The Association between the ALDH2 rs671 Polymorphism and Athletic Performance in Japanese Power and Strength Athletes
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Athletic Performance
2.3. Genotyping
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Folland, P.J.; Williams, A.G. The Adaptations to Strength Training: Morphological and Neurological Contributions to Increased Strength. Sports Med. 2007, 37, 145–168. [Google Scholar] [CrossRef] [PubMed]
- Fry, A.C.; Webber, J.M.; Weiss, L.W.; Harber, M.P.; Vaczi, M.; Pattison, N.A. Muscle Fiber Characteristics of Competitive Power Lifters. J. Strength Cond. Res. 2003, 17, 402–410. [Google Scholar] [PubMed]
- Fry, A.C.; Schilling, B.K.; Staron, R.S.; Hagerman, F.C.; Hikida, R.S.; Thrush, J.T. Muscle Fiber Characteristics and Performance Correlates of Male Olympic-Style Weightlifters. J. Strength Cond. Res. 2003, 17, 746–754. [Google Scholar] [PubMed]
- Noriyuki, F.; Kumagai, H.; Ahmetov, I.I. Chapter Fourteen—Genetics of Muscle Fiber Composition. In Sports, Exercise, and Nutritional Genomics; Debmalya, B., Ahmetov, I.I., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 295–314. [Google Scholar]
- Storey, A.; Smith, H.K. Unique Aspects of Competitive Weightlifting: Performance, Training and Physiology. Sports Med. 2012, 42, 769–790. [Google Scholar] [CrossRef] [PubMed]
- Ahmetov, O.L.; Vinogradova, I.I.; Williams, A.G. Gene Polymorphisms and Fiber-Type Composition of Human Skeletal Muscle. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 292–303. [Google Scholar] [CrossRef]
- Stepto, N.K.; Coffey, V.G.; Carey, A.L.; Ponnampalam, A.P.; Canny, B.J.; Powell, D.; Hawley, J.A. Global Gene Expression in Skeletal Muscle from Well-Trained Strength and Endurance Athletes. Med. Sci. Sports Exerc. 2009, 41, 546–565. [Google Scholar] [CrossRef]
- Zempo, H.; Miyamoto-Mikami, E.; Kikuchi, N.; Fuku, N.; Miyachi, M.; Murakami, H. Heritability Estimates of Muscle Strength-Related Phenotypes: A Systematic Review and Meta-Analysis. Scand. J. Med. Sci. Sports 2017, 27, 1537–1546. [Google Scholar] [CrossRef]
- Roth, S.M. Genetic Aspects of Skeletal Muscle Strength and Mass with Relevance to Sarcopenia. Bonekey Rep. 2012, 1, 58. [Google Scholar] [CrossRef]
- Maciejewska-Skrendo, A.; Cieszczyk, P.; Chycki, J.; Sawczuk, M.; Smolka, W. Genetic Markers Associated with Power Athlete Status. J. Hum. Kinet. 2019, 68, 17–36. [Google Scholar] [CrossRef]
- Weyerstraß, J.; Stewart, K.; Wesselius, A.; Zeegers, M. Nine Genetic Polymorphisms Associated with Power Athlete Status—A Meta-Analysis. J. Sci. Med. Sport 2018, 21, 213–220. [Google Scholar] [CrossRef]
- Xiao, Q.; Weiner, H.; Crabb, D.W. The Mutation in the Mitochondrial Aldehyde Dehydrogenase (Aldh2) Gene Responsible for Alcohol-Induced Flushing Increases Turnover of the Enzyme Tetramers in a Dominant Fashion. J. Clin. Investig. 1996, 98, 2027–2032. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Zheng, W.; Okada, Y.; Takeuchi, F.; Tabara, Y.; Hwang, J.Y.; Dorajoo, R.; Li, H.; Tsai, F.J.; Yang, X.; et al. Meta-Analysis of Genome-Wide Association Studies in East Asian-Ancestry Populations Identifies Four New Loci for Body Mass Index. Hum. Mol. Genet. 2014, 23, 5492–5504. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ni, J.; Cai, X.; Lian, F.; Ma, H.; Xu, L.; Yang, L. Positive Association between Aldh2 Rs671 Polymorphism and Essential Hypertension: A Case-Control Study and Meta-Analysis. PLoS ONE 2017, 12, e0177023. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.; Zhan, Z.; Ma, L.; Bai, W.; Zeng, S. Effect of Aldh2 Polymorphism on Cancer Risk in Asians: A Meta-Analysis. Medicine 2019, 98, e14855. [Google Scholar] [CrossRef]
- Wakabayashi, Y.; Tamura, Y.; Kouzaki, K.; Kikuchi, N.; Hiranuma, K.; Menuki, K.; Tajima, T.; Yamanaka, Y.; Sakai, A.; Nakayama, K.I.; et al. Acetaldehyde Dehydrogenase 2 Deficiency Increases Mitochondrial Reactive Oxygen Species Emission and Induces Mitochondrial Protease Omi/Htra2 in Skeletal Muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R677–R690. [Google Scholar] [CrossRef]
- Kikuchi, N.; Tajima, T.; Tamura, Y.; Yamanaka, Y.; Menuki, K.; Okamoto, T.; Sakamaki-Sunaga, M.; Sakai, A.; Hiranuma, K.; Nakazato, K. The Aldh2 Rs671 Polymorphism Is Associated with Athletic Status and Muscle Strength in a Japanese Population. Biol. Sport 2022, 39, 429–434. [Google Scholar] [CrossRef]
- Bloomer, R.J.; Goldfarb, A.H. Anaerobic Exercise and Oxidative Stress: A Review. Can. J. Appl. Physiol. 2004, 29, 245–263. [Google Scholar] [CrossRef]
- Jurgenson, J.; Serg, M.; Kampus, P.; Kals, J.; Zagura, M.; Viru, M.; Zilmer, K.; Zilmer, M.; Eha, J.; Unt, E. Oxidative Stress Parameters and Its Associations with Arterial Stiffness in Competitive Powerlifting Athletes after 12-Week Supervised Strength Training. J. Strength Cond. Res. 2019, 33, 1816–1822. [Google Scholar] [CrossRef]
- Liu, J.F.; Chang, W.Y.; Chan, K.H.; Tsai, W.Y.; Lin, C.L.; Hsu, M.C. Blood Lipid Peroxides and Muscle Damage Increased Following Intensive Resistance Training of Female Weightlifters. Ann. N. Y. Acad. Sci. 2005, 1042, 255–261. [Google Scholar] [CrossRef]
- Ahmetov, I.I.; Naumov, V.A.; Donnikov, A.E.; Maciejewska-Karlowska, A.; Kostryukova, E.S.; Larin, A.K.; Maykova, E.V.; Alexeev, D.G.; Fedotovskaya, O.N.; Generozov, E.V.; et al. Sod2 Gene Polymorphism and Muscle Damage Markers in Elite Athletes. Free Radic. Res. 2014, 48, 948–955. [Google Scholar] [CrossRef]
- Romanello, V.; Sandri, M. Mitochondrial Quality Control and Muscle Mass Maintenance. Front. Physiol. 2015, 6, 422. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Nakamura, S.; Sato, Y.; Kobayashi, T.; Miyamoto, K.; Oya, A.; Matsumoto, M.; Nakamura, M.; Kanaji, A.; Miyamoto, T. Aldh2 Mutation Promotes Skeletal Muscle Atrophy in Mice Via Accumulation of Oxidative Stress. Bone 2021, 142, 115739. [Google Scholar] [CrossRef] [PubMed]
- Kasai, A.; Jee, E.; Tamura, Y.; Kouzaki, K.; Kotani, T.; Nakazato, K. Aldehyde Dehydrogenase 2 Deficiency Promotes Skeletal Muscle Atrophy in Aged Mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2022, 322, R511–R525. [Google Scholar] [CrossRef]
- Moreland, E.; Borisov, O.V.; Semenova, E.A.; Larin, A.K.; Andryushchenko, O.N.; Andryushchenko, L.B.; Generozov, E.V.; Williams, A.G.; Ahmetov, I.I. Polygenic Profile of Elite Strength Athletes. J. Strength Cond. Res. 2020, 36, 2509–2514. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.C.; Keiller, D.R.; Roberts, J.D.; Gordon, D.A. Do Exercise-Associated Genes Explain Phenotypic Variance in the Three Components of Fitness? A Systematic Review & Meta-Analysis. PLoS ONE 2021, 16, e0249501. [Google Scholar]
- Kregel, K.C.; Zhang, H.J. An Integrated View of Oxidative Stress in Aging: Basic Mechanisms, Functional Effects, and Pathological Considerations. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2007, 292, R18–R36. [Google Scholar] [CrossRef]
- Guilherme, J.P.L.F.; Semenova, E.A.; Borisov, O.V.; Larin, A.K.; Moreland, E.; Generozov, E.V.; Ahmetov, I.I. Genomic Predictors of Testosterone Levels Are Associated with Muscle Fiber Size and Strength. Eur. J. Appl. Physiol. 2022, 122, 415–423. [Google Scholar] [CrossRef]
- Lin, C.L.; Chien, R.N.; Chen, L.W.; Huang, T.S.; Shyu, Y.C.; Yeh, C.T.; Liang, K.H. The Aldehyde Dehydrogenase Aldh2*2 Allele, Associated with Alcohol Drinking Behavior, Dates Back to Prehistoric Times. Biomolecules 2021, 11, 1376. [Google Scholar] [CrossRef]
- Otis, J.S.; Brown, L.A.; Guidot, D.M. Oxidant-Induced Atrogin-1 and Transforming Growth Factor-Beta1 Precede Alcohol-Related Myopathy in Rats. Muscle Nerve 2007, 36, 842–848. [Google Scholar] [CrossRef]
- Caceres-Ayala, C.; Pautassi, R.M.; Acuña, M.J.; Cerpa, W.; Rebolledo, D.L. The Functional and Molecular Effects of Problematic Alcohol Consumption on Skeletal Muscle: A Focus on Athletic Performance. Am. J. Drug Alcohol Abuse 2022, 48, 133–147. [Google Scholar] [CrossRef]
| Genotype | Allele | p Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | GG | GA | AA | G | A | Genotype | Dominant | Recessive | Allele | |
| n (%) | n (%) | n (%) | n (%) | n (%) | ||||||
| All power/strength athletes | 253 | 140 (56) | 93 (38) | 20 (6) | 532 (75) | 182 (25) | 0.688 | 0.502 | 0.465 | 0.384 |
| Powerlifters | 84 | 50 (60) | 28 (33) | 6 (7) | 128 (76) | 40 (24) | 0.929 | 0.866 | 0.785 | 0.879 |
| Weightlifters | 169 | 90 (53) | 65 (39) | 14 (8) | 245 (73) | 93 (27) | 0.491 | 0.454 | 0.264 | 0.224 |
| controls | 721 | 418 (58) | 255 (35) | 48 (7) | 1091 (76) | 351 (24) | ||||
| GG (n = 50) | GA (n = 28) | AA (n = 6) | GA + AA (n = 34) | p Value (Genotype) | ES (Genotype) | p Value (Recessive) | ES (Recessive) | |
|---|---|---|---|---|---|---|---|---|
| Sex | ||||||||
| Men | n = 44 (88%) | n = 22 (79%) | n = 5 (83%) | n = 27 (79%) | 0.541 * | 0.285 * | ||
| Women | n = 6 (12%) | n = 6 (21%) | n = 1 (17%) | n = 7 (21%) | ||||
| Athletic status | ||||||||
| International | n = 16 (32%) | n = 12 (43%) | n = 1 (17%) | n = 13 (38%) | 0.158 * | 0.112 * | ||
| National | n = 25 (50%) | n = 16 (57%) | n = 4 (66%) | n = 20 (59%) | ||||
| Regional | n = 9 (18%) | n = 0 (0%) | n = 1 (17%) | n = 1 (3%) | ||||
| Age, yr | 28.8 ± 7.7 | 29.9 ± 12.1 | 30.1 ± 5.3 | 30.0 ± 11.2 | 0.841 | 0.004 | 0.556 | 0.004 |
| Height, cm | 167.2 ± 7.1 | 166.6 ± 8.1 | 168.3 ± 10.2 | 166.9 ± 8.4 | 0.871 | 0.003 | 0.873 | 0.0003 |
| Body mass, kg | 76.2 ± 17.3 | 78.2 ± 21.1 | 82.9 ± 26.0 | 79.1 ± 21.7 | 0.691 | 0.009 | 0.503 | 0.005 |
| Powerlifting experience, yr | 4.9 ± 5.0 | 4.5 ± 5.2 | 5.7 ± 6.4 | 4.8 ± 5.4 | 0.862 | 0.004 | 0.890 | 0.0002 |
| Squat, kg | 211.9 ± 47.6 | 196.1 ± 54.1 | 216.2 ± 59.2 | 199.7 ± 54.7 | 0.553 † | 0.02 | 0.665 † | 0.01 |
| Bench press, kg | 145.1 ± 35.6 | 129.6 ± 38.1 | 159.5 ± 51.4 | 134.9 ± 41.6 | 0.049† | 0.05 | 0.554 † | 0.01 |
| Deadlift, kg | 236.4 ± 43.1 | 220.4 ± 50.6 | 225.8 ± 50.3 | 221.4 ± 49.9 | 0.620 † | 0.02 | 0.313 † | 0.02 |
| Squat, kg/BW | 2.7 ± 0.3 | 2.5 ± 0.4 | 2.6 ± 0.5 | 2.5 ± 0.4 | 0.032† | 0.09 | 0.011† | 0.08 |
| Bench press, kg/BW | 1.9 ± 0.3 | 1.6 ± 0.3 | 1.9 ± 0.5 | 1.7 ± 0.4 | 0.025† | 0.09 | 0.047† | 0.06 |
| Deadlift, kg/BW | 3.1 ± 0.4 | 2.8 ± 0.5 | 2.8 ± 0.5 | 2.8 ± 0.5 | 0.071† | 0.07 | 0.023† | 0.07 |
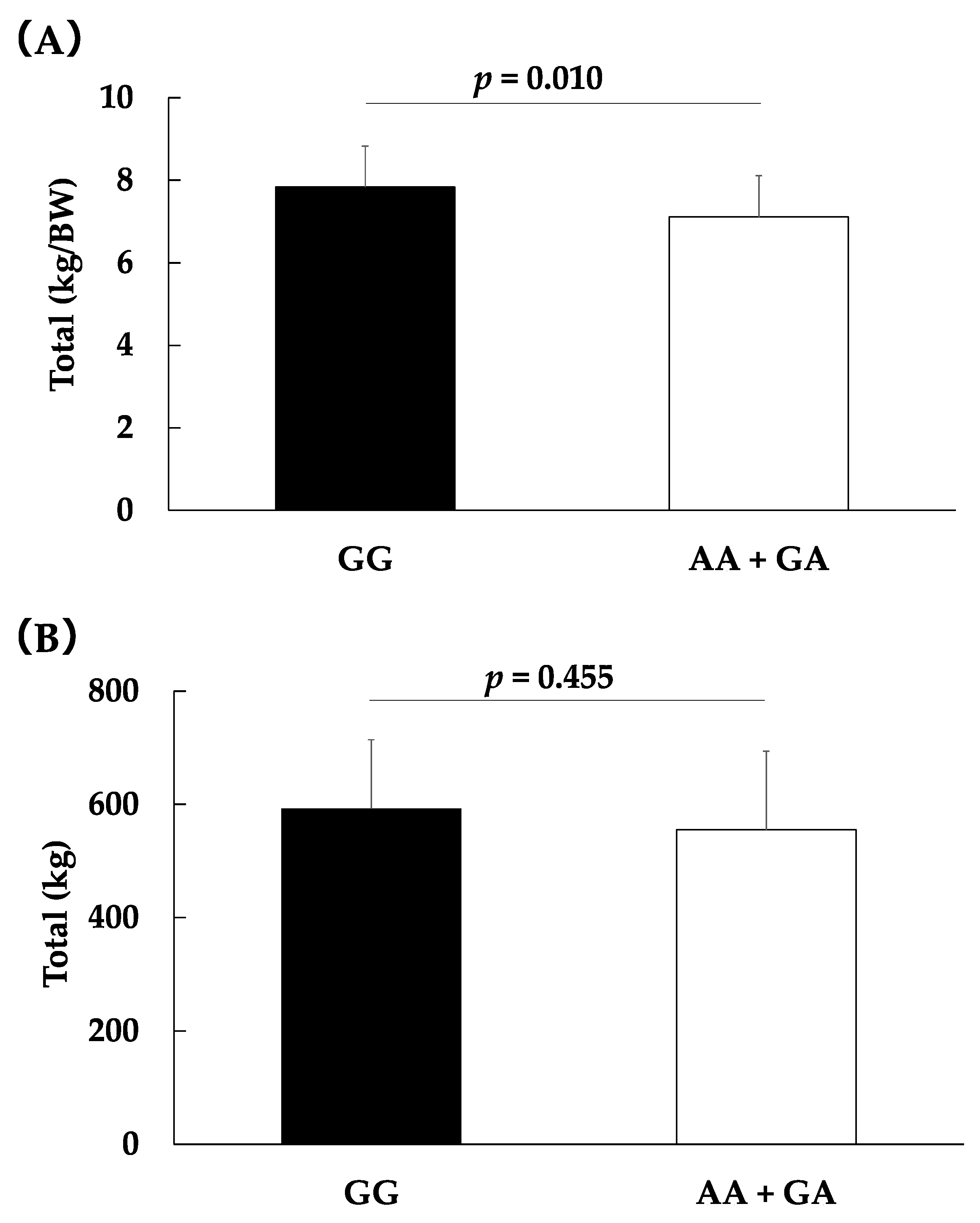
| Total, kg | 592.7 ± 121.7 | 545.6 ± 137.6 | 600.8 ± 146.2 | 555.3 ± 138.5 | 0.363 | 0.03 | 0.455 | 0.02 |
| Total, kg/BW | 7.8 ± 1.0 | 7.0 ± 1.2 | 7.4 ± 1.3 | 7.1 ± 1.2 | 0.031† | 0.09 | 0.011† | 0.08 |
| GG (n = 90) | GA (n = 65) | AA (n = 14) | GA + AA (n = 79) | p Value (Genotype) | ES (Genotype) | p Value (Recessive) | ES (Recessive) | |
|---|---|---|---|---|---|---|---|---|
| Sex | ||||||||
| Men | n = 53 (59%) | n = 37 (57%) | n = 6 (43%) | n = 43 (54%) | 0.530 * | 0.559 * | ||
| Women | n = 37 (41%) | n = 28 (43%) | n = 8 (57%) | n = 36 (46%) | ||||
| Athletic status | ||||||||
| International | n = 34 (38%) | n = 24 (37%) | n = 5 (36%) | n = 29 (37%) | 0.999 * | 0.973 * | ||
| National | n = 39 (43%) | n = 28 (43%) | n = 6 (43%) | n = 34 (43%) | ||||
| Regional | n = 17 (19%) | n = 13 (20%) | n = 3 (21%) | n = 16 (20%) | ||||
| Age, yr | 20.8 ± 3.1 | 21.0 ± 4.5 | 20.9 ± 4.0 | 21.0 ± 4.4 | 0.902 | 0.001 | 0.666 | 0.001 |
| Height, cm | 163.4 ± 8.5 | 161.5 ± 8.7 | 162.6 ± 9.2 | 161.7 ± 8.7 | 0.406 | 0.01 | 0.201 | 0.01 |
| Body mass, kg | 72.7 ± 20.0 | 71.4 ± 19.2 | 67.9 ± 14.4 | 70.8 ± 18.4 | 0.673 | 0.005 | 0.520 | 0.002 |
| weightlifting experience, yr | 5.7 ± 2.6 | 6.1 ± 3.7 | 5.9 ± 3.8 | 6.1 ± 3.6 | 0.695 | 0.004 | 0.411 | 0.004 |
| Snatch, kg | 103.6 ± 29.1 | 99.7 ± 27.0 | 94.6 ± 21.4 | 98.8 ± 26.0 | 0.570 † | 0.01 | 0.294 † | 0.008 |
| Clean and Jerk, kg | 128.4 ± 34.3 | 125.3 ± 32.4 | 116.2 ± 25.0 | 123.7 ± 31.3 | 0.726 † | 0.01 | 0.458 † | 0.005 |
| Snatch, kg/BW | 1.4 ± 0.2 | 1.4 ± 0.3 | 1.4 ± 0.2 | 1.4 ± 0.2 | 0.873 † | 0.002 | 0.753 † | 0.002 |
| Clean and Jerk, kg/BW | 1.7 ± 0.3 | 1.7 ± 0.3 | 1.7 ± 0.3 | 1.7 ± 0.3 | 0.995 † | 0.001 | 0.922 † | 0.0001 |
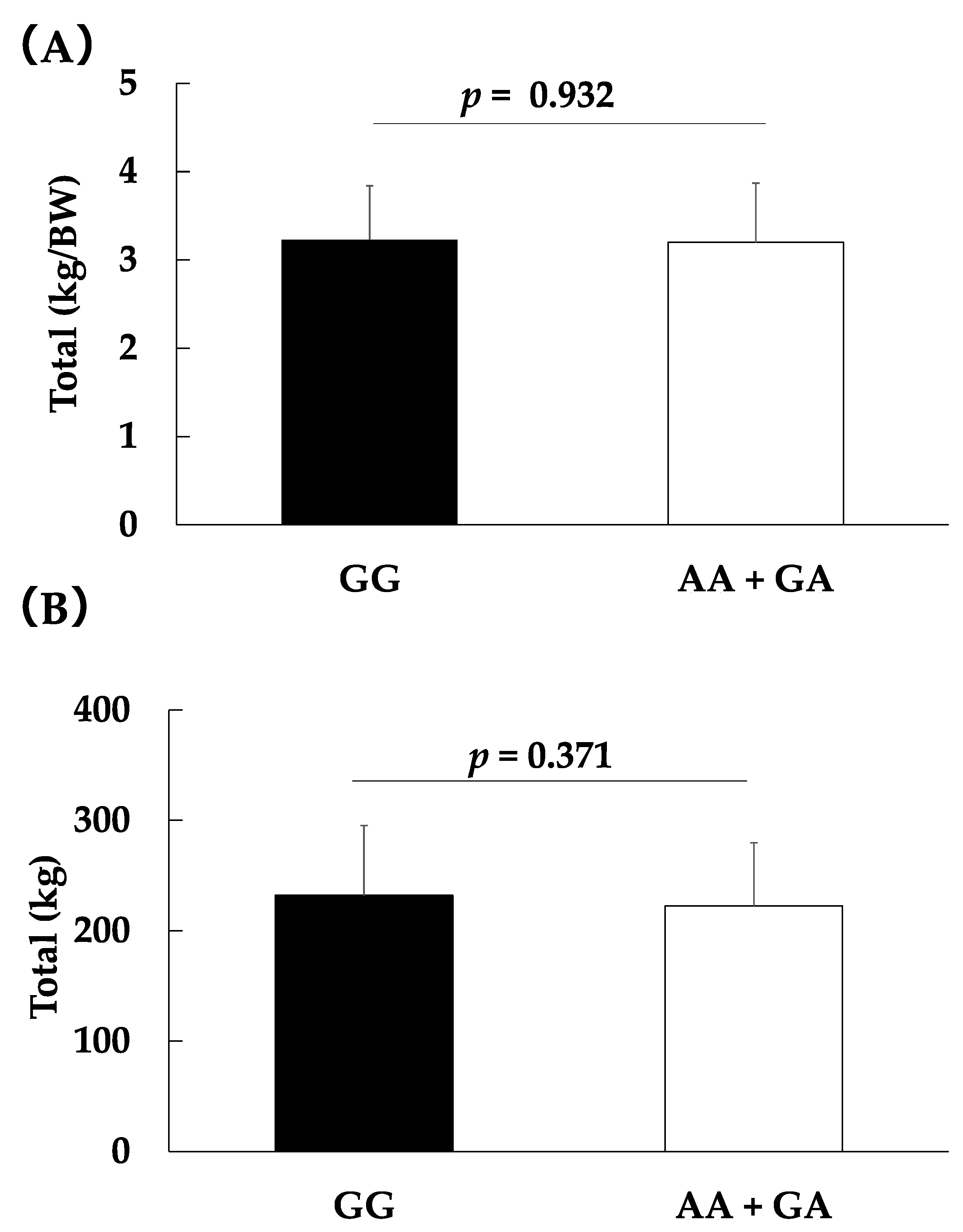
| Total, kg | 232.1 ± 63.2 | 225.1 ± 59.1 | 210.2 ± 25.0 | 222.6 ± 57.0 | 0.669 † | 0.01 | 0.371 † | 0.006 |
| Total, kg/BW | 3.2 ± 0.6 | 3.2 ± 0.6 | 3.1 ± 0.6 | 3.2 ± 0.6 | 0.928 † | 0.001 | 0.932 † | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saito, A.; Saito, M.; Almeida, K.Y.d.; Homma, H.; Deguchi, M.; Kozuma, A.; Kobatake, N.; Okamoto, T.; Nakazato, K.; Kikuchi, N. The Association between the ALDH2 rs671 Polymorphism and Athletic Performance in Japanese Power and Strength Athletes. Genes 2022, 13, 1735. https://doi.org/10.3390/genes13101735
Saito A, Saito M, Almeida KYd, Homma H, Deguchi M, Kozuma A, Kobatake N, Okamoto T, Nakazato K, Kikuchi N. The Association between the ALDH2 rs671 Polymorphism and Athletic Performance in Japanese Power and Strength Athletes. Genes. 2022; 13(10):1735. https://doi.org/10.3390/genes13101735
Chicago/Turabian StyleSaito, Aoto, Mika Saito, Kathleen Y. de Almeida, Hiroki Homma, Minoru Deguchi, Ayumu Kozuma, Naoyuki Kobatake, Takanobu Okamoto, Koichi Nakazato, and Naoki Kikuchi. 2022. "The Association between the ALDH2 rs671 Polymorphism and Athletic Performance in Japanese Power and Strength Athletes" Genes 13, no. 10: 1735. https://doi.org/10.3390/genes13101735
APA StyleSaito, A., Saito, M., Almeida, K. Y. d., Homma, H., Deguchi, M., Kozuma, A., Kobatake, N., Okamoto, T., Nakazato, K., & Kikuchi, N. (2022). The Association between the ALDH2 rs671 Polymorphism and Athletic Performance in Japanese Power and Strength Athletes. Genes, 13(10), 1735. https://doi.org/10.3390/genes13101735







