Establishment of an Efficient In Vitro Propagation of Cnidium officinale Makino and Selection of Superior Clones through Flow Cytometric Assessment of DNA Content
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Plant Material and Induction of Microshoots from Rhizome Buds
2.2. Flow Cytometry
2.3. Assessment of in Vitro Growth of Regenerated Plants
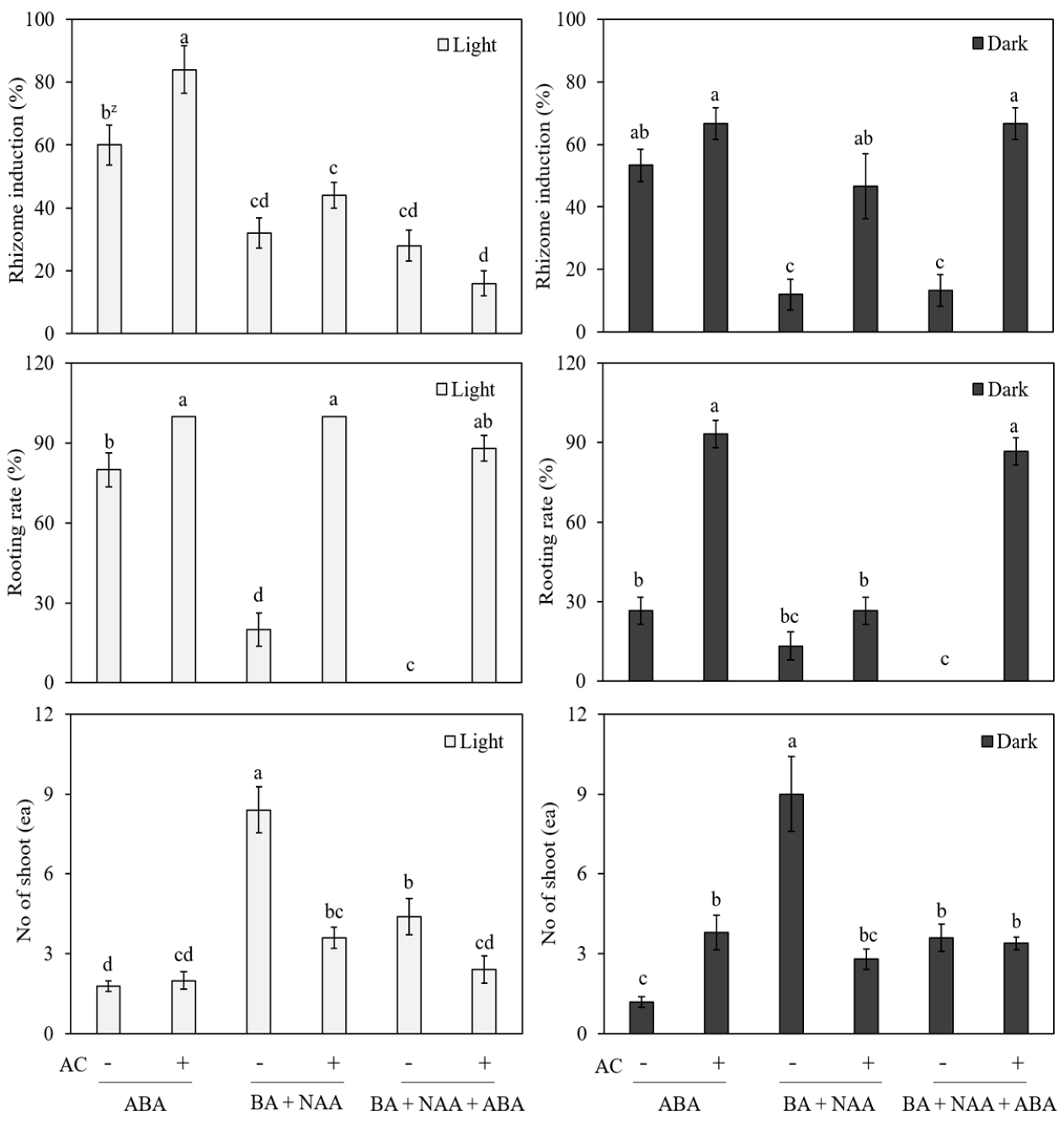
2.4. In Vitro Induction of Rhizome
2.5. Acclimatization
2.6. Statistical Analysis
3. Results
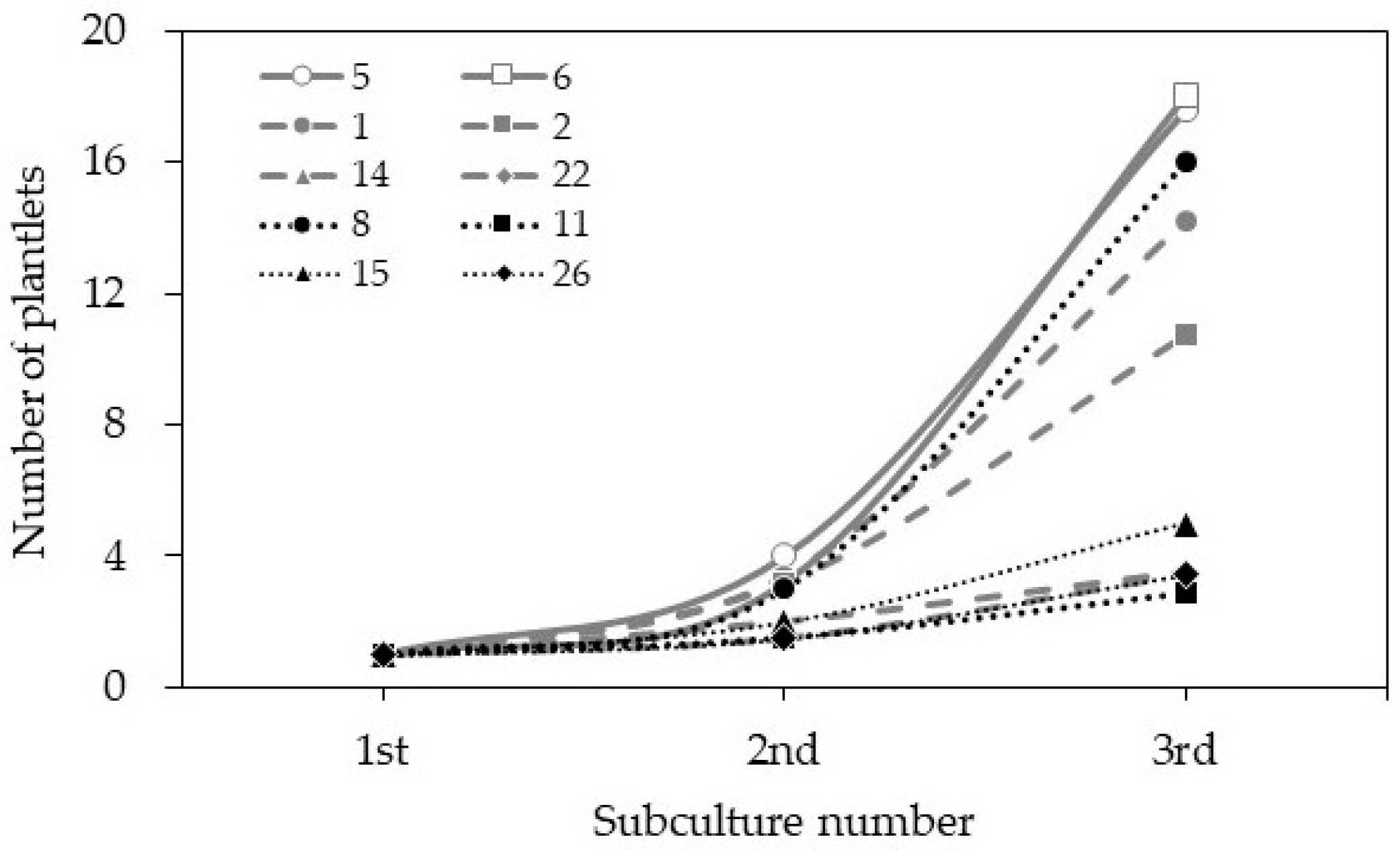
3.1. Establishment of Culture
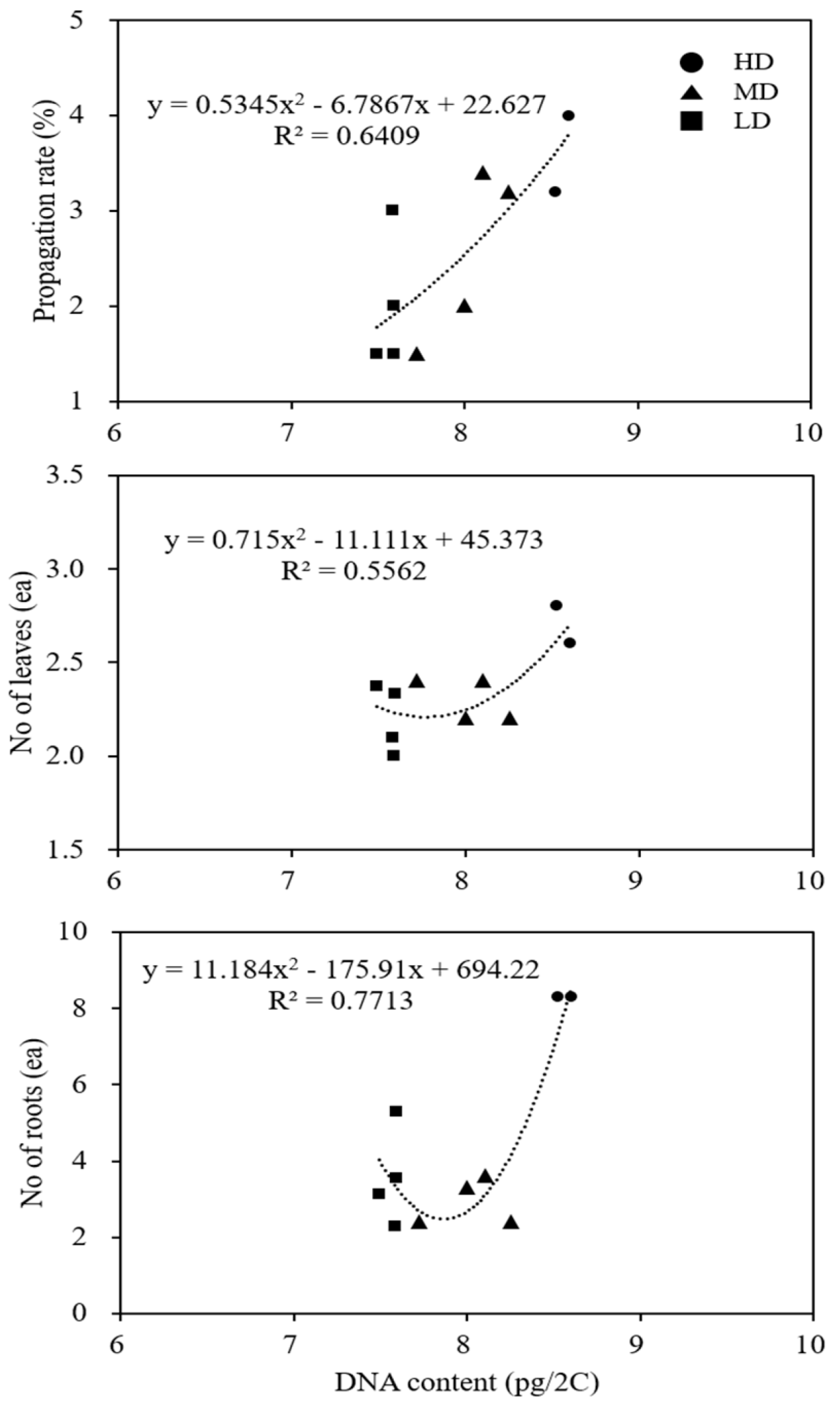
3.2. Flow Cytometric Analysis of DNA Content in Different Clones of Cnidium officinale and Selection of Superior Clones with the Highest Regeneration Potential
3.3. Comparison of Growth Characteristics of Different Clones
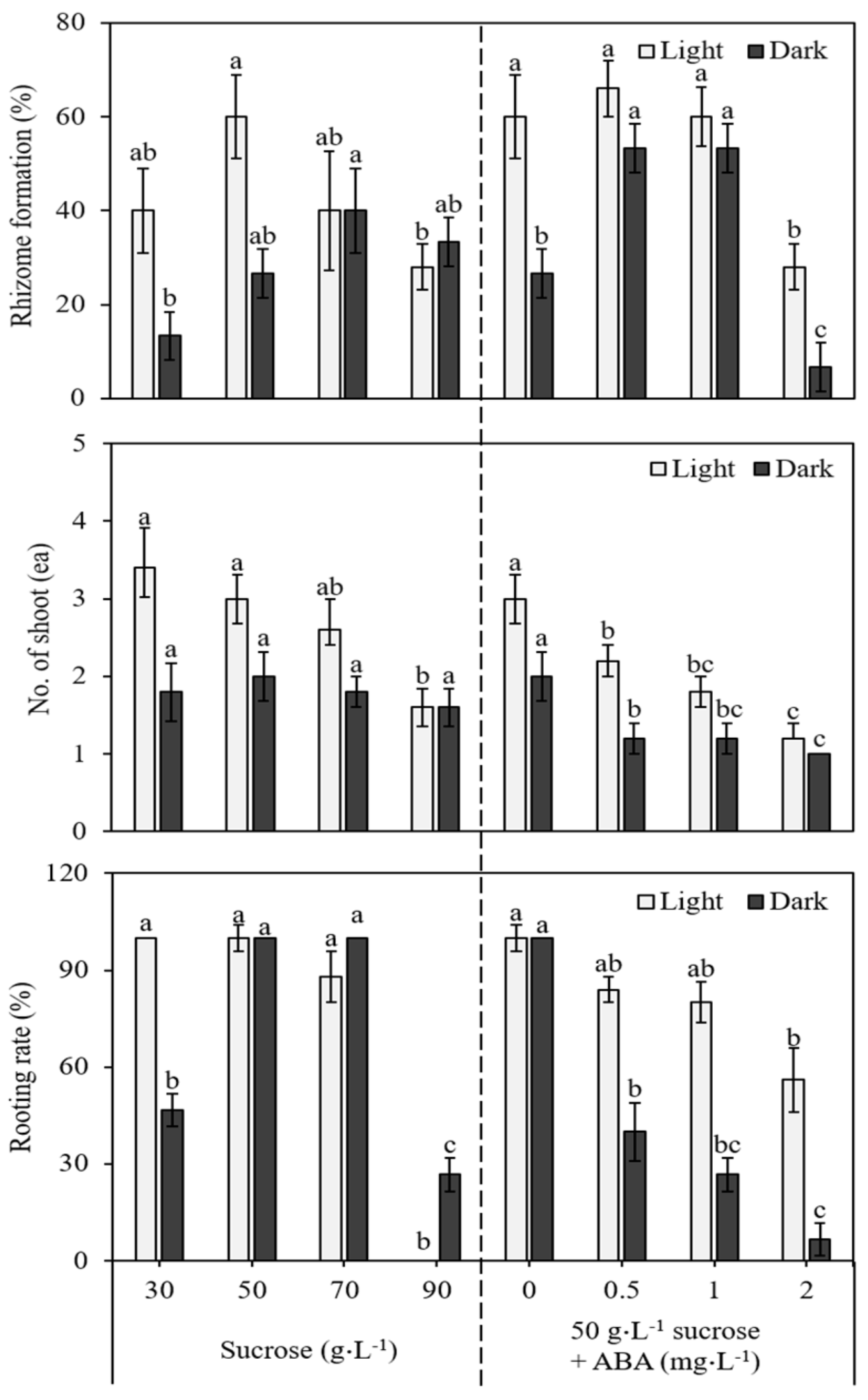
3.4. In Vitro Induction of Rhizome
3.5. Acclimatization
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, C.Y.; Kim, Y.K.; Kim, Y.S.; Suh, S.Y.; Lee, S.Y.; Park, S.U. Somatic embryogenesis and plant regeneration in Cnidium officinale Makino. J. Med. Plant Res. 2009, 3, 96–100. [Google Scholar]
- Tomoda, M.; Ohara, N.; Gonda, R.; Shimizu, N.; Takada, K.; Satoh, Y.; Shirai, S. An acidic polysaccharide having immunological activities from the rhizome of Cnidium officinale. Chem. Pharm. Bull. 1992, 40, 3025–3029. [Google Scholar] [CrossRef][Green Version]
- Tomoda, M.; Ohara, N.; Shimizu, N.; Gonda, R. Characterization of a novel glucan, which exhibits reticuloendothelial system-potentiating and anti-complementary activities, from the rhizome of Cnidium officinale. Chem. Pharm. Bull. 1994, 42, 630–633. [Google Scholar] [CrossRef][Green Version]
- Tomoda, M.; Ohara, N.; Shimizu, N.; Gonda, R. Characterization of a novel heteroglucan from the rhizome of Cnidium officinale exhibiting high reticuloendothelial system-potentiating and anti-complementary activities. Biol. Pharm. Bull. 1994, 17, 973–976. [Google Scholar] [CrossRef][Green Version]
- Kobayashi, M.; Fujita, M.; Mitsuhashi, H. Components of Cnidium officinale Makino: Occurrence of pregneolone, coniferyl ferulate, and hydoxyphthlides. Chem. Pharm. Bull. 1984, 32, 3779. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Kim, M.S.L.; Sawamura, M. Constituents of the essential oil of Cnidium officinale Makino, a Korean medicinal plant. Flavour Frag. J. 2001, 17, 49–53. [Google Scholar] [CrossRef]
- Kwak, D.H.; Kim, J.K.; Kim, J.Y.; Jeong, H.Y.; Keum, K.S.; Han, S.H.; Rho, Y.I.; Woo, W.H.; Jung, K.Y.; Choi, B.K.; et al. Anti-angiogenic activities of Cnidium officinale Makino and Tabanus bovinus. J. Ethnopharmacol. 2002, 81, 373–379. [Google Scholar] [CrossRef]
- Lee, K.Y.; Kim, J.H.; Kim, E.Y.; Yeom, M.; Jung, H.S.; Sohn, Y. Water extract of Cnidii Rhizoma suppresses RANKL-induced osteoclastogenesis in RAW 264.7 cell by inhibiting NFATc1/c-Fos signaling and prevents ovariectomized bone loss in SD-rat. BMC Complement. Altern. Med. 2019, 19, 207–219. [Google Scholar] [CrossRef]
- Cruz, J.D.L.; Kim, D.J.; Hwang, S.G. Anti-cancer effects of Cnidium officinale Makino extract mediated through apoptosis and cell cycle arrest in the HT-29 human colorectal cancer cell line. Asian Pac. J. Cancer Prev. 2014, 15, 5117–5121. [Google Scholar] [CrossRef]
- Hong, H.; Cheolan, J.; Curz, J.F.D.L.; Hwang, S.G. Cnidium officinale Makino extract induces apoptosis through activation of caspase-3 and p53 in human liver cancer HepG2 cells. Exp. Ther. Med. 2017, 14, 3191–3197. [Google Scholar] [CrossRef][Green Version]
- Jeong, S.I.; Kwak, D.H.; Lee, S.; Choo, Y.K.; Woo, W.H.; Keum, K.S.; Choi, B.K.; Jung, K.Y. Inhibitory effects of Cnidium officinale Makino and Tabanus fulvas Meigan on the high glucose-induced proliferation of glomerular mesangial cells. Phytomedicine 2005, 12, 648–655. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.H.; Oh, E.Y.; Kim, G.Y.; Choi, B.T.; Kim, C.; Choi, Y.H. Ethanol extract of Cnidium officinale exhibits anti-inflammatory effects in BV2 microglial cells by suppressing NF-kB nuclear translocation and activation of the PI3K/Kkt singling pathway. Int. J. Mol. Med. 2013, 32, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.N.K.; Cao, T.Q.; Kim, J.A.; Youn, U.Y.; Kim, S.; Woo, M.H.; Min, B.S. Anti-inflammatory activity of compounds from the rhizome of Cnidium officinale. Arch. Pharm. Res. 2018, 41, 977–985. [Google Scholar] [CrossRef]
- Lim, E.Y.; Kim, J.G.; Lee, J.; Lee, C.; Shim, J.; Kim, Y.T. Analgesic effect of Cnidium officinale extracts on postoperative, neuropathic, and menopausal pain in rat models. Evid.-Based Complement. Altern. Med. 2019, 2019, 9698727. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Shim, Y.S.; An, S.Y.; Kang, M.K. Surface characterization, biocompatibility and antifungal efficacy of a denature-lining material containing Cnidium officinale extracts. Molecules 2021, 26, 1440. [Google Scholar] [CrossRef]
- Lee, M.J.; Kang, M.K. Analysis of the antimicrobial, cytotoxic and antioxidant activities of Cnidium officinale extracts. Plants 2020, 9, 988. [Google Scholar] [CrossRef]
- Yang, H.; Jung, D.H.; Lee, H.W. Therapeutic effect of Cnidium officinale Makino extract on ovariectomized hand-limb ischemic mice. Int. Med. Res. 2019, 8, 107–115. [Google Scholar]
- Kim, Y.J. Inhibition effect of Cnidium officinale Makino extracts on MMP1 expression in human dermal fibroblasts. Asian J. Beauty Cosmetol. 2018, 16, 131–138. [Google Scholar] [CrossRef]
- Cha, H.J. Cnidium officinale Makino extracts inhibit α-MSH-induced melanogenesis in B16F10 mouse melanoma cells. Asian J. Beauty Cosmetol. 2018, 16, 122–130. [Google Scholar] [CrossRef]
- Cho, D.Y.; Lee, E.K.; Soh, W.Y. Cotyledon structure and germinability if somatic embryos formed from inflorescence explants of Cnidium officinale M. Korean J. Plant Tissue Cult. 2000, 27, 137–142. [Google Scholar]
- Pant, B.; Kohda, H.; Namera, A. Clonal propagation of Cnidium officinale by shoot tip culture. Planta Med. 1996, 62, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Chirangini, P.; Sinha, S.K.; Sharma, G.J. In vitro propagation and mirorhizome induction in Kaempferia galanga Linn. and K. rotunda Linn. Ind. J. Biotechnol. 2005, 4, 404–408. [Google Scholar]
- Nayak, S.; Naik, P.K. Factors affecting in vitro mirorhizome formation and growth in Curcuma longa L. and improved field performance of micropropagated plants. ScienceAsia 2006, 32, 31–37. [Google Scholar] [CrossRef]
- Kapoor, P.; Usha Rao, I. In vitro rhizome induction and plantlet formation from multiple shoots in Bambusa bambos var. gigantea Bennet and Gaur by using growth regulators and sucrose. Plant Cell Tiss. Organ Cult. 2006, 85, 211–217. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Planta. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Rice, A.; Glick, L.; Abadi, S.; Einhorn, M.; Kopelaman, N.M.; Salman-Minkov, A.; Mayzel, J.; Mayrose, I. The Chromosome Counts Database (CCDB)—A community resource of plant chromosome numbers. New Phytol. 2015, 206, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Adil, M.; Kang, D.I.; Jeong, B.R. Data on recurrent somatic embryogenesis and in vitro micropropagtion of Cnidium officinale Makino. Data Br. 2018, 19, 2311–2314. [Google Scholar] [CrossRef] [PubMed]
- Sliwinska, E.; Thiem, B. Genome size stability in six medicinal plants species propagated in vitro. Biol. Plant. 2007, 51, 556–558. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, J.; Sun, K.; Chang, D.; Bai, S.; Shen, Y.; Huang, L.; Zhang, J.; Zhang, Y.; Dong, Y. Ploidy level and DNA content of Erianthus arundinaceus as determined by flow cytometry and the association with biological characteristics. PLoS ONE 2016, 11, e0151948. [Google Scholar] [CrossRef] [PubMed]
- Zafer, N.; Mujib, A.; Aali, M.; Tonk, D.; Gulzar, B.; Malik, M.; Sayeed, R.; Mamgain, J. Genome size analysis of field grown and tissue culture regenerated Rauvolfia serpentina (L.) by flow cytometry: Histology and scanning electron microscopic study for in vitro morphogenesis. Ind. Corps Prod. 2019, 128, 545–555. [Google Scholar] [CrossRef]
- Sharma, T.R.; Singh, B.M. In vitro microrhizome production in Zingiber officinale Rosc. Plant Cell Rep. 1995, 15, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Gopal, J.; Minocha, J.L.; Dhaliwal, H.S. Microtuerization in potato (Solanum tuberosum L.). Plant Cell Rep. 1998, 17, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhu, L.; Pan, C.; Xu, L.; Liu, Y.; Ke, W.; Yang, P. Transcriptomic analysis of the regulation of rhizome formation in temperate and tropical lotus (Nelumbo nucifera). Sci. Rep. 2015, 5, 13059. [Google Scholar] [CrossRef] [PubMed]
- Sakamura, F.; Ogihara, K.; Suga, T.; Taniguchi, K.; Tanaka, R. Volatile constituents of Zingiber officinalae rhizomes produced by in vitro shoot tip culture. Phytochemistry 1986, 25, 1333–1335. [Google Scholar] [CrossRef]
- Shimasaki, K.; Uemoto, S. Rhizome induction and plantlet regeneration of Cymbidium goeringii from flower bud cultures in vitro. Plant Cell Tiss. Organ Cult. 1991, 25, 49–52. [Google Scholar] [CrossRef]

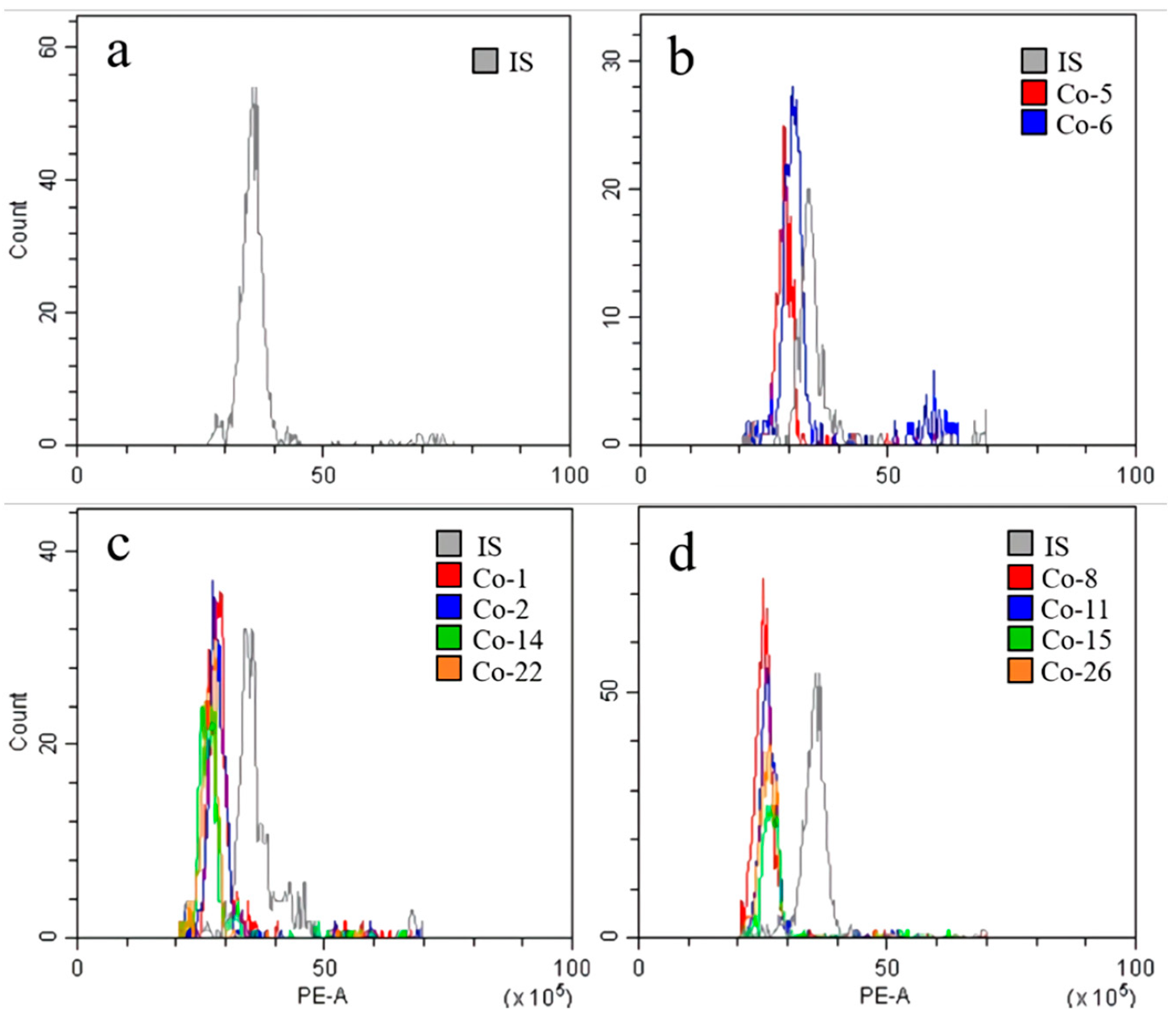

| Group | Clone | Median | CV | DNA Content z (pg/2C) |
|---|---|---|---|---|
| HD y | 5 | 2,988,019.06 | 5.9% | 8.60 ± 0.15a x |
| HD | 6 | 2,961,047.90 | 6.0% | 8.52 ± 0.20ab |
| MD | 1 | 2,816,142.06 | 5.8% | 8.10 ± 0.10abc |
| MD | 2 | 2,869,026.08 | 5.8% | 8.26 ± 0.41abc |
| MD | 14 | 2,779,891.16 | 6.0% | 8.00 ± 0.24abc |
| MD | 22 | 2,683,071.82 | 6.8% | 7.72 ± 0.28bc |
| LD | 8 | 2,634,563.66 | 6.8% | 7.58 ± 0.23c |
| LD | 11 | 2,602,247.30 | 6.3% | 7.49 ± 0.20c |
| LD | 15 | 2,638,274.26 | 6.7% | 7.59 ± 0.38c |
| LD | 26 | 2,636,395.84 | 6.1% | 7.68 ± 0.18c |
| Clone | Plant Height (mm) | Fresh Weight (mg/Plantlet) | No. of Leaves (ea/Plantlet) | No. of Roots (ea/Plantlet) | Root Length (mm) |
|---|---|---|---|---|---|
| 1 | 24.5 ± 0.9bc z | 168.0 ± 12.1ab | 2.4 ± 0.3 | 3.6 ± 0.4bc | 11.1 ± 1.3a |
| 2 | 18.9 ± 0.7ef | 160.0 ± 18.5ab | 2.2 ± 0.2 | 2.4 ± 0.3c | 4.6 ± 0.3d |
| 5 | 21.4 ± 1.2cde | 230.0 ± 23.3a | 2.6 ± 0.5 | 8.3 ± 0.3a | 6.4 ± 0.9cd |
| 6 | 22.1 ± 1.0bcde | 198.0 ± 18.6ab | 2.8 ± 0.2 | 8.3 ± 1.0a | 10.3 ± 0.6ab |
| 8 | 19.9 ± 0.9def | 206.0 ± 14.6a | 2.1 ± 0.2 | 2.3 ± 0.6c | 5.9 ± 1.7cd |
| 11 | 17.6 ± 0.8f | 202.0 ± 13.4a | 2.4 ± 0.2 | 3.1 ± 0.9bc | 6.3 ± 0.5cd |
| 14 | 25.3 ± 1.6ab | 204.0 ± 23.4a | 2.2 ± 0.3 | 3.3 ± 0.5bc | 7.2 ± 1.3cd |
| 15 | 20.8 ± 1.2def | 210.0 ± 29.4a | 2.3 ± 0.2 | 3.6 ± 0.5bc | 8.0 ± 0.7bc |
| 22 | 27.9 ± 1.2a | 208.0 ± 15.3a | 2.4 ± 0.3 | 2.4 ± 0.3c | 8.2 ± 1.0abc |
| 26 | 22.4 ± 1.3bcd | 134.0 ± 15.1b | 2.0 ± 0.1 | 5.3 ± 0.8b | 7.9 ± 1.2bc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-E.; Han, J.-E.; Lee, H.; Murthy, H.N.; Kwon, H.-J.; Lee, G.-M.; Park, S.-Y. Establishment of an Efficient In Vitro Propagation of Cnidium officinale Makino and Selection of Superior Clones through Flow Cytometric Assessment of DNA Content. Genes 2022, 13, 1815. https://doi.org/10.3390/genes13101815
Kim H-E, Han J-E, Lee H, Murthy HN, Kwon H-J, Lee G-M, Park S-Y. Establishment of an Efficient In Vitro Propagation of Cnidium officinale Makino and Selection of Superior Clones through Flow Cytometric Assessment of DNA Content. Genes. 2022; 13(10):1815. https://doi.org/10.3390/genes13101815
Chicago/Turabian StyleKim, Hyung-Eun, Jong-Eun Han, Hyoshin Lee, Hosakatte Niranjana Murthy, Hyuk-Joon Kwon, Gun-Myung Lee, and So-Young Park. 2022. "Establishment of an Efficient In Vitro Propagation of Cnidium officinale Makino and Selection of Superior Clones through Flow Cytometric Assessment of DNA Content" Genes 13, no. 10: 1815. https://doi.org/10.3390/genes13101815
APA StyleKim, H.-E., Han, J.-E., Lee, H., Murthy, H. N., Kwon, H.-J., Lee, G.-M., & Park, S.-Y. (2022). Establishment of an Efficient In Vitro Propagation of Cnidium officinale Makino and Selection of Superior Clones through Flow Cytometric Assessment of DNA Content. Genes, 13(10), 1815. https://doi.org/10.3390/genes13101815







