acid and aa sequences are summarized in Supplementary Excel File S1. Metagenome-assembled genomes (MAGs) were not considered in further analyses. Degraded van loci (lacking either vanH, vanA, or vanX homologs), such as those previously observed in Desulfitobacterium (Dsf.) hafniense strains [52] and in some Bacilli spp. [25], were omitted to avoid overcomplicating the general picture.
Routine analysis with all amino and nucleic acid sequences was conducted using Geneious 4.8.5 [60].
2.2. Mapping MGE-Related Genes
To discover and map MGE-related genes co-localized with van loci, aa sequences coded by ORFs from ca. 50 kbp (or less if not available) regions up- and downstream of van loci were analyzed with either CD-Search or Batch CD-Search [61,62]. This allowed us to identify proteins involved in DNA transfer and recombination or prophage-related proteins. Proteins that carried conserved domains typical for IS transposases were further used as queries for BLASTP (default algorithm parameters) against the ISfinder database [63], allowing us to identify those belonging to different IS families. If necessary, inverted repeat regions were identified using the EMBOSS einverted tool [64] with the default algorithm parameters, although the “maximum extent of repeats” parameter was manually adjusted for each search to cover the whole region of interest. Putative prophage regions were inspected using Phaster [65] and PhisDetector [66] web servers under default search conditions.
The accession numbers for MGE-related genes and proteins, as well as additional information are given in Supplementary Excel File S1.
2.3. Phylogenetic Reconstruction
Mega 11 (v.11.0.13) [67] software was used for the phylogenetic analysis. All trees were generated using the neighbor-joining method [68]. The confidence of the branching was estimated using an interior-branch test (1000 replicates) [69]. The evolutionary distances were computed using the JTT matrix-based method [70]. Ambiguous positions were removed with the pairwise deletion option. The rate variation among sites was modeled with a 5-parameter σ distribution. The phylogeny of VanH proteins was built using 269 aa sequences, including SCO2118 (putative d-Lac dehydrogenase from S. coelicolor A3(2)) as an outgroup (Supplementary FASTA File S1); the phylogeny of VanA was built using 265 aa sequences proteins, including SCO5560 (putative d-Ala-d-Ala ligase from S. coelicolor A3(2)) as an outgroup (Supplementary FASTA File S2); the phylogeny of VanHA concatenates was constructed by using 262 aa sequences and included concatenated SCO2118-SCO5560 as an outgroup (Supplementary FASTA File S3).
3. Results
Our screening of publicly available genomic records revealed the presence of vanHAXRS and related genes in four classes of Gram-positive bacteria beyond the phylum Actinobacteria, i.e., Bacilli, Clostridia, Erysipelotrichia, and Ktedonobacteria, as well as in one class of Gram-negative bacteria—Anaerolineae (Table 1)—all belonging to the Terrabacteria superphylum [71]. To our best knowledge, van genes have not been previously reported in representatives of the last three classes. Notably, we did not include van loci found in MAGs in this analysis. For example, at least one MAG containing van loci was also found in the classes Acidobacteria, Fusobacteria, Negativicutes, and Thermotogae. However, the analysis of genes up- and downstream of these van loci showed them to be identical or homologous to genes from different Actinobacteria spp. (namely, micrococci). This allowed us to reasonably suspect anomalies in the MAG assembly, presuming that the final genome reconstruction was “contaminated” with nucleic acid sequences from other bacteria.

Table 1.
List of bacterial strains from Anaerolineae, Bacilli, Clostridia, Erysipelotrichia, and Ktedonobacteria classes found to carry van loci; more detailed information, as well as accession numbers for nucleic and aa sequences, is given in Supplementary Excel File S1.
In the following subsections, the organization and genetic background of vanHAXRS for each taxonomical class is further investigated.
3.1. van Genes in Class Anaerolineae
A single set of van genes was discovered in one of the species from a still poorly investigated class of Gram-negative bacteria—Anaerolineae [53]. Here, the organization of van genes in Al. lenta MO-CFX2 SCF03 (vanRSWHAX, Figure 1a, Supplementary Excel File S1 “Anaerolineae” worksheet) resembled the one described in the Ecc. faecalis BM4382 pIP834+ plasmid-borne Tn1549 transposon, although it lacked vanY homolog. In addition, a set of genes coding for putative transposases, recombinases, and integrases was found near the van locus (see Supplementary Excel File S1 “Anaerolineae MGERG”). However, none of them showed significant similarity to the counterparts from previously published MGEs carrying van genes (summarized in ESM Table S1).
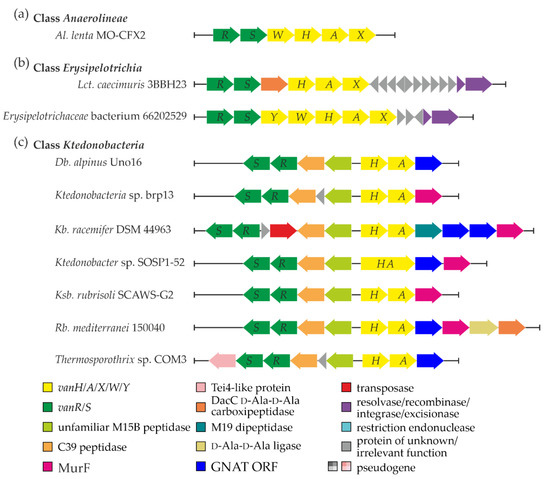
Figure 1.
Graphical summary of van and other relevant co-localized genes discovered in Anaerolineae (a), Erysipelotrichia (b), and Ktedonobacteria (c) classes. The color coding of the schemes is explained in the legend of the figure. Please see the main text for more details.
3.2. van Genes in Class Erysipelotrichia
Most studied representatives of the class Erysipelotrichia belong to the gut microbiota, while the class itself was recently delineated as a sister taxon of the Clostridia class within the phylum Firmicutes [72]. Our screen indicated the presence of van genes in the genomes of Lct. caecimuris 3BBH23 (isolated from healthy human feces [73]) and of unclassified clinical Erysipelotrichaceae isolate 66202529 (CP046174, Supplementary Excel File S1 “Erysipelotrichia”). Both vanRS and vanHAX operons were found in Lct. caecimuris 3BBH23, with a gene coding for a VanYD DacC-like carboxypeptidase [74] in between (Figure 1b). The chromosome of Erysipelotrichaceae isolate 66202529 carried a set of vanRSYWHAX genes (Figure 1b), like in the Ecc. faecium MLG229 Tn1549-like transposon. In both Lct. caecimuris 3BBH23 and isolate 66202529, we found a pair of genes coding for excisionase and a tyrosine-based site-specific integrase downstream of van loci (Figure 1b), as well as multiple other MGE-related genes (Supplementary Excel File S1 “Erysipelotrichia”). Peculiarly, the integrase coded downstream of van loci in Lct. caecimuris 3BBH23 lacked any significant similarity to homologs in previously published MGEs carrying van genes (summarized in ESM Table S1). Thus, van loci in Lct. caecimuris 3BBH23 are likely to be a part of a novel MGE. On the contrary, the integrase from isolate 66202529 appeared to share 100% aa sequence identity with the integrases found downstream of van loci in Ecc. faecium MLG229 and Clostridium sp. MLG245 Tn1549-like transposons, implying that isolate 66202529 carries the latter MGE.
3.3. Peculiarities of Organization and Genetic Context of van Genes in Class Ktedonobacteria
For the first time, our screen revealed that van genes are present in representatives of the class Ktedonobacteria (phylum Chloroflexi) (Table 1, Supplementary Excel File S1 “Ktedonobacteria”). These enigmatic organisms belong to an unusual group of filamentous Gram-positive soil dwellers. Ktedonobacteria spp. resemble filamentous sporulating actinomycetes in certain aspects of morphology and lifestyle [55,75], implying that a sophisticated machinery for cell-wall rearrangement might exist there.
The arrangements and genetic contexts of van genes in Ktedonobacteria spp. were unprecedented: in all of them, we observed a tendency to lose vanX (Figure 1c, Supplementary Excel File S1 “Ktedonobacteria”). However, this loss was correlated with the gain of a novel gene pair positioned between vanRS and vanHA (Figure 1c). Remarkably, these two genes were found to code for peptidases. One peptidase belonged to the MEROPS [76] M15B subfamily of metallopeptidases, while the second resembled peptidases of the MEROPS C39 family. Typical VanX proteins also belong to the M15B subfamily but are quite different from M15B metallopeptidases from the Ktedonobacteria spp. mentioned above. VanX from S. coelicolor A3(2) (taken as a reference) shares only ca. 15% aa sequence identity with any of the Ktedonobacteria spp. M15B metallopeptidases, and it is shorter (202 aa vs. ca. > 320 aa). Moreover, Kb. racemifer DSM 44963 contained an additional gene coding for a MEROPS M19 family dipeptidase just after vanA (thus directly replacing the canonical position of vanX, Figure 1c). Other relevant genes were co-localized with van loci in Ktedonobacteria spp., including genes for MurF (a UDP-N-acetylmuramoyl-tripeptide ligase), a GCN5-related N-acetyltransferase (GNAT), a VanYD-like DacC d-Ala-d-Ala carboxypeptidase, and a d-Ala-d-Ala ligase (Figure 1c, Supplementary Excel File S1). Another interesting feature was observed in Ktedonobacter sp. SOSP1-52, where vanH and vanA were fused (Figure 1c); however, it remains unknown whether the fusion protein is produced or whether what we observed is just an error of genome sequencing and annotation.
Further analysis of the close genomic neighborhood of van loci in Ktedonobacteria spp. (except Db. alpinus Uno16) revealed a striking number of genes coding for transposases (often incomplete or frameshifted, Supplementary Excel File S1 “Ktedonobacteria MGERG”) belonging to different insertion sequence (IS) families. Most of them had DDE catalytic residues [77]. In addition, we discovered multiple short and long inverted repeats. Genes coding for recombinase/integrase-like enzymes were also found (Supplementary Excel File S1 “Ktedonobacteria MGERG”), although they did not show repetitive patterns across different Ktedonobacteria spp.
To study the last aspect more comprehensively, we created a detailed map of the genomic neighborhood of van loci in Kb. racemifer DSM 44963 (given in Figure 2). The examined region (ca. 50 kbp up- and downstream of van loci) contained 21 genes for transposases (4 of them were pseudogenes), 2 genes for integrase-like enzymes, and one gene for a restriction endonuclease. Three segments surrounded by large inverted repeat regions were found upstream of the van loci (Figure 2): in two cases, these inverted repeats were duplicated genes for transposases, while the third case involved the duplication and inversion of the vanRS locus. Moreover, two intact ISKra4-like IS elements and one ISKra7-like IS element were found up- and downstream of the van loci, respectively. Another putative IS element composed of a gene for an IS1380-family transposase with 36 bp inverted repeats was found downstream of van loci; however, neither the transposase nor the repeat regions were significantly similar to any entries of the ISfinder database [63]. Finally, two peptidase genes (M15B and C39, see above) might also belong to an unknown IS element in Kb. racemifer DSM 44963. Both genes were co-localized with an IS200/IS605-family transposase gene and flanked with 58 bp inverted repeats (Figure 2). Interestingly, the same inverted repeats were found flanking M15B- and C39-peptidase genes in other Ktedonobacteria spp., although the IS200/IS605-family transposase gene was present only in Kb. racemifer DSM 44963.
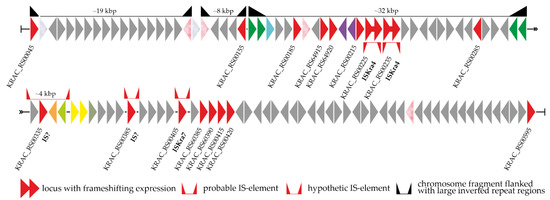
Figure 2.
Detailed map of the genomic context of van loci in Kb. racemifer DSM 44963. The color coding of the scheme is explained in the Figure 1 legend. Please see the main text for more details.
3.4. Updates on vanHAXRS Gene Distribution in Bacilli Class
Bacteria belonging to the Bacilli class are well known to carry vanHAXRS genes; these are either opportunistic pathogens such as staphylococci and enterococci or non-harmful soil dwellers (see summary in ESM Table S1). Since our previous bioinformatic analysis [25], multiple novel genomic records for Bacilli spp. have become available in GenBank and RefSeq. This allowed us to significantly update the picture of the vanHAXRS distribution in the Bacilli class: vanHAXRS genes were discovered in 48 novel strains from this class (Table 1, Supplementary Excel File S1“Bacilli”).
The arrangements of van genes varied across different strains and are summarized in Figure 3. The simplest one—only vanHAX genes with no MGE-related genes nearby—was discovered in Pba. dendritiformis J6TS7 (Figure 3a, Supplementary Excel File S1 “Bacilli”). An additional vanS pseudogene was present in Pba. flagellatus DXL2 (Figure 3b, Supplementary Excel File S1 “Bacilli”); however, vanS was found at the 5′-end of the available contig, suggesting that it could be intact and likely followed by a vanR gene. No adjacent MGE-related genes were discovered in Pba. flagellatus DXL2, either. The van genes in Brevibacillus sp. SKDU10 also lacked the vanRS locus, and they were arranged as vanHAXYZ. A vanH pseudogene, another copy of vanZ, and a transposase pseudogene (Figure 3c, Supplementary Excel File S1 “Bacilli”, “Bacilli MGERG”) were discovered further downstream.
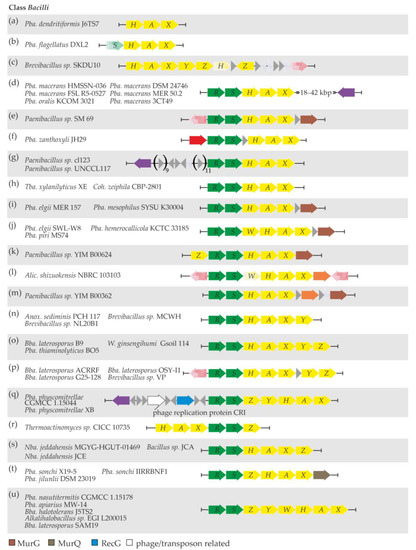
Figure 3.
Schematic representation of the arrangement patterns observed in the van and relevant co-localized genes from the Bacilli class. Twenty one arrangement patterns (a–u) were determined and are discussed in the main text. To interpret the color coding of Figure 3, please also refer to the legend in Figure 1.
A set of five Pba. macerans strains, together with Pba. oralis KCOM 3021 (Figure 3d), carried vanRSHAX genes with a highly conserved gene for a tyrosine-type site-specific recombinase lying downstream. This recombinase did not share any similarity with the recombinases already reported to be co-localized with van loci. Some other MGE-related genes were also discovered up- and downstream of van loci in these six strains, although they were not strictly conserved (Supplementary Excel File S1 “Bacilli MGERG”). Furthermore, van genes were organized as vanRSHAX in eight additional strains (Figure 3e–i, Supplementary Excel File S1 “Bacilli”). In addition, van loci were co-localized with transposase genes in Paenibacillus sp. SM 69 and Pba. zanthoxyli JH29 and with a gene for an unprecedented tyrosine-type site-specific recombinase in Paenibacillus sp. cl123 and Paenibacillus sp. UNCCL117 (Supplementary Excel File S1 “Bacilli MGERG”). Notably, in Paenibacillus sp. SM 69, Pba. elgii MER 157, and Pba. mesophilus SYSU K30004, a gene for UDP-N-acetylglucosamine-N-acetylmuramyl-(pentapeptide) pyrophosphoryl-undecaprenol N-acetylglucosamine transferase (MurG) was found downstream of van loci (Figure 3e,i). A slightly different arrangement—vanRSWHAX—was observed in three other Paenibacillus spp. (Figure 3j). Here, no MGE-related genes were discovered nearby, but a gene for MurG was again found downstream of van loci (Supplementary Excel File S1 “Bacilli”).
In Paenibacillus sp. YIM B00624, van genes were organized as vanZRSHAX (Figure 3k), again with no MGE-related genes nearby, but followed by a gene for MurG (Supplementary Excel File S1 “Bacilli”).
Only two strains, namely, Alic. shizuokensis NBRC 103103 and Paenibacillus sp. YIM B00362, were found to carry a gene for a DacC-like VanYD carboxypeptidase within van loci, organized as vanRS(W pseudogene)HAX(YD) and vanRSHAX(YD), respectively (Figure 3l,m). In Alic. shizuokensis NBRC 103103, van loci were surrounded by two transposase pseudogenes, while Paenibacillus sp. YIM B00362 had no MGE-related genes near van loci (Supplementary Excel File S1 “Bacilli MGERG”). As in some of the other abovementioned Paenibacillus spp., Paenibacillus sp. YIM B00362 had a gene for MurG just downstream of van genes (Supplementary Excel File S1 “Bacilli”).
Next, in Anox. sediminis PCH 117, Brevibacillus sp. MCWH, and Brevibacillus sp. NL20B1 van genes were organized as vanRSHAXY (Figure 3n). No MGE-related genes were found to co-localize with van genes in these three strains. One additional gene—vanZ—was found downstream of vanRSHAXY in Bba. laterosporus B9, Pba. thiaminolyticus BO5, and W. ginsengihumi Gsoil 114 (Figure 3o). Notably, in Bba. laterosporus B9, van loci were carried on a plasmid. Another group of four Brevibacillus spp. had a similar arrangement of van genes: vanRSHAX and vanYZ loci separated by a non-correlated gene (Figure 3p), also sharing a transposase pseudogene just upstream of vanR (Supplementary Excel File S1 “Bacilli MGERG”).
Pba. physcomitrellae CGMCC 1.15044 and Pba. physcomitrellae XB represented a rather unique case among Bacilli spp., where van genes were organized as vanRSZYHAX, and a set of three MGE-related genes was found upstream (Figure 3q). These three genes code for an ATP-dependent DNA helicase RecG, a CRI phage replication protein, and an unprecedented serine-type recombinase (Supplementary Excel File S1 “Bacilli MGERG”). The RecG and recombinase proteins shared high similarity with counterparts in the genomes of phages of the order Caudovirales [78].
Three distinct combinations of vanH, A, X, R, S, and Z genes were observed in the following strains: vanHAXRSZ in Thermoactinomyces sp. CICC 10735 (Figure 3r); vanRSHAXZ in Nba. jeddahensis MGYG-HGUT-01469, Nba. jeddahensis JCE, and Bacillus sp. JCA (Figure 3s); and vanRSZHAX in Pba. sonchi X19-5, Pba. sonchi IIRRBNF1, and Pba. jilunlii DSM 23019 (Figure 3t). In Thermoactinomyces sp. CICC 10735, Nba. jeddahensis MGYG-HGUT-01469, Nba. jeddahensis JCE, Pba. sonchi X19-5, and Pba. jilunlii DSM 23019, van loci were not accompanied by MGE-related genes, while the genomic neighborhood of van genes in Pba. sonchi IIRRBNF1 and Bacillus sp. JCA was enriched with genes and pseudogenes for transposases of different types (Supplementary Excel File S1 “Bacilli MGERG”). In Pba. sonchi X19-5, Pba. sonchi IIRRBNF1, and Pba. jilunlii DSM 23019, a gene coding for an N-acetylmuramic acid 6-phosphate etherase (MurQ) was found just downstream of vanX.
Finally, the vanRSZYWHAX arrangement was discovered in Pba. nasutitermitis CGMCC 1.15178, Pba. apiarius MW-14, Bba. halotolerans J5TS2, Alkalihalobacillus sp. EGI L200015, and Bba. laterosporus SAM19 (Figure 3u). In each of these strains, van loci were accompanied by variable sets of genes and pseudogenes for transposases of different types (Supplementary Excel File S1 “Bacilli MGERG”). Notably, a gene for an unprecedented tyrosine-type site-specific integrase was found downstream of van loci in Pba. apiarius MW-14.
3.5. Updating the Picture of vanHAXRS Gene Distribution in Clostridia Class
To date, at least two Clostridia spp. carrying vanHAX and vanRS loci as a part of a Tn1549-like transposon have been reported (ESM Table S1). Bizarrely recombined van loci lacking vanH orthologs were also discovered in different strains of Dsf. hafniense [52] (ESM Table S1). Our screen added new results to previous data: vanHAX, vanRS, and related genes were found within the genomic records of 23 strains from the Clostridia class (Table 1, Supplementary Excel File S1 “Clostridia”). The arrangements of vanHAX, vanRS, and related genes were found to be quite variable.
The simplest one—the vanHAX locus only—was observed in Am. terrae CBA3637 and Bl. producta ER3 (Figure 4a, Supplementary Excel File S1 “Clostridia”). Additionally, in Am. terrae CBA3637, genes for UDP-N-acetylmuramoyl-l-alanyl-d-glutamate-2,6-diaminopimelate ligase (MurE) and a serine-based recombinase were found upstream of vanHAX (Figure 4a, Supplementary Excel File S1 “Clostridia” and “Clostridia MGERG”). This recombinase did not share any similarity with other van-loci-associated recombinases published previously.
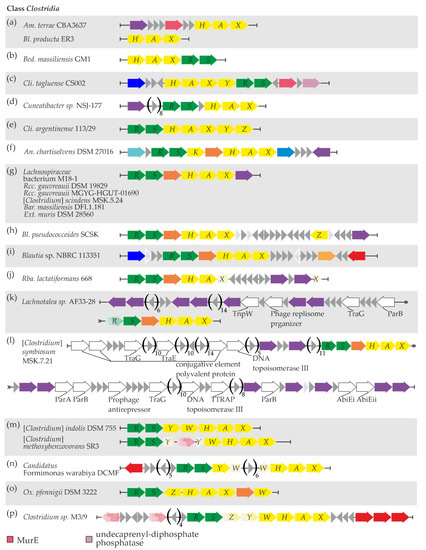
Figure 4.
Sixteen (a–p) arrangement patterns of van genes and relevant neighboring genes were discovered in bacteria from the Clostridia class. The contents of the figure are discussed in the main text. To interpret the color coding of Figure 4, please also refer to the legends in Figure 1 and Figure 3.
The vanHAXRS arrangement was found in Bed. massiliensis GM1 (Figure 4b, Supplementary Excel File S1 “Clostridia”), although no MGE-related genes were found nearby.
Next, in Cli. tagluense CS002, van genes were arranged as vanHAXYRS with additional genes for MurE and undecaprenyl-diphosphate phosphatase downstream, and no MGE-related genes were found nearby (Figure 4c, Supplementary Excel File S1 “Clostridia”).
The vanRSHAX arrangement was observed in Cuneatibacter sp. NSJ-177, with an upstream gene coding for a tyrosine-based site-specific recombinase/integrase (Figure 4d, Supplementary Excel File S1 “Clostridia” and “Clostridia MGERG”). Again, this recombinase shared no similarity to the previously reported ones associated with van loci.
Cli. argentinense 113/29 exhibited the vanRSHAXYZ arrangement of van genes, with no MGE-related genes nearby (Figure 4e, Supplementary Excel File S1 “Clostridia”).
In An. chartisolvens DSM 27016, we found the vanRS locus, followed by vanK, vanYD (coding for a DacC-like carboxypeptidase), and vanHAX (Figure 4f, Supplementary Excel File S1 “Clostridia”); the van genes were surrounded by genes coding for a RecG-like DNA helicase and a restriction endonuclease. Finally, a gene coding for a serine-based recombinase was found downstream of van loci; as in the cases above, this recombinase shared no similarity with previously published van-loci-associated recombinases (Supplementary Excel File S1 “Clostridia MGERG”).
A vanRS(YD)HAX arrangement was found within the genomic records of several Clostridia spp. The first group of them included Lachnospiraceae bacterium M18-1, Rcc. gauvreauii DSM 19829, Rcc. gauvreauii MGYG-HGUT-01690, [Clostridium] scindens MSK.5.24, Bar. massiliensis DFI.1.181, and Ext. muris DSM 28560 (Figure 4g, Supplementary Excel File S1 “Clostridia”). All six of these bacterial strains shared a common gene for an unknown highly conserved tyrosine-based site-specific recombinase/integrase just after vanX. In addition, the genomic neighborhood of van loci in Lachnospiraceae bacterium M18-1 and Ext. muris DSM 28560 contained a rich repertoire of putative MGE-related genes (Supplementary Excel File S1 “Clostridia MGERG”). Although many of them were homologous to genes found in phages from the order Caudovirales [78], neither Phaster nor PhisDetector tools were able to identify prophage regions near van loci in M18-1 and Ext. muris DSM 28560.
The same arrangement of van genes was observed in Bl. pseudococcoides SCSK and Blautia sp. NBRC 113351 (Figure 4h,i). In the first strain, an additional vanZ gene was found downstream of the main van loci (Figure 4h, Supplementary Excel File S1 “Clostridia”), as well as a gene for an unprecedented serine-based recombinase (Supplementary Excel File S1 “Clostridia MGERG”). At the same time, a gene for an IS110-family transposase was downstream of van loci in Blautia sp. NBRC 113351 (Supplementary Excel File S1 “Clostridia MGERG”). A peculiar case was observed for Rba. lactatiformans 668, where the vanRS(YD)HAX arrangement was interrupted by the insertion of ten genes into the open reading frame (ORF) of vanX (Figure 4j, Supplementary Excel File S1 “Clostridia”). Two of these genes code for a MobM-like relaxase and a serine-based recombinase, respectively (Supplementary Excel File S1 “Clostridia MGERG”); neither of them was similar to the MGE-related genes found before in association with van loci. Finally, the vanRS(YD)HAX arrangement was also found in Lachnotalea sp. AF33-28 and [Clostridium] symbiosum MSK.7.21 (Figure 4k,l). The genetic context of van loci in both of these strains was quite interesting. Multiple genes coding for MGE-related proteins were found upstream of van loci in Lachnotalea sp. AF33-28 (Supplementary Excel File S1 “Clostridia MGERG”). All of these proteins lacked counterparts in previously published MGEs carrying van genes but appeared similar (≥56% aa sequence identity) to proteins encoded in genomes of phages from the Caudovirales order [78]. When prophage identification analysis was performed on Lachnotalea sp. AF33-28 using Phaster, an incomplete prophage region was indeed identified upstream of van loci. The most similar phage for this region was identified as Faecalibacterium phage FP_Brigit (NC_047909). A similar situation was observed in [Clostridium] symbiosum MSK.7.21 (Supplementary Excel File S1 “Clostridia MGERG”). However, neither Phaster nor PhisDetector prophage detection tools were able to discover prophages near van loci in [Clostridium] symbiosum MSK.7.21.
Another arrangement—vanRSYWHAX—was discovered in [Clostridium] indolis DSM 755, [Clostridium] methoxybenzovorans SR3 (Figure 4m, Supplementary Excel File S1 “Clostridia”), and Candidatus Formimonas warabiya DCMF (Figure 4n). Notably, in [Clostridium] methoxybenzovorans SR3, the vanY ORF was interrupted by a transposase pseudogene, while in Candidatus Formimonas warabiya DCMF, the vanW ORF contained a six-gene insertion. An IS1634-family transposase gene was also found upstream of van loci in the latter strain (Supplementary Excel File S1 “Clostridia MGERG”).
Ox. pfennigii DSM 3222 carried a vanRSZHAX(YD)W arrangement (Figure 4o), with no MGE-related genes nearby.
Finally, a vanRSZYWHAX arrangement was observed in Clostridium sp. M3/9, with vanZ and vanY genes containing mutations that interrupt ORFs (Figure 4p, Supplementary Excel File S1 “Clostridia”). A set of pseudo- and functional genes for transposases was found up- and downstream of van loci (Supplementary Excel File S1 “Clostridia MGERG”).
3.6. Building a Consensus Scheme for Phylogenetic Relations between Newly Discovered van Proteins and Those from Phylum Actinobacteria
Thus, our screen revealed multiple novel van genes from representatives of five bacterial classes. To integrate this information, we further aimed to fulfill two goals: (a) create a reliable phylogenetic tree representing the evolutionary interrelationships among Van proteins encoded by different bacterial classes; (b) see whether the genetic context of van genes (i.e., co-localized MGE-related genes) correlates with their phylogeny.
To achieve these goals, we first reconstructed separate phylogenies for VanH and VanA (i) encoded within the genomes of Actinobacteria spp. (using datasets from our previous work [25]), (ii) from nucleic acid sequences of pathogens and non-harmful soil bacteria published before (see ESM Table S1), and (iii) discovered in the current work. Separate phylogenetic reconstructions for the two proteins were coherent (see ESM Figures S1 and S2), allowing us to reasonably assume that vanHA (and corresponding proteins) evolved as a single unit (with insignificant exceptions). We moved onward to the creation of a representative phylogenetic tree using concatenated sequences of VanH and VanA proteins, encoded in a single locus. Unfortunately, we could not also use VanX sequences, since all of the representatives of the Ktedonobacteria class lack the corresponding genes (see Section 3.3). Sequences from Dsf. hafniense strains were also not included, since corresponding van loci lack vanH orthologs [52].
The final tree is shown in Figure 5. An analysis of its topology allowed us to make some important observations. At first glance, a very recent horizontal gene transfer (HGT) event involving van genes from Actinobacteria seemed evident: VanHA sequences from all Ktedonobacteria spp. and from some Clostridia spp. (namely, An. chartisolvens DSM 27016, Cli. tagluense CS002, Ox. pfennigii DSM 3222, and Candidatus Formimonas warabiya DCMF) rooted deep in the core Actinobacteria clade (further referred to as “Core Actinobacteria”) (Figure 5), being a sister clade to VanHA from Stackebrandtia nassauensis DSM 44728. Notably, VanA sequences from Dsf. hafniense strains also belonged to an analogous clade of the VanA tree (ESM Figure S2). The repertoire of MGE-related genes co-localized with van loci in all of these species was variable, hindering the comprehension of transmission vectors (Figure 5, Supplementary Excel File S1 “Clostridia MGERG” and “Ktedonobacteria MGERG”). However, in Ktedonobacteria spp., multiple IS transposase genes were co-localized with van loci, as well as with seemingly intact IS elements (Supplementary Excel File S1 “Ktedonobacteria MGERG”).
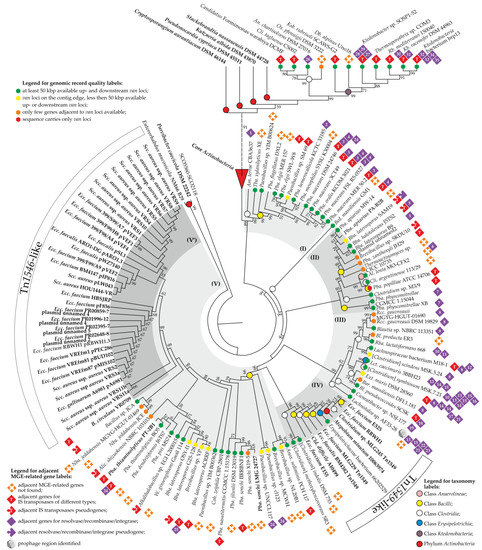
Figure 5.
Rooted neighbor-joining phylogenetic tree of 261 VanHA sequences from Actinobacteria, Anaerolineae, Bacilli, Clostridia, Erysipelotrichia, and Ktedonobacteria, together with the sequence of SCO5560-SCO2118 used as an outgroup. The tree was generated as described in Section 2. For its better representability, branch lengths were ignored, while the “Core Actinobacteria” clade was collapsed; the same tree completely expanded and drawn to scale is given in ESM Figure S3. Numbers at nodes indicate bootstrap-support percentages derived from 1000 replications (shown only for ≥ 15% values). Five clades (I–V) discussed in the main text are highlighted in gray, while the labels are interpreted above and below the tree. Taxa known to carry van loci from previous works are given in bold. The following MGE-related enzymes are numbered 1-35 (if a numbered label is found near few taxa, the accession number would be given only for “prototype”, implying that its counterparts are close homologs sharing ≥ 50% aa sequence identity): (1) WP_162363028—serine-based recombinase/integrase; (2) WP_139603211—relaxase/mobilisation nuclease; (3) WP_124332115—tyrosine-based recombinase/integrase; (4) WP_036622915—tyrosine-based recombinase/integrase; (5) AKF95793—tyrosine-based recombinase/integrase; (6) WP_119072093—serine-based recombinase/integrase; (7) WP_124332115—serine-based recombinase/integrase; (8) WP_028530137—tyrosine-based recombinase/integrase; (9) WP_225305221—serine-based recombinase/integrase; (10) WP_016316877—relaxase/mobilisation nuclease; (11) WP_016316878—serine-based recombinase/integrase; (12) EOS38773—serine-based recombinase/integrase; (13) WP_213540055 –relaxase/mobilisation nuclease; (14) WP_213540032—tyrosine-based recombinase/integrase; (15) WP_213540058—relaxase/mobilisation nuclease; (16) WP_173892454—serine-based recombinase/integrase; (17) WP_132278971—relaxase/mobilisation nuclease; (18) WP_065543677—tyrosine-type recombinase/integrase; (19) WP_244802434—tyrosine-type recombinase/integrase; (20) WP_002569238—relaxase/mobilisation nuclease; (21) WP_117962491—serine-based recombinase/integrase; (22) WP_117761817—serine-based recombinase/integrase; (23) WP_117962497—relaxase/mobilisation nuclease; (24) WP_072327346—tyrosine-based recombinase/integrase; (25) WP_161555111—tyrosine-based recombinase/integrase; (26) WP_170138094—serine-based recombinase/integrase; (27) GHO64028—tyrosine-based recombinase/integrase; (28) GHO64041—phage integrase; (29) GHO64148—serine-based recombinase/integrase; (30) WP_007903552—tyrosine-based recombinase/integrase; (31) WP_075164122—tyrosine-based recombinase/integrase; (32) BCL79139—tyrosine-based recombinase/integrase; (33) BCL79135—serine-based recombinase/integrase; (34) BCL79150—tyrosine-based recombinase/integrase; (35) BCL79151—tyrosine-based recombinase/integrase.
In addition to the “Core Actinobacteria”, five major clades were defined within the tree. Clade (I) (Figure 5) appeared to be a sister clade of the “Core Actinobacteria”. It consisted of sequences from 12 strains of Paenibacillus, Tba. xylanilyticus XE (all 13 belonging to the class Bacilli), and Am. terrae CBA3637 (class Clostridia)—the last one being a basal taxon of clade (I). In all of these species, MGE-related genes co-localized with van loci were quite different (Figure 5, Supplementary Excel File S1 “Clostridia MGERG” and “Bacilli MGERG”). Clade (II) was composed of VanHA sequences mainly from Bacilli spp. These were six strains of the genus Paenibacillus, four strains of Brevibacillus, Thermoactinomyces sp. CICC 10735, two strains of the genus Clostridium, and Al. lenta MO-CFX2 (class Anaerolineae) (Figure 5). VanHA sequences from Pba. physcomitrellae strains formed the basal branch of clade (II). Again, here, we did not observe any reproducible pattern of MGE-related genes co-localized with van loci (Figure 5, Supplementary Excel File S1). Notably, it appeared that one of the clade (II) species, namely, Bba. laterosporus B9, carries van loci on a plasmid. Unfortunately, the quality of genome assemblies of another clade (II) species did not allow us to understand whether they carry van loci on plasmids as well.
VanHA sequences from Clostridia spp. (with the single exception of Lct. caecimuris 3BBH23, class Erysipelotrichia) formed clade (III) (Figure 5). The basal taxon of clade (III) was Lachnotalea sp. AF33-28. In contrast to clade (II), we observed a certain similarity in the repertoire of MGE-related genes co-localized with van loci in strains forming clade (III) (Figure 5). In some of the strains, most notably in Lachnotalea sp. AF33-28, [Clostridium] symbiosum MSK.7.21, Lachnospiraceae bacterium M18-1, and Ext. muris DSM 28560, large assemblages of putative MGE-related genes were associated with van loci (see Section 3.5). These assemblages resembled prophages, although an incomplete prophage region was identified only in Lachnotalea sp. AF33-28.
The last two clades—(IV) and (V)—deserve special attention. Clade (IV) was composed of VanHA sequences from [Clostridium] methoxybenzovorans SR3, [Clostridium] indolis DSM 755, Erysipelotrichaceae bacterium 66202529 (class Erysipelotrichia), and a set of strains known to carry van loci as a part of Tn1549-like transposons, namely, Atopobium (Atpb.) minutum 10063974 (phylum Actinobacteria), Clostridium sp. MLG245, Cld. difficile AI0499, Ecc. faecium E525, Ecc. faecium RBWH1, Ecc. faecium MLG229, Ecc. faecalis BM4382, and Ecc. faecium E155 (see ESM Table S1 for references) (Figure 5). While Erysipelotrichaceae bacterium 66202529 clearly carried genes from the Tn1549-like transposon near van loci (see Section 3.2), no MGE-related genes were discovered near van loci in [Clostridium] methoxybenzovorans SR3 and [Clostridium] indolis DSM 755.
Clade (V) included a large portion of VanHA sequences from different Bacilli spp.; the repertoire of MGE-related genes co-localized with van loci was rather poor in species belonging to this clade (Figure 5). Finally, a subclade of clade (V)—(V’)—appeared to be composed of VanHA sequences from plasmids carrying Tn1546-like transposons, isolated from clinically relevant enterococci and staphylococci from all over the world (Figure 5, see also ESM Table S1 for references). Bacillus circulans VR0709 was a basal taxon of (V’) (Figure 5), where a single MGE-related gene was found adjacent to the van loci [50,79]. This was a gene for an IS200/IS605-family (Y1) transposase (CAB61226) that had no homologs in other clade (V’) strains. Peculiarly, Ecc. faecium RBWH1 pRBWH1.3+ [32] appeared to carry two sets of van genes simultaneously: one as a part of the Tn1546-like transposon on the plasmid (pRBWH1.3), and another as a part of the Tn1549-like transposon integrated into the chromosome.
Concluding the analysis of the tree, it seems interesting to note that VanHA sequences encoded in putative MGEs from two actinobacteria—Parvibacter (Prv.) caecicola DSM 22242 and Enterorhabdus (Enr.) mucosicola NM66_B29—formed the basal branch of the whole tree (Figure 5).
4. Discussion
The results obtained in the current work indicate that van genes are more widely distributed among bacteria than expected before: for the first time, van genes were discovered in the genomic records of organisms from still poorly studied classes such as Anaerolineae, Erysipelotrichia, and Ktedonobacteria. Most likely, the spread of van genes among these different bacteria was determined by multiple HGT events. This idea is not new [26,52,80,81], but the abundance of genomic data available now allows us to outline some of these HGT events more precisely. Although the overall picture is quite puzzling and we are only at the beginning of such types of investigations, in the discussion, we propose a speculative scenario of how van genes might have disseminated (Figure 6).
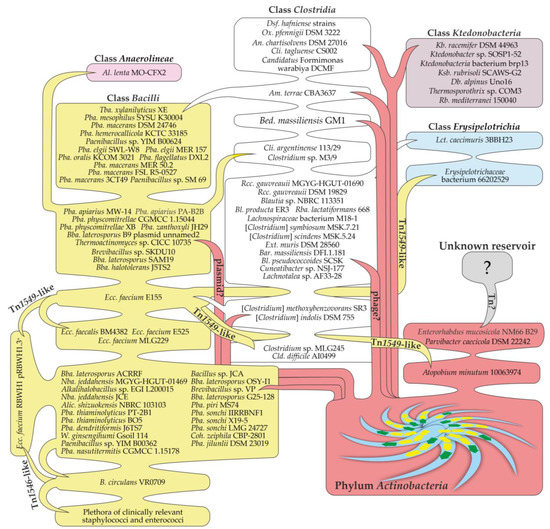
Figure 6.
A scheme depicting a possible scenario for the transmission of vanHAX genes from Actinobacteria spp. to representatives of other classes. The scheme is discussed in the main text.
As noted in the previous section, the most recent HGT event may have led to the transmission of van genes from actinobacteria to the Ktedonobacteria class and to some members of the Clostridia class (see scheme in Figure 6). The corresponding proteins did not diverge much from the actinobacterial ones and were found deep in the “core Actinobacteria” clade (Figure 5). However, the G-C content of these van loci (<50%) is definitely lower than that of actinobacteria (>60%), indicating that the corresponding genes were eventually adapted to the translation machinery of the novel hosts. Most of these Ktedonobacteria and Clostridia spp. were isolated from soil biotopes [55], likely sharing their ecological niches with Actinobacteria spp.—the source of van loci. Notable exceptions were Ox. pfennigii DSM 3222, isolated from cattle rumen [82] (a biotope also inhabited by different actinobacteria [83]), and Cli. tagluense CS002—a causative agent of meat spoilage [84]. Although no MGE-related genes were discovered near van loci in Ox. pfennigii DSM 3222 and only a single ISNCY-family transposase was encoded near van loci in Cli. tagluense CS002, these examples chart new possible routes for the further spread of van genes in antropocenoses. The vehicle/vehicles of such putative HGT events remain elusive, although multiple genes for transposases and resolvases/recombinases/integrases were found to co-localize with van loci in these Ktedonobacteria and Clostridia spp. It seems that in all of these cases, van loci were located on the chromosome; consequently, it is also possible that MGE-related genes might have been partially or completely lost upon integration, eroding the genomic landscapes of putative vehicles carrying van genes.
IS-like elements might have played some role in the HGT of van loci into Ktedonobacteria spp., since, in these microbes, an immense number of transposase genes and intact IS elements are found nearby. Notably, van loci in Ktedonobacteria spp. underwent peculiar modifications: genes coding for VanX were lost, while novel genes for other types of peptidases were gained. Some evidence exists that at least some of these new genes were introduced as “passenger genes” in IS-like elements (see Section 3.3). As already mentioned above, Ktedonobacteria spp. have an actinomycete-like lifestyle [55]. Accordingly, these microbes might have recruited van loci for protection against dalbaheptide producers (inhabiting the same biotopes), self-immunity against certain endogenous secondary metabolites, or morphogenesis-related cell-wall remodeling. Overall, the case of van genes in Ktedonobacteria spp. is of utmost interest and requires further experimental investigation.
Aside from VanHA sequences rooted deep in “Core Actinobacteria”, our reference tree demonstrated the presence of five well-defined clades (99% bootstrap support) and a monotaxon branch (Bed. massiliensis GM1). For further interpretation, we would like to make two assumptions: (a) each of these clades (and Bed. massiliensis GM1 branch) emerged as a result of a single HGT event from Actinobacteria; (b) the basal taxa of each clade represent a starting point in which van loci were delivered by an initial HGT event (before further spreading to the other taxa of the clade). Following these assumptions, an interesting speculative yet plausible scenario for van gene dissemination can be proposed (Figure 6).
In clade (I), a HGT event likely introduced van loci to some Clostridia spp. since the basal taxon of this clade is Am. terrae CBA3637, belonging to the class Clostridia. Am. terrae CBA3637 was isolated from river sediments [85], which are also inhabited by a plethora of actinobacteria [86]. Further, van genes spread to other clade (I) taxa—Tba. xylanilyticus XE and Paenibacillus spp. (Figure 6).
Clade (II) points to several HGT events in addition to the putative initial one. Here, the majority of taxa belong to the Bacilli class. However, sequences from an Anaerolineae class bacterium—Al. lenta MO-CFX2—are positioned far from the base of clade (II), suggesting a putative HGT event delivering van genes from clade (II) Bacilli spp. to Al. lenta MO-CFX2. Notably, Al. lenta MO-CFX2 is the first reported Gram-negative bacterium [87] carrying van loci. The same scenario also works for the two Clostridia spp. found in clade (II)—Clostridium sp. M3/9 and Cli. argentinense 113/29. In at least one clade (II) taxon, Bba. laterosporus B9, van loci are located on a plasmid, implying that a possible vehicle bringing van genes to clade (II) might have been a plasmid.
Except for one, all taxa forming clade (III) belong to Clostridia spp. An exception is the VanHA sequences of Lct. caecimuris 3BBH23 from the Erysipelotrichia class, rooted deep in clade (III). Such a topology most likely indicates that Lct. caecimuris 3BBH23 received van loci from some clade (III) Clostridia spp. as a result of an additional HGT event (Figure 6). It is also important to note that van loci in the basal taxon of the clade (III)—Lachnotalea sp. AF33-28—are located downstream of an incomplete prophage region. Thus, the vehicles that delivered van genes to clade (III) organisms might have been some phages.
VanHA sequences from the classes Bacilli, Erysipelotrichia, Clostridia and the phylum Actinobacteria composed clade (IV). The basal branch of clade (IV) was represented by sequences from [Clostridium] indolis DSM 755 and [Clostridium] methoxybenzovorans SR3, isolated from soil [88] and wastewater [89], respectively. No MGE-related genes accompanied van loci in these two organisms. However, in all of the other taxa of clade (III), van genes were found as a part of Tn1549-like transposons. Such evidence induces us to speculate that an unknown vehicle delivered van genes to [Clostridium] indolis DSM 755 and [Clostridium] methoxybenzovorans SR3 (or ancestral organisms) in an initial HGT event, leading to the emergence of clade (III) (Figure 6). In the following HGT events, van loci were mobilized by a Tn1549-like transposon and then spread to other clade (III) taxa. These taxa included several clinical and human isolates: an organism from the Erysipelotrichia class (Erysipelotrichaceae bacterium 66202529), Cld. difficile AI0499, Clostridium sp. MLG245, several strains of enterococci, and the actinobacterium Atpb. minutum 10063974 (Figure 6). Overall, we might conclude that two bacteria from the Erysipelotrichia class obtained van genes in two independent HGT events, while the Tn1549-like transposon mediated the reverse transition of van genes to the Actinobacteria phylum in the case of Atpb. minutum 10063974.
Unlike all other clades, clade (V) was composed exclusively of Bacilli spp. The basal taxa of clade (V) were mostly soil dwellers and did not show any clear repetitive patterns of MGE-related genes co-localized with van loci (Figure 5). Herein, subclade (V’) was the most interesting, being composed principally of VanHA sequences encoded on plasmids carrying Tn1546-like transposons from different clinical isolates of staphylococci and enterococci. At the basal branch of subclade (V’), there are VanHA sequences from a clinical isolate of the soil-dwelling opportunistic human pathogen B. circulans VR0709 [50]. Overall, the topology of clade (V) suggests that an unknown vehicle delivered van loci to B. circulans VR0709 from a gene pool existing in basal clade (V) taxa. Consequentially, a Tn1546-containing plasmid mobilized van loci from B. circulans VR0709 and mediated further dissemination in nosocomial environments among staphylococci and enterococci (Figure 6). This last observation actually corroborates a previous conclusion made from a phylogenetic analysis conducted more than ten years ago on a less extensive sample of available van gene sequences [80].
Finally, we would also like to comment on van genes from the two actinobacterial strains—Prv. caecicola DSM 22242 and Enr. mucosicola NM66_B29. VanHA sequences from these two organisms were the most divergent, being an outgroup for our reference tree (Figure 5). In our previous work, we demonstrated that van loci in Prv. caecicola DSM 22242 and Enr. mucosicola NM66_B29 are co-localized with an assemblage of MGE-related genes resembling a transposon of unknown type [25]. Notably, both strains were isolated from the intestines of laboratory mice [90,91]. Keeping in mind the case of Atpb. minutum 10063974 (see above), it is tempting to suppose that Prv. caecicola DSM 22242 and Enr. mucosicola NM66_B29 represent another example of a reverse HGT event that brought van genes back to the Actinobacteria phylum. However, the source of the putative transposons found in Prv. caecicola DSM 22242 and Enr. mucosicola NM66_B29 remains unknown (Figure 6).
Our results demonstrate that van genes from the Actinobacteria phylum represent an interesting and widespread part of the Terrabacteria mobilome. The flow of van genes is a continuous process that occurs actively, as demonstrated by the example of Ktedonobacteria spp. Likely carried by different vehicles, van genes spread from one taxon to another, creating a puzzling network of consequential HGT events (Figure 6). Bacteria from the Bacilli and Clostridia classes play an important role in the spread of van genes and might represent their source for classes such as Anaerolineae and Erysipelotrichia. We are aware that our scenario depicting the spread of vanHAX genes is speculative and that it will probably change when more genomic information becomes available. However, we believe that at the current point, it provides a useful interpretation of the overall picture to start from in future investigations.
5. Conclusions
Through extensive bioinformatic work, we discovered that van genes are present in three classes of eubacteria—Anaerolineae, Erysipelotrichia, and Ktedonobacteria—as well as in new genera belonging to the Clostridia and Bacilli classes. The majority of these van genes tended to be co-localized with MGE-related genes of various types. Based on phylogenetic reconstruction, we could assume that the class Anaerolineae recently obtained van genes from Bacilli spp., while two recent independent HGT events delivered van genes to Erysipelotrichia from Clostridia and Bacilli spp., respectively. In addition, the most recent HGT event spread van genes from Actinobacteria directly to species belonging to the Ktedonobacteria class. Ktedonobacteria tended to lose vanX and gain novel genes coding for carboxypeptidases. Finally, a Tn1549-like transposon likely mobilized van genes from Clostridia spp., while a Tn1546-like transposon mobilized van genes from Bacilli spp.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/genes13111960/s1.
Author Contributions
Conceptualization, O.Y.; Investigation, O.Y. and E.B.; Supervision, F.M. and V.F.; Writing—Original Draft, O.Y.; Writing—Review and Editing, F.M., E.B. and V.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the public grant “Fondo di Ateneo per la Ricerca” 2020 and 2021 to F.M. and by the BG-09F grant of the Ministry of Education and Science of Ukraine to V.F.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data obtained in the study are presented in either the main text or Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nicolaou, K.C.; Boddy, C.N.C.; Bräse, S.; Winssinger, N. Chemistry, biology, and medicine of the glycopeptide antibiotics. Angew. Chem. Int. Ed. 1999, 38, 2096–2152. [Google Scholar] [CrossRef]
- Parenti, F.; Cavalleri, B. Proposal to name the vancomycin-ristocetin like glycopeptides as dalbaheptides. J. Antibiot. 1989, 42, 1882–1883. [Google Scholar] [CrossRef] [PubMed]
- Kaniusaite, M.; Tailhades, J.; Kittilä, T.; Fage, C.D.; Goode, R.J.A.; Schittenhelm, R.B.; Cryle, M.J. Understanding the early stages of peptide formation during the biosynthesis of teicoplanin and related glycopeptide antibiotics. FEBS J. 2021, 288, 507–529. [Google Scholar] [CrossRef]
- Stegmann, E.; Frasch, H.J.; Wohlleben, W. Glycopeptide biosynthesis in the context of basic cellular functions. Curr. Opin. Microbiol. 2010, 13, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Thaker, M.N.; Wright, G.D. Opportunities for synthetic biology in antibiotics: Expanding glycopeptide chemical diversity. ACS Synth. Biol. 2015, 4, 195–206. [Google Scholar] [CrossRef]
- Yushchuk, O.; Ostash, B. Glycopeptide antibiotics: Genetics, chemistry, and new screening approaches. In Natural Products from Actinomycetes; Springer: Singapore, 2022; pp. 411–444. [Google Scholar] [CrossRef]
- Yushchuk, O.; Zhukrovska, K.; Berini, F.; Fedorenko, V.; Marinelli, F. Genetics behind the glycosylation patterns in the biosynthesis of dalbaheptides. Front. Chem. 2022, 10, 858708. [Google Scholar] [CrossRef]
- Marcone, G.L.; Binda, E.; Berini, F.; Marinelli, F. Old and new glycopeptide antibiotics: From product to gene and back in the post-genomic era. Biotechnol. Adv. 2018, 36, 534–554. [Google Scholar] [CrossRef]
- Marschall, E.; Cryle, M.J.; Tailhades, J. Biological, chemical, and biochemical strategies for modifying glycopeptide antibiotics. J. Biol. Chem. 2019, 294, 18769–18783. [Google Scholar] [CrossRef]
- Müller, A.; Klöckner, A.; Schneider, T. Targeting a cell wall biosynthesis hot spot. Nat. Prod. Rep. 2017, 34, 909–932. [Google Scholar] [CrossRef]
- Jovetic, S.; Zhu, Y.; Marcone, G.L.; Marinelli, F.; Tramper, J. β-Lactam and glycopeptide antibiotics: First and last line of defense? Trends Biotechnol. 2010, 28, 596–604. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Calic, D.; Schweizer, F.; Zelenitsky, S.; Adam, H.; Lagacé-Wiens, P.R.S.; Rubinstein, E.; Gin, A.S.; Hoban, D.J.; Karlowsky, J.A. New lipoglycopeptides: A comparative review of dalbavancin, oritavancin and telavancin. Drugs 2010, 70, 859–886. [Google Scholar] [CrossRef] [PubMed]
- Binda, E.; Marinelli, F.; Marcone, G.L. Old and new glycopeptide antibiotics: Action and resistance. Antibiotics 2014, 3, 572–594. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, P.; Yarlagadda, V.; Ghosh, C.; Haldar, J. A review on cell wall synthesis inhibitors with an emphasis on glycopeptide antibiotics. Medchemcomm 2017, 8, 516–533. [Google Scholar] [CrossRef]
- Vollmer, W.; Blanot, D.; De Pedro, M.A. Peptidoglycan structure and architecture. FEMS Microbiol. Rev. 2008, 32, 149–167. [Google Scholar] [CrossRef] [PubMed]
- Nitanai, Y.; Kikuchi, T.; Kakoi, K.; Hanamaki, S.; Fujisawa, I.; Aoki, K. Crystal structures of the complexes between vancomycin and cell-wall precursor analogs. J. Mol. Biol. 2009, 385, 1422–1432. [Google Scholar] [CrossRef]
- Williams, D.H. The glycopeptide story—How to kill the deadly “superbugs. ” Nat. Prod. Rep. 1996, 13, 469–477. [Google Scholar] [CrossRef]
- Binda, E.; Marcone, G.L.; Pollegioni, L.; Marinelli, F. Characterization of VanYn, a novel d,d-peptidase/d,d-carboxypeptidase involved in glycopeptide antibiotic resistance in Nonomuraea sp. ATCC 39727. FEBS J. 2012, 279, 3203–3213. [Google Scholar] [CrossRef]
- Marcone, G.L.; Beltrametti, F.; Binda, E.; Carrano, L.; Foulston, L.; Hesketh, A.; Bibb, M.; Marinelli, F. Novel mechanism of glycopeptide resistance in the A40926 producer Nonomuraea sp. ATCC 39727. Antimicrob. Agents Chemother. 2010, 54, 2465–2472. [Google Scholar] [CrossRef]
- Yushchuk, O.; Homoniuk, V.; Ostash, B.; Marinelli, F.; Fedorenko, V. Genetic insights into the mechanism of teicoplanin self-resistance in Actinoplanes teichomyceticus. J. Antibiot. 2020, 73, 255–259. [Google Scholar] [CrossRef]
- Kilian, R.; Frasch, H.J.; Kulik, A.; Wohlleben, W.; Stegmann, E. The VanRS homologous two-component system VnlRSAb of the glycopeptide producer Amycolatopsis balhimycina activates transcription of the vanHAXSc genes in Streptomyces coelicolor, but not in A. balhimycina. Microb. Drug Resist. 2016, 22, 499–509. [Google Scholar] [CrossRef]
- Yushchuk, O.; Binda, E.; Marinelli, F. Glycopeptide antibiotic resistance genes: Distribution and function in the producer actinomycetes. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Stegmann, E.; Frasch, H.J.; Kilian, R.; Pozzi, R. Self-resistance mechanisms of actinomycetes producing lipid II-targeting antibiotics. Int. J. Med. Microbiol. 2015, 305, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.J.; Hutchings, M.I.; Neu, J.M.; Wright, G.D.; Paget, M.S.B.; Buttner, M.J. Characterization of an inducible vancomycin resistance system in Streptomyces coelicolor reveals a novel gene (vanK) required for drug resistance. Mol. Microbiol. 2004, 52, 1107–1121. [Google Scholar] [CrossRef] [PubMed]
- Andreo-Vidal, A.; Binda, E.; Fedorenko, V.; Marinelli, F.; Yushchuk, O. Genomic insights into the distribution and phylogeny of glycopeptide resistance determinants within the actinobacteria phylum. Antibiotics 2021, 10, 1533. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.G.; Broadhead, G.; Leskiw, B.K.; Wright, G.D. d-Ala-d-Ala ligases from glycopeptide antibiotic-producing organisms are highly homologous to the enterococcal vancomycin-resistance ligases VanA and VanB. Proc. Natl. Acad. Sci. USA 1997, 94, 6480–6483. [Google Scholar] [CrossRef]
- Rushton-Green, R.; Darnell, R.L.; Taiaroa, G.; Carter, G.P.; Cook, G.M.; Morgan, X.C. Agricultural origins of a highly persistent lineage of vancomycin-resistant Enterococcus faecalis in New Zealand. Appl. Environ. Microbiol. 2019, 85, e00137-19. [Google Scholar] [CrossRef]
- Lim, S.K.; Tanimoto, K.; Tomita, H.; Ike, Y. Pheromone-responsive conjugative vancomycin resistance plasmids in Enterococcus faecalis isolates from humans and chicken feces. Appl. Environ. Microbiol. 2006, 72, 6544–6553. [Google Scholar] [CrossRef]
- Mello, S.S.; Van Tyne, D.; Lebreton, F.; Silva, S.Q.; Nogueira, M.C.L.; Gilmore, M.S.; Camargo, I.L.B.C. A mutation in the glycosyltransferase gene lafB causes daptomycin hypersusceptibility in Enterococcus faecium. J. Antimicrob. Chemother. 2020, 75, 36–45. [Google Scholar] [CrossRef]
- Ballard, S.A.; Pertile, K.K.; Lim, M.; Johnson, P.D.R.; Grayson, M.L. Molecular characterization of vanB elements in naturally occurring gut anaerobes. Antimicrob. Agents Chemother. 2005, 49, 1688–1694. [Google Scholar] [CrossRef]
- Kinnear, C.L.; Hansen, E.; Morley, V.J.; Tracy, K.C.; Forstchen, M.; Read, A.F.; Woods, R.J. Daptomycin treatment impacts resistance in off-target populations of vancomycin-resistant Enterococcus faecium. PLoS Biol. 2020, 18, e3000987. [Google Scholar] [CrossRef]
- Bohlmann, L.; De Oliveira, D.M.P.; El-Deeb, I.M.; Brazel, E.B.; Harbison-Price, N.; Ong, C.L.Y.; Rivera-Hernandez, T.; Ferguson, S.A.; Cork, A.J.; Phan, M.D.; et al. Chemical synergy between ionophore PBT2 and zinc reverses antibiotic resistance. MBio 2018, 9, e02391-18. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Sung, K.; Marasa, B.; Min, S.; Kweon, O.; Nawaz, M.; Cerniglia, C. Draft genome sequence of multidrug-resistant Enterococcus faecium clinical isolate VRE3, with a sequence type 16 pattern and novel structural arrangement of Tn1546. Genome Announc. 2015, 3, 1000000. [Google Scholar] [CrossRef] [PubMed]
- Melegh, S.; Nyul, A.; Kovács, K.; Kovács, T.; Ghidán, Á.; Dombrádi, Z.; Szabó, J.; Berta, B.; Lesinszki, V.; Pászti, J.; et al. Dissemination of VanA-type Enterococcus faecium isolates in Hungary. Microb. Drug Resist. 2018, 24, 1376–1390. [Google Scholar] [CrossRef] [PubMed]
- Eshaghi, A.; Shahinas, D.; Li, A.; Kariyawasam, R.; Banh, P.; Desjardins, M.; Melano, R.G.; Patel, S.N. Characterization of an Enterococcus gallinarum isolate carrying a dual vanA and vanB cassette. J. Clin. Microbiol. 2015, 53, 2225–2229. [Google Scholar] [CrossRef][Green Version]
- Zaheer, R.; Cook, S.R.; Barbieri, R.; Goji, N.; Cameron, A.; Petkau, A.; Polo, R.O.; Tymensen, L.; Stamm, C.; Song, J.; et al. Surveillance of Enterococcus spp. reveals distinct species and antimicrobial resistance diversity across a One-Health continuum. Sci. Rep. 2020, 10, 3937. [Google Scholar] [CrossRef]
- Weigel, L.M.; Clewell, D.B.; Gill, S.R.; Clark, N.C.; McDougal, L.K.; Flannagan, S.E.; Kolonay, J.F.; Shetty, J.; Killgore, G.E.; Tenover, F.C. Genetic analysis of a high-level vancomycin-resistant isolate of Staphylococcus aureus. Science 2003, 302, 1569–1571. [Google Scholar] [CrossRef] [PubMed]
- Panesso, D.; Planet, P.J.; Diaz, L.; Hugonnet, J.E.; Tran, T.T.; Narechania, A.; Munita, J.M.; Rincon, S.; Carvajal, L.P.; Reyes, J.; et al. Methicillin-susceptible, vancomycin-resistant Staphylococcus aureus, Brazil. Emerg. Infect. Dis. 2015, 21, 1844–1848. [Google Scholar] [CrossRef]
- Zhu, W.; Murray, P.R.; Huskins, W.C.; Jernigan, J.A.; McDonald, L.C.; Clark, N.C.; Anderson, K.F.; McDougal, L.K.; Hageman, J.C.; Olsen-Rasmussen, M.; et al. Dissemination of an Enterococcus Inc18-like vanA plasmid associated with vancomycin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2010, 54, 4314–4320. [Google Scholar] [CrossRef]
- Kos, V.N.; Desjardins, C.A.; Griggs, A.; Cerqueira, G.; Van Tonder, A.; Holden, M.T.G.; Godfrey, P.; Palmer, K.L.; Bodi, K.; Mongodin, E.F.; et al. Comparative genomics of vancomycin-resistant Staphylococcus aureus strains and their positions within the clade most commonly associated with methicillin-resistant S. aureus hospital-acquired infection in the United States. MBio 2012, 3, e00112-12. [Google Scholar] [CrossRef]
- Garnier, F.; Taourit, S.; Glaser, P.; Courvalin, P.; Galimand, M. Characterization of transposon Tn1549, conferring VanB-type resistance in Enterococcus spp. Microbiology 2000, 146, 1481–1489. [Google Scholar] [CrossRef]
- De Been, M.; Van Schaik, W.; Cheng, L.; Corander, J.; Willems, R.J. Recent recombination events in the core genome are associated with adaptive evolution in Enterococcus faecium. Genome Biol. Evol. 2013, 5, 1524–1535. [Google Scholar] [CrossRef] [PubMed]
- Sletvold, H.; Johnsen, P.J.; Wikmark, O.G.; Simonsen, G.S.; Sundsfjord, A.; Nielsen, K.M. Tn1546 is part of a larger plasmid-encoded genetic unit horizontally disseminated among clonal Enterococcus faecium lineages. J. Antimicrob. Chemother. 2010, 65, 1894–1906. [Google Scholar] [CrossRef] [PubMed]
- Halvorsen, E.M.; Williams, J.J.; Bhimani, A.J.; Billings, E.A.; Hergenrother, P.J. Txe, an endoribonuclease of the enterococcal Axe-Txe toxin-antitoxin system, cleaves mRNA and inhibits protein synthesis. Microbiology 2011, 157, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Szakacs, T.A.; Kalan, L.; McConnell, M.J.; Eshaghi, A.; Shahinas, D.; McGeer, A.; Wright, G.D.; Low, D.E.; Patel, S.N. Outbreak of vancomycin-susceptible Enterococcus faecium containing the wild-type vanA gene. J. Clin. Microbiol. 2014, 52, 1682–1686. [Google Scholar] [CrossRef] [PubMed]
- Sletvold, H.; Johnsen, P.J.; Simonsen, G.S.; Aasnæs, B.; Sundsfjord, A.; Nielsen, K.M. Comparative DNA analysis of two vanA plasmids from Enterococcus faecium strains isolated from poultry and a poultry farmer in Norway. Antimicrob. Agents Chemother. 2007, 51, 736–739. [Google Scholar] [CrossRef]
- Sletvold, H.; Johnsen, P.J.; Hamre, I.; Simonsen, G.S.; Sundsfjord, A.; Nielsen, K.M. Complete sequence of Enterococcus faecium pVEF3 and the detection of an ω-ε-ζ toxin-antitoxin module and an ABC transporter. Plasmid 2008, 60, 75–85. [Google Scholar] [CrossRef]
- Guardabassi, L.; Perichon, B.; Van Heijenoort, J.; Blanot, D.; Courvalin, P. Glycopeptide resistance vanA operons in Paenibacillus strains isolated from soil. Antimicrob. Agents Chemother. 2005, 49, 4227–4233. [Google Scholar] [CrossRef]
- Fraimow, H.; Knob, C.; Herrero, I.A.; Patel, R. Putative VanRS-like two-component regulatory system associated with the inducible glycopeptide resistance cluster of Paenibacillus popilliae. Antimicrob. Agents Chemother. 2005, 49, 2625–2633. [Google Scholar] [CrossRef][Green Version]
- Ligozzi, M.; Lo Cascio, G.; Fontana, R. vanA gene cluster in a vancomycin-resistant clinical isolate of Bacillus circulans. Antimicrob. Agents Chemother. 1998, 42, 2055–2059. [Google Scholar] [CrossRef]
- Knight, D.R.; Androga, G.O.; Ballard, S.A.; Howden, B.P.; Riley, T.V. A phenotypically silent vanB2 operon carried on a Tn1549-like element in Clostridium difficile. Msphere 2016, 1, e00177-16. [Google Scholar] [CrossRef]
- Kruse, T.; Levisson, M.; de Vos, W.M.; Smidt, H. vanI: A novel d-Ala-d-Lac vancomycin resistance gene cluster found in Desulfitobacterium hafniense. Microb. Biotechnol. 2014, 7, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Sekiguchi, Y. Cultivation of uncultured Chloroflexi subphyla: Significance and ecophysiology of formerly uncultured Chloroflexi “subphylum I” with natural and biotechnological relevance. Microbes Environ. 2009, 24, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.J.; Xia, F.; Overbeek, R.A.; Olsen, G.J. Genomes of the class Erysipelotrichia clarify the firmicute origin of the class Mollicutes. Int. J. Syst. Evol. Microbiol. 2013, 63, 2727–2741. [Google Scholar] [CrossRef]
- Yabe, S.; Sakai, Y.; Abe, K.; Yokota, A. Diversity of Ktedonobacteria with actinomycetes-like morphology in terrestrial environments. Microbes Environ. 2017, 32, 61–70. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Schoch, C.L.; Ciufo, S.; Domrachev, M.; Hotton, C.L.; Kannan, S.; Khovanskaya, R.; Leipe, D.; McVeigh, R.; O’Neill, K.; Robbertse, B.; et al. NCBI Taxonomy: A comprehensive update on curation, resources and tools. Database 2020, 2020, baaa062. [Google Scholar] [CrossRef]
- Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2016, 44, D67–D72. [Google Scholar] [CrossRef]
- O’Leary, N.A.; Wright, M.W.; Brister, J.R.; Ciufo, S.; Haddad, D.; McVeigh, R.; Rajput, B.; Robbertse, B.; Smith-White, B.; Ako-Adjei, D.; et al. Reference sequence (RefSeq) database at NCBI: Current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 2016, 44, D733–D745. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Bryant, S.H. CD-Search: Protein domain annotations on the fly. Nucleic Acids Res. 2004, 32, 327–331. [Google Scholar] [CrossRef]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef]
- Siguier, P.; Perochon, J.; Lestrade, L.; Mahillon, J.; Chandler, M. ISfinder: The reference centre for bacterial insertion sequences. Nucleic Acids Res. 2006, 34, 32–36. [Google Scholar] [CrossRef]
- Rice, P.; Longden, L.; Bleasby, A. EMBOSS: The European Molecular Biology Open Software Suite. Trends Genet. 2000, 16, 276–277. [Google Scholar] [CrossRef]
- Arndt, D.; Grant, J.R.; Marcu, A.; Sajed, T.; Pon, A.; Liang, Y.; Wishart, D.S. PHASTER: A better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016, 44, W16–W21. [Google Scholar] [CrossRef]
- Zhou, F.; Gan, R.; Zhang, F.; Ren, C.; Yu, L.; Si, Y.; Huang, Z. PHISDetector: A tool to detect diverse in silico phage–host interaction signals for virome studies. Genom. Proteom. Bioinform. 2022, in press. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Sitnikova, T.; Rzhetsky, A.; Nei, M. Interior-branch and bootstrap tests of phylogenetic trees. Mol. Biol. Evol. 1995, 12, 319–333. [Google Scholar] [CrossRef][Green Version]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The rapid generation of mutation data matrices from protein sequences. Bioinformatics. 1992, 8, 275–282. [Google Scholar] [CrossRef]
- Battistuzzi, F.U.; Feijao, A.; Hedges, S.B. A genomic timescale of prokaryote evolution: Insights into the origin of methanogenesis, phototrophy, and the colonization of land. BMC Evol. Biol. 2004, 4, 44. [Google Scholar] [CrossRef]
- Schleifer, K.H. Phylum XIII. Firmicutes Gibbons and Murray 1978, 5 (Firmacutes [sic] Gibbons and Murray 1978, 5). In Bergey’s Manual® of Systematic Bacteriology; Springer: Berlin/Heidelberg, Germany, 2009; Volume 3, p. 1450. [Google Scholar]
- Ogata, Y.; Sakamoto, M.; Ohkuma, M.; Hattori, M.; Suda, W. Complete genome sequence of Longicatena caecimuris strain 3BBH23, isolated from healthy Japanese feces. Microbiol. Resour. Announc. 2021, 10, e00282-21. [Google Scholar] [CrossRef]
- Reynolds, P.E.; Ambur, O.H.; Casadewall, B.; Courvalin, P. The VanYD d,d-carboxypeptidase of Enterococcus faecium BM4339 is a penicillin-binding protein. Microbiology 2001, 147, 2571–2578. [Google Scholar] [CrossRef]
- Cavaletti, L.; Monciardini, P.; Bamonte, R.; Schumann, P.; Ronde, M.; Sosio, M.; Donadio, S. New lineage of filamentous, spore-forming, Gram-positive bacteria from soil. Appl. Environ. Microbiol. 2006, 72, 4360–4369. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Waller, M.; Barrett, A.J.; Bateman, A. MEROPS: The database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 2014, 42, D503–D509. [Google Scholar] [CrossRef]
- Siguier, P.; Gourbeyre, E.; Varani, A.; Ton-Hoang, B.; Chandler, M. Everyman’s guide to bacterial insertion sequences. Microbiol. Spectr. 2015, 3, 1–35. [Google Scholar] [CrossRef]
- Maniloff, J.; Ackermann, H.W. Taxonomy of bacterial viruses: Establishment of tailed virus genera and the order Caudovirales. Arch. Virol. 1998, 143, 2051–2063. [Google Scholar] [CrossRef]
- Fontana, R.; Ligozzi, M.; Pedrotti, C.; Padovani, E.M.; Cornaglia, G. Vancomycin-resistant Bacillus circulans carrying the vanA gene responsible for vancomycin resistance in enterococci. Eur. J. Clin. Microbiol. Infect. Dis. 1997, 16, 473–474. [Google Scholar] [CrossRef]
- Aminov, R.I.; Mackie, R.I. Evolution and ecology of antibiotic resistance genes. FEMS Microbiol. Lett. 2007, 271, 147–161. [Google Scholar] [CrossRef]
- Heaton, M.P.; Discotto, L.F.; Pucci, M.J.; Handwerger, S. Mobilization of vancomycin resistance by transposon-mediated fusion of a VanA plasmid with an Enterococcus faecium sex pheromone-response plasmid. Gene 1996, 171, 9–17. [Google Scholar] [CrossRef]
- Krumholz, L.R.; Bryant, M.P. Clostridium pfennigii sp. nov. uses methoxyl groups of monobenzenoids and produces butyrate. Int. J. Syst. Bacteriol. 1985, 35, 454–456. [Google Scholar] [CrossRef]
- Šul’ák, M.; Sikorová, L.; Jankuvová, J.; Javorský, P.; Pristaš, P. Variability of Actinobacteria, a minor component of rumen microflora. Folia Microbiol. 2012, 57, 351–353. [Google Scholar] [CrossRef]
- Wambui, J.; Stevens, M.J.A.; Cernela, N.; Stephan, R. Unraveling the genotypic and phenotypic diversity of the psychrophilic Clostridium estertheticum complex, a meat spoilage agent. Front. Microbiol. 2022, 13, 856810. [Google Scholar] [CrossRef]
- Kim, Y.B.; Kim, J.Y.; Kim, J.; Song, H.S.; Whon, T.W.; Lee, S.H.; Yoo, S.R.; Myoung, J.; Son, H.S.; Roh, S.W. Aminipila terrae sp. nov., a strictly anaerobic bacterium isolated from river sediment. Arch. Microbiol. 2021, 203, 3163–3169. [Google Scholar] [CrossRef]
- Passari, A.K.; Leo, V.V.; Chandra, P.; Kumar, B.; Nayak, C.; Hashem, A.; Abd Allah, E.F.; Alqarawi, A.A.; Singh, B.P. Bioprospection of actinobacteria derived from freshwater sediments for their potential to produce antimicrobial compounds. Microb. Cell Fact. 2018, 17, 68. [Google Scholar] [CrossRef]
- Nakahara, N.; Nobu, M.K.; Takaki, Y.; Miyazaki, M.; Tasumi, E.; Sakai, S.; Ogawara, M.; Yoshida, N.; Tamaki, H.; Yamanaka, Y.; et al. Aggregatilinea lenta gen. nov., sp. nov., a slow-growing, facultatively anaerobic bacterium isolated from subseafloor sediment, and proposal of the new order Aggregatilineales ord. nov. within the class Anaerolineae of the phylum Chloroflexi. Int. J. Syst. Evol. Microbiol. 2019, 69, 1185–1194. [Google Scholar] [CrossRef]
- Biddle, A.S.; Leschine, S.; Huntemann, M.; Han, J.; Chen, A.; Kyrpides, N.; Markowitz, V.; Palaniappan, K.; Ivanova, N.; Mikhailova, N.; et al. The complete genome sequence of Clostridium indolis DSM 755T. Stand. Genom. Sci. 2015, 9, 1089–1104. [Google Scholar] [CrossRef]
- Mechichi, T.; Labat, M.; Patel, B.K.C.; Woo, T.H.; Thomas, P.; Garcia, J. New aromatic O-demethylating homoacetogen from an olive mill wastewater treatment digester. Int. J. Syst. Bacteriol. 1997, 49, 1201–1209. [Google Scholar] [CrossRef]
- Danylec, N.; Stoll, D.A.; Huch, M. Draft genome sequences of type strains of Gordonibacter faecihominis, Paraeggerthella hongkongensis, Parvibacter caecicola, Slackia equolifaciens, Slackia faecicanis, and Slackia isoflavoniconvertens. Microbiol. Resour. Announc. 2019, 36, e01532-18. [Google Scholar] [CrossRef]
- Clavel, T.; Charrier, C.; Braune, A.; Wenning, M.; Blaut, M.; Haller, D. Isolation of bacteria from the ileal mucosa of TNFdeltaARE mice and description of Enterorhabdus mucosicola gen. nov., sp. nov. Int. J. Syst. Evol. Microbiol. 2009, 59, 1805–1812. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).