Evaluation of Mean Percentage of Full-Length SMN Transcripts as a Molecular Biomarker of Spinal Muscular Atrophy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. RNA Isolation and cDNA Synthesis
2.3. Real-Time PCR
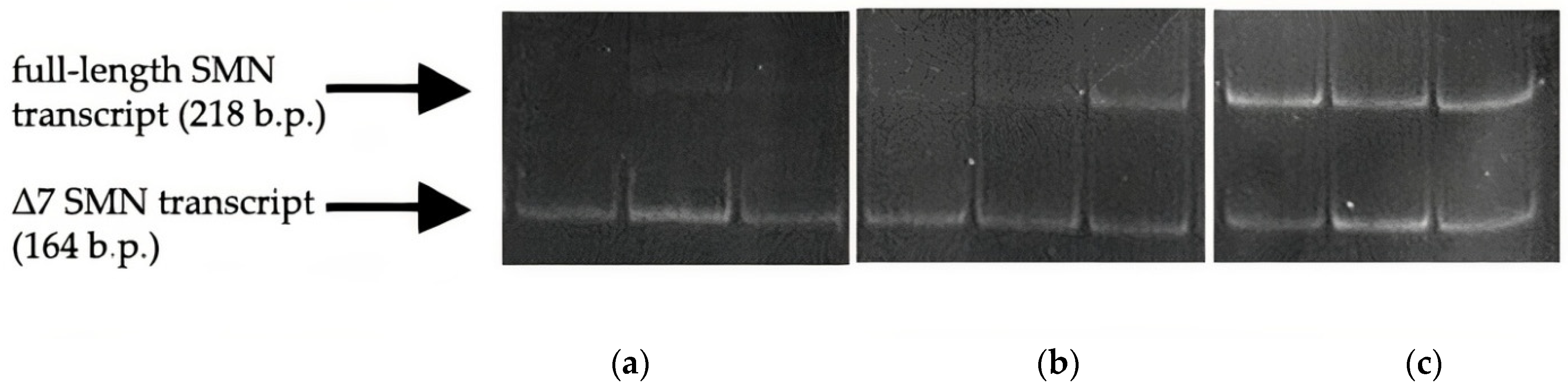
2.4. Semiquantitative and Quantitative Fluorescence RT-PCR
2.5. Transfection
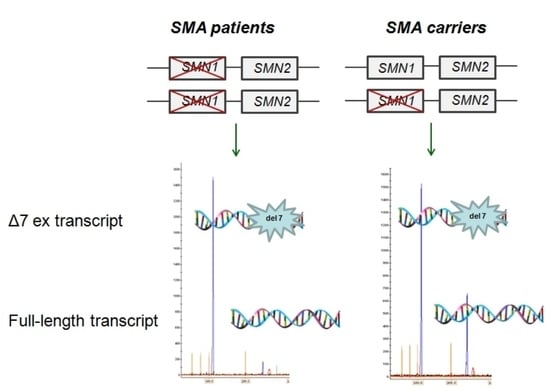
3. Results
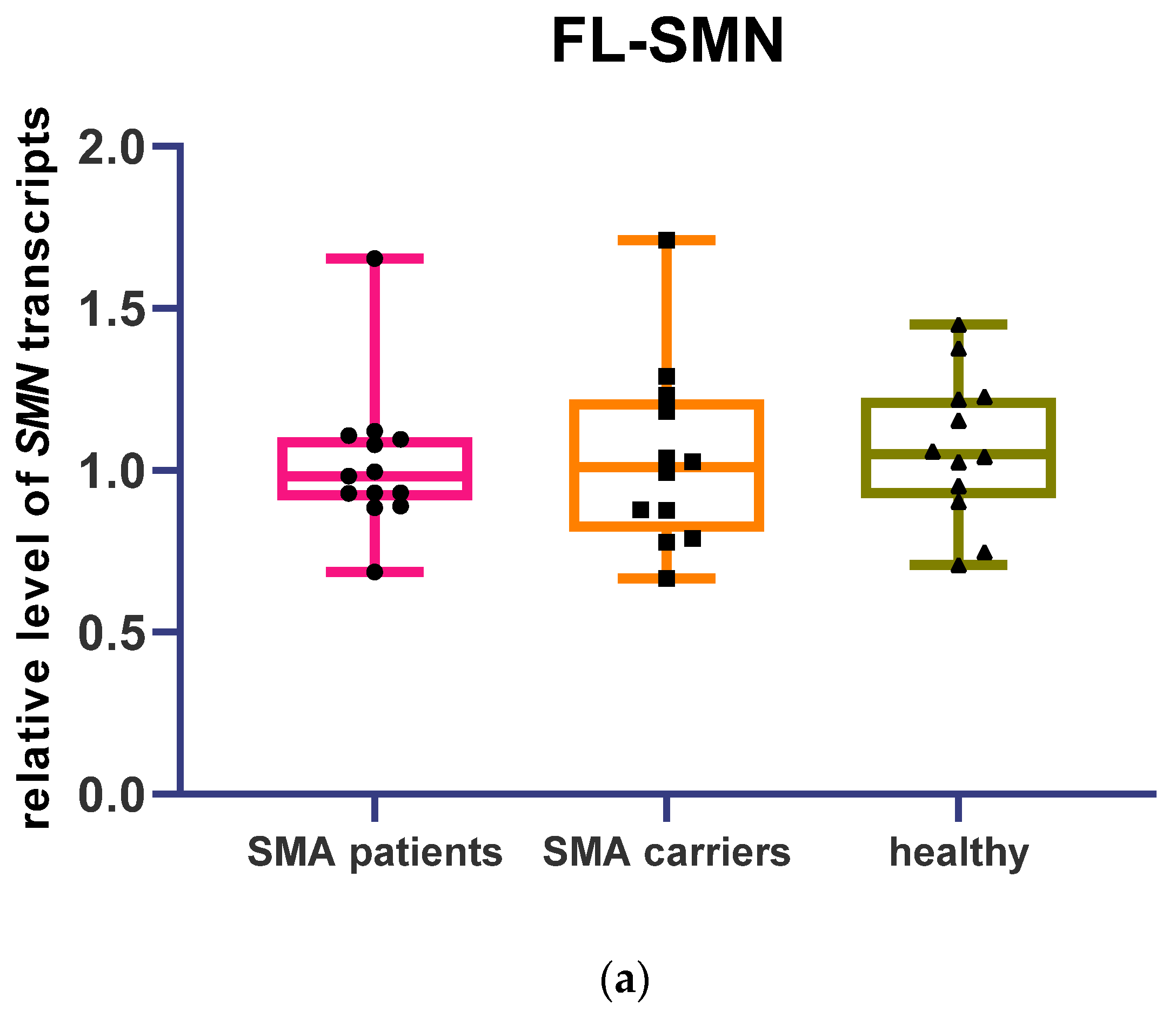
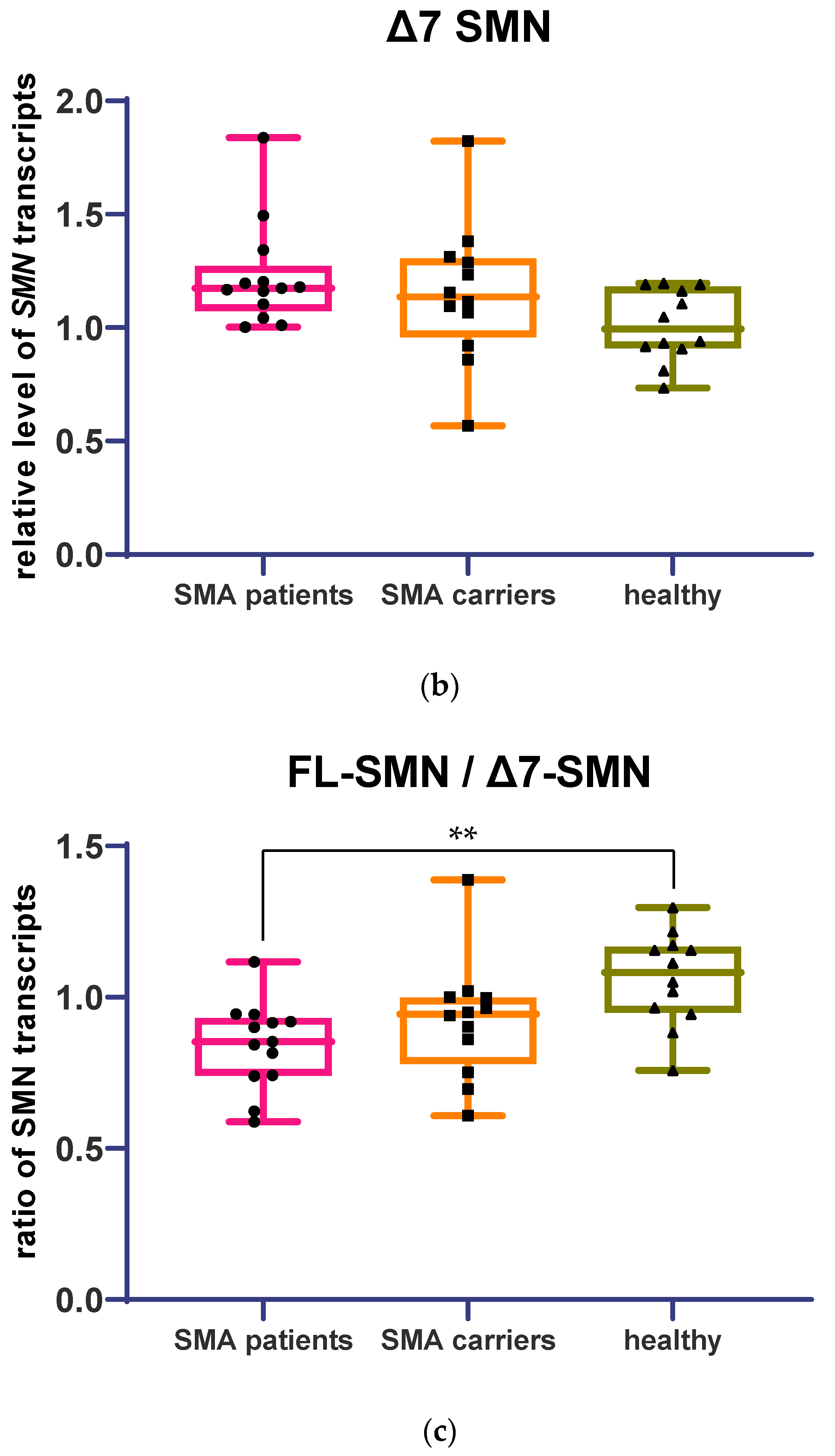
3.1. Determination of Relative Full-Length and Δ7 SMN Transcripts Level in Patients, SMA Carriers, and Healthy Individuals by Means of Quantitative Real-Time PCR
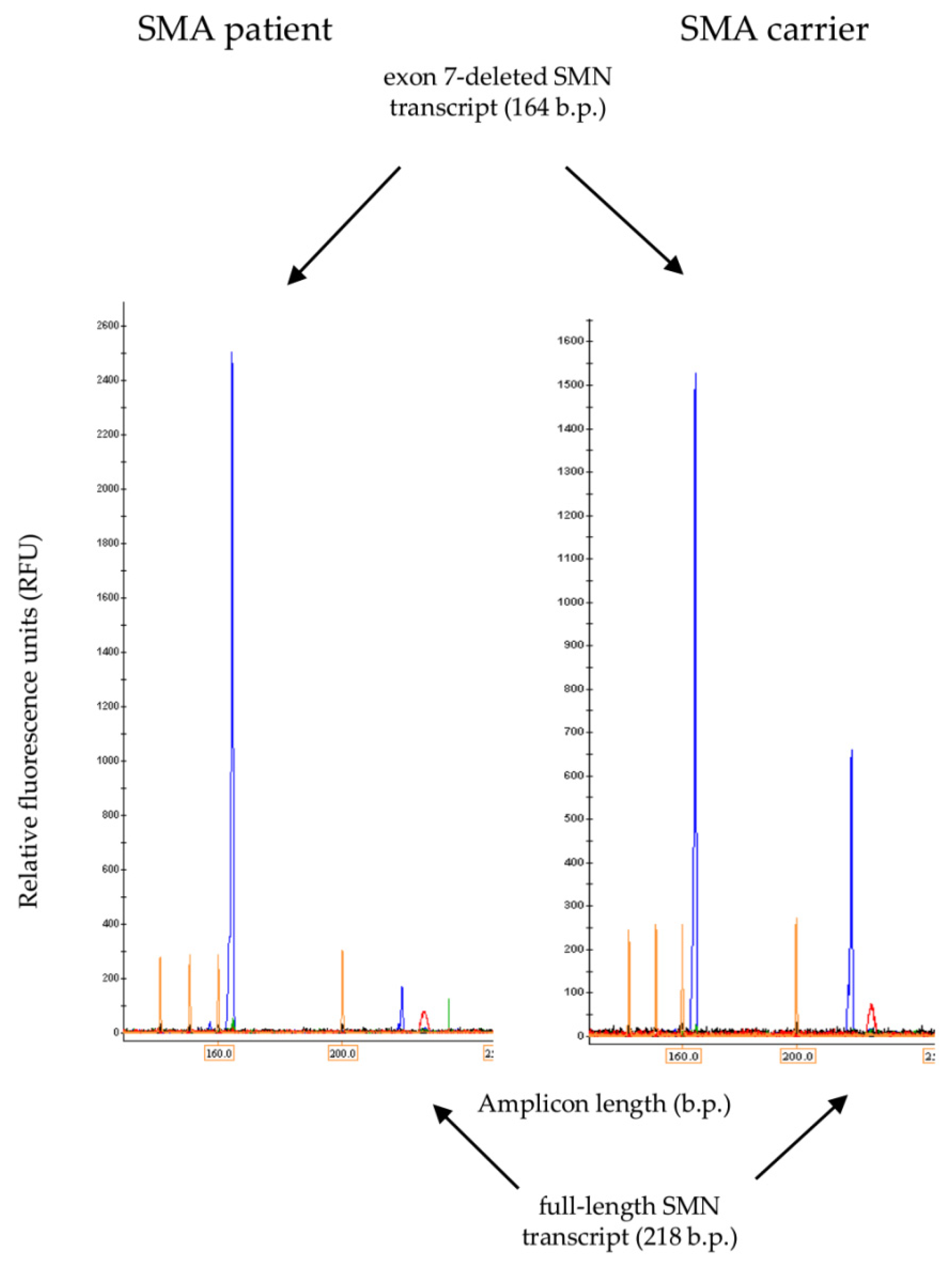
3.2. Full-Length and Δ7 SMN Transcripts Percentage Detected in Patients, SMA Carriers, and Healthy Individuals by Semiquantitative and Quantitative Fluorescence RT-PCR
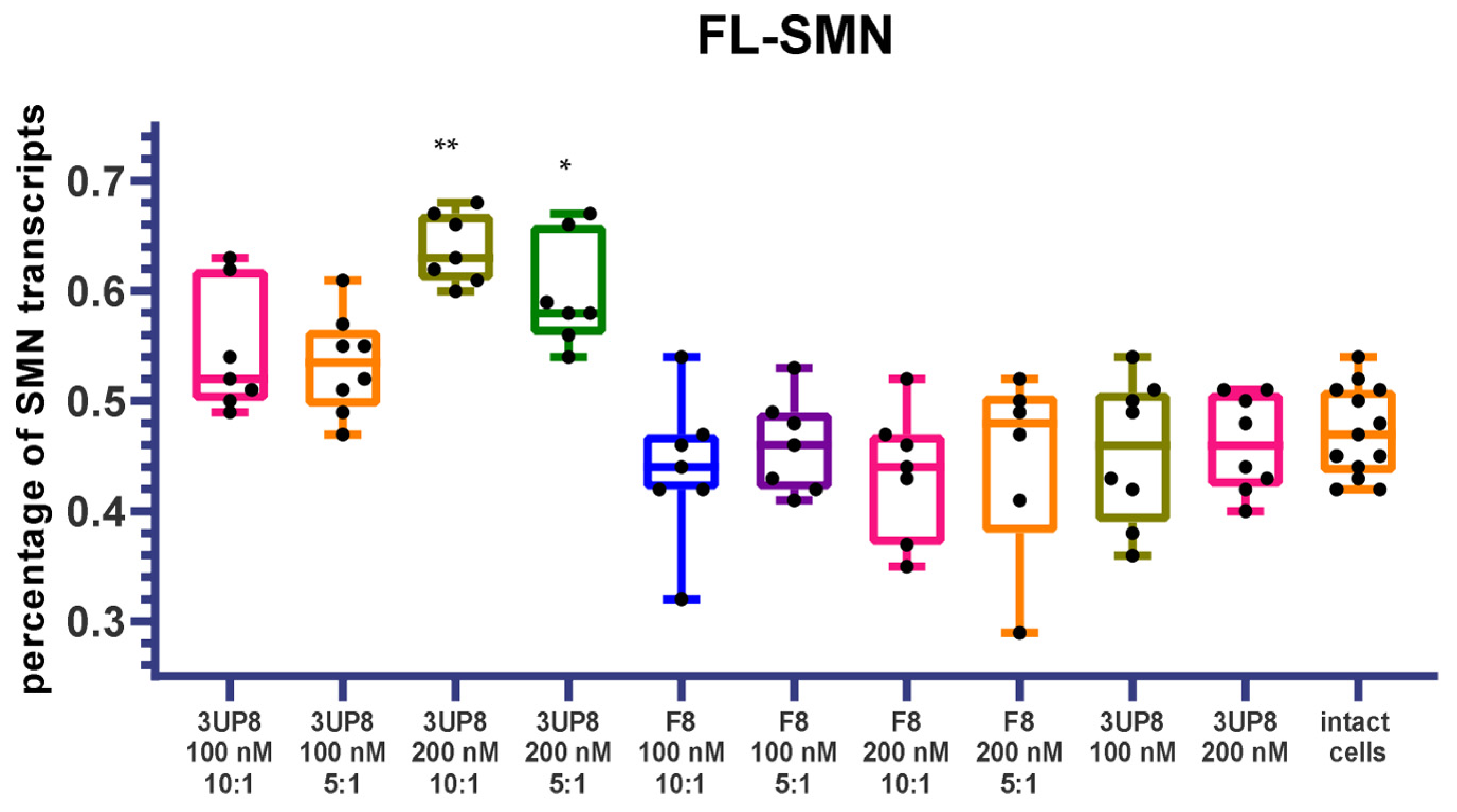
3.3. Full-Length SMN Transcripts Percentage Increase after SMN2 Splicing Correction
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lefebvre, S.; Bürglen, L.; Reboullet, S.; Clermont, O.; Burlet, P.; Viollet, L.; Benichou, B.; Cruaud, C.; Millasseau, P.; Zeviani, M.; et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell 1995, 80, 155–165. [Google Scholar] [CrossRef]
- Monani, U.R.; Lorson, C.L.; Parsons, D.W.; Prior, T.W.; Androphy, E.J.; Burghes, A.H.M.; McPherson, J.D. A single nucleotide difference that alters splicing patterns distinguishes the SMA gene SMN1 from the copy gene SMN. Hum. Mol. Genet. 1999, 8, 1177–1183. [Google Scholar] [CrossRef]
- Zheleznyakova, G.Y.; Kiselev, A.V.; Vakharlovsky, V.G.; Rask-Andersen, M.; Chavan, R.; Egorova, A.A.; Schiöth, H.B.; Baranov, V.S. Genetic and expression studies of SMN2 gene in Russian patients with spinal muscular atrophy type II and III. BMC Med. Genet. 2011, 12, 96. [Google Scholar] [CrossRef] [PubMed]
- Maretina, M.A.; Zheleznyakova, G.Y.; Lanko, K.M.; Egorova, A.A.; Baranov, V.S.; Kiselev, A.V. Molecular Factors Involved in Spinal Muscular Atrophy Pathways as Possible Disease-modifying Candidates. Curr. Genomics 2018, 19, 339–355. [Google Scholar] [CrossRef] [PubMed]
- Maretina, M.A.; Kiselev, A.V.; Ilina, A.V.; Egorova, A.A.; Glotov, A.S.; Bespalova, O.N.; Baranov, V.S.; Kogan, I.Y. Current Trends in the Diagnosis, Screening and Treatment of Spinal Muscular Atrophy. Ann. Russ. Acad. Med. Sci. 2022, 77, 87–96. [Google Scholar] [CrossRef]
- Finkel, R.S.; Mercuri, E.; Darras, B.T.; Connolly, A.M.; Kuntz, N.L.; Kirschner, J.; Chiriboga, C.A.; Saito, K.; Servais, L.; Tizzano, E.; et al. Nusinersen versus Sham Control in Infantile-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- De Vivo, D.C.; Bertini, E.; Swoboda, K.J.; Hwu, W.-L.; Crawford, T.O.; Finkel, R.S.; Kirschner, J.; Kuntz, N.L.; Parsons, J.A.; Ryan, M.M.; et al. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: Interim efficacy and safety results from the Phase 2 NURTURE study. Neuromuscul. Disord. 2019, 29, 842–856. [Google Scholar] [CrossRef] [PubMed]
- Kichula, E.A.; Proud, C.M.; Farrar, M.A.; Kwon, J.M.; Saito, K.; Desguerre, I.; McMillan, H.J. Expert recommendations and clinical considerations in the use of onasemnogene abeparvovec gene therapy for spinal muscular atrophy. Muscle Nerve 2021, 64, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.N.; Shishimorova, M.; Cao, L.C.; Gangwani, L.; Singh, R.N. A short antisense oligonucleotide masking a unique intronic motif prevents skipping of a critical exon in spinal muscular atrophy. RNA Biol. 2009, 6, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Sahashi, K.; Hung, G.; Rigo, F.; Passini, M.A.; Bennett, C.F.; Krainer, A.R. Antisense correction of SMN2 splicing in the CNS rescues necrosis in a type III SMA mouse model. Genes Dev. 2010, 24, 1634–1644. [Google Scholar] [CrossRef] [PubMed]
- Brichta, L.; Holker, I.; Haug, K.; Klockgether, T.; Wirth, B. In vivo activation of SMN in spinal muscular atrophy carriers and patients treated with valproate. Ann. Neurol. 2006, 59, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Tiziano, F.D.; Pinto, A.M.; Fiori, S.; Lomastro, R.; Messina, S.; Bruno, C.; Pini, A.; Pane, M.; D’Amico, A.; Ghezzo, A.; et al. SMN transcript levels in leukocytes of SMA patients determined by absolute real-time PCR. Eur. J. Hum. Genet. 2010, 18, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Crawford, T.O.; Paushkin, S.V.; Kobayashi, D.T.; Forrest, S.J.; Joyce, C.L.; Finkel, R.S.; Kaufmann, P.; Swoboda, K.J.; Tiziano, D.; Lomastro, R.; et al. Evaluation of SMN protein, transcript, and copy number in the biomarkers for spinal muscular atrophy (BforSMA) clinical study. PLoS ONE 2012, 7, 33572. [Google Scholar] [CrossRef] [PubMed]
- Vezain, M.; Saugier-Veber, P.; Melki, J.; Toutain, A.; Bieth, E.; Husson, M.; Pedespan, J.M.; Viollet, L.; Pénisson-Besnier, I.; Fehrenbach, S.; et al. A sensitive assay for measuring SMN mRNA levels in peripheral blood and in muscle samples of patients affected with spinal muscular atrophy. Eur. J. Hum. Genet. 2007, 15, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Mattis, V.B.; Rai, R.; Wang, J.; Chang, C.W.T.; Coady, T.; Lorson, C.L. Novel aminoglycosides increase SMN levels in spinal muscular atrophy fibroblasts. Hum. Genet. 2006, 120, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Grigor’eva, E.V.; Valetdinova, K.R.; Ustyantseva, E.I.; Shevchenko, A.I.; Medvedev, S.P.; Mazurok, N.A.; Maretina, M.A.; Kuranova, M.L.; Kiselev, A.V.; Baranov, V.S.; et al. Neural differentiation of patient-specific induced pluripotent stem cells from patients with a hereditary form of spinal muscular atrophy. Genes Cells 2016, 11, 70–79. [Google Scholar]
- Bustin, S.A. Absolute quantification of mrna using real-time reverse transcription polymerase chain reaction assays. J. Mol. Endocrinol. 2000, 25, 169–193. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, G.; Burghes, A.H.M.; Hsieh, C.; Do, J.; Chu, B.T.T.; Perry, S.; Barkho, B.; Kaufmann, P.; Sproule, D.M.; Feltner, D.E.; et al. Biodistribution of onasemnogene abeparvovec DNA, mRNA and SMN protein in human tissue. Nat. Med. 2021, 27, 1701–1711. [Google Scholar] [CrossRef] [PubMed]
- Baranello, G.; Darras, B.T.; Day, J.W.; Deconinck, N.; Klein, A.; Masson, R.; Mercuri, E.; Rose, K.; El-Khairi, M.; Gerber, M.; et al. Risdiplam in Type 1 Spinal Muscular Atrophy. N. Engl. J. Med. 2021, 384, 915–923. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maretina, M.; Egorova, A.; Lanko, K.; Baranov, V.; Kiselev, A. Evaluation of Mean Percentage of Full-Length SMN Transcripts as a Molecular Biomarker of Spinal Muscular Atrophy. Genes 2022, 13, 1911. https://doi.org/10.3390/genes13101911
Maretina M, Egorova A, Lanko K, Baranov V, Kiselev A. Evaluation of Mean Percentage of Full-Length SMN Transcripts as a Molecular Biomarker of Spinal Muscular Atrophy. Genes. 2022; 13(10):1911. https://doi.org/10.3390/genes13101911
Chicago/Turabian StyleMaretina, Marianna, Anna Egorova, Kristina Lanko, Vladislav Baranov, and Anton Kiselev. 2022. "Evaluation of Mean Percentage of Full-Length SMN Transcripts as a Molecular Biomarker of Spinal Muscular Atrophy" Genes 13, no. 10: 1911. https://doi.org/10.3390/genes13101911
APA StyleMaretina, M., Egorova, A., Lanko, K., Baranov, V., & Kiselev, A. (2022). Evaluation of Mean Percentage of Full-Length SMN Transcripts as a Molecular Biomarker of Spinal Muscular Atrophy. Genes, 13(10), 1911. https://doi.org/10.3390/genes13101911