Genetics of Oocyte Maturation Defects and Early Embryo Development Arrest
Abstract
1. Introduction
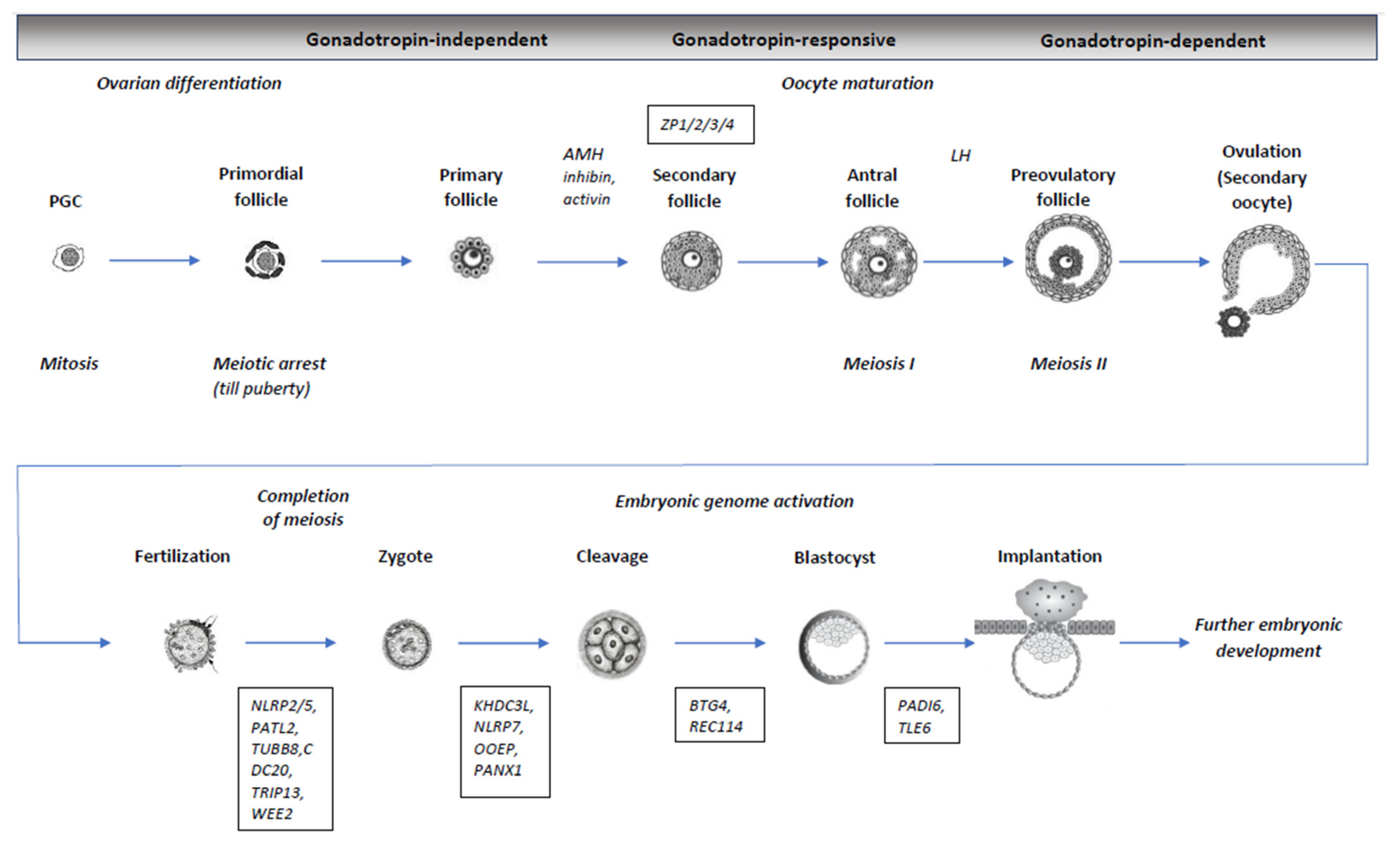
2. Oogenesis, Meiosis, and Oocyte Maturation
3. Maternal Effect Genes (MEGs)
4. Genes of SCMC Components
5. Genes Involved in the Formation of the Zona Pellucida and Associated Disorders
6. Other Genes Associated with Female Infertility
| Gene Symbol | Locus | Function | Phenotype | OMIM | Inheritance | References |
|---|---|---|---|---|---|---|
| TUBB8 CDC20 TRIP13 | 10p15.3 1p34.2 5p15.33 | Oocyte meiotic spindle assembly Components of spindle assembly checkpoints | OMA, IF, FF, EEDA, ZCF | 616768 603618 604507 | AD, AR AR AR | [21,24,32,35,37,38,96] |
| 4242PATL2 | 15q21.1 | mRNA binding/inhibition of post-transcription translation | OMA, IF, FF, EEDA | 614661 | AR | [40,97] |
| BTG4 | 11q23.1 | Maternal mRNA decay | ZCF | 605673 | AR | [47] |
| KHDC3L NLRP2 NLRP5 NLRP7 PADI6 TLE6 OOEP | 6q13 19q13.42 19.13.43 19q13.42 1p36.13 19p13.3 6q13 | SCMC member SCMC member SCMC member SCMC member SCMC member SCMC member SCMC member | EEDA, RHM, RPL EEDA, MLID EEDA, MLID EEDA, RHM EEDA, ZCF, RHM, MLID EEDA, IF EEDA, MLID | 611687 609364 609658 609661 610363 612399 611689 | AR AR AR AR AR AR AR | [50,51,58,68,71,98,99,100] |
| ZP1 ZP2 ZP3 ZP4 | 11q12.2 16p12.3-p12.2 7q11.23 1q43 | Formation of zona pellucida (ZP) | EFS, AZPF | 195000 182888 182889 613514 | AR AD, AR AD AR | [77,101,102,103,104,105] |
| WEE2 | 7q34 | Regulator of meiosis during Prophase I and Metaphase II | OMA, FF | 614084 | AR | [106,107] |
| PANX1 | 11q21 | Oocyte maturation | ODP | 608420 | AD | [90,108] |
| REC114 | 15q24.1 | Initiation of double-strand breaks and homologous recombination of DNA | ODP/EEDA, RHM | 618421 | AR | [94,109] |
7. mtDNA, Oocyte Maturation and Embryo Developmental Competence
8. Genes Involved in Oocyte Maturation Defects and Early Embryo Lethality as Cause of IVF/ICSI Failures
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mascarenhas, M.N.; Flaxman, S.R.; Boerma, T.; Vanderpoel, S.; Stevens, G.A. National, Regional, and Global Trends in Infertility Prevalence Since 1990: A Systematic Analysis of 277 Health Surveys. PLoS Med. 2012, 9, e1001356. [Google Scholar] [CrossRef]
- Carson, S.A.; Kallen, A.N. Diagnosis and Management of Infertility. JAMA 2021, 326, 65. [Google Scholar] [CrossRef] [PubMed]
- Yatsenko, S.A.; Rajkovic, A. Genetics of Human Female Infertility. Biol. Reprod. 2019, 101, 549–566. [Google Scholar] [CrossRef]
- Mallepaly, R.; Butler, P.R.; Herati, A.S.; Lamb, D.J. Genetic Basis of Male and Female Infertility. In Monographs in Human Genetics; Karger Publishers: Basel, Switzerland, 2017; Volume 21, pp. 1–16. [Google Scholar]
- Pisarska, M.D.; Chan, J.L.; Lawrenson, K.; Gonzalez, T.L.; Wang, E.T. Genetics and Epigenetics of Infertility and Treatments on Outcomes. J. Clin. Endocrinol. Metab. 2019, 104, 1871–1886. [Google Scholar] [CrossRef] [PubMed]
- Ben Maamar, M.; Nilsson, E.E.; Skinner, M.K. Epigenetic Transgenerational Inheritance, Gametogenesis and Germline Development†. Biol. Reprod. 2021, 105, 570–592. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Iaquinta, P.J.; Xia, N.; Liu, L.; Diao, L.; Reijo Pera, R.A. Transcriptional Control of Human Gametogenesis. Hum. Reprod. Update 2022, 28, 313–345. [Google Scholar] [CrossRef]
- Liu, L.; Kong, N.; Xia, G.; Zhang, M. Molecular Control of Oocyte Meiotic Arrest and Resumption. Reprod. Fertil. Dev. 2013, 25, 463. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, C.-Y.; Zhao, Y.; Dean, J. FIGLA, LHX8 and SOHLH1 Transcription Factor Networks Regulate Mouse Oocyte Growth and Differentiation. Nucleic Acids Res. 2020, 48, 3525–3541. [Google Scholar] [CrossRef] [PubMed]
- França, M.M.; Mendonca, B.B. Genetics of Primary Ovarian Insufficiency in the Next-Generation Sequencing Era. J. Endocr. Soc. 2020, 4, bvz037. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ouyang, H.; Xia, G. The Signal Pathway of Gonadotrophins-Induced Mammalian Oocyte Meiotic Resumption. Mol. Hum. Reprod. 2009, 15, 399–409. [Google Scholar] [CrossRef]
- Mehlmann, L.M. Stops and Starts in Mammalian Oocytes: Recent Advances in Understanding the Regulation of Meiotic Arrest and Oocyte Maturation. Reproduction 2005, 130, 791–799. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, L.; Wang, J.; Luo, G.; Xi, Q.; Zhou, X.; Li, Z.; Yang, X.; Duan, J.; Jin, L.; et al. Novel Homozygous Mutations in PATL2 Lead to Female Infertility with Oocyte Maturation Arrest. J. Assist. Reprod. Genet. 2020, 37, 841–847. [Google Scholar] [CrossRef]
- Lin, J.; Xu, H.; Chen, B.; Wang, W.; Wang, L.; Sun, X.; Sang, Q. Expanding the Genetic and Phenotypic Spectrum of Female Infertility Caused by TLE6 Mutations. J. Assist. Reprod. Genet. 2020, 37, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Sang, Q.; Li, B.; Kuang, Y.; Wang, X.; Zhang, Z.; Chen, B.; Wu, L.; Lyu, Q.; Fu, Y.; Yan, Z.; et al. Homozygous Mutations in WEE2 Cause Fertilization Failure and Female Infertility. Am. J. Hum. Genet. 2018, 102, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Sang, Q.; Kuang, Y.; Sun, X.; Yan, Z.; Zhang, S.; Shi, J.; Tian, G.; Luchniak, A.; Fukuda, Y.; et al. Mutations in TUBB8 and Human Oocyte Meiotic Arrest. N. Engl. J. Med. 2016, 374, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Begemann, M.; Rezwan, F.I.; Beygo, J.; Docherty, L.E.; Kolarova, J.; Schroeder, C.; Buiting, K.; Chokkalingam, K.; Degenhardt, F.; Wakeling, E.L.; et al. Maternal Variants in NLRP and Other Maternal Effect Proteins Are Associated with Multilocus Imprinting Disturbance in Offspring. J. Med. Genet. 2018, 55, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Janke, C.; Magiera, M.M. The Tubulin Code and Its Role in Controlling Microtubule Properties and Functions. Nat. Rev. Mol. Cell Biol. 2020, 21, 307–326. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Hu, H.; Zhang, S.; Xu, X.; Gao, Y.; Gong, F.; Lu, G.; Lin, G. The Comprehensive Variant and Phenotypic Spectrum of TUBB8 in Female Infertility. J. Assist. Reprod. Genet. 2021, 38, 2261–2272. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Li, K.; Zheng, C.; Tang, Y.; Bai, D.; Yin, J.; Chi, F.; Zhang, Y.; Li, Y.; Tu, Z.; et al. Identification and Rescue of a Novel TUBB8 Mutation That Causes the First Mitotic Division Defects and Infertility. J. Assist. Reprod. Genet. 2020, 37, 2713–2722. [Google Scholar] [CrossRef]
- Wang, A.-C.; Zhang, Y.-S.; Wang, B.-S.; Zhao, X.-Y.; Wu, F.-X.; Zhai, X.-H.; Sun, J.-X.; Mei, S.-Y. Mutation Analysis of the TUBB8 Gene in Primary Infertile Women with Arrest in Oocyte Maturation. Gynecol. Endocrinol. 2018, 34, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Sha, Q.; Zheng, W.; Feng, X.; Yuan, R.; Hu, H.; Gong, F.; Hu, L.; Lin, G.; Ou, X. Novel Mutations in TUBB8 Expand the Mutational and Phenotypic Spectrum of Patients with Zygotes Containing Multiple Pronuclei. Gene 2021, 769, 145227. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wang, W.; Peng, X.; Jiang, H.; Zhang, S.; Li, D.; Li, B.; Fu, J.; Kuang, Y.; Sun, X.; et al. The Comprehensive Mutational and Phenotypic Spectrum of TUBB8 in Female Infertility. Eur. J. Hum. Genet. 2019, 27, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xi, Q.; Zhu, L.; Yang, X.; Jin, L.; Wang, J.; Zhang, T.; Zhou, X.; Zhang, D.; Peng, X.; et al. TUBB8 Mutations Cause Female Infertility with Large Polar Body Oocyte and Fertilization Failure. Reprod. Sci. 2021, 28, 2942–2950. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Li, B.; Li, D.; Yan, Z.; Mao, X.; Xu, Y.; Mu, J.; Li, Q.; Jin, L.; He, L.; et al. Novel Mutations and Structural Deletions in TUBB8: Expanding Mutational and Phenotypic Spectrum of Patients with Arrest in Oocyte Maturation, Fertilization or Early Embryonic Development. Hum. Reprod. 2017, 32, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Zheng, L.; Liang, H.; Li, Y.; Zhao, H.; Li, R.; Lai, L.; Zhang, Q.; Wang, W. A Novel Mutation in the TUBB8 Gene Is Associated with Complete Cleavage Failure in Fertilized Eggs. J. Assist. Reprod. Genet. 2018, 35, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Guo, J.; Xu, Y.; Lin, X.; Deng, W.; Cheng, L.; Zhao, H.; Jiang, S.; Gao, M.; Huang, J.; et al. Two Mutations in TUBB8 Cause Developmental Arrest in Human Oocytes and Early Embryos. Reprod. Biomed. Online 2021, 43, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.; Jacobsen, F.W.; Hsu-Chen, J.; Wu, T.; Baum, L.G. A Novel Mammalian Protein, P55CDC, Present in Dividing Cells Is Associated with Protein Kinase Activity and Has Homology to the Saccharomyces Cerevisiae Cell Division Cycle Proteins Cdc20 and Cdc4. Mol. Cell. Biol. 1994, 14, 3350–3363. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.-M. The Anaphase-Promoting Complex. Mol. Cell 2002, 9, 931–943. [Google Scholar] [CrossRef]
- Baumgarten, A.J.; Felthaus, J.; Wäsch, R. Strong Inducible Knockdown of APC/C Cdc20 Does Not Cause Mitotic Arrest in Human Somatic Cells. Cell Cycle 2009, 8, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Hamada, M.; Malureanu, L.; Jeganathan, K.B.; Zhou, W.; Morbeck, D.E.; van Deursen, J.M. Cdc20 Is Critical for Meiosis I and Fertility of Female Mice. PLoS Genet. 2010, 6, e1001147. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Xue, S.; Yao, Z.; Shi, J.; Chen, B.; Wu, L.; Sun, L.; Xu, Y.; Yan, Z.; Li, B.; et al. Biallelic Mutations in CDC20 Cause Female Infertility Characterized by Abnormalities in Oocyte Maturation and Early Embryonic Development. Protein Cell 2020, 11, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Guan, Y.; Meng, Q.; Wang, W.; Wu, L.; Chen, B.; Hu, J.; Zhu, J.; Zhang, Z.; Mu, J.; et al. Identification of Novel Mutations in CDC20: Expanding the Mutational Spectrum for Female Infertility. Front. Cell Dev. Biol. 2021, 9, 647130. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fan, L.; Peng, N.; Yang, L.; Mou, L.; Huang, W. R383C Mutation of Human CDC20 Results in Idiopathic Non-Obstructive Azoospermia. Oncotarget 2017, 8, 99816–99824. [Google Scholar] [CrossRef] [PubMed]
- Biswas, L.; Tyc, K.; El Yakoubi, W.; Morgan, K.; Xing, J.; Schindler, K. Meiosis Interrupted: The Genetics of Female Infertility via Meiotic Failure. Reproduction 2021, 161, R13–R35. [Google Scholar] [CrossRef]
- Ma, H.T.; Poon, R.Y.C. TRIP13 Regulates Both the Activation and Inactivation of the Spindle-Assembly Checkpoint. Cell Rep. 2016, 14, 1086–1099. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, S.; Guo, J.; Meng, F.; Chen, X.; Gong, F.; Lu, G.; Zheng, W.; Lin, G. Identification of Novel Variants of Thyroid Hormone Receptor Interaction Protein 13 That Cause Female Infertility Characterized by Zygotic Cleavage Failure. Front. Physiol. 2022, 13, 899149. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, B.; Fu, J.; Li, R.; Diao, F.; Li, C.; Chen, B.; Du, J.; Zhou, Z.; Mu, J.; et al. Bi-Allelic Missense Pathogenic Variants in TRIP13 Cause Female Infertility Characterized by Oocyte Maturation Arrest. Am. J. Hum. Genet. 2020, 107, 15–23. [Google Scholar] [CrossRef]
- Yost, S.; de Wolf, B.; Hanks, S.; Zachariou, A.; Marcozzi, C.; Clarke, M.; de Voer, R.M.; Etemad, B.; Uijttewaal, E.; Ramsay, E.; et al. Biallelic TRIP13 Mutations Predispose to Wilms Tumor and Chromosome Missegregation. Nat. Genet. 2017, 49, 1148–1151. [Google Scholar] [CrossRef]
- Christou-Kent, M.; Kherraf, Z.; Amiri-Yekta, A.; Le Blévec, E.; Karaouzène, T.; Conne, B.; Escoffier, J.; Assou, S.; Guttin, A.; Lambert, E.; et al. PATL2 Is a Key Actor of Oocyte Maturation Whose Invalidation Causes Infertility in Women and Mice. EMBO Mol. Med. 2018, 10, e8515. [Google Scholar] [CrossRef]
- Cao, Q.; Zhao, C.; Wang, C.; Cai, L.; Xia, M.; Zhang, X.; Han, J.; Xu, Y.; Zhang, J.; Ling, X.; et al. The Recurrent Mutation in PATL2 Inhibits Its Degradation Thus Causing Female Infertility Characterized by Oocyte Maturation Defect Through Regulation of the Mos-MAPK Pathway. Front. Cell Dev. Biol. 2021, 9, 628649. [Google Scholar] [CrossRef]
- Maddirevula, S.; Coskun, S.; Alhassan, S.; Elnour, A.; Alsaif, H.S.; Ibrahim, N.; Abdulwahab, F.; Arold, S.T.; Alkuraya, F.S. Female Infertility Caused by Mutations in the Oocyte-Specific Translational Repressor PATL2. Am. J. Hum. Genet. 2017, 101, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, X.; Shi, J.; Yu, X.; Zhang, X.; Zhu, K.; Yi, Z.; Duan, E.; Li, L. BTG4 Is a Key Regulator for Maternal MRNA Clearance during Mouse Early Embryogenesis. J. Mol. Cell Biol. 2016, 8, 366–368. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Ji, S.-Y.; Sha, Q.-Q.; Dang, Y.; Zhou, J.-J.; Zhang, Y.-L.; Liu, Y.; Wang, Z.-W.; Hu, B.; Sun, Q.-Y.; et al. BTG4 Is a Meiotic Cell Cycle–Coupled Maternal-Zygotic-Transition Licensing Factor in Oocytes. Nat. Struct. Mol. Biol. 2016, 23, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Sha, Q.Q.; Zhu, Y.Z.; Li, S.; Jiang, Y.; Chen, L.; Sun, X.H.; Shen, L.; Ou, X.H.; Fan, H.Y. Characterization of Zygotic Genome Activation-Dependent Maternal MRNA Clearance in Mouse. Nucleic Acids Res. 2020, 48, 879. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, M.; Pfender, S.; Santhanam, B.; Schuh, M. The BTG4 and CAF1 Complex Prevents the Spontaneous Activation of Eggs by Deadenylating Maternal MRNAs. Open Biol. 2016, 6, 905–913. [Google Scholar] [CrossRef]
- Zheng, W.; Zhou, Z.; Sha, Q.; Niu, X.; Sun, X.; Shi, J.; Zhao, L.; Zhang, S.; Dai, J.; Cai, S.; et al. Homozygous Mutations in BTG4 Cause Zygotic Cleavage Failure and Female Infertility. Am. J. Hum. Genet. 2020, 107, 24–33. [Google Scholar] [CrossRef]
- Li, L.; Baibakov, B.; Dean, J. A Subcortical Maternal Complex Essential for Preimplantation Mouse Embryogenesis. Dev. Cell 2008, 15, 416–425. [Google Scholar] [CrossRef]
- Lu, X.; Gao, Z.; Qin, D.; Li, L. A Maternal Functional Module in the Mammalian Oocyte-To-Embryo Transition. Trends Mol. Med. 2017, 23, 1014–1023. [Google Scholar] [CrossRef]
- Mahadevan, S.; Sathappan, V.; Utama, B.; Lorenzo, I.; Kaskar, K.; Van den Veyver, I.B. Maternally Expressed NLRP2 Links the Subcortical Maternal Complex (SCMC) to Fertility, Embryogenesis and Epigenetic Reprogramming. Sci. Rep. 2017, 7, 44667. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, Z.; Zhang, D.; Zhao, B.; Liu, L.; Xie, Z.; Yao, Y.; Zheng, P. KHDC3L Mutation Causes Recurrent Pregnancy Loss by Inducing Genomic Instability of Human Early Embryonic Cells. PLOS Biol. 2019, 17, e3000468. [Google Scholar] [CrossRef]
- Judson, H.; Hayward, B.E.; Sheridan, E.; Bonthron, D.T. A Global Disorder of Imprinting in the Human Female Germ Line. Nature 2002, 416, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, M.; Nguyen, N.M.P.; Foroughinia, L.; Dash, P.; Ahmadpour, F.; Verma, I.C.; Slim, R.; Fardaei, M. Two Novel Mutations in the KHDC3L Gene in Asian Patients with Recurrent Hydatidiform Mole. Hum. Genome Var. 2016, 3, 16027. [Google Scholar] [CrossRef]
- Fallahi, J.; Anvar, Z.; Razban, V.; Momtahan, M.; Namavar-Jahromi, B.; Fardaei, M. Founder Effect of KHDC3L, p.M1V Mutation, on Iranian Patients with Recurrent Hydatidiform Moles. IJMS 2020, 45, 118–124. [Google Scholar] [CrossRef]
- Meyer, E.; Lim, D.; Pasha, S.; Tee, L.J.; Rahman, F.; Yates, J.R.W.; Woods, C.G.; Reik, W.; Maher, E.R. Germline Mutation in NLRP2 (NALP2) in a Familial Imprinting Disorder (Beckwith-Wiedemann Syndrome). PLoS Genet. 2009, 5, e1000423. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Wang, W.; Chen, B.; Wu, L.; Li, B.; Mao, X.; Zhang, Z.; Fu, J.; Kuang, Y.; Sun, X.; et al. Mutations in NLRP2 and NLRP5 Cause Female Infertility Characterised by Early Embryonic Arrest. J. Med. Genet. 2019, 56, 471–480. [Google Scholar] [CrossRef]
- Sparago, A.; Verma, A.; Patricelli, M.G.; Pignata, L.; Russo, S.; Calzari, L.; De Francesco, N.; Del Prete, R.; Palumbo, O.; Carella, M.; et al. The Phenotypic Variations of Multi-Locus Imprinting Disturbances Associated with Maternal-Effect Variants of NLRP5 Range from Overt Imprinting Disorder to Apparently Healthy Phenotype. Clin. Epigenetics 2019, 11, 190. [Google Scholar] [CrossRef] [PubMed]
- Docherty, L.E.; Rezwan, F.I.; Poole, R.L.; Turner, C.L.S.; Kivuva, E.; Maher, E.R.; Smithson, S.F.; Hamilton-Shield, J.P.; Patalan, M.; Gizewska, M.; et al. Mutations in NLRP5 Are Associated with Reproductive Wastage and Multilocus Imprinting Disorders in Humans. Nat. Commun. 2015, 6, 8086. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, J.; Razban, V.; Momtahan, M.; Akbarzadeh-Jahromi, M.; Namavar-Jahromi, B.; Anvar, Z.; Fardaei, M. A Novel Mutation in NLRP7 Related to Recurrent Hydatidiform Mole and Reproductive Failure. Int. J. Fertil. Steril. 2019, 13, 135–138. [Google Scholar] [CrossRef]
- Singer, H.; Biswas, A.; Nuesgen, N.; Oldenburg, J.; El-Maarri, O. NLRP7, Involved in Hydatidiform Molar Pregnancy (HYDM1), Interacts with the Transcriptional Repressor ZBTB16. PLoS ONE 2015, 10, e0130416. [Google Scholar] [CrossRef]
- Cubellis, M.V.; Pignata, L.; Verma, A.; Sparago, A.; Del Prete, R.; Monticelli, M.; Calzari, L.; Antona, V.; Melis, D.; Tenconi, R.; et al. Loss-of-Function Maternal-Effect Mutations of PADI6 Are Associated with Familial and Sporadic Beckwith-Wiedemann Syndrome with Multi-Locus Imprinting Disturbance. Clin. Epigenetics 2020, 12, 139. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, R.; Pang, Z.; Wei, Z.; Sun, L.; Li, S.; Wang, G.; Liu, Y.; Zhou, Y.; Ye, H.; et al. Novel Homozygous PADI6 Variants in Infertile Females with Early Embryonic Arrest. Front. Cell Dev. Biol. 2022, 10, 819667. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Shi, Y.; Fu, J.; Yu, M.; Feng, R.; Sang, Q.; Liang, B.; Chen, B.; Qu, R.; Li, B.; et al. Mutations in PADI6 Cause Female Infertility Characterized by Early Embryonic Arrest. Am. J. Hum. Genet. 2016, 99, 744–752. [Google Scholar] [CrossRef]
- Eggermann, T.; Kadgien, G.; Begemann, M.; Elbracht, M. Biallelic PADI6 Variants Cause Multilocus Imprinting Disturbances and Miscarriages in the Same Family. Eur. J. Hum. Genet. 2021, 29, 575–580. [Google Scholar] [CrossRef]
- Zheng, W.; Chen, L.; Dai, J.; Dai, C.; Guo, J.; Lu, C.; Gong, F.; Lu, G.; Lin, G. New Biallelic Mutations in PADI6 Cause Recurrent Preimplantation Embryonic Arrest Characterized by Direct Cleavage. J. Assist. Reprod. Genet. 2020, 37, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tan, Z.; He, J.; Jin, T.; Han, Y.; Hu, L.; Huang, S. Two Novel Mutations in PADI6 and TLE6 Genes Cause Female Infertility Due to Arrest in Embryonic Development. J. Assist. Reprod. Genet. 2021, 38, 1551–1559. [Google Scholar] [CrossRef]
- Yu, X.-J.; Yi, Z.; Gao, Z.; Qin, D.; Zhai, Y.; Chen, X.; Ou-Yang, Y.; Wang, Z.-B.; Zheng, P.; Zhu, M.-S.; et al. The Subcortical Maternal Complex Controls Symmetric Division of Mouse Zygotes by Regulating F-Actin Dynamics. Nat. Commun. 2014, 5, 4887. [Google Scholar] [CrossRef] [PubMed]
- Alazami, A.M.; Awad, S.M.; Coskun, S.; Al-Hassan, S.; Hijazi, H.; Abdulwahab, F.M.; Poizat, C.; Alkuraya, F.S. TLE6 Mutation Causes the Earliest Known Human Embryonic Lethality. Genome Biol. 2015, 16, 240. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, C.; Chen, B.; Lv, M.; Zou, H.; Liu, Y.; Gao, Y.; Wang, T.; Xing, Q.; Zhu, Y.; et al. Identification of Novel Biallelic TLE6 Variants in Female Infertility With Preimplantation Embryonic Lethality. Front. Genet. 2021, 12, 666136. [Google Scholar] [CrossRef]
- He, D.-J.; Wang, L.; Zhang, Z.-B.; Guo, K.; Li, J.-Z.; He, X.-C.; Cui, Q.-H.; Zheng, P. Maternal Gene Ooep May Participate in Homologous Recombination-Mediated DNA Double-Strand Break Repair in Mouse Oocytes. Zool. Res. 2018, 39, 387–395. [Google Scholar] [CrossRef]
- Tong, X.; Jin, J.; Hu, Z.; Zhang, Y.; Fan, H.; Zhang, Y.; Zhang, S. Mutations in OOEP and NLRP5 Identified in Infertile Patients with Early Embryonic Arrest. Hum. Mutat. 2022, 43, 1–12. [Google Scholar] [CrossRef]
- Gupta, S.K.; Bansal, P.; Ganguly, A.; Bhandari, B.; Chakrabarti, K. Human Zona Pellucida Glycoproteins: Functional Relevance during Fertilization. J. Reprod. Immunol. 2009, 83, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K. The Human Egg’s Zona Pellucida. In Current Topics in Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 130, pp. 379–411. [Google Scholar]
- Lefievre, L. Four Zona Pellucida Glycoproteins Are Expressed in the Human. Hum. Reprod. 2004, 19, 1580–1586. [Google Scholar] [CrossRef] [PubMed]
- Litscher, E.S.; Wassarman, P.M. Zona Pellucida Genes and Proteins and Human Fertility. Trends Dev. Biol. 2020, 13, 21–33. [Google Scholar] [PubMed]
- Avella, M.A.; Baibakov, B.; Dean, J. A Single Domain of the ZP2 Zona Pellucida Protein Mediates Gamete Recognition in Mice and Humans. J. Cell Biol. 2014, 205, 801–809. [Google Scholar] [CrossRef]
- Cao, Q.; Zhao, C.; Zhang, X.; Zhang, H.; Lu, Q.; Wang, C.; Hu, Y.; Ling, X.; Zhang, J.; Huo, R. Heterozygous Mutations in ZP1 and ZP3 Cause Formation Disorder of ZP and Female Infertility in Human. J. Cell. Mol. Med. 2020, 24, 8557–8566. [Google Scholar] [CrossRef]
- Lv, C.; Huang, H.-L.; Yi, D.-J.; Peng, T.-L.; Tan, H.-J.; Quan, R.-P.; Deng, H.-W.; Xiao, H.-M. Mutant Zp1 Impedes Incorporation of ZP3 and ZP4 in the Zona Pellucida, Resulting in Zona Absence and Female Infertility in Rats. Biol. Reprod. 2021, 104, 1262–1270. [Google Scholar] [CrossRef]
- Jovine, L.; Darie, C.C.; Litscher, E.S.; Wassarman, P.M. Zona Pellucida Domain Proteins. Annu. Rev. Biochem. 2005, 74, 83–114. [Google Scholar] [CrossRef]
- Zeng, M.-H.; Wang, Y.; Huang, H.-L.; Quan, R.-P.; Yang, J.-T.; Guo, D.; Sun, Y.; Lv, C.; Li, T.-Y.; Wang, L.; et al. Zp4 Is Completely Dispensable for Fertility in Female Rats. Biol. Reprod. 2021, 104, 1282–1291. [Google Scholar] [CrossRef]
- Han, S.J.; Conti, M. New Pathways from PKA to the Cdc2/Cyclin B Complex in Oocytes: Wee1B as a Potential PKA Substrate. Cell Cycle 2006, 5, 227–231. [Google Scholar] [CrossRef]
- Schall, P.Z.; Latham, K.E. Essential Shared and Species-Specific Features of Mammalian Oocyte Maturation-Associated Transcriptome Changes Impacting Oocyte Physiology. Am. J. Physiol. Cell Physiol. 2021, 321, C3–C16. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, T.; Yu, M.; Bian, Y.; Cao, Y.; Ning, Y.; Su, S.; Zhang, J.; Zhao, S. Novel WEE2 Gene Variants Identified in Patients with Fertilization Failure and Female Infertility. Fertil. Steril. 2019, 111, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Zheng, W.; Dai, C.; Guo, J.; Lu, C.; Gong, F.; Li, Y.; Zhou, Q.; Lu, G.; Lin, G. New Biallelic Mutations in WEE2: Expanding the Spectrum of Mutations That Cause Fertilization Failure or Poor Fertilization. Fertil. Steril. 2019, 111, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Shu, L.; Cai, L.; Sun, X.; Cui, Y.; Liu, J. Homozygous Missense Mutation Arg207Cys in the WEE2 Gene Causes Female Infertility and Fertilization Failure. J. Assist. Reprod. Genet. 2019, 36, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Mu, J.; Zhao, J.; Zhou, Z.; Chen, B.; Wu, L.; Yan, Z.; Wang, W.; Zhao, L.; Dong, J.; et al. Novel Mutations in WEE2: Expanding the Spectrum of Mutations Responsible for Human Fertilization Failure. Clin. Genet. 2019, 95, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Dahl, G. The Pannexin1 Membrane Channel: Distinct Conformations and Functions. FEBS Lett. 2018, 592, 3201–3209. [Google Scholar] [CrossRef] [PubMed]
- Baranova, A.; Ivanov, D.; Petrash, N.; Pestova, A.; Skoblov, M.; Kelmanson, I.; Shagin, D.; Nazarenko, S.; Geraymovych, E.; Litvin, O.; et al. The Mammalian Pannexin Family Is Homologous to the Invertebrate Innexin Gap Junction Proteins. Genomics 2004, 83, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Penuela, S.; Gehi, R.; Laird, D.W. The Biochemistry and Function of Pannexin Channels. Biochim. Biophys. Acta-Biomembr. 2013, 1828, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Qu, R.; Dou, Q.; Wu, F.; Wang, W.; Chen, B.; Mu, J.; Zhang, Z.; Zhao, L.; Zhou, Z.; et al. Homozygous Variants in PANX1 Cause Human Oocyte Death and Female Infertility. Eur. J. Hum. Genet. 2021, 29, 1396–1404. [Google Scholar] [CrossRef]
- Sang, Q.; Zhang, Z.; Shi, J.; Sun, X.; Li, B.; Yan, Z.; Xue, S.; Ai, A.; Lyu, Q.; Li, W.; et al. A Pannexin 1 Channelopathy Causes Human Oocyte Death. Sci. Transl. Med. 2019, 11, eaav8731. [Google Scholar] [CrossRef]
- Zhang, Y.; Suzuki, T.; Li, K.; Gothwal, S.K.; Shinohara, M.; Shinohara, A. Genetic Interactions of Histone Modification Machinery Set1 and PAF1C with the Recombination Complex Rec114-Mer2-Mei4 in the Formation of Meiotic DNA Double-Strand Breaks. Int. J. Mol. Sci. 2020, 21, 2679. [Google Scholar] [CrossRef]
- Maleki, S.; Neale, M.J.; Arora, C.; Henderson, K.A.; Keeney, S. Interactions between Mei4, Rec114, and Other Proteins Required for Meiotic DNA Double-Strand Break Formation in Saccharomyces Cerevisiae. Chromosoma 2007, 116, 471–486. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Oliver, C.; Brun, C.; Juarez-Martinez, A.B.; Tarabay, Y.; Kadlec, J.; de Massy, B. Mouse REC114 Is Essential for Meiotic DNA Double-Strand Break Formation and Forms a Complex with MEI4. Life Sci. Alliance 2018, 1, e201800259. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.M.P.; Ge, Z.-J.; Reddy, R.; Fahiminiya, S.; Sauthier, P.; Bagga, R.; Sahin, F.I.; Mahadevan, S.; Osmond, M.; Breguet, M.; et al. Causative Mutations and Mechanism of Androgenetic Hydatidiform Moles. Am. J. Hum. Genet. 2018, 103, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhu, X.; Wang, M.; Cai, L.; Ge, Q.; Fu, Y.; Jin, L. The Homozygous p.Tyr228Cys Variant in CDC20 Causes Oocyte Maturation Arrest: An Additional Evidence Supporting the Causality between CDC20 Mutation and Female Infertility. J. Assist. Reprod. Genet. 2021, 38, 2219–2222. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Tong, X.; Wang, F.; Luo, L.; Jin, R.; Fu, Y.; Zhou, G.; Li, D.; Song, G.; Liu, Y.; et al. Novel Mutations in PATL2 Cause Female Infertility with Oocyte Germinal Vesicle Arrest. Hum. Reprod. 2018, 33, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Demond, H.; Anvar, Z.; Jahromi, B.N.; Sparago, A.; Verma, A.; Davari, M.; Calzari, L.; Russo, S.; Jahromi, M.A.; Monk, D.; et al. A KHDC3L Mutation Resulting in Recurrent Hydatidiform Mole Causes Genome-Wide DNA Methylation Loss in Oocytes and Persistent Imprinting Defects Post-Fertilisation. Genome Med. 2019, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, H.; He, Y.; Zeng, J.; Zhao, J.; Xia, Q.; Wu, L.; Yao, Z.; Li, Y. A Novel Homozygous Mutation in the PADI6 Gene Causes Early Embryo Arrest. Reprod. Health 2022, 19, 190. [Google Scholar] [CrossRef]
- Li, G.; Tian, X.; Lv, D.; Zhang, L.; Zhang, Z.; Wang, J.; Yang, M.; Tao, J.; Ma, T.; Wu, H.; et al. NLRP7 Is Expressed in the Ovine Ovary and Associated with in Vitro Pre-Implantation Embryo Development. Reproduction 2019, 158, 415–427. [Google Scholar] [CrossRef]
- Wang, J.; Yang, X.; Sun, X.; Ma, L.; Yin, Y.; He, G.; Zhang, Y.; Zhou, J.; Cai, L.; Liu, J.; et al. A Novel Homozygous Nonsense Mutation in Zona Pellucida 1 (ZP1) Causes Human Female Empty Follicle Syndrome. J. Assist. Reprod. Genet. 2021, 38, 1459–1468. [Google Scholar] [CrossRef]
- Luo, G.; Zhu, L.; Liu, Z.; Yang, X.; Xi, Q.; Li, Z.; Duan, J.; Jin, L.; Zhang, X. Novel Mutations in ZP1 and ZP2 Cause Primary Infertility Due to Empty Follicle Syndrome and Abnormal Zona Pellucida. J. Assist. Reprod. Genet. 2020, 37, 2853–2860. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zeng, Y.; Chen, H.; Zhou, Z.; Fu, J.; Sang, Q.; Wang, L.; Sun, X.; Chen, B.; Xu, C. A Novel Homozygous Variant in ZP2 Causes Abnormal Zona Pellucida Formation and Female Infertility. J. Assist. Reprod. Genet. 2021, 38, 1239–1245. [Google Scholar] [CrossRef]
- Gao, L.-L.; Zhou, C.-X.; Zhang, X.-L.; Liu, P.; Jin, Z.; Zhu, G.-Y.; Ma, Y.; Li, J.; Yang, Z.-X.; Zhang, D. ZP3 Is Required for Germinal Vesicle Breakdown in Mouse Oocyte Meiosis. Sci. Rep. 2017, 7, 41272. [Google Scholar] [CrossRef] [PubMed]
- Lamas-Toranzo, I.; Fonseca Balvís, N.; Querejeta-Fernández, A.; Izquierdo-Rico, M.J.; González-Brusi, L.; Lorenzo, P.L.; García-Rebollar, P.; Avilés, M.; Bermejo-Álvarez, P. ZP4 Confers Structural Properties to the Zona Pellucida Essential for Embryo Development. Elife 2019, 8, 48904. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wang, G.; Wang, J.; Mu, X.; Chen, H.; Song, X.; Bai, X. Novel Compound Heterozygous Mutation in WEE2 Is Associated with Fertilization Failure: Case Report of an Infertile Woman and Literature Review. BMC Womens. Health 2020, 20, 246. [Google Scholar] [CrossRef]
- Jin, J.; Tong, X.; Zhang, Y.-L.; Yang, W.; Ma, Y.; Ren, P.; Zhou, F.; Zhang, S. Novel WEE2 Compound Heterozygous Mutations Identified in Patients with Fertilization Failure or Poor Fertilization. J. Assist. Reprod. Genet. 2021, 38, 2861–2869. [Google Scholar] [CrossRef] [PubMed]
- Kordowitzki, P.; Sokołowska, G.; Wasielak-Politowska, M.; Skowronska, A.; Skowronski, M.T. Pannexins and Connexins: Their Relevance for Oocyte Developmental Competence. Int. J. Mol. Sci. 2021, 22, 5918. [Google Scholar] [CrossRef] [PubMed]
- Boekhout, M.; Karasu, M.E.; Wang, J.; Acquaviva, L.; Pratto, F.; Brick, K.; Eng, D.Y.; Xu, J.; Camerini-Otero, R.D.; Patel, D.J.; et al. REC114 Partner ANKRD31 Controls Number, Timing, and Location of Meiotic DNA Breaks. Mol. Cell 2019, 74, 1053–1068.e8. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.; Vissing, J. Paternal Inheritance of Mitochondrial DNA. N. Engl. J. Med. 2002, 347, 576–580. [Google Scholar] [CrossRef] [PubMed]
- McWilliams, T.; Suomalainen, A. Mitochondrial DNA Can Be Inherited from Fathers, Not Just Mothers. Nature 2019, 565, 296–297. [Google Scholar] [CrossRef]
- Luo, S.; Valencia, C.A.; Zhang, J.; Lee, N.-C.; Slone, J.; Gui, B.; Wang, X.; Li, Z.; Dell, S.; Brown, J.; et al. Biparental Inheritance of Mitochondrial DNA in Humans. Proc. Natl. Acad. Sci. USA 2018, 115, 13039–13044. [Google Scholar] [CrossRef] [PubMed]
- Khrapko, K.; Coller, H.A.; André, P.C.; Li, X.-C.; Hanekamp, J.S.; Thilly, W.G. Mitochondrial Mutational Spectra in Human Cells and Tissues. Proc. Natl. Acad. Sci. USA 1997, 94, 13798–13803. [Google Scholar] [CrossRef]
- Shigenaga, M.K.; Hagen, T.M.; Ames, B.N. Oxidative Damage and Mitochondrial Decay in Aging. Proc. Natl. Acad. Sci. USA 1994, 91, 10771–10778. [Google Scholar] [CrossRef]
- Stewart, J.B.; Freyer, C.; Elson, J.L.; Wredenberg, A.; Cansu, Z.; Trifunovic, A.; Larsson, N.-G. Strong Purifying Selection in Transmission of Mammalian Mitochondrial DNA. PLoS Biol. 2008, 6, e10. [Google Scholar] [CrossRef] [PubMed]
- Wai, T.; Ao, A.; Zhang, X.; Cyr, D.; Dufort, D.; Shoubridge, E.A. The Role of Mitochondrial DNA Copy Number in Mammalian Fertility. Biol. Reprod. 2010, 83, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Colagar, A.H.; Mosaieby, E.; Seyedhassani, S.M.; Mohajerani, M.; Arasteh, A.; Kamalidehghan, B.; Houshmand, M. T4216C Mutation in NADH Dehydrogenase I Gene Is Associated with Recurrent Pregnancy Loss. Mitochondrial DNA 2013, 24, 610–612. [Google Scholar] [CrossRef] [PubMed]
- Rebolledo-Jaramillo, B.; Su, M.S.W.; Stoler, N.; McElhoe, J.A.; Dickins, B.; Blankenberg, D.; Korneliussen, T.S.; Chiaromonte, F.; Nielsen, R.; Holland, M.M.; et al. Maternal Age Effect and Severe Germ-Line Bottleneck in the Inheritance of Human Mitochondrial DNA. Proc. Natl. Acad. Sci. USA 2014, 111, 15474–15479. [Google Scholar] [CrossRef]
- Ma, H.; Hayama, T.; Van Dyken, C.; Darby, H.; Koski, A.; Lee, Y.; Gutierrez, N.M.; Yamada, S.; Li, Y.; Andrews, M.; et al. Deleterious MtDNA Mutations Are Common in Mature Oocytes. Biol. Reprod. 2020, 102, 607–619. [Google Scholar] [CrossRef]
- Lopes, F.C.A. Mitochondrial Metabolism and DNA Methylation: A Review of the Interaction between Two Genomes. Clin. Epigenetics 2020, 12, 182. [Google Scholar] [CrossRef]
- Keefe, D.; Kumar, M.; Kalmbach, K. Oocyte Competency Is the Key to Embryo Potential. Fertil. Steril. 2015, 103, 317–322. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Sci. 80-. 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Van Blerkom, J. Mitochondria in Human Oogenesis and Preimplantation Embryogenesis: Engines of Metabolism, Ionic Regulation and Developmental Competence. Reproduction 2004, 128, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Waymire, K.G.; Narula, N.; Li, P.; Rocher, C.; Coskun, P.E.; Vannan, M.A.; Narula, J.; MacGregor, G.R.; Wallace, D.C. A Mouse Model of Mitochondrial Disease Reveals Germline Selection Against Severe MtDNA Mutations. Sci. 80-. 2008, 319, 958–962. [Google Scholar] [CrossRef]
- Seyedhassani, S.M.; Houshmand, M.; Kalantar, S.M.; Modabber, G.; Aflatoonian, A. No Mitochondrial DNA Deletions but More D-Loop Point Mutations in Repeated Pregnancy Loss. J. Assist. Reprod. Genet. 2010, 27, 641–648. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solovova, O.A.; Chernykh, V.B. Genetics of Oocyte Maturation Defects and Early Embryo Development Arrest. Genes 2022, 13, 1920. https://doi.org/10.3390/genes13111920
Solovova OA, Chernykh VB. Genetics of Oocyte Maturation Defects and Early Embryo Development Arrest. Genes. 2022; 13(11):1920. https://doi.org/10.3390/genes13111920
Chicago/Turabian StyleSolovova, Olga Aleksandrovna, and Vyacheslav Borisovich Chernykh. 2022. "Genetics of Oocyte Maturation Defects and Early Embryo Development Arrest" Genes 13, no. 11: 1920. https://doi.org/10.3390/genes13111920
APA StyleSolovova, O. A., & Chernykh, V. B. (2022). Genetics of Oocyte Maturation Defects and Early Embryo Development Arrest. Genes, 13(11), 1920. https://doi.org/10.3390/genes13111920






