Abstract
The mitochondrial 1555A>G mutation plays a critical role in aminoglycoside-induced and non-syndromic hearing loss (AINSHL). Previous studies have suggested that mitochondrial secondary variants may modulate the clinical expression of m.1555A>G-induced deafness, but the molecular mechanism has remained largely undetermined. In this study, we investigated the contribution of a deafness-associated tRNAGln 4394C>T mutation to the clinical expression of the m.1555A>G mutation. Interestingly, a three-generation family with both the m.1555A>G and m.4394C>T mutations exhibited a higher penetrance of hearing loss than another family harboring only the m.1555A>G mutation. At the molecular level, the m.4394C>T mutation resides within a very conserved nucleotide of tRNAGln, which forms a new base-pairing (7T-66A) and may affect tRNA structure and function. Using trans-mitochondrial cybrid cells derived from three subjects with both the m.1555A>G and m.4394C>T mutations, three patients with only the m.1555A>G mutation and three control subjects without these primary mutations, we observed that cells with both the m.1555A>G and m.4394C>T mutations exhibited more severely impaired mitochondrial functions than those with only the m.1555A>G mutation. Furthermore, a marked decrease in mitochondrial RNA transcripts and respiratory chain enzymes was observed in cells harboring both the m.1555A>G and m.4394C>T mutations. Thus, our data suggest that the m.4394C>T mutation may play a synergistic role in the m.1555A>G mutation, enhancing mitochondrial dysfunctions and contributing to a high penetrance of hearing loss in families with both mtDNA pathogenic mutations.
1. Introduction
Deafness is a common sensory defect, with an incidence of 2.8 in 1000 children [1]. This disease is related to single-gene mutations or environmental risk factors such as aminoglycoside antibiotics (AmAn). In fact, ~50% of AINSHL cases have a genetic etiology, and >150 nuclear genes, together with >1200 mutations, have been confirmed to be associated with hearing loss (http://deafnessvariationdatabase.org/ (accessed on 11 September 2022) [2,3]. Mitochondrial DNA (mtDNA) mutations have been thought to be associated with both syndromic and nonsyndromic hearing loss [4,5]. Among these, the m.1555A>G and m.1494C>T mutations in the extremely conserved A-site of 12S rRNA have been found to be associated with AINSHL in many families worldwide [6,7,8]. Without the use of AmAn, individuals with the m.1555A>G mutation exhibit a variable degree of hearing impairment, from profound to normal [9,10]. Furthermore, functional characterization of cell lines derived from subjects with the m.1555A>G mutation shows that it confers a mild impairment of mitochondrial function and is sensitive to AmAn [11,12], suggesting that this mutation is insufficient by itself to form enough phenotypes, and that therefore a combination of environmental factors, nuclear genes and mitochondrial genetic variants may contribute to deafness expression [13,14,15]. In particular, it has been documented that mt-tRNAIle 4317A>G, tRNAGlu 14693A>G and tRNACys 5802T>C variants may increase the penetrance and expressivity of the m.1555A>G mutation [16,17,18]. Nevertheless, the pathogenesis of mtDNA secondary variants remains unclear.
Previously, we established a new multiplex allele-specific PCR (MAS-PCR) that could screen for the presence of deafness-related m.1555A>G or m.1494C>T mutations. This MAS-PCR is a simple, inexpensive, fast and reliable method, which can be used to detect the deafness-associated 12S rRNA mutations in the general population [19]. To further assess its accuracy, we screened for the presence of the m.1555A>G and m.1494C>T mutations in a cohort of 500 deaf patients and 300 controls [20]. During that process, we identified a novel tRNAGln 4394C>T mutation, together with the m.1555A>G mutation, in a Chinese family with maternal transmission of AINSHL (ID: HZD510), which manifested a much higher penetrance of hearing impairment (50% including AmAn and 10% excluding AmAn) than another pedigree (ID: HZD055) with only the m.1555A>G mutation (30% including AmAn and 0% excluding AmAn) which harbored the same mitochondrial haplogroup D4g2a as HZD510 [21]. Intriguingly, the m.4394C>T mutation was located at conventional position 7 in the Acceptor arm of tRNAGln, which was conserved from various vertebrates. Importantly, the m.4394C>T mutation formed a novel nucleotide pairing (7T-66A), while the m.4269A>G mutation, which occurred at the same position in the tRNAIle had been linked to cardiomyopathy [22,23]. Thus, we hypothesized that the m.4394C>T mutation, which may be similar to the m.4269A>G mutation, could also impair the mitochondrial function that is involved in deafness expression. To further understand the functional roles of the m.4394C>T mutation, trans-mitochondrial cells (cybrids) were generated in three patients from the HZD510 pedigree harboring both the m.1555A>G and m.4394C>T mutations, three patients from the HZD055 pedigree with only the m.1555A>G mutation and three healthy subjects without these mtDNA mutations. We found that the m.4394C>T mutation could enhance mitochondrial dysfunctions due to the m.1555A>G mutation.
2. Materials and Methods
2.1. Subjects and Audiological Assessments
Two Han Chinese families (HZD055 and HZD510) with hearing impairment were selected from Hangzhou First People’s Hospital (Figure 1). The patients’ demographics and family medical history were gathered, and blood samples were collected in our laboratory. Our study was approved by the Ethics Committee of Hangzhou First People’s Hospital (No: 2020-285-01), each subject providing his/her written informed consent. Furthermore, the consent for publication of their cases was also obtained from each individual. In addition, 300 healthy subjects (169 males and 131 females), aged from 19 to 55, with an average age of 39 years, were enrolled as controls.
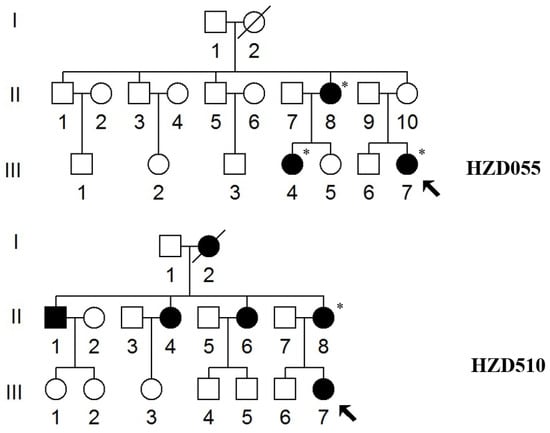
Figure 1.
Two Han Chinese pedigrees with AINSHL. Arrows indicate the probands, * indicates the individuals who had a history of using AmAn.
In addition, pure-tone audiometry (PTA) was carried out in a sound-controlled room at frequencies ranging from 250 to 8000 Hz, as suggested in our recent study [24]. The levels of hearing loss were divided into five grades: normal: <20 decibels (dB); mild: 20–40 dB; moderate: 41–70 dB; severe: 71–95 dB; and profound >95 dB, according to the study as previously described [25].
2.2. mtDNA Analysis
The genomic DNA of the affected matrilineal relatives of these pedigrees was isolated using Puregene DNA isolation kits (Biomega). 24 PCR primers spanning complete mtDNA genes were used to amplify the mitochondrial genomes, as suggested in a previous study [26]. The PCR products were purified and analyzed, and subsequently compared with an updated version of the human mitochondrial genome sequence to detect the mutations (GenBank Accessible No: NC_012920.1) [27,28].
2.3. Data Analyses
The ClustalW program (http://www.ebi.ac.uk/Tools/msa/clustalw2/ (accessed on 11 September 2022) was used to align the mtDNA sequences. A total of 14 species were selected for this analysis. The conservation index (CI) was measured by comparing the human mtDNA with that of other vertebrates. CI ≥ 75% was considered to have functional significance [29]. Moreover, the Phylotree (http://www.phylotree.org/ (accessed on 11 September 2022)) and East Asia phylogeny were used to determine the mtDNA haplogroups of these two pedigrees [21,30].
2.4. Screening for Nuclear Gene Mutations
To see the contributions of common nuclear modified genes (GJB2, GJB3, GJB6, SLC26A4 and TRMU) to m.1555A>G-induced deafness, we conducted mutational screening for these genes in two pedigrees (HZD055 and HZD510). PCR-Sanger sequencing was performed using the method described previously [31,32].
2.5. Generation of Cell Lines and Culture Conditions
Nine trans-mitochondrial cells which derived from three patients with both the m.1555A>G and m.4394C>T mutations (HZD510: II-6, II-8 and III-7), three individuals with only the m.1555A>G mutation (HZD055: II-8, III-4 and III-7) and three controls without these mtDNA mutations (C1, C2 and C3). These were constructed by introducing mitochondria from immortalized lymphoblastoid cell lines into human ρ° cells, which were generated from bromodeoxyuridine (BrdU)-resistant 143B cells, according to the protocols described elsewhere [33]. All cells were cultured in RPMI1640 medium (Invitrogen), supplemented with 10% cosmic calf serum.
2.6. ATP Analysis
The intracellular ATP levels in cell lines derived from the six deaf patients (HZD510: II-6, II-8 and III-7; HZD055: II-8, III-4 and III-7), together with the three controls (C1, C2 and C3), were determined using Cell Titer-Glo® Luminescent Cell Viability Assay (Promega), according to the manufacturer’s protocols [34].
2.7. Analysis of Mitochondrial Membrane Potential (MMP)
The levels of MMP in nine cybrid cells were evaluated using JC-1 dye (Life Technology, Carlsbad, CA, USA). At a low concentration, JC-1 existed as a monomer and was detected as green fluorescence, while at a high concentration, JC-1 existed as a multimer and was detected as red fluorescence [35]. To measure the MMP, JC-1 staining buffer was first added to the cells for 20 min. Subsequently, the solution was removed, and the cells were washed with JC-1 twice. After adding PBS per well, the fluorescence signal of the cells was observed under flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA) [36].
2.8. Mitochondrial Reactive Oxygen Species (ROS) Analysis
A total of 2 × 106 cells were maintained in fluorescent probe 2′-7′dichlorofluorescin diacetate (DCFH-DA) with a final concentration of 10 μM at 37 °C for 0.5 h. Subsequently, the cells were washed twice with PBS and analyzed using a fluorescence plate reader (Millipore, Burlington, MA, USA) at 485 nm excitation and 535 nm emission [37].
2.9. mtDNA Quantification
Quantitative PCR (qPCR) was applied to quantify the mtDNA content. The mtDNA ND1 was used to determine the mtDNA copy numbers, and the nuclear β-globin gene was used as a control. The primer sequence for the amplification of the mt-ND1 was: forward: 5′-AACATACCCATGGCCAACCT-3′; reverse: 5′-AGCGAAGGGTTGTAGTAGCCC-3′. The primer for the genetic amplification of the β-globin gene was: forward: 5′-GAAGAGCCAAGGACAGGTAC-3′; reverse: 5′-CAACTTCATCCACGTTCACC-3′. The qPCR data were normalized to β-globin, then compared with the wild-type control values and analyzed using a relative quantification method (2−ΔΔCT) [38].
2.10. Measurement of mt-RNA Transcription
The total RNA was isolated from nine cybrids using the TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA). In brief, 500 ng of RNA was used with a reverse transcription kit (Takara, Kusatsu, Shiga, Japan). Then, fluorogenic SYBR Green (Bio-Rad, Hercules, CA, USA) was used for qPCR, following the protocol suggested previously [39]. The primers for the qPCR amplification are listed in Table 1.

Table 1.
Oligonucleotide primers for mtDNA transcription.
2.11. Assays of Activities of Respiratory Chain Complexes
The enzymatic activities of complexes I, II, III and IV were assayed as detailed elsewhere [16], and their activities were normalized by citrate synthase activity.
2.12. Qualification of 8-Hydroxy-2′-Deoxyguanosine (8-OHdG)
The presence of 8-OHdG is commonly regarded as an indicator of oxidative stress and mitochondrial dysfunction [40]. The plasma concentrations of 8-OHdG in three subjects with both the m.1555A>G and m.4394C>T mutations, three subjects with only the m.1555A>G mutation and three controls were analyzed using enzyme-linked immunosorbent assays (ELISA), according to the protocol provided by the manufacturer (Nikken Foods, St. Louis, MO, USA).
2.13. Assigning Pathogenicity to the mt-tRNAGln Mutation
The updated pathogenicity scoring system was used to assess the m.4394C>T mutation [41]. The mutation was classified as “probably pathogenic” with a score of ≥11 points, “possibly pathogenic” with a score of 7–10 points and a “neutral polymorphism” with a score of ≤6 points.
2.14. Computer Analysis
All data were expressed as the mean ± standard deviation (SD). The statistical analyses were carried out using the unpaired, two-tailed Student’s t test contained in the GraphPad Prism 5 program (GraphPad Software). p < 0.05 was believed to be statistically significant.
3. Results
3.1. Clinical Features of HZD055 and HZD510 Pedigrees
We examined two Chinese families with maternally transmitted AINSHL in the Otology Clinic of Hangzhou First People’s Hospital (Figure 1). In the HZD055 pedigree, the proband (III-7) lived in Hangzhou city of Zhejiang Province. She had a history of using gentamycin when she was 19 years old, and she began to suffer from bilateral hearing loss two months after she started using gentamycin. Clinical examinations revealed that she exhibited bilateral hearing loss (106 dB for the right ear, 86 dB for left ear). Interestingly, her matrilineal relatives (II-8 and III-4) also had a history of using AmAn. As shown in Figure 2, they had profound hearing impairment.
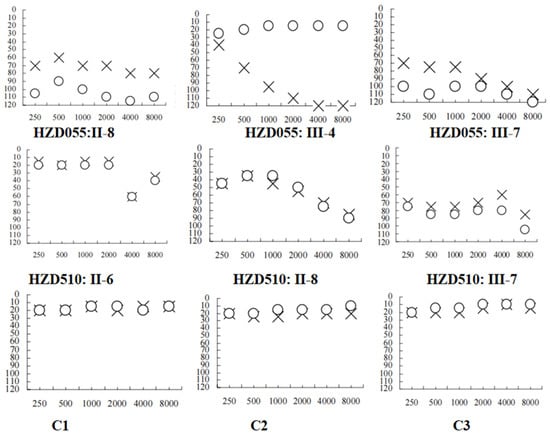
Figure 2.
Audiograms of several matrilineal relatives in two Chinese families with AINSHL. X: left ear; O: right ear; C1: HZD055: II-6; C2: HZD055: III-1; C3: HZD055: III-3.
In the HZD510 pedigree, five of the ten matrilineal relatives exhibited deafness as a sole clinical phenotype. Genetic counseling suggested that the matrilineal relatives (II-1, II-4, II-6, II-8 and III-7) were deaf patients. Audiological examinations revealed that subject II-6 had mild hearing loss, subject II-8 had moderate hearing loss and proband (III-7) developed severe hearing impairment (Figure 2 and Table 2). A comprehensive genetic counseling revealed that these subjects did not have any other clinical abnormalities, such as cardiovascular disease, visual loss, trauma, infectious diseases or neurological disorders.

Table 2.
Summary of the clinical data of several members in two pedigrees with hearing impairment.
As shown in Figure 1, the family histories of HZD055 and HZD510 were consistent with maternal inheritance. Notably, the penetrance of hearing loss in HZD055 and HZD510 was 30% and 50% (AmAn included), respectively; when the AmAn was excluded, the penetrance of these pedigrees was 0% and 10%, respectively. The age at onset of deafness in HZD055 and HZD510 varied from 19 to 45, with an average of 27 years.
3.2. Detecting the Deafness-Associated mtDNA Mutations
We used PCR and Sanger sequencing technologies to screen mtDNA mutations/variants in the matrilineal relatives of the HZD055 and HZD510 pedigrees. Compared with the rCRS, we identified a set of mtDNA mutations which belonged to East Asian mitochondrial haplogroup D4g2a [21]. As shown in Table 3, there were 14 variants in D-loop, 4 variants in 12S rRNA, 3 variants in 16S rRNA, 1 mutation in tRNAGln (4394C>T), as well as the 9-bp deletion in the nucleotide 8271–8279, whereas the rest were located at oxidative phosphorylation (OXPHOS)-related genes. In addition, ten missense mutations were found in this study, including ND2 4491G>A (p.V8I), 5178C>A (p.L237M), COII 7976G>A (p.G131S), A8 8414C>T (p.L17F), 8584G>A (p.A20T), A6 8701A>G (p.T59A), 8860A>G (p.T112A), ND3 10398A>G (p.T112A), CytB 14766C>T (p.T7I) and 15326A>G (p.T194A). These mtDNA variants were further assessed by evolutionary conservation analysis in four species: human [28], bovine [42], mouse [43] and Xenopus laevis [44]. We found that aside from the m.1555A>G and m.4394C>T mutations (Figure 3), other variants were not well conserved. In addition, the m.1555A>G and m.4394C>T mutations were not detected in the 300 controls, suggesting that they may be associated with AINSHL.

Table 3.
mtDNA sequence variants in two pedigrees with hearing loss.
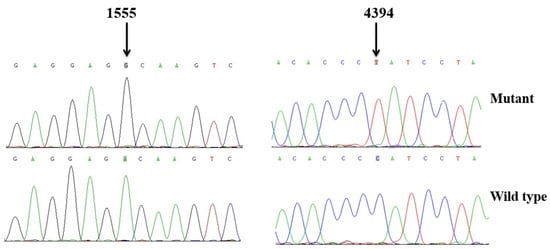
Figure 3.
Identification of the m.1555A>G and m.4394C>T mutations via Sanger sequencing, partial sequence chromatograms of 12S rRNA and tRNAGln gene from affected individual and a Chinese control. Arrows indicate the location of the base changes at positions 1555 and 4394.
As shown in Figure 4 and Figure 5, the homoplasmic m.4394C>T mutation resided at position 7 in the Acceptor arm of tRNAGln, which was extremely conserved from various species. Importantly, the m.4394C>T mutation formed a new 7T-66A base-pairing and may have a structural and functional impact on tRNAGln.
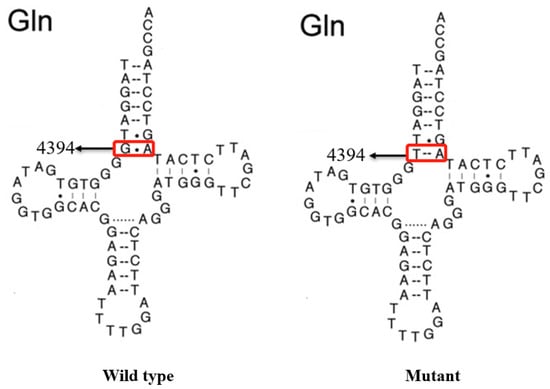
Figure 4.
Secondary structure of mt-tRNAGln with and without the m.4394C>T mutation, which is derived from Mitomap database (www.mitomap.org). Arrows denoted the position of the m.4394C>T mutation.
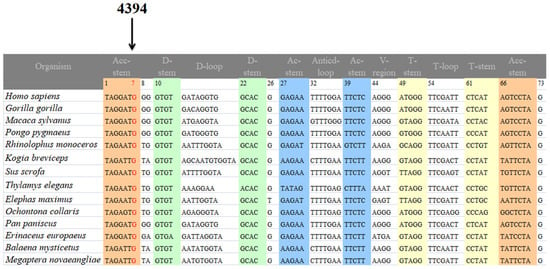
Figure 5.
Sequence alignment of the mt-tRNAGln gene from 14 species. The arrow indicates the m.4394C>T mutation.
3.3. Construction of Cybrid Cells from Two Chinese Families with AINSHL
Immortalized lymphoblastoid cell lines were generated from three matrilineal relatives (HZD510: II-6, II-8 and III-7) harboring both the m.1555A>G and m.4394C>T mutations, three subjects (HZD055: II-8, III-4 and III-7) belonging to the mtDNA haplogroup D4g2a, and three controls without these pathogenic mutations (C1, C2 and C3) (Figure 6). The cybrid cells were used to further the biochemical analysis.
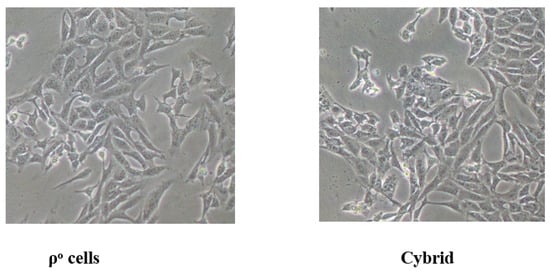
Figure 6.
The cellular morphology of human ρ° and cybrids cells.
3.4. Decreased in ATP Production
ATP is the key energy-carrying molecule in all cells. As shown in Figure 7A, the ATP levels in cell lines with both the m.1555A>G and m.4394C>T mutations and the cells with only the m.1555A>G mutation were 48% (p < 0.001) and 74% (p = 0.0012) of the average value measured in the control cell lines, respectively.
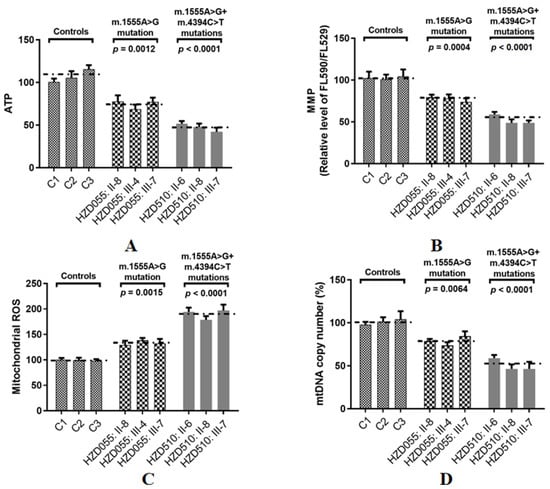
Figure 7.
Assessments of mitochondrial functions in three patients with both the m.1555A>G and m.4394C>T mutations, three patients with only the m.1555A>G mutation and three controls. (A) Analysis of ATP production in nine cybrids; (B) MMP analysis in cybrids; (C) Qualification of ROS levels; (D) Determining the mtDNA content.
3.5. Reductions in MMP
Depolarization of MMP is often associated with apoptosis by the cytochrome c released from mitochondria [45]. To see whether the m.4394C>T mutation affected MMP, the JC-1 assay was used to evaluate the MMP in both mutant and control cells. As shown in Figure 7B, the levels of MMP in the mutant cell lines carrying both the m.1555A>G and m.4394C>T mutations and the cells carrying only the m.1555A>G mutation were 53% (p < 0.0001) and 74% (p = 0.0004) of the mean values measured in the control cell lines, respectively.
3.6. The Increase in ROS Production
ROS plays an active role in many cellular process [46]. As illustrated in Figure 7C, the levels of ROS generation in mutant cell lines with both the m.1555A>G and m.4394C>T mutations and the cells carrying only the m.1555A>G mutation were 192% (p < 0.0001) and 137% (p = 0.0015) of the mean values measured in the control cell lines, respectively.
3.7. mtDNA Copy Number Decrease
The mtDNA copy number is a promising biomarker of mitochondrial dysfunction [47]. To see whether the m.4394C>T mutation affected mtDNA content, Qpcr was used to assess the mtDNA copy number variations. As shown in Figure 7D, the levels of the mtDNA copy number in the mutant cell lines carrying both the m.1555A>G and m.4394C>T mutations and the cells carrying only the m.1555A>G mutation were 52% (p < 0.0001) and 78% (p = 0.0064) of the mean values measured in control group, respectively.
3.8. Effect of the m.4394C>T Mutation on mt-RNA Transcription
To explore the synergistic roles of the m.4394C>T mutation on m.1555A>G-induced mitochondrial dysfunction, we evaluated the mt-RNA transcription in patient-derived cell lines and found that significantly lower levels of ND1, ND2, ND3, ND4, A6 and A8 mRNA were present in the cell lines carrying both the m.1555A>G and m.4394C>T mutations when compared with the cells carrying only the m.1555A>G mutation and controls without these primary mutations (Figure 8). This result indicated that the m.4394C>T mutation might, at least partially, block mt-RNA transcription.
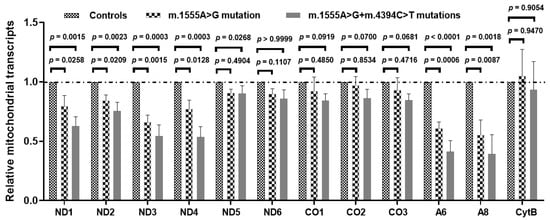
Figure 8.
Mt-RNA transcription analysis in three patients with both the m.1555A>G and m.4394C>T mutations, three patients with only the m.1555A>G mutation and three controls.
3.9. Reduced Activities of Complexes I~IV
To see the effect of the m.4394C>T mutation on OXPHOS functions, we analyzed the activities of respiratory complexes in three cell lines with both the m.1555A>G and m.4394C>T mutations, three cell lines derived from patients with only the m.1555A>G mutation, and three cells without these mutations. As shown in Figure 9, we noticed that Complexes I~IV were markedly decreased in cells with both mtDNA mutations, as compared with controls (p < 0.05 for all).
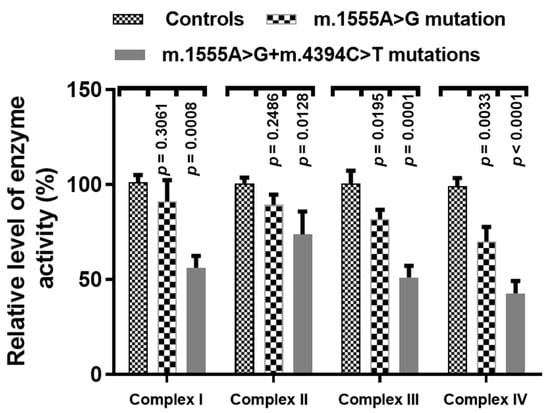
Figure 9.
Enzymatic activities of respiratory chain complexes in three patients with both the m.1555A>G and m.4394C>T mutations, three patients with only the m.1555A>G mutation and three controls.
3.10. The m.4394C>T Mutation Increases DNA Damage
To see whether the m.4394C>T mutation enhanced the DNA damage, the serum 8-OHdG levels were measured using ELISA. As shown in Figure 10, we found that subjects with both the m.1555A>G and m.4394C>T mutations exhibited higher levels of 8-OHdG than patients with only the m.1555A>G mutation (p = 0.0002) and controls without those mutations (p < 0.0001).
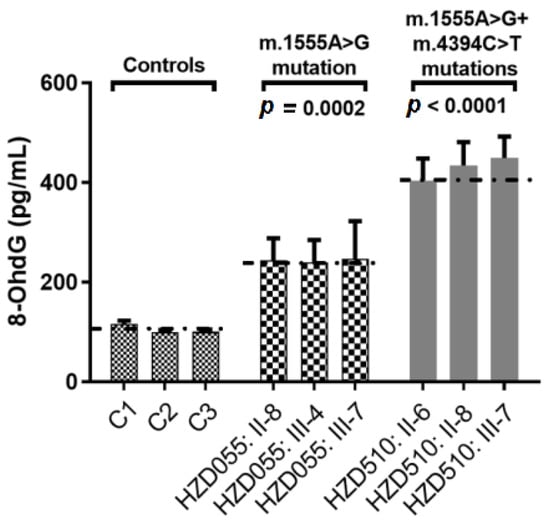
Figure 10.
Qualification of serum 8-OHdG levels in three patients with both the m.1555A>G and m.4394C>T mutations, three patients with only the m.1555A>G mutation and three controls.
3.11. Mutational Screening for Common Deafness-Associated Nuclear Genes
To uncover the roles of nuclear gene mutations in deafness, we screened for the mutations in common deafness-associated genes (GJB2, GJB3, GJB6 SLC26A4 and TRMU) in the matrilineal relatives of the HZD055 and HZD510 pedigrees. However, PCR and Sanger sequencing revealed that no functional variants could be identified in these genes, suggesting that nuclear modified genes may not play an active role in m.1555A>G-induced deafness.
3.12. Possible Pathogenic Effect of m.4394C>T in AINSHL
As shown in Table 4, according to the pathogenicity scoring system [41], the total score of the m.4394C>T mutation was 8 points, meaning it is “possibly pathogenic” and may cause AINSHL.

Table 4.
The predicted pathogenicity of the mt-tRNAGln 4394C>T mutation.
4. Discussion
The present study has explored the synergistic role of the m.4394C>T mutation in clinical manifestations of deafness-associated m.1555A>G mutation. Hearing loss as a sole clinical phenotype only presented in matrilineal relatives, but not in other members in these pedigrees, suggesting that mitochondrial dysfunctions caused by mtDNA mutations are the molecular basis for this disease. In fact, the m.1555A>G mutation was originally identified in Arab-Israeli families with AmAn-associated hearing loss [48]. Without the usage of AmAn, subjects with the m.1555A>G mutation exhibited a wide range of clinical phenotypes that ranged from mild to severe hearing loss, and even normal hearing, indicating that while the m.1555A>G mutation is a major risk factor for hearing loss, by itself it is not sufficient to produce the phenotype [49], for which it requires other factors such as nuclear genes, AmAn or mtDNA secondary variants [50,51]. In particular, mtDNA haplogroup B4C1C exhibited a higher penetrance and expressivity of m.1555A>G-induced deafness [52]. Recent experimental studies indicate that mitochondrial haplogroup B enhances the risk for hearing impairment in Chinese patients carrying the m.1555A>G mutation [53]. Moreover, mitochondrial haplogroup D4 specific m.5802T>C, m.10454T>C, m.12224C>T and m.11696G>A mutations may enhance m.1555A>G-induced deafness [15]. Sequence characterization of the complete mitochondrial genome of matrilineal relatives from these pedigrees suggested the presence of sets of polymorphisms belonging to mtDNA haplogroup D4g2a [21]. We noticed that the penetrance of hearing loss in HZD055 was 50% including AmAn and 10% excluding AmAn, while in HZD510 it was 30% including AmAn and 0% excluding AmAn. Compared with previous studies, the penetrances of m.1555A>G-induced hearing loss ranged from 22.2% to 73.9% (average: 50.9%, including AmAn), whereas excluding the effects of AmAn, the penetrances varied from 11.1% to 60.8% (average: 30.8%, Table 5) [15,16,20,54].

Table 5.
Summary of clinical and molecular data for 12 families with hearing loss.
The m.4394C>T mutation resided at position 7 in the 5′ end of tRNAGln, which was well conserved from different vertebrates. Notably, the m.4394C>T mutation introduced a novel 7T-66A base-pairing and was critical for the steady-state level of the tRNAGln. Interestingly, nucleotides at the same position in tRNAGly (9997T>C) and in tRNAIle (4269A>G) were regarded as pathogenic mutations for cardiomyopathy [55,56]. Hence, the m.4394C>T mutation may be similar to the m.9997T>C or m.4269A>G mutation, and cause mitochondrial dysfunction that is involved in hearing impairment.
Trans-mitochondrial technology was frequently used to assess the contribution of mtDNA genetic polymorphisms to OXPHOS function since noise from the nuclear genetic background was adjusted. We demonstrated that cybrid cells bearing both the m.1555A>G and m.4394C>T mutations exhibited much more severe effects on mitochondrial dysfunctions than those with only the m.1555A>G mutation and controls. Specifically, we observed drops of approximately 52% and 26% in ATP production in mutant cells carrying both the m.1555A>G and m.4394C>T mutations or mutant cell lines with only the m.1555A>G mutation, respectively, which are below the threshold ATP level for developing a clinical phenotype [57]. Notably, the deficiency of mitochondrial respiratory chain functions will frequently affect MMP, and in this study, approximately 47% and 26% reductions in MMP were found in cell lines carrying both the mtDNA mutations, and in cell lines with only the m.1555A>G mutation, respectively. It had been recognized that loss of MMP may cause cytochrome c to release into the cytoplasm and then induce programmed cell death [58]. Moreover, MMP constitutes an important component of mitochondrial respiration complexes and is associated with many mitochondrial functions, such as ATP formation and mitochondrial protein import [59,60]. In addition, a much lower level in mtDNA content was found in patients with both mutations than in patients with only the m.1555A>G mutation. Indeed, the mtDNA copy number is an important element for mitochondrial replication and transcription, which is critical for cell functions [61]. Alternations in mtDNA copy number are associated with diverse human pathologies [62]. Thus, the abnormal mtDNA copy number and MMP may lead to an increased in ROS production. ROS are the by-products of oxidative stress, and include peroxides, superoxides, hydroxyl radicals and singlet oxygen [63]. It has been documented that ROS plays a crucial role in human physiological and pathophysiological processes [64]. High ROS concentration will react with proteins, lipids, RNA or DNA, and frequently lead to irreversible functional alterations, including regulating mitochondrial functions, apoptosis and signaling transduction [65]. These results indicated that the ATP, MMP, ROS and mtDNA copy number were impaired in cells with both mtDNA mutations.
Next, examination of the RNA level of mtDNA-encoded OXPHOS subunits revealed that the mRNA levels of ND1, ND2, ND3, ND4, A6 and A8 were lower in cybrids carrying both mtDNA mutations than in cybrids with only the m.1555A>G mutation (p < 0.05 for all). This result indicates that defective mt-RNA translation caused by both the m.1555A>G and m.4394C>T mutations may be responsible for multiple OXPHOS complex deficiencies in cybrid cells. The altered tRNAGln metabolism caused by the m.4394C>T mutation led to a defect in mitochondrial translation. In fact, cell lines with both the m.1555A>G and m.4394C>T mutations would perturb the activities of Complexes I–IV and then worsen the defects in mitochondrial respiratory phenotypes associated with the m.1555A>G mutation, as in the case of the m.14692A>G mutation [66].
In addition, the level of serum 8-OHdG was much higher in patients with both the m.1555A>G and m.4394C>T mutations, suggesting that the mitochondrial dysfunctions caused by the m.1555A>G mutation may be worsened by the m.4394C>T mutation. Furthermore, the absent of any functional variants in deafness-related common nuclear genes (GJB2, GJB3, GJB6, SLC26A4 and TRMU) in the matrilineal relatives in these pedigrees suggests that nuclear modified genes may not play an active role in deafness expression, hence, the mt-tRNAGln 4394C>T mutation may enhance the penetrance of m.1555A>G-induced deafness.
5. Conclusions
In conclusion, the mt-tRNAGln 4394C>T mutation may aggravate mitochondrial dysfunction and increase the penetrance of hearing impairment caused by the m.1555A>G mutation.
Author Contributions
Conceptualization, Y.D.; methodology, Y.D.; formal analysis, Y.D. and J.L.; investigation, Y.D., Y.T. and Q.G.; funding acquisition, Y.D.; writing—original draft, Y.D.; visualization Y.D. and Y.T.; data curation, Y.T. and Q.G.; validation, Y.T.; writing—review and editing, J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Health Commission of Zhejiang Province (2021RC022), Hangzhou Bureau of Science and Technology (20201203B210) and Hangzhou Municipal Health Commission (ZD20220010).
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Hangzhou First People’s Hospital (Approval No: 2020-285-01).
Informed Consent Statement
Written informed consent to publish this paper has been obtained from the patients.
Data Availability Statement
All the data supporting the results of this study are included in the article.
Acknowledgments
We thank Hezhi Fang from Wenzhou Medical University for providing the human ρ° cells.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Korver, A.M.; Smith, R.J.; Van Camp, G.; Schleiss, M.R.; Bitner-Glindzicz, M.A.; Lustig, L.R.; Usami, S.I.; Boudewyns, A.N. Congenital hearing loss. Nat. Rev. Dis. Prim. 2017, 3, 16094. [Google Scholar] [CrossRef] [PubMed]
- Willems, P.J. Genetic causes of hearing loss. N. Engl. J. Med. 2000, 342, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Bitner-Glindzicz, M. Hereditary deafness and phenotyping in humans. Br. Med. Bull. 2002, 63, 73–94. [Google Scholar] [CrossRef] [PubMed]
- Fischel-Ghodsian, N. Mitochondrial deafness mutations reviewed. Hum. Mutat. 1999, 13, 261–270. [Google Scholar] [CrossRef]
- Ding, Y.; Leng, J.; Fan, F.; Xia, B.; Xu, P. The role of mitochondrial DNA mutations in hearing loss. Biochem. Genet. 2013, 51, 588–602. [Google Scholar] [CrossRef]
- Kullar, P.J.; Gomez-Duran, A.; Gammage, P.A.; Garone, C.; Minczuk, M.; Golder, Z.; Wilson, J.; Montoya, J.; Häkli, S.; Kärppä, M.; et al. Heterozygous SSBP1 start loss mutation co-segregates with hearing loss and the m.1555A>G mtDNA variant in a large multigenerational family. Brain 2018, 141, 55–62. [Google Scholar] [CrossRef]
- Rahman, S.; Ecob, R.; Costello, H.; Sweeney, M.G.; Duncan, A.J.; Pearce, K.; Strachan, D.; Forge, A.; Davis, A.; Bitner-Glindzicz, M. Hearing in 44-45 year olds with m.1555A>G, a genetic mutation predisposing to aminoglycoside-induced deafness: A population based cohort study. BMJ Open 2012, 2, e000411. [Google Scholar] [CrossRef]
- Subathra, M.; Ramesh, A.; Selvakumari, M.; Karthikeyen, N.P.; Srisailapathy, C.R. Genetic Epidemiology of mitochondrial pathogenic variants causing nonsyndromic hearing loss in a large cohort of south Indian hearing impaired individuals. Ann. Hum. Genet. 2016, 80, 257–273. [Google Scholar] [CrossRef]
- Prezant, T.R.; Agapian, J.V.; Bohlman, M.C.; Bu, X.; Oztas, S.; Qiu, W.Q.; Arnos, K.S.; Cortopassi, G.A.; Jaber, L.; Rotter, J.I.; et al. Mitochondrial ribosomal RNA mutation associated with both antibiotic-induced and non-syndromic deafness. Nat. Genet. 1993, 4, 289–294. [Google Scholar] [CrossRef]
- Tang, X.; Yang, L.; Zhu, Y.; Liao, Z.; Wang, J.; Qian, Y.; Tao, Z.; Hu, L.; Wu, G.; Lan, J.; et al. Very low penetrance of hearing loss in seven Han Chinese pedigrees carrying the deafness-associated 12S rRNA A1555G mutation. Gene 2007, 393, 11–19. [Google Scholar] [CrossRef]
- Guan, M.X.; Fischel-Ghodsian, N.; Attardi, G. Nuclear background determines biochemical phenotype in the deafness-associated mitochondrial 12S rRNA mutation. Hum. Mol. Genet. 2001, 10, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.X.; Fischel-Ghodsian, N.; Attardi, G. A biochemical basis for the inherited susceptibility to aminoglycoside ototoxicity. Hum. Mol. Genet. 2000, 9, 1787–1793. [Google Scholar] [CrossRef] [PubMed]
- Dillard, L.K.; Martinez, R.X.; Perez, L.L.; Fullerton, A.M.; Chadha, S.; McMahon, C.M. Prevalence of aminoglycoside-induced hearing loss in drug-resistant tuberculosis patients: A systematic review. J. Infect. 2021, 83, 27–36. [Google Scholar] [CrossRef]
- Adeyemo, A.; Faridi, R.; Chattaraj, P.; Yousaf, R.; Tona, R.; Okorie, S.; Bharadwaj, T.; Nouel-Saied, L.M.; Acharya, A.; Schrauwen, I.; et al. Genomic analysis of childhood hearing loss in the Yoruba population of Nigeria. Eur. J. Hum. Genet. 2022, 30, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Qian, Y.; Li, Z.; Yang, A.; Zhu, Y.; Li, R.; Yang, L.; Tang, X.; Chen, B.; Ding, Y.; et al. Mitochondrial haplotypes may modulate the phenotypic manifestation of the deafness-associated 12S rRNA 1555A>G mutation. Mitochondrion 2010, 10, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; He, Z.; Tang, X.; Zheng, J.; Jin, X.; Zhu, Y.; Ren, X.; Zhou, M.; Wang, M.; Gong, S.; et al. Contribution of the tRNAIle 4317A→G mutation to the phenotypic manifestation of the deafness-associated mitochondrial 12S rRNA 1555A→G mutation. J. Biol. Chem. 2018, 293, 3321–3334. [Google Scholar] [CrossRef]
- Ding, Y.; Li, Y.; You, J.; Yang, L.; Chen, B.; Lu, J.; Guan, M.X. Mitochondrial tRNA(Glu) A14693G variant may modulate the phenotypic manifestation of deafness-associated 12S rRNA A1555G mutation in a Han Chinese family. J. Genet. Genom. 2009, 36, 241–250. [Google Scholar] [CrossRef]
- Chen, B.; Sun, D.; Yang, L.; Zhang, C.; Yang, A.; Zhu, Y.; Zhao, J.; Chen, Y.; Guan, M.; Wang, X.; et al. Mitochondrial ND5 T12338C, tRNA(Cys) T5802C, and tRNA(Thr) G15927A variants may have a modifying role in the phenotypic manifestation of deafness-associated 12S rRNA A1555G mutation in three Han Chinese pedigrees. Am. J. Med. Genet. A 2008, 146A, 1248–1258. [Google Scholar] [CrossRef]
- Qi, L.; Ping, L.; Xue-jun, D.; Guo-can, Y.; Qing, W.; Yu, D. A Novel Method for Detection the Deafness-Associated Mitochondrial A1555G and C1494T Mutations. Clin. Lab. 2016, 62, 477–481. [Google Scholar] [CrossRef]
- Ding, Y.; Lang, J.; Zhang, J.; Xu, J.; Lin, X.; Lou, X.; Zheng, H.; Huai, L. Screening for deafness-associated mitochondrial 12S rRNA mutations by using a multiplex allele-specific PCR method. Biosci. Rep. 2020, 40, BSR20200778. [Google Scholar] [CrossRef]
- Kong, Q.P.; Bandelt, H.J.; Sun, C.; Yao, Y.G.; Salas, A.; Achilli, A.; Wang, C.Y.; Zhong, L.; Zhu, C.L.; Wu, S.F.; et al. Updating the East Asian mtDNA phylogeny: A prerequisite for the identification of pathogenic mutations. Hum. Mol. Genet. 2006, 15, 2076–2086. [Google Scholar] [CrossRef] [PubMed]
- Yasukawa, T.; Hino, N.; Suzuki, T.; Watanabe, K.; Ueda, T.; Ohta, S. A pathogenic point mutation reduces stability of mitochondrial mutant tRNA(Ile). Nucleic Acids Res. 2000, 28, 3779–3784. [Google Scholar] [CrossRef] [PubMed]
- Degoul, F.; Brulé, H.; Cepanec, C.; Helm, M.; Marsac, C.; Leroux, J.; Giegé, R.; Florentz, C. Isoleucylation properties of native human mitochondrial tRNAIle and tRNAIle transcripts. Implications for cardiomyopathy-related point mutations (4269, 4317) in the tRNAIle gene. Hum. Mol. Genet. 1998, 7, 347–354. [Google Scholar] [CrossRef]
- Yu, X.; Li, S.; Ding, Y. Maternally transmitted nonsyndromic hearing impairment may be associated with mitochondrial tRNAAla 5601C>T and tRNALeu(CUN) 12311T>C mutations. J. Clin. Lab. Anal. 2022, 36, e24298. [Google Scholar] [CrossRef] [PubMed]
- Mazzoli, M.; Guy Van, C.; Newton, V.; Giarbini, N. Recommendations for the Description of Genetic and Audiological Data for Families with Nonsyndromic Hereditary Hearing Impairment. Audiol. Med. 2009, 1, 148–150. [Google Scholar] [CrossRef]
- Ding, Y.; Teng, Y.S.; Zhuo, G.C.; Xia, B.H.; Leng, J.H. The Mitochondrial tRNAHis G12192A Mutation May Modulate the Clinical Expression of Deafness-Associated tRNAThr G15927A Mutation in a Chinese Pedigree. Curr. Mol. Med. 2019, 19, 136–146. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, B.; Xia, W.W.; Fang, B.; Ding, X.X.; Hu, G.W. The mitochondrial transfer RNAAsp A7551G mutation may contribute to the clinical expression of deafness associated with the A1555G mutation in a pedigree with hearing impairment. Mol. Med. Rep. 2019, 19, 1797–1802. [Google Scholar] [CrossRef] [PubMed]
- Andrews, R.M.; Kubacka, I.; Chinnery, P.F.; Lightowlers, R.N.; Turnbull, D.M.; Howell, N. Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nat. Genet. 1999, 23, 147. [Google Scholar] [CrossRef] [PubMed]
- Levin, L.; Zhidkov, I.; Gurman, Y.; Hawlena, H.; Mishmar, D. Functional recurrent mutations in the human mitochondrial phylogeny: Dual roles in evolution and disease. Genome Biol. Evol. 2013, 5, 876–890. [Google Scholar] [CrossRef] [PubMed]
- Van Oven, M.; Kayser, M. Updated comprehensive phylogenetic tree of global human mitochondrial DNA variation. Hum. Mutat. 2009, 30, E386–E394. [Google Scholar] [CrossRef]
- Yang, L.; Guo, Q.; Leng, J.; Wang, K.; Ding, Y. Late onset of type 2 diabetes is associated with mitochondrial tRNATrp A5514G and tRNASer(AGY) C12237T mutations. J. Clin. Lab. Anal. 2022, 36, e24102. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, C.; Li, M.; Zhao, Z.; Tian, S.; Xia, H.; Liu, P.; Han, Y.; Ren, R.; Chen, J.; et al. Mutation analysis of common deafness genes among 1,201 patients with non-syndromic hearing loss in Shanxi Province. Mol. Genet. Genom. Med. 2019, 7, e537. [Google Scholar] [CrossRef] [PubMed]
- King, M.P.; Attadi, G. Mitochondria-mediated transformation of human rho(0) cells. Methods Enzymol. 1996, 264, 13–34. [Google Scholar] [CrossRef]
- Ding, Y.; Xia, B.H.; Zhuo, G.C.; Zhang, C.J.; Leng, J.H. Premature ovarian insufficiency may be associated with the mutations in mitochondrial tRNA genes. Endocr. J. 2019, 6, 81–88. [Google Scholar] [CrossRef]
- Perelman, A.; Wachtel, C.; Cohen, M.; Haupt, S.; Shapiro, H.; Tzur, A. JC-1: Alternative excitation wavelengths facilitate mitochondrial membrane potential cytometry. Cell Death Dis. 2012, 3, e430. [Google Scholar] [CrossRef]
- Reers, M.; Smiley, S.T.; Mottola-Hartshorn, C.; Chen, A.; Lin, M.; Chen, L.B. Mitochondrial membrane potential monitored by JC-1 dye. Methods Enzymol. 1995, 260, 406–417. [Google Scholar] [CrossRef]
- Ding, Y.; Xia, B.H.; Zhang, C.J.; Zhuo, G.C. Mitochondrial tRNALeu(UUR) C3275T, tRNAGln T4363C and tRNALys A8343G mutations may be associated with PCOS and metabolic syndrome. Gene 2018, 642, 299–306. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, Q.; Xiong, J.; Lou, X.; Han, Q.; Wei, X.; Xie, J.; Li, X.; Zhou, H.; Shen, L.; et al. Systematic analysis of a mitochondrial disease-causing ND6 mutation in mitochondrial deficiency. Mol. Genet. Genom. Med. 2020, 8, e1199. [Google Scholar] [CrossRef]
- Chen, J.W.; Ma, P.W.; Yuan, H.; Wang, W.L.; Lu, P.H.; Ding, X.R.; Lun, Y.Q.; Yang, Q.; Lu, L.J. mito-TEMPO Attenuates Oxidative Stress and Mitochondrial Dysfunction in Noise-Induced Hearing Loss via Maintaining TFAM-mtDNA Interaction and Mitochondrial Biogenesis. Front. Cell. Neurosci. 2022, 16, 803718. [Google Scholar] [CrossRef]
- Yarham, J.W.; Al-Dosary, M.; Blakely, E.L.; Alston, C.L.; Taylor, R.W.; Elson, J.L.; McFarland, R. A comparative analysis approach to determining the pathogenicity of mitochondrial tRNA mutations. Hum. Mutat. 2011, 32, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Gadaleta, G.; Pepe, G.; De Candia, G.; Quagliariello, C.; Sbisà, E.; Saccone, C. The complete nucleotide sequence of the Rattus norvegicus mitochondrial genome: Cryptic signals revealed by comparative analysis between vertebrates. J. Mol. Evol. 1989, 28, 497–516. [Google Scholar] [CrossRef] [PubMed]
- Bibb, M.J.; Van Etten, R.A.; Wright, C.T.; Walberg, M.W.; Clayton, D.A. Sequence and gene organization of mouse mitochondrial DNA. Cell 1981, 26, 167–180. [Google Scholar] [CrossRef]
- Roe, B.A.; Ma, D.P.; Wilson, R.K.; Wong, J.F. The complete nucleotide sequence of the Xenopus laevis mitochondrial genome. J. Biol. Chem. 1985, 260, 9759–9774. [Google Scholar] [CrossRef]
- Esmekaya, M.A.; Canseven, A.G.; Kayhan, H.; Tuysuz, M.Z.; Sirav, B.; Seyhan, N. Mitochondrial hyperpolarization and cytochrome-c release in microwave-exposed MCF-7 cells. Gen. Physiol. Biophys. 2017, 36, 211–218. [Google Scholar] [CrossRef]
- Sena, L.A.; Chandel, N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell. 2012, 48, 158–167. [Google Scholar] [CrossRef]
- Castellani, C.A.; Longchamps, R.J.; Sun, J.; Guallar, E.; Arking, D.E. Thinking outside the nucleus: Mitochondrial DNA copy number in health and disease. Mitochondrion 2020, 53, 214–223. [Google Scholar] [CrossRef]
- Qian, Y.; Guan, M.X. Interaction of aminoglycosides with human mitochondrial 12S rRNA carrying the deafness-associated mutation. Antimicrob. Agents Chemother. 2009, 53, 4612–4618. [Google Scholar] [CrossRef]
- Chen, C.; Chen, Y.; Guan, M.X. A peep into mitochondrial disorder: Multifaceted from mitochondrial DNA mutations to nuclear gene modulation. Protein Cell 2015, 6, 862–870. [Google Scholar] [CrossRef]
- Fischel-Ghodsian, N. Genetic factors in aminoglycoside toxicity. Pharmacogenomics 2005, 6, 27–36. [Google Scholar] [CrossRef]
- Guan, M.X.; Fischel-Ghodsian, N.; Attardi, G. Biochemical evidence for nuclear gene involvement phenotype of non-syndromic deafness associated with mitochondrial 12 S rRNA mutation. Hum. Mol. Genet. 1996, 5, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Wang, Z.; Dai, W.; Li, Q.; Chen, G.; Cong, N.; Guan, M.; Li, H. A six-generation Chinese family in haplogroup B4C1C exhibits high penetrance of 1555A > G-induced hearing Loss. BMC Med. Genet. 2010, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Ying, Z.; Zheng, J.; Cai, Z.; Liu, L.; Dai, Y.; Yao, J.; Wang, H.; Gao, Y.; Zheng, B.; Tang, X.; et al. Mitochondrial haplogroup B increases the risk for hearing loss among the Eastern Asian pedigrees carrying 12S rRNA 1555A>G mutation. Protein Cell 2015, 6, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Liu, Q.; Jiang, L.; Liu, C.; Ou, Q. Mitochondrial COX2 G7598A mutation may have a modifying role in the phenotypic manifestation of aminoglycoside antibiotic-induced deafness associated with 12S rRNA A1555G mutation in a Han Chinese pedigree. Genet. Test Mol. Biomark. 2013, 17, 122–130. [Google Scholar] [CrossRef]
- Merante, F.; Tein, I.; Benson, L.; Robinson, B.H. Maternally inherited hypertrophic cardiomyopathy due to a novel T-to-C transition at nucleotide 9997 in the mitochondrial tRNA(glycine) gene. Am. J. Hum. Genet. 1994, 55, 437–446. [Google Scholar]
- Kaido, M.; Fujimura, H.; Taniike, M.; Yoshikawa, H.; Toyooka, K.; Yorifuji, S.; Inui, K.; Okada, S.; Sparaco, M.; Yanagihara, T. Focal cytochrome c oxidase deficiency in the brain and dorsal root ganglia in a case with mitochondrial encephalomyopathy (tRNA(Ile) 4269 mutation): Histochemical, immunohistochemical, and ultrastructural study. J. Neurol. Sci. 1995, 131, 170–176. [Google Scholar] [CrossRef]
- Houstek, J.; Klement, P.; Floryk, D.; Antonická, H.; Hermanská, J.; Kalous, M.; Hansíková, H.; Hout’ková, H.; Chowdhury, S.K.; Rosipal, T.; et al. A novel deficiency of mitochondrial ATPase of nuclear origin. Hum. Mol. Genet. 1999, 8, 1967–1974. [Google Scholar] [CrossRef][Green Version]
- Mohamed, M.S.; Abdelhamid, A.O.; Almutairi, F.M.; Ali, A.G.; Bishr, M.K. Induction of apoptosis by pyrazolo[3,4-d]pyridazine derivative in lung cancer cells via disruption of Bcl-2/Bax expression balance. Bioorg. Med. Chem. 2018, 26, 623–629. [Google Scholar] [CrossRef]
- Pfanner, N.; Craig, E.A.; Meijer, M. The protein import machinery of the mitochondrial inner membrane. Trends Biochem. Sci. 1994, 19, 368–372. [Google Scholar] [CrossRef]
- Yamaki, M.; Umehara, T.; Chimura, T.; Horikoshi, M. Cell death with predominant apoptotic features in Saccharomyces cerevisiae mediated by deletion of the histone chaperone ASF1/CIA1. Genes Cells 2001, 6, 1043–1054. [Google Scholar] [CrossRef]
- Clay Montier, L.L.; Deng, J.J.; Bai, Y. Number matters: Control of mammalian mitochondrial DNA copy number. J. Genet. Genom. 2009, 36, 125–131. [Google Scholar] [CrossRef]
- Fontana, G.A.; Gahlon, H.L. Mechanisms of replication and repair in mitochondrial DNA deletion formation. Nucleic Acids Res. 2020, 48, 11244–11258. [Google Scholar] [CrossRef] [PubMed]
- Mao, M.; Zhang, T.; Wang, Z.; Wang, H.; Xu, J.; Yin, F.; Wang, G.; Sun, M.; Wang, Z.; Hua, Y.; et al. Glaucocalyxin A-induced oxidative stress inhibits the activation of STAT3 signaling pathway and suppresses osteosarcoma progression in vitro and in vivo. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1214–1225. [Google Scholar] [CrossRef] [PubMed]
- D’Autréaux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Brieger, K.; Schiavone, S.; Miller, F.J., Jr.; Krause, K.H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef]
- Wang, M.; Liu, H.; Zheng, J.; Chen, B.; Zhou, M.; Fan, W.; Wang, H.; Liang, X.; Zhou, X.; Eriani, G.; et al. A Deafness- and Diabetes-associated tRNA Mutation Causes Deficient Pseudouridinylation at Position 55 in tRNAGlu and Mitochondrial Dysfunction. J. Biol. Chem. 2016, 291, 21029–21041. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).