Osteogenesis Imperfecta: Search for Mutations in Patients from the Republic of Bashkortostan (Russia)
Abstract
:1. Introduction
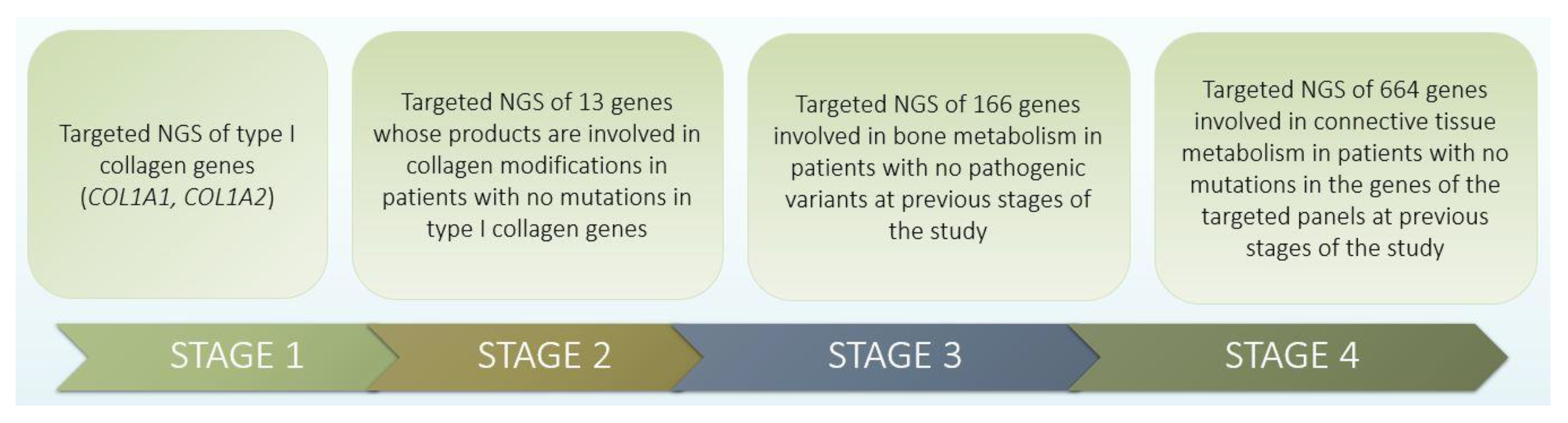
2. Materials and Methods
3. Results
3.1. Identification of Pathogenic Changes in OI Families
3.2. Clinical and Genetic Characteristics of Patients with Identified Mutations
4. Discussion
4.1. Mutations of the COL1A1 Gene
4.2. Mutations of the COL1A2 Gene
4.3. Mutations in the P3H1 Gene
4.4. Mutation of the IFITM5 Gene
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Forlino, A.; Marini, J.C. Osteogenesis imperfecta. Lancet 2016, 387, 1657–1671. [Google Scholar] [CrossRef]
- Van Dijk, F.; Sillence, D. Osteogenesis imperfecta: Clinical diagnosis, nomenclature and severity assessment. Am. J. Med. Genet. Part A 2014, 164, 1470–1481. [Google Scholar] [CrossRef] [Green Version]
- Zaripova, A.R.; Khusainova, R.I. Modern classification and molecular-genetic aspects of osteogenesis imperfecta. Vavilov J. Genet. Breed. 2020, 24, 219–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindahl, K.; Åström, E.; Rubin, C.-J.; Grigelioniene, G.; Malmgren, B.; Ljunggren, Ö.; Kindmark, A. Genetic epidemiology, prevalence, and genotype—Phenotype correlations in the Swedish population with osteogenesis imperfecta. Eur. J. Hum. Genet. 2015, 23, 1042–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marini, J.C.; Forlino, A.; Cabral, W.A.; Barnes, A.; Antonio, J.D.S.; Milgrom, S.; Hyland, J.C.; Körkkö, J.; Prockop, D.J.; De Paepe, A.; et al. Consortium for osteogenesis imperfecta mutations in the helical domain of type I collagen: Regions rich in lethal mutations align with collagen binding sites for integrins and proteoglycans. Hum. Mutat. 2007, 28, 209–221. [Google Scholar] [CrossRef]
- Available online: https://oi.gene.le.ac.uk/ (accessed on 9 December 2021).
- Skryabin, N.A.; Vasilyeva, O.Y.; Sivtsev, A.A.; Zhalsanova, I.Z.; Postrigan, A.E.; Minaicheva, L.I.; Agafonova, A.A.; Pe-trova, V.V.; Nazarenko, L.P.; Seitova, G.N.; et al. Analysis of the composition of COL1A1 and COL1A2 gene mutations using massive parallel sequencing in patients with osteogenesis imperfecta in the Tomsk region. Med. Genet. 2020, 19, 38–45. [Google Scholar] [CrossRef]
- Available online: http://www.openbioinformatics.org/annovar (accessed on 9 December 2021).
- Available online: www.hgvs.org (accessed on 9 December 2021).
- Available online: http://www.ncbi.nlm.nih.gov/RefSeq/ (accessed on 9 December 2021).
- Available online: http://gnomad.broadinsitute.org (accessed on 9 December 2021).
- Zhang, Z.-L.; Zhang, H.; Ke, Y.-H.; Yue, H.; Xiao, W.-J.; Yu, J.-B.; Gu, J.-M.; Hu, W.-W.; Wang, C.; He, J.-W.; et al. The identification of novel mutations in COL1A1, COL1A2, and LEPRE1 genes in Chinese patients with osteogenesis imperfecta. J. Bone Miner. Metab. 2012, 30, 69–77. [Google Scholar] [CrossRef]
- Venturi, G.; Tedeschi, E.; Mottes, M.; Valli, M.; Camilot, M.; Viglio, S.; Antoniazzi, F.; Tatò, L. Osteogenesis imperfecta: Clinical, biochemical and molecular findings. Clin. Genet. 2006, 70, 131–139. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Chuang, C.-K.; Su, Y.-N.; Chen, M.-R.; Chiu, H.-C.; Niu, D.-M.; Lin, S.-P. Genotype and phenotype analysis of Taiwanese patients with osteogenesis imperfecta. Orphanet J. Rare Dis. 2015, 10, 152. [Google Scholar] [CrossRef] [Green Version]
- Nicholls, A.C.; Oliver, J.; McCarron, S.; Winter, G.B.; Pope, F.M. Splice site mutation causing deletion of exon 21 sequences from the pro α 2(I) chain of type I collagen in a patient with severe dentinogenesis imperfecta but very mild osteogenesis imper-fecta. Hum. Mutat. 1996, 7, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, F.K.; De Leenheer, E.M.; Coucke, P.J.; Cremers, C.W.; Dhooge, I.J. Audiometric, surgical, and genetic findings in 15 ears of patients with osteogenesis imperfecta. Laryngoscope 2009, 119, 1171–1179. [Google Scholar] [CrossRef]
- Fuccio, A.; Iorio, M.; Amato, F.; Elce, A.; Ingino, R.; Filocamo, M.; Castaldo, G.; Salvatore, F.; Tomaiuolo, R. A Novel DHPLC-Based Procedure for the Analysis of COL1A1 and COL1A2 Mutations in Osteogenesis Imperfecta. J. Mol. Diagn. 2011, 13, 648–656. [Google Scholar] [CrossRef]
- Ries-Levavi, L.; Ish-Shalom, T.; Frydman, M.; Lev, D.; Cohen, S.; Barkai, G.; Goldman, B.; Byers, P.; Friedman, E. Genetic and biochemical analyses of Israeli osteogenesis imperfecta patients. Hum. Mutat. 2004, 23, 399–400. [Google Scholar] [CrossRef] [PubMed]
- Zhytnik, L.; Maasalu, K.; Pashenko, A.; Khmyzov, S.; Reimann, E.; Prans, E.; Kõks, S.; Märtson, A. COL1A1/2 Pathogenic Variants and Phenotype Characteristics in Ukrainian Osteogenesis Imperfecta Patients. Front. Genet. 2019, 10, 722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rolvien, T.; Stürznickel, J.; Schmidt, F.N.; Butscheidt, S.; Schmidt, T.; Busse, B.; Mundlos, S.; Schinke, T.; Kornak, U.; Amling, M.; et al. Comparison of Bone Microarchitecture Between Adult Osteogenesis Imperfecta and Early-Onset Osteoporosis. Calcif. Tissue Int. 2018, 103, 512–521. [Google Scholar] [CrossRef] [Green Version]
- Ries, L.; Frydman, M.; Barkai, G.; Goldman, B.; Friedman, E. Prenatal diagnosis of a novelCOL1A1 mutation in osteogenesis imperfecta type I carried through full term pregnancy. Prenat. Diagn. 2000, 20, 876–880. [Google Scholar] [CrossRef]
- Hartikka, H.; Kuurila, K.; Körkkö, J.; Kaitila, I.; Grénman, R.; Pynnönen, S.; Hyland, J.C.; Ala-Kokko, L. Lack of correlation between the type ofCOL1A1orCOL1A2mutation and hearing loss in osteogenesis imperfecta patients. Hum. Mutat. 2004, 24, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Gentile, F.V.; Zuntini, M.; Parra, A.; Battistelli, L.; Pandolfi, M.; Pals, G.; Sangiorgi, L. Validation of a quantitative PCR-high-resolution melting protocol for simultaneous screening ofCOL1A1andCOL1A2point mutations and large rearrangements: Application for diagnosis of osteogenesis imperfecta. Hum. Mutat. 2012, 33, 1697–1707. [Google Scholar] [CrossRef]
- Niramitmahapanya, S.; Anusornvongchai, T.; Pingsuthiwong, S.; Sarinnapakorn, V.; Deerochanawong, C.; Sunthornthepvarakul, T. Novel COL1A1 gene mutation (R1026X) of type I osteogenesis imperfecta: A first case report. J. Med. Assoc. Thail. Chotmaihet Thangphaet 2013, 96, 23682531. [Google Scholar]
- Kaneto, C.M.; Lima, P.S.; Zanette, D.L.; Prata, K.L.; Neto, J.M.P.; de Paula, F.J.; A Silva, W. COL1A1and miR-29b show lower expression levels during osteoblast differentiation of bone marrow stromal cells from Osteogenesis Imperfecta patients. BMC Med. Genet. 2014, 15, 45. [Google Scholar] [CrossRef] [Green Version]
- Duan, H.; Yan, Z.; Lu, Y.; Cheng, J.; Zhang, D.; Yuan, H.; Han, D. Identification of two recurrent mutations of COL1A1 gene in Chinese Van der Hoeve syndrome patients. Acta Oto-Laryngol. 2016, 136, 786–791. [Google Scholar] [CrossRef]
- Beck, K.; Chan, V.C.; Shenoy, N.; Kirkpatrick, A.; Ramshaw, J.A.M.; Brodsky, B. Destabilization of osteogenesis imperfecta collagen-like model peptides correlates with the identity of the residue replacing glycine. Proc. Natl. Acad. Sci. USA 2000, 97, 4273–4278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryan, M.A.; Cheng, H.; Brodsky, B. Sequence environment of mutation affects stability and folding in collagen model peptides of osteogenesis imperfecta. Biopolymer 2011, 96, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Makareeva, E.; Mertz, E.L.; Kuznetsova, N.V.; Sutter, M.B.; DeRidder, A.M.; Cabral, W.A.; Barnes, A.; McBride, D.J.; Marini, J.C.; Leikin, S. Structural Heterogeneity of Type I Collagen Triple Helix and Its Role in Osteogenesis Imperfecta. J. Biol. Chem. 2008, 283, 4787–4798. [Google Scholar] [CrossRef] [Green Version]
- Persikov, A.V.; Pillitteri, R.J.; Amin, P.; Schwarze, U.; Byers, P.H.; Brodsky, B. Stability related bias in residues replacing glycines within the collagen triple helix (Gly-Xaa-Yaa) in inherited connective tissue disorders. Hum. Mutat. 2004, 24, 330–337. [Google Scholar] [CrossRef]
- Xiao, J.; Cheng, H.; Silva, T.; Baum, J.; Brodsky, B. Osteogenesis Imperfecta Missense Mutations in Collagen: Structural Consequences of a Glycine to Alanine Replacement at a Highly Charged Site. Biochemistry 2011, 50, 10771–10780. [Google Scholar] [CrossRef] [Green Version]
- Körkkö, J.; Ala-Kokko, L.; De Paepe, A.; Nuytinck, L.; Earley, J.; Prockop, D.J. Analysis of the COL1A1 and COL1A2 Genes by PCR Amplification and Scanning by Conformation-Sensitive Gel Electrophoresis Identifies Only COL1A1 Mutations in 15 Patients with Osteogenesis Imperfecta Type I: Identification of Common Sequences of Null-Allele Mutations. Am. J. Hum. Genet. 1998, 62, 98–110. [Google Scholar] [CrossRef] [Green Version]
- Benusiené, E.; Kucinskas, V. COL1A1 mutation analysis in Lithuanian patients with osteogenesis imperfecta. J. Appl. Genet. 2003, 44, 95–102. [Google Scholar] [PubMed]
- Roschger, P.; Fratzl-Zelman, N.; Misof, B.M.; Glorieux, F.H.; Klaushofer, K.; Rauch, F. Evidence that Abnormal High Bone Mineralization in Growing Children with Osteogenesis Imperfecta is not Associated with Specific Collagen Mutations. Calcif. Tissue Int. 2008, 82, 263–270. [Google Scholar] [CrossRef]
- Van Dijk, F.; Cobben, J.; Kariminejad, A.; Maugeri, A.; Nikkels, P.; van Rijn, R.; Pals, G. Osteogenesis Imperfecta: A Review with Clinical Examples. Mol. Syndr. 2011, 2, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Lund, A.M.; Skovby, F.; Schwartz, M. Serine for glycine substitutions in the C-terminal third of the α 1(I) chain of collagen I in five patients with nonlethal osteogenesis imperfecta. Hum. Mutat. 1997, 9, 378–382. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, D.-L.; Hu, J.-Y.; Liao, Y.-H.; Yang, Z.; Liang, Q.; Wang, L.-T. Gene mutation analysis of a Chinese family with osteogenesis imperfecta. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2006, 23, 192–194. [Google Scholar]
- Lee, K.-S.; Song, H.-R.; Cho, T.-J.; Kim, H.J.; Lee, T.-M.; Jin, H.-S.; Park, H.-Y.; Kang, S.; Jung, S.-C.; Koo, S.K. Mutational spectrum of type I collagen genes in Korean patients with osteogenesis imperfecta. Hum. Mutat. 2006, 27, 599. [Google Scholar] [CrossRef]
- Nawawi, N.M.; Selveindran, N.M.; Rasat, R.; Chow, Y.P.; Latiff, Z.A.; Zakaria, S.Z.S.; Jamal, R.; Murad, N.A.A.; Aziz, B.B.A. Genotype-phenotype correlation among Malaysian patients with osteogenesis imperfecta. Clin. Chim. Acta 2018, 484, 141–147. [Google Scholar] [CrossRef]
- Kloen, P.; Donders, J.C.; Eekhoff, E.M.W.; Hamdy, R.C. Pauwels Osteotomy for Femoral Neck Nonunion in Two Adult Siblings with Osteogenesis Imperfecta. Hip Pelvis 2018, 30, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Duy, B.H.; Zhytnik, L.; Maasalu, K.; Kändla, I.; Prans, E.; Reimann, E.; Märtson, A.; Kõks, S. Mutation analysis of the COL1A1 and COL1A2 genes in Vietnamese patients with osteogenesis imperfecta. Hum. Genom. 2016, 10, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Cui, Y.; Zhou, X.; Han, J. Development of a High-Throughput Resequencing Array for the Detection of Pathogenic Mutations in Osteogenesis Imperfecta. PLoS ONE 2015, 10, e0119553. [Google Scholar] [CrossRef] [PubMed]
- Forlino, A.; D’Amato, E.; Valli, M.; Camera, G.; Hopkins, E.; Marini, J.C.; Cetta, G.; Coviello, D. Phenotypic Comparison of an Osteogenesis Imperfecta Type IV Proband with a de Novoα2(I) Gly922 → Ser Substitution in Type I Collagen and an Unrelated Patient with an Identical Mutation. Biochem. Mol. Med. 1997, 62, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Barkova, E.; Mohan, U.; Chitayat, D.; Keating, S.; Toi, A.; Frank, J.; Frank, R.; Tomlinson, G.; Glanc, P. Fetal skeletal dysplasias in a tertiary care center: Radiology, pathology, and molecular analysis of 112 cases. Clin. Genet. 2014, 87, 330–337. [Google Scholar] [CrossRef]
- Marini, J.C.; Lewis, M.B.; Wang, Q.; Chen, K.J.; Orrison, B.M. Serine for glycine substitutions in type I collagen in two cases of type IV osteogenesis imperfecta (OI). Additional evidence for a regional model of OI pathophysiology. J. Biol. Chem. 1993, 268, 2667–2673. [Google Scholar] [CrossRef]
- Sztrolovics, R.; Glorieux, F.; Van Der Rest, M.; Roughley, P. Identification of type I collagen gene (COL1A2) mutations in nonlethal osteogenesis imperfecta. Hum. Mol. Genet. 1993, 2, 1319–1321. [Google Scholar] [CrossRef]
- Stephen, J.; Girisha, K.M.; Dalal, A.; Shukla, A.; Shah, H.; Srivastava, P.; Kornak, U.; Phadke, S.R. Mutations in patients with osteogenesis imperfecta from consanguineous Indian families. Eur. J. Med. Genet. 2015, 58, 21–27. [Google Scholar] [CrossRef]
- Ben Amor, I.M.; Roughley, P.; Glorieux, F.H.; Rauch, F. Skeletal clinical characteristics of osteogenesis imperfecta caused by haploinsufficiency mutations in COL1A1. J. Bone Miner. Res. 2013, 28, 2001–2007. [Google Scholar] [CrossRef]
- Baldridge, D.; Schwarze, U.; Morello, R.; Lennington, J.; Bertin, T.K.; Pace, J.M.; Pepin, M.G.; Weis, M.; Eyre, D.R.; Walsh, J.; et al. CRTAP and LEPRE1 mutations in recessive osteogenesis imperfecta. Hum. Mutat. 2008, 29, 1435–1442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myllyharju, J. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 2004, 20, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Cabral, W.A.; Chang, W.; Barnes, A.M.; Weis, M.; Scott, M.A.; Leikin, S.; Makareeva, E.; Kuznetsova, N.V.; Rosenbaum, K.N.; Tifft, C.J.; et al. Prolyl 3-hydroxylase 1 deficiency causes a recessive metabolic bone disorder resembling lethal/severe osteogenesis imperfecta. Nat. Genet. 2007, 39, 359–365. [Google Scholar] [CrossRef]
- Pepin, M.G.; Schwarze, U.; Singh, V.; Romana, M.; Jones-LeCointe, A.; Byers, P.H. Allelic background of LEPRE1 mutations that cause recessive forms of osteogenesis imperfecta in different populations. Mol. Genet. Genom. Med. 2013, 1, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, S.; McInerney-Leo, A.M.; McKenzie, F.A.; Baynam, G.; Broley, S.; Cavan, B.; Munns, C.F.; Pruijs, J.E.; Sillence, D.; Terhal, P.A.; et al. The IFITM5 mutation c.-14C> T results in an elongated transcript expressed in human bone; and causes varying phenotypic severity of osteogenesis imperfecta type V. BMC Musculoskelet Disord. 2014, 15, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semler, O.; Garbes, L.; Keupp, K.; Swan, D.; Zimmermann, K.; Becker, J.; Iden, S.; Wirth, B.; Eysel, P.; Koerber, F.; et al. A Mutation in the 5′-UTR of IFITM5 Creates an In-Frame Start Codon and Causes Autosomal-Dominant Osteogenesis Imperfecta Type V with Hyperplastic Callus. Am. J. Hum. Genet. 2012, 91, 349–357. [Google Scholar] [CrossRef] [Green Version]
- Cho, T.-J.; Lee, K.-E.; Lee, S.-K.; Song, S.J.; Kim, K.J.; Jeon, D.; Lee, G.; Kim, H.-N.; Lee, H.R.; Eom, H.-H.; et al. A Single Recurrent Mutation in the 5′-UTR of IFITM5 Causes Osteogenesis Imperfecta Type V. Am. J. Hum. Genet. 2012, 91, 343–348. [Google Scholar] [CrossRef] [Green Version]
- Grover, M.; Campeau, P.M.; Lietman, C.D.; Lu, J.T.; Gibbs, R.A.; Schlesinger, A.E.; Lee, B.H. Osteogenesis imperfecta without features of type V caused by a mutation in the IFITM5 gene. J. Bone Miner. Res. 2013, 28, 2333–2337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takagi, M.; Sato, S.; Hara, K.; Tani, C.; Miyazaki, O.; Nishimura, G.; Hasegawa, T. A recurrent mutation in the 5′-UTR of IFITM5 causes osteogenesis imperfecta type V. Am. J. Med. Genet. Part A 2013, 161, 1980–1982. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, J.R.; Lietman, C.; Grover, M.; Lu, J.T.; Nagamani, S.C.; Dawson, B.C.; Baldridge, D.M.; Bainbridge, M.N.; Cohn, D.H.; Blazo, M.; et al. Phenotypic Variability of Osteogenesis Imperfecta Type V Caused by an IFITM 5 Mutation. J. Bone Miner. Res. 2013, 28, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
| Family | Patient | Mutation | Inheritance Type | OI Type | Sex | Age | Blue Sclera | Fractures |
|---|---|---|---|---|---|---|---|---|
| COL1A1 | ||||||||
| Family 1 | Patient 1 | c.358C>T, p. Arg120* | de novo | 1 | F | 68 | + | 18 |
| Family 2 | Patient 2 | c.375dupC, p. Ala126fs | de novo | 1 | F | 54 | + | 11 |
| Family 3 | Patient 3 | c.407dupG, p.Gly136fs | de novo | 1 | F | 5 | + | 3 |
| Family 4 | Patient 4 | c.579delT, p.Gly194fs | de novo | 1 | M | 19 | + | 33 |
| Family 5 | Patient 5 | c.579delT, p.Gly194fs | AD * | 1 | M | 26 | + | 15 |
| Patient 6 | 1 | F | 59 | − | 3 | |||
| Family 6 | Patient 7 | c.658C>T, p.Arg220* | no parents’ DNA | 1 | M | 9 | + | 11 |
| Family 7 | Patient 8 | c.858+1G>A | no parents’ DNA | 1 | M | 32 | + | 15 |
| Family 8 | Patient 9 | c.967G>T, p.Gly323* | AD | 1 | F | 30 | + | 8 |
| Patient 10 | 1 | F | 52 | + | 20 | |||
| Family 9 | Patient 11 | c.1081C>T, p.Arg361* | AD | 3 | M | 29 | + | 19 |
| Patient 12 | 3 | M | 52 | − | − | |||
| Family 10 | Patient 13 | c.1243C>T, p.Arg415* | de novo | 1 | F | 28 | + | 10 |
| Family 11 | Patient 14 | c.2444delG, p.Gly815fs | no parents’ DNA | 1 | F | 27 | + | 13 |
| Family 12 | Patient 15 | c.2461G>A, p.Gly821Ser | no parents’ DNA | 3 | F | 28 | + | 15 |
| Family 13 | Patient 16 | c.2569G>T, p.Gly857Cys | no parents’ DNA | 3 | F | 12 | + | 50 |
| Family 14 | Patient 17 | c.2869C>T, p.Gln957* | AD | 1 | F | 17 | + | 14 |
| Patient 18 | 1 | M | 46 | − | − | |||
| Family 15 | Patient 19 | c.3076C>T, p.Arg1026* | de novo | 1 | M | 9 | + | 7 |
| Family 16 | Patient 20 | c.3076C>T, p.Arg1026* | no parents’ DNA | 1 | M | 17 | + | 12 |
| Family 17 | Patient 21 | c.3792delG, p.Met1264fs | de novo | 4 | M | 6 | − | 4 |
| Family 18 | Patient 22 | c.1354-12G>A | AD | 1 | M | 10 | + | 6 |
| Patient 23 | 1 | F | 36 | − | 3 | |||
| COL1A2 | ||||||||
| Family 1 | Patient 24 | c.647G>A, p. Arg216His | no parents’ DNA | 4 | M | 12 | − | 3 |
| Family 2 | Patient 25 | c.874G>A, p. Gly292Ser | no parents’ DNA | 1 | M | 20 | + | 7 |
| Family 3 | Patient 26 | c.1826G>A, p. Arg609Gln | AD | 1 | M | 11 | + | 5 |
| Patient 27 | 1 | M | 41 | − | − | |||
| Family 4 | Patient 28 | c.1897_1902dupGCTGGT, p. Ala633_Gly634dup | no parents’ DNA | 1 | F | 20 | + | 5 |
| Family 5 | Patient 29 | c.2341G>C, p. Gly781Arg | de novo | 1 | M | 4 | + | 5 |
| Family 6 | Patient 30 | c.2756G>A, p. Gly919Asp | AD | 3 | M | 23 | + | 10 |
| Patient 31 | 3 | F | 52 | − | − | |||
| Family 7 | Patient32 | c.2971G>C, p. Gly991Arg | AD | 4 | M | 34 | − | 7 |
| Patient 33 | 4 | M | 58 | − | 5 | |||
| Family 8 | Patient 34 | c.3034G>A, p. Gly1012Ser | AD | 3 | F | 6 | + | 5 |
| Patient 35 | 3 | F | 32 | + | 7 | |||
| Family 9 | Patient 36 | c.3277G>A, p. Gly1093Ser | de novo | 1 | F | 14 | + | 90 |
| Family 10 | Patient 37 | c.3977A>G, p. Lys1326Arg | AD | 4 | M | 4 | − | 4 |
| Patient 38 | 4 | M | 45 | − | − | |||
| Family | Patient | Mutation | Inheritance Type | OI Type | Sex | Age | Blue Sclera | Fractures |
|---|---|---|---|---|---|---|---|---|
| P3H1 mutations | ||||||||
| Family 1 | Patient 39 | c.1051G>T, p. Glu351* | de novo | 3 | M | 24 | + | 12 |
| IFITM5 mutations | ||||||||
| Family 2 | Patient 40 | c.-14C>T | de novo | 5 | F | 27 | + | 15 |
| Family 3 | Patient 41 | c.-14C>T | de novo | 5 | F | 26 | + | 50 |
| Family 4 | Patient 42 | c.-14C>T | de novo | 5 | M | 10 | + | 10 |
| No. | Patient | Mutation | Inheritance Type | Age | Sex | Blue Sclera | Number of Fractures | Disorders |
|---|---|---|---|---|---|---|---|---|
| 1 | Patient 43 | ALOX12B: c.526G>A, p. Glu176Lys | de novo | 12 | M | - | 4 | Hyperkeratosis, metatarsal and metacarpal fractures |
| 2 | Patient 44 | PLEKHM1: c.2902-9C>T | de novo | 15 | F | + | >9 | Sensorineural hearing loss, decreased Bone mineral density (BMD), myopia, arthropathy |
| 3 | Patient 45 | ERCC4: c.2395C>T, p. Arg799Trp | de novo | 5 | F | + | 1 | Simple farsighted astigmatism, mild anemia |
| 4 | Patient 46 | ARSB: c.454C>T,p. Arg152Trp | de novo | 12 | M | + | 18 | He started talking late. The gait is limping. There are no visible deformations. |
| 5 | Patient 47 | PTH1R: c.342C>A, p. His114Gln | de novo | 8 | M | + | >5 | BMD decrease, kyphoscoliotic deformity of the chest, fractures of the metacarpal bones |
| 6 | Patient 48 | CLCN7: c.141+4A>C | de novo | 31 | M | + | >11 | Decrease in BMD, facial phenotypes (frontal bumps), fractures of the bones of the hand, foot |
| 7 | Patient 49 | TGFB1: c.945G>C, p. Lys315Asn | de novo | 29 | F | + | >17 | Short stature, varus deformity of the limbs, sabre-shaped deformity of the shins, kyphoscoliotic deformity of the chest, curvature of the left forearm, high palate. |
| Family /Patient | Mutation 1 | Mutation 2 | Inheritance Type | Age | OI Type | Sex | Blue Sclera | Fractures | Disorders | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Patient 17 | c.2869C>T, p. Gln957* in COL1A1 gene | c.1197+5G>A in COL1A2 gene | AD * | 17 | 1 | F | + | 14 | Knee joint deformities, saber-shaped deformity of the hips |
| 2 | Patient 4 | c.579delT, p. Gly194fs in COL1A1 gene | c.1197+5G>A in COL1A2 gene | de novo | 19 | 1 | M | + | 33 | Hypermobility of joints, deformity of the elbow joint |
| 3 | Patient 33 | c.2971G>C, p. Gly991Arg in COL1A2 gene | c.212G>C, p.Ser71Thr in FGF23 gene | AD | 58 | 4 | M | − | 5 | Short stature |
| 4 | Patient 40 | c.-14C>T in IFITM5 gene | c.1903C>T, p. Arg635* in LAMB3 gene | de novo | 27 | 5 | F | − | 15 | Kyphoscoliotic deformity of the spine and chest, platyspondylia, hypermobility of joints, arched curvature of the bones of both legs |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nadyrshina, D.; Zaripova, A.; Tyurin, A.; Minniakhmetov, I.; Zakharova, E.; Khusainova, R. Osteogenesis Imperfecta: Search for Mutations in Patients from the Republic of Bashkortostan (Russia). Genes 2022, 13, 124. https://doi.org/10.3390/genes13010124
Nadyrshina D, Zaripova A, Tyurin A, Minniakhmetov I, Zakharova E, Khusainova R. Osteogenesis Imperfecta: Search for Mutations in Patients from the Republic of Bashkortostan (Russia). Genes. 2022; 13(1):124. https://doi.org/10.3390/genes13010124
Chicago/Turabian StyleNadyrshina, Dina, Aliya Zaripova, Anton Tyurin, Ildar Minniakhmetov, Ekaterina Zakharova, and Rita Khusainova. 2022. "Osteogenesis Imperfecta: Search for Mutations in Patients from the Republic of Bashkortostan (Russia)" Genes 13, no. 1: 124. https://doi.org/10.3390/genes13010124
APA StyleNadyrshina, D., Zaripova, A., Tyurin, A., Minniakhmetov, I., Zakharova, E., & Khusainova, R. (2022). Osteogenesis Imperfecta: Search for Mutations in Patients from the Republic of Bashkortostan (Russia). Genes, 13(1), 124. https://doi.org/10.3390/genes13010124






