Transcriptomic Analysis of the Pistacia vera (L.) Fruits Enable the Identification of Genes and Hormone-Related Gene Linked to Inflorescence Bud Abscission
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection, Sequencing, and Pre-Processing
2.2. Transcriptomic Analysis, Annotation, and Evaluation
2.3. Differentially Expressed Genes (DEGs) between “ON” and “OFF”
2.4. Functional and Gene Enrichment Analysis
- (1)
- Nearly identical: Score ≥ 1000 and e-value = 0.
- (2)
- Highly similar: Score ≥ 1000 and e-value ≠ 0 OR (Score ≥ 500 & Score < 1000) and e-value = 0.
- (3)
- Moderately similar: (Score ≥ 200 & Score < 1000) and e-value ≠ 0.
- (4)
- Weakly similar: (Score ≠ 100 & score < 200).
- (5)
- Very weakly similar: (Score < 100) based on the blastx score and e-value.
3. Results
3.1. Transcriptomic Assembly and Annotation Results
3.2. Effect of Crop Load on Photosynthesis in Fruits of “OFF” vs. “ON” Branches
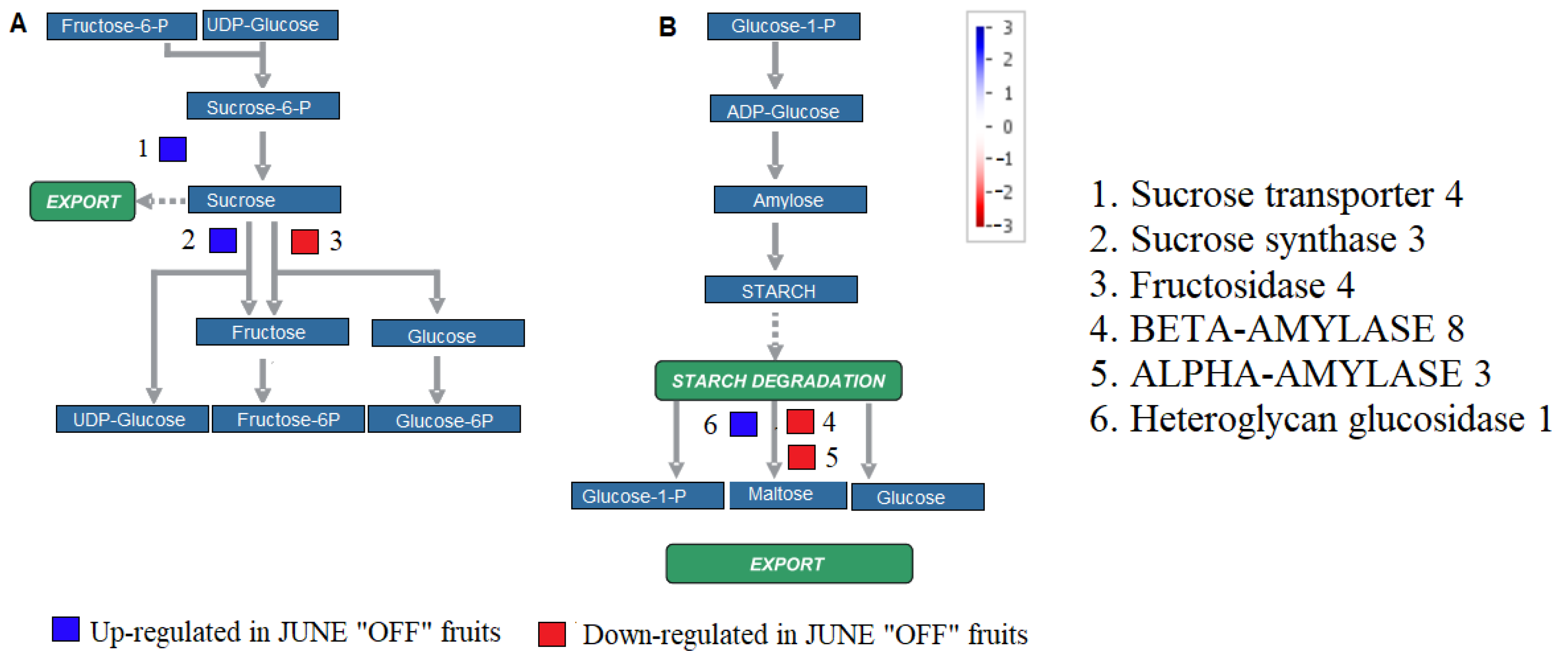
3.3. Effect of Crop Load on Starch Metabolism in Fruits of “OFF” vs. “ON” Branches
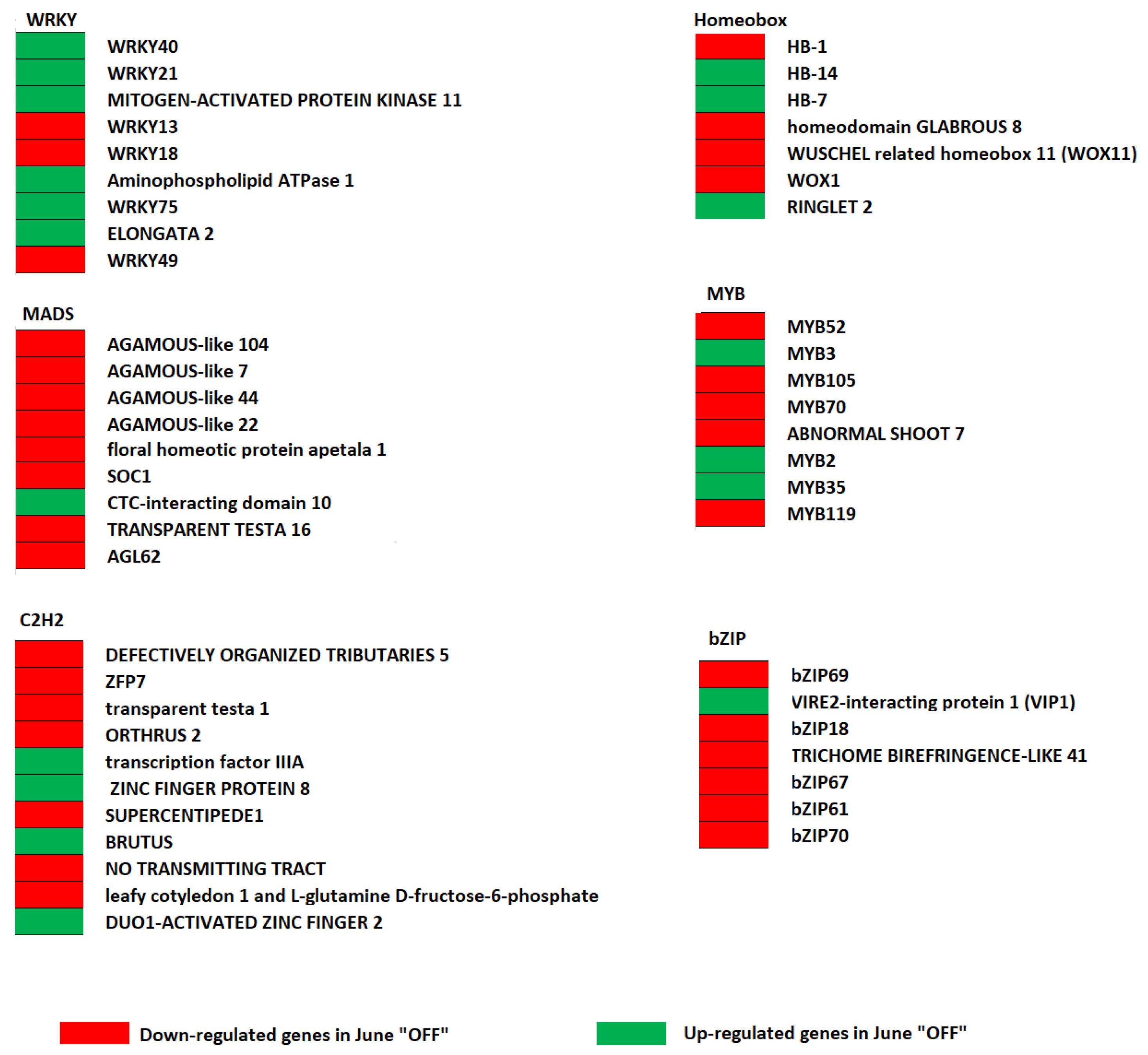
3.4. Effect of Crop Load Status on Transcription Factors in Fruits of “OFF” vs. “ON” Branches
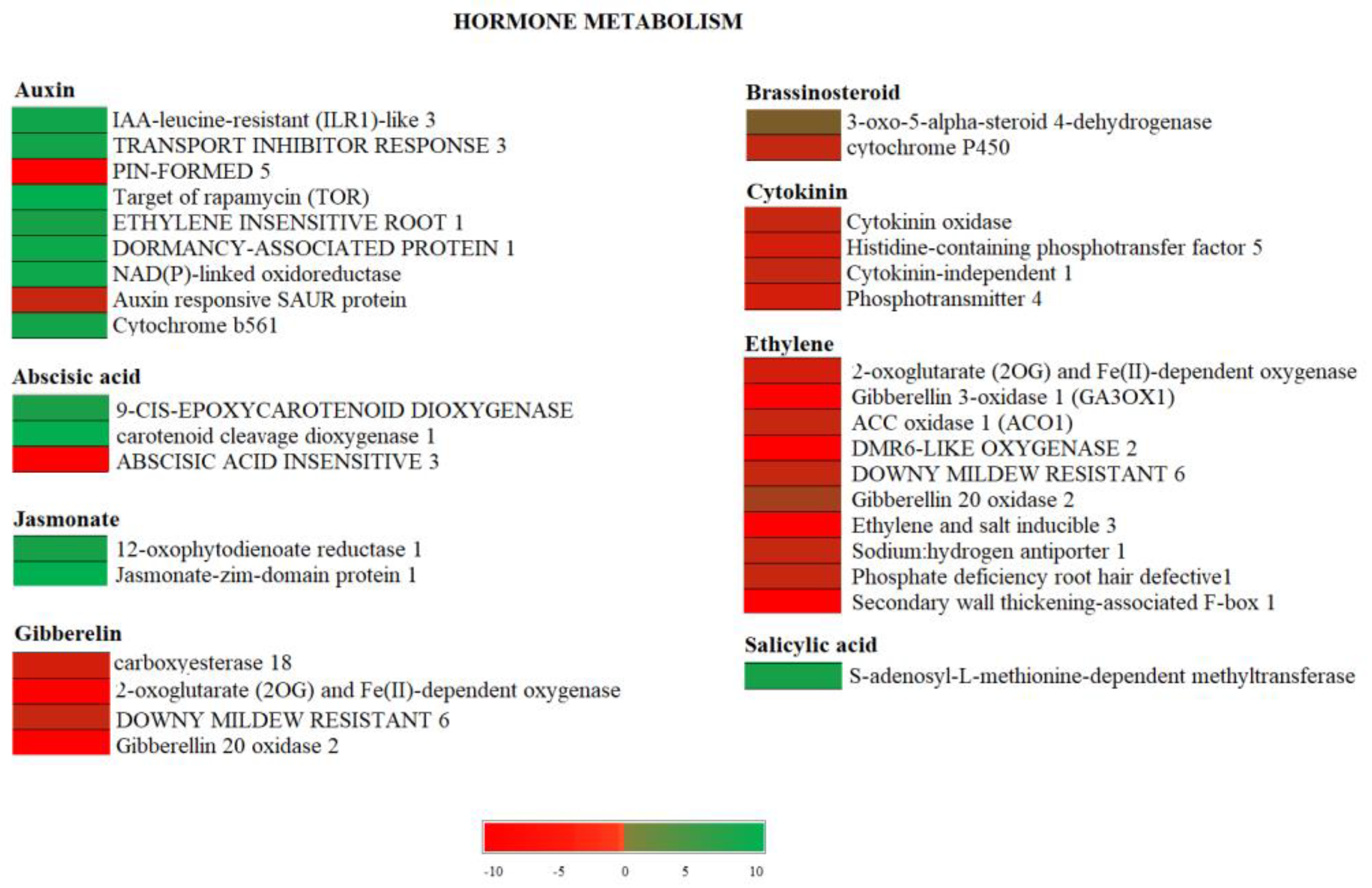
3.5. Effect of Crop Load on Hormone Metabolism in Fruits of “OFF” vs. “ON” Branches
3.6. Effect of Crop Load Status on Polyamines in Fruits of “OFF” vs. “ON” Branches
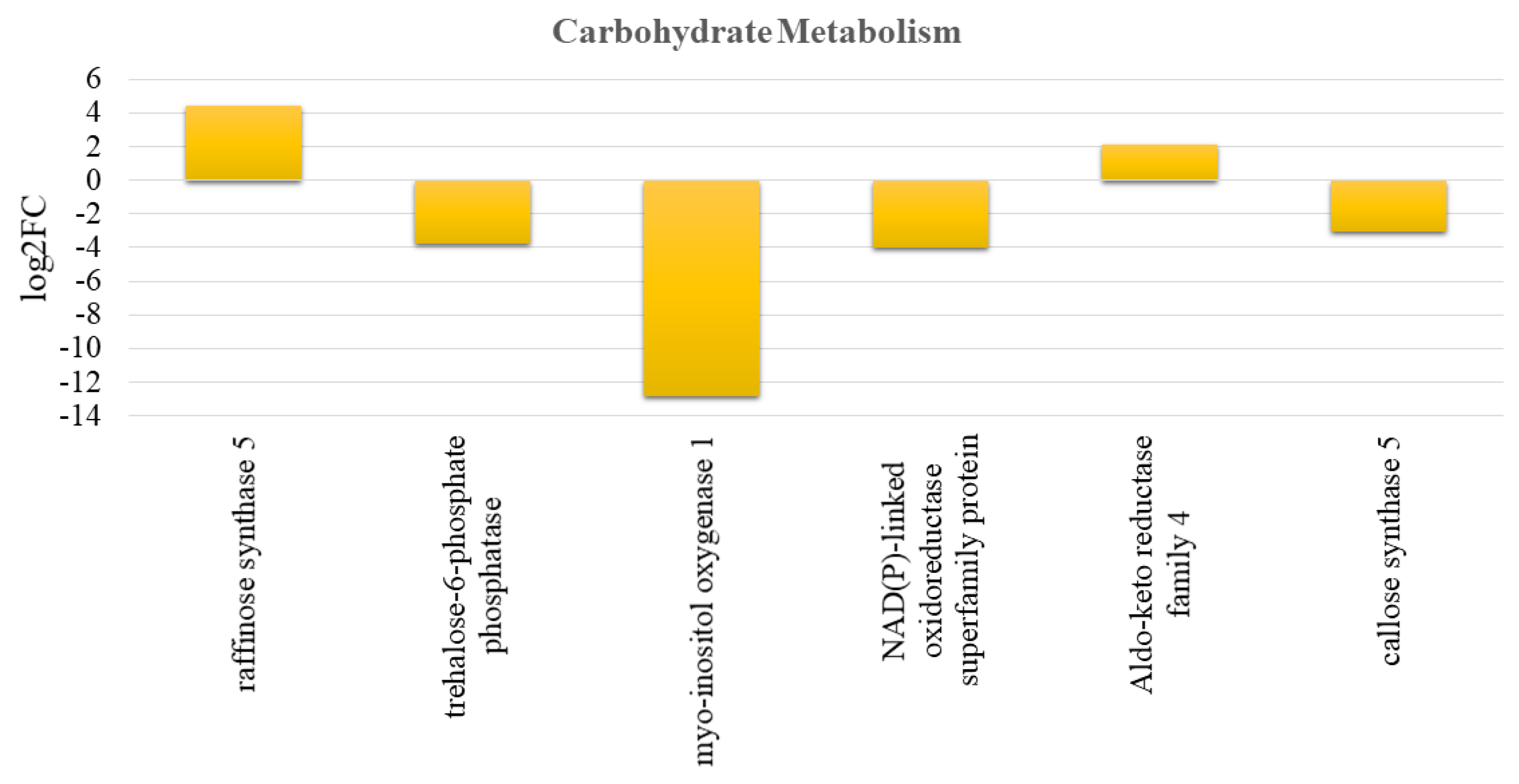
3.7. Effect of Crop Load on Carbohydrate Metabolism and Mobilization in Fruits of “OFF” vs. “ON” Branches
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crane, J.C.; Nelson, M.M. Effect of crop load, girdling and auxin application on alternate bearing of the pistachio. Am. Soc. Hort. Sci. J. 1972, 97, 337–339. [Google Scholar]
- Crane, J.C.; Iwakiri, B.T. Reconsideration of the cause of inflorescence bud abscission in Pistachio. HortScience 1987, 22, 1315–1316. [Google Scholar]
- Nzima, M.D.S.; Martin, G.C.; Nishijima, C. Leaf Development, Dry Matter Accumulation, and Distribution within Branches of Alternate-bearing ‘Kerman’ Pistachio Trees. J. Am. Soc. Hortic. Sci. 1997, 122, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Caruso, T.; Fabbri, A.; Giovannini, D. Inflorescence bud growth, development and abscission in shoots of bearing and disbudded ‘Bianca’ pistachio trees. J. Hortic. Sci. 1995, 70, 857–866. [Google Scholar] [CrossRef]
- Vemmos, S.; Pontikis, C.; Tolza-Marioli, A. Respiration rate and ethylene production in inflorescence buds of pistachio in relation to alternate bearing. Sci. Hortic. 1994, 57, 165–172. [Google Scholar] [CrossRef]
- Khezri, M.; Heerema, R.; Brar, G.; Ferguson, L. Alternate bearing in pistachio (Pistacia vera L.): A review. Trees 2020, 34, 855–868. [Google Scholar] [CrossRef]
- Vemmos, S.N.; Papagiannopoulou, A.; Coward, S. Effects of shoot girdling on photosynthetic capacity, leaf carbohydrate, and bud abscission in pistachio (Pistacia vera L.). Photosynthetica 2012, 50, 35–48. [Google Scholar] [CrossRef]
- Baninasab, B.; Rahemi, M.; Shariatmadari, H. Seasonal Changes in Mineral Content of Different Organs in the Alternate Bearing of Pistachio Trees. Commun. Soil Sci. Plant Anal. 2007, 38, 241–258. [Google Scholar] [CrossRef]
- Spann, T.M.; Beede, R.H.; Dejong, T.M. Seasonal carbohydrate storage and mobilization in bearing and non-bearing pistachio (Pistacia vera) trees. Tree Physiol. 2008, 28, 207–213. [Google Scholar] [CrossRef] [Green Version]
- Gündeşli, M.A.; Kafkas, S.; Zarifikhosroshahi, M.; Kafkas, N.E. Role of endogenous polyamines in the alternate bearing phenomenon in pistachio. Turk. J. Agric. For. 2019, 43, 265–274. [Google Scholar] [CrossRef]
- Benny, J.; Marra, F.P.; Giovino, A.; Balan, B.; Caruso, T.; Martinelli, F.; Marchese, A. Transcriptome Analysis of Pistacia vera Inflorescence Buds in Bearing and Non-Bearing Shoots Reveals the Molecular Mechanism Causing Premature Flower Bud Abscission. Genes 2020, 11, 851. [Google Scholar] [CrossRef] [PubMed]
- Yanofsky, M.F. Floral Meristems to Floral Organs: Genes Controlling Early Events in Arabidopsis Flower Development. Annu. Rev. Plant Biol. 1995, 46, 167–188. [Google Scholar] [CrossRef]
- Kobayashi, Y. A Pair of Related Genes with Antagonistic Roles in Mediating Flowering Signals. Science 1999, 286, 1960–1962. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Fambuena, N.; Mesejo, C.; González-Mas, M.C.; Primo-Millo, E.; Agustí, M.; Iglesias, D.J. Fruit load modulates flowering-related gene expression in buds of alternate-bearing ‘Moncada’ mandarin. Ann. Bot. 2012, 110, 1109–1118. [Google Scholar] [CrossRef] [Green Version]
- Boss, P.K.; Bastow, R.M.; Mylne, J.S.; Dean, C. Multiple Pathways in the Decision to Flower: Enabling, Promoting, and Resetting. Plant Cell 2004, 16, S18–S31. [Google Scholar] [CrossRef] [Green Version]
- Guitton, B.; Kelner, J.-J.; Velasco, R.; Gardiner, S.E.; Chagné, D.; Costes, E. Genetic control of biennial bearing in apple. J. Exp. Bot. 2011, 63, 131–149. [Google Scholar] [CrossRef] [Green Version]
- Figueroa, C.M.; Lunn, J.E. A Tale of Two Sugars: Trehalose 6-Phosphate and Sucrose. Plant Physiol. 2016, 172, 7–27. [Google Scholar] [CrossRef] [Green Version]
- Crepin, N.; Rolland, F. SnRK1 activation, signaling, and networking for energy homeostasis. Curr. Opin. Plant Biol. 2019, 51, 29–36. [Google Scholar] [CrossRef]
- Schepetilnikov, M.; Ryabova, L.A. Auxin Signaling in Regulation of Plant Translation Reinitiation. Front. Plant Sci. 2017, 8, 1014. [Google Scholar] [CrossRef] [Green Version]
- Dobrenel, T.; Caldana, C.; Hanson, J.; Robaglia, C.; Vincentz, M.; Veit, B.; Meyer, C. TOR signaling and nutrient sensing. Annu. Rev. Plant Biol. 2016, 67, 261–285. [Google Scholar] [CrossRef]
- Martinelli, F.; Marchese, A.; Balan, B.; Giovino, A.; Caruso, T.; Fretto, S.; Marra, F.P. RNA-Seq analysis to investigate alternate bearing mechanism in Pistacia vera L. Acta Hortic. 2018, 1229, 71–78. [Google Scholar] [CrossRef]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Godzik, A. Cd-hit: A fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 2006, 22, 1658–1659. [Google Scholar] [CrossRef] [Green Version]
- Simão, F.A.; Waterhouse, R.M.; Ioannidis, P.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 2015, 31, 3210–3212. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2009, 26, 139–140. [Google Scholar] [CrossRef] [Green Version]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Thimm, O.; Bläsing, O.; Gibon, Y.; Nagel, A.; Meyer, S.; Krüger, P.; Selbig, J.; Müller, L.A.; Rhee, S.Y.; Stitt, M. Mapman: A user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. Plant J. 2004, 37, 914–939. [Google Scholar] [CrossRef]
- Huang, D.; Sherman, B.T.; Tan, Q.; Collins, J.R.; Alvord, W.G.; Roayaei, J.; Stephens, R.; Baseler, M.W.; Lane, H.C.; Lempicki, R.A. The DAVID Gene Functional Classification Tool: A novel biological module-centric algorithm to functionally analyze large gene lists. Genome Biol. 2007, 8, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Marino, G.; Ferguson, L.; Caruso, T.; Roxas, A.A.; Marra, F. A carbon budget model to predict branch carbohydrate deficiencies as a function of water stress and crop load in pistachio (Pistacia vera L.). Acta Hortic. 2018, 1229, 183–188. [Google Scholar] [CrossRef]
- Marra, F.; Barone, E.; Motisi, A.; Sidari, M.; Caruso, T. Dry Matter Accumulation and Carbohydrate Content Within Branches Of Fruiting And Deblossomed Pistachio (Pistacia vera L.) Trees. Acta Hortic. 1998, 470, 331–339. [Google Scholar] [CrossRef]
- Hong, Y.-F.; Ho, T.-H.D.; Wu, C.-F.; Ho, S.-L.; Yeh, R.-H.; Lu, C.-A.; Chen, P.-W.; Yu, L.-C.; Chao, A.; Yu, S.-M. Convergent Starvation Signals and Hormone Crosstalk in Regulating Nutrient Mobilization upon Germination in Cereals. Plant Cell 2012, 24, 2857–2873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponnu, J.; Wahl, V.; Schmid, M. Trehalose-6-Phosphate: Connecting Plant Metabolism and Development. Front. Plant Sci. 2011, 2, 70. [Google Scholar] [CrossRef] [Green Version]
- Tsai, A.Y.-L.; Gazzarrini, S. Trehalose-6-phosphate and SnRK1 kinases in plant development and signaling: The emerging picture. Front. Plant Sci. 2014, 5, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wingler, A. Transitioning to the Next Phase: The Role of Sugar Signaling throughout the Plant Life Cycle. Plant Physiol. 2018, 176, 1075–1084. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-R.; Lee, K.-W.; Chen, C.-Y.; Hong, Y.-F.; Chen, J.-L.; Lu, C.-A.; Chen, K.-T.; Ho, T.-H.D.; Yu, S.-M. SnRK1A-Interacting Negative Regulators Modulate the Nutrient Starvation Signaling Sensor SnRK1 in Source-Sink Communication in Cereal Seedlings under Abiotic Stress. Plant Cell 2014, 26, 808–827. [Google Scholar] [CrossRef] [Green Version]
- Alford, S. myo-Inositol oxygenase is required for responses to low energy conditions in Arabidopsis thaliana. Front. Plant Sci. 2012, 3, 69. [Google Scholar] [CrossRef] [Green Version]
- Munir, S.; Mumtaz, M.A.; Ahiakpa, J.K.; Liu, G.; Chen, W.; Zhou, G.; Zheng, W.; Ye, Z.; Zhang, Y. Genome-wide analysis of Myo-inositol oxygenase gene family in tomato reveals their involvement in ascorbic acid accumulation. BMC Genom. 2020, 21, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Zhai, H.; Wang, F.; Si, Z.; Huo, J.; Xing, L.; An, Y.; He, S.; Liu, Q. Amyo-inositol-1-phosphate synthase gene, IbMIPS1, enhances salt and drought tolerance and stem nematode resistance in transgenic sweet potato. Plant Biotechnol. J. 2015, 14, 592–602. [Google Scholar] [CrossRef]
- Noctor, G. NAD(P) synthesis and pyridine nucleotide cycling in plants and their potential importance in stress conditions. J. Exp. Bot. 2006, 57, 1603–1620. [Google Scholar] [CrossRef] [Green Version]
- Potters, G.; Horemans, N.; Jansen, M.A.K. The cellular redox state in plant stress biology—A charging concept. Plant Physiol. Biochem. 2010, 48, 292–300. [Google Scholar] [CrossRef]
- Ohto, M.; Fischer, R.L.; Goldberg, R.B.; Nakamura, K.; Harada, J.J. Control of seed mass by APETALA2. Proc. Natl. Acad. Sci. USA 2005, 102, 3123–3128. [Google Scholar] [CrossRef] [Green Version]
- Cernac, A.; Benning, C. WRINKLED1 encodes an AP2/EREB domain protein involved in the control of storage compound biosynthesis in Arabidopsis. Plant J. 2004, 40, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Snell, P.; Grimberg, Å.; Carlsson, A.S.; Hofvander, P. WRINKLED1 Is Subject to Evolutionary Conserved Negative Autoregulation. Front. Plant Sci. 2019, 10, 387. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Liu, H.; Shanklin, J. Phosphorylation of WRINKLED1 by KIN10 Results in Its Proteasomal Degradation, Providing a Link between Energy Homeostasis and Lipid Biosynthesis. Plant Cell 2017, 29, 871–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, Z.; Keereetaweep, J.; Liu, H.; Feil, R.; Lunn, J.E.; Shanklin, J. Trehalose 6-Phosphate Positively Regulates Fatty Acid Synthesis by Stabilizing WRINKLED1. Plant Cell 2018, 30, 2616–2627. [Google Scholar] [CrossRef] [Green Version]
- Maeo, K.; Tokuda, T.; Ayame, A.; Mitsui, N.; Kawai, T.; Tsukagoshi, H.; Ishiguro, S.; Nakamura, K. An AP2-type transcription factor, WRINKLED1, of Arabidopsis thaliana binds to the AW-box sequence conserved among proximal upstream regions of genes involved in fatty acid synthesis. Plant J. 2009, 60, 476–487. [Google Scholar] [CrossRef]
- Athenstaedt, K.; Daum, G. The life cycle of neutral lipids: Synthesis, storage and degradation. Cell. Mol. Life Sci. 2006, 63, 1355–1369. [Google Scholar] [CrossRef]
- Smaczniak, C.; Immink, R.G.; Angenent, G.C.; Kaufmann, K. Developmental and evolutionary diversity of plant MADS-domain factors: Insights from recent studies. Development 2012, 139, 3081–3098. [Google Scholar] [CrossRef] [Green Version]
- Ito, T.; Ng, K.-H.; Lim, T.-S.; Yu, H.; Meyerowitz, E.M. The Homeotic Protein AGAMOUS Controls Late Stamen Development by Regulating a Jasmonate Biosynthetic Gene in Arabidopsis. Plant Cell 2007, 19, 3516–3529. [Google Scholar] [CrossRef] [Green Version]
- Raza, A.; Charagh, S.; Zahid, Z.; Mubarik, M.S.; Javed, R.; Siddiqui, M.H.; Hasanuzzaman, M. Jasmonic acid: A key frontier in conferring abiotic stress tolerance in plants. Plant Cell Rep. 2021, 40, 1513–1541. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Liu, W.; Hu, W.; Liu, G.; Wu, C.; Liu, W.; Zeng, H.; He, C.; Shi, H. Genome-wide analysis of autophagy-related genes in banana highlights MaATG8s in cell death and autophagy in immune response to Fusarium wilt. Plant Cell Rep. 2017, 36, 1237–1250. [Google Scholar] [CrossRef] [PubMed]
- Takeda, F.; Crane, J.C. Abscisic-Acid in Pistachio as Related to Inflorescence Bud Abscission. J. Am. Soc. Hortic 1980, 105, 573–576. [Google Scholar]
- Gündeşli, M.A. Endogenous gibberellins and abscisic acid-metabolites: Their role for during flower bud abscission and embryo development in pistachio. Turk. J. Agric. For. 2020, 44, 290–300. [Google Scholar] [CrossRef]
- Gündeşli, M.A.; Kafkas, S.; Guney, M.; Kafkas, N.E. Changes in endogenous auxin level during flower bud abscission process in Pistachio (Pistacia vera L.). Turk. J. Agric. For. 2020, 44, 71–82. [Google Scholar] [CrossRef]
- Gundesli, M.A.; Kafkas, S.; Guney, M.; Kafkas, N.E. Identification of the profile of endogenous cytokinin-like compounds during different plant growth stages and their effects on flower bud abscission in pistachio (Pistacia vera L.). Folia Hortic. 2020, 32, 21–35. [Google Scholar] [CrossRef]
- Reguera, M.; Peleg, Z.; Abdel-Tawab, Y.M.; Tumimbang, E.B.; Delatorre, C.A.; Blumwald, E. Stress-Induced Cytokinin Synthesis Increases Drought Tolerance through the Coordinated Regulation of Carbon and Nitrogen Assimilation in Rice. Plant Physiol. 2013, 163, 1609–1622. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-M.; Forney, C.; Bondada, B.; Leng, F.; Xie, Z.-S. The Molecular Regulation of Carbon Sink Strength in Grapevine (Vitis vinifera L.). Front. Plant Sci. 2021, 11, 606918. [Google Scholar] [CrossRef]
- Khezri, M.; Talaie, A.; Javanshah, A.; Hadavi, F. Effect of exogenous application of free polyamines on physiological disorders and yield of ‘Kaleh-Ghoochi’ pistachio shoots (Pistacia vera L.). Sci. Hortic. 2010, 125, 270–276. [Google Scholar] [CrossRef]
- Benny, J.; Marchese, A.; Giovino, A.; Marra, F.P.; Perrone, A.; Caruso, T.; Martinelli, F. Gaining Insight into Exclusive and Common Transcriptomic Features Linked to Drought and Salinity Responses across Fruit Tree Crops. Plants 2020, 9, 1059. [Google Scholar] [CrossRef]
- Giovino, A.; Bertolini, E.; Fileccia, V.; Al Hassan, M.; Labra, M.; Martinelli, F. Transcriptome analysis of Phoenix canariensis Chabaud in response to Rhynchophorus ferrugineus Olivier attacks. Front. Plant Sci. 2015, 8, 817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| June (%) | SE | July (%) | SE | September (%) | SE | |
|---|---|---|---|---|---|---|
| ON | 7.4 | ±4 | 54.7 | ±10 | 78.3 | ±11 |
| OFF | 0 | ±0 | 25.4 | ±3 | 31 | ±5 |
| Comparison | Differentially Expressed Genes | Up-Regulated | Down-Regulated |
|---|---|---|---|
| June “OFF” vs. June “ON” | 1536 | 702 | 834 |
| July “OFF” vs. July “ON” | 950 | 482 | 468 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benny, J.; Giovino, A.; Marra, F.P.; Balan, B.; Martinelli, F.; Caruso, T.; Marchese, A. Transcriptomic Analysis of the Pistacia vera (L.) Fruits Enable the Identification of Genes and Hormone-Related Gene Linked to Inflorescence Bud Abscission. Genes 2022, 13, 60. https://doi.org/10.3390/genes13010060
Benny J, Giovino A, Marra FP, Balan B, Martinelli F, Caruso T, Marchese A. Transcriptomic Analysis of the Pistacia vera (L.) Fruits Enable the Identification of Genes and Hormone-Related Gene Linked to Inflorescence Bud Abscission. Genes. 2022; 13(1):60. https://doi.org/10.3390/genes13010060
Chicago/Turabian StyleBenny, Jubina, Antonio Giovino, Francesco Paolo Marra, Bipin Balan, Federico Martinelli, Tiziano Caruso, and Annalisa Marchese. 2022. "Transcriptomic Analysis of the Pistacia vera (L.) Fruits Enable the Identification of Genes and Hormone-Related Gene Linked to Inflorescence Bud Abscission" Genes 13, no. 1: 60. https://doi.org/10.3390/genes13010060
APA StyleBenny, J., Giovino, A., Marra, F. P., Balan, B., Martinelli, F., Caruso, T., & Marchese, A. (2022). Transcriptomic Analysis of the Pistacia vera (L.) Fruits Enable the Identification of Genes and Hormone-Related Gene Linked to Inflorescence Bud Abscission. Genes, 13(1), 60. https://doi.org/10.3390/genes13010060








